Abstract
We hypothesize that nitroglycerin (NTG) causes direct oxidation of multiple cellular sulfhydryl (SH) proteins and that manipulation of SH redox status affects NTG tolerance. In LLC-PK1 cells, we found that nitrate tolerance, as indicated by cGMP accumulation toward NTG, was accompanied by increased protein [35S]cysteine incorporation, significant S-glutathionylation of multiple proteins, and decreased metabolic activity of several SH-sensitive enzymes, including creatine kinase, xanthine oxidoreductase, and glutaredoxin (GRX). Cells overexpressing GRX exhibited reduced cellular protein S-glutathionylation (PSSG) and absence of NTG tolerance, whereas those with silenced GRX showed increased extent of NTG-induced tolerance. Incubation of LLC-PK1 cells with oxidized glutathione led to several major observations associated with nitrate tolerance, namely, reduced cGMP accumulation, PSSG formation, superoxide accumulation, and the attenuation of these events by vitamin C. Aortic S-glutathionylated proteins increased approximately 3-fold in rats made tolerant in vivo to NTG and showed significant negative correlation with vascular responsiveness ex vivo. NTG incubation in EA.hy926 endothelial cells and LLC-PK1 cells led to increased S-glutathionylation and activity of p21ras, a known mediator of cellular signaling. These results indicate that the hallmark events of NTG tolerance, such as reduced bioactivation and redox signaling, are associated with GRX-dependent protein deglutathionylation.
Pharmacological tolerance toward organic nitrates such as nitroglycerin
(NTG) has been known for over a century. This phenomenon is associated with an
array of events, including immediate consequences such as reduction in
vascular response and metabolic bioactivation, as well as downstream events
such as enhanced vasoconstrictiveness, withdrawal rebound, and gene regulation
(for reviews, see Fung, 2004;
Münzel et al., 2005).
Several major hypotheses have been advanced to explain this phenomenon,
including sulfhydryl depletion (Needleman
and Johnson, 1973), superoxide
(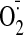 )
generation leading to oxidative stress
(Münzel et al., 1995),
and inactivation of mitochondrial aldehyde dehydrogenase (ALDH2)
(Chen et al., 2002). Recently,
Wenzel et al. (2007) observed
that “mitochondrial oxidative stress (especially superoxide and
peroxynitrite) in response to organic nitrate treatment may inactivate ALDH2,
thereby leading to nitrate tolerance.” These investigators therefore
viewed oxidative stress in nitrate tolerance to precede ALDH2
inactivation.
)
generation leading to oxidative stress
(Münzel et al., 1995),
and inactivation of mitochondrial aldehyde dehydrogenase (ALDH2)
(Chen et al., 2002). Recently,
Wenzel et al. (2007) observed
that “mitochondrial oxidative stress (especially superoxide and
peroxynitrite) in response to organic nitrate treatment may inactivate ALDH2,
thereby leading to nitrate tolerance.” These investigators therefore
viewed oxidative stress in nitrate tolerance to precede ALDH2
inactivation.
We showed recently (Tsou et al.,
2008) that
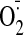 generation, particularly through the NADPH oxidase (NOX) system, cannot
account for nitrate tolerance initiation. Therefore, in our view, the
initiating biochemical reactions that lead to NTG-induced oxidative stress are
still undefined. We have shown previously that NTG reacts nonselectively with
thiol compounds, producing its dinitrate metabolites
(Chong and Fung, 1989). NTG
also oxidizes the SH groups of ALDH2
(Wenzel et al., 2007) and
glutathione transferase (Lee and Fung,
2003). Thus, NTG-mediated protein SH oxidation may not be
selective. Recently, protein S-glutathionylation (PSSG), an oxidative
reaction between cellular proteins and glutathione (GSH), has been identified
as an important mechanism in redox regulation under physiological conditions
(Klatt and Lamas, 2000). For
example, S-glutathionylation of p21ras at cysteine 118 has
been shown to mediate its activation and downstream signaling in endothelial
cells (Clavreul et al.,
2006a). Deglutathionylation is efficiently catalyzed by the
thiol-disulfide oxidoreductase glutaredoxins (GRX)
(Fernandes and Holmgren,
2004).
generation, particularly through the NADPH oxidase (NOX) system, cannot
account for nitrate tolerance initiation. Therefore, in our view, the
initiating biochemical reactions that lead to NTG-induced oxidative stress are
still undefined. We have shown previously that NTG reacts nonselectively with
thiol compounds, producing its dinitrate metabolites
(Chong and Fung, 1989). NTG
also oxidizes the SH groups of ALDH2
(Wenzel et al., 2007) and
glutathione transferase (Lee and Fung,
2003). Thus, NTG-mediated protein SH oxidation may not be
selective. Recently, protein S-glutathionylation (PSSG), an oxidative
reaction between cellular proteins and glutathione (GSH), has been identified
as an important mechanism in redox regulation under physiological conditions
(Klatt and Lamas, 2000). For
example, S-glutathionylation of p21ras at cysteine 118 has
been shown to mediate its activation and downstream signaling in endothelial
cells (Clavreul et al.,
2006a). Deglutathionylation is efficiently catalyzed by the
thiol-disulfide oxidoreductase glutaredoxins (GRX)
(Fernandes and Holmgren,
2004).
Based on these considerations, we hypothesize that nitrate tolerance may be
initiated by nonselective SH oxidation of multiple cellular proteins [protein
sulfhydryls (PSH)] that contain sensitive thiol groups. Depending on the
protein affected, different events will result. Thus, SH oxidation of ALDH2
and other metabolizing enzymes for NTG will lead to reduced bioactivation of
the organic nitrate to NO (Beretta et al.,
2008), whereas oxidation of the xanthine oxidoreductase (XOR)
system leads to the activation of xanthine oxidase (XO), resulting in
increased
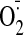 accumulation (Tsou et al.,
2008). Glutathionylation of SH-sensitive signaling proteins such
as p21ras, we surmise, may account for the downstream effects of
nitrate tolerance, such as withdrawal rebound and vascular gene
regulation.
accumulation (Tsou et al.,
2008). Glutathionylation of SH-sensitive signaling proteins such
as p21ras, we surmise, may account for the downstream effects of
nitrate tolerance, such as withdrawal rebound and vascular gene
regulation.
The objective of this study, therefore, was to examine the relevance of multiple protein S-glutathionylation in nitrate tolerance. We first examined whether multiple protein S-oxidation occurred under in vitro and in vivo nitrate tolerance conditions. Next, we studied whether altering the extent of S-glutathionylation would affect the extent of NTG-induced tolerance. This alteration was achieved by manipulating the expression of the cellular GRX system or by external addition of oxidized glutathione (GSSG), an agent known to promote S-glutathionylation.
Materials and Methods
Detailed materials and methods approximately cell culture, cell tolerance induction, protein [35S]cysteine incorporation, Western blots, enzyme activity assays, and superoxide measurement can be found in the Supplemental Data at http://jpet.aspetjournals.org/.
Overexpression of GRX. When LLC-PK1 cells became approximately 50% confluent, they were passaged and grown in media without antibiotics before the transfection. Cells were transfected with 5 μg of either the pcDNA3-His-GRX vector (kindly donated by Dr. Yong J. Lee, University of Pittsburgh, Pittsburgh, PA) or a sham, empty pcDNA3.1(+) expression vector using Lipofectamine reagent (Invitrogen, Carlsbad, CA), which was diluted with Opti-MEM I reduced serum media (Invitrogen). Five micrograms of vector was diluted in 300 μl of Opti-MEM I. The Lipofectamine reagent mixture and vector DNA mixture were combined and incubated at room temperature for 45 min to allow the complex to form. After 45 min, an equal volume of Opti-MEM I was added to the complex mixture. Diluted complex mixture (1.2 ml) and 1.2 ml of blank Ham's F-12 media were then added to each flask of cells. The cells were incubated at 37°C with 5% CO2 for 20 to 24 h. The transfection reaction was stopped by adding 2.4 ml of Ham's F-12 media with twice the normal concentration of FBS (30%) without antibiotics. Cells were harvested 24 h after terminating the transfection reaction. At the end of the experiment, cells were lysed using a Triton X lysis buffer containing 2% Triton X and 1:10 dilution of protease inhibitor cocktail in distilled water. The supernatant fractions were used for Western blotting and activity assay.
Silencing of GRX Expression. RNA silencing technique was used to suppress GRX expression in LLC-PK1 cells. GRX siRNA (5′-GCUCCUCAGCCAAUUGCCCUUCAAA-3′) was designed and manufactured by Invitrogen. A scrambled sequence containing the same GC content as the GRX siRNA (5′-GCUACUCCGUAACGUUCCCUCCAAA-3′) was used as negative control. LLC-PK1 cells from a 50% confluent T75 flask were plated in 6 T25 flasks with F-12 medium containing 15% FBS without antibiotics. They were grown overnight in 37°C, 5% CO2 incubator until ∼20 to 50% confluent. On the day of transfection, the siRNAs were diluted in Opti-MEM I reduced serum medium to make 1 to 3 μM (250–750 pmol). For each flask, Lipofectamine 2000 (final concentration, 2 μg/ml) was diluted in Opti-MEM I reduced serum medium and incubated for 5 min at room temperature. After the incubation, it was mixed with the diluted oligonucleotides for 20 min at room temperature to allow complex formation to occur. The complexes were then added to each flask containing 4 ml of antibiotic-free F-12 medium containing 15% FBS. The cells were allowed to grow for 24 to 72 h and harvested for GRX activity assay at 24, 48, and 72 h after transfection. The cells treated with 3 μM siRNA for 24 h were found to have the best inhibitory effect, and this condition was chosen for subsequent tolerance studies.
In Vivo Tolerance Induction. All procedures were performed according to protocols approved by the SUNY Institutional Animal Care and Use Committee. Mini osmotic pumps (2ML1) primed with NTG solution (5 mg/ml) were implanted on the back of Sprague-Dawley rats (200–225 g) slightly posterior to the scapulae to achieve subcutaneous delivery of the drug. According to the manufacturer, the flow rate was set at 10 μl/h, and the amount of NTG released was anticipated to be 50 μg/h. At 72 h after implantation, each animal was anesthetized with a mixture of ketamine (90 mg/kg) and xylazine (9 mg/kg), and the thoracic aorta was collected for vascular relaxation studies. The rest of the aorta, along with the abdominal artery, was saved for PSSG enzyme-linked immunosorbent assay (ELISA). Where applicable, cellular GSH and GSSG concentrations were measured by high-performance liquid chromatography (Ayala-Fierro and Carter, 2000).
Protein S-Glutathionylation ELISA. Frozen rat blood vessels or cell lysates were homogenized in lysis buffer containing 2% Triton X and protease inhibitors. After centrifugation, the supernatant was collected for protein assay and PSSG determination by ELISA. Lysates were diluted to 10 μg/ml with phosphate-buffered and were incubated in 96-well plates (100 μl/well) overnight at 4°C. After washing with phosphate-buffered saline/Tween 20, the plate was incubated with antibodies against S-glutathionylated proteins (diluted 1:1000) for 2 h at room temperature. The plate was washed and incubated with anti-mouse IgG-alkaline phosphatase conjugate (1: 500) for 1 h at room temperature. The absorbance was monitored for 10 min at 405 nm after adding the substrate p-nitrophenyl phosphate (SIGMAFAST p-nitrophenyl phosphate tablets, final concentration, 1 mg/ml; Sigma-Aldrich, St. Louis, MO). The slope (Abs/min) of the absorbance-time plot was used for comparison. The assay was validated using S-glutathionylated creatine kinase (CK) as positive controls. There was excellent correlation between the slope and the S-glutathionylated CK concentrations, with r2 = 0.97.
S-Glutathionylation of p21ras by NTG. The human vascular endothelial cell line EA.hy926 (gift from Dr. M. Edgell, University of North Carolina, Chapel Hill, NC) was cultured in high glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). Cells grown to confluence were washed with serum-free medium and incubated in serum-free media containing 0.1 mM NTG (American Regent, Shirley, NY) or in solvent control (30% propylene glycol/30% ethanol) for 48 h. Tolerance was induced in LLC-PK1 cells by incubating with 0.1 mM NTG for 5 h. Activation of p21ras was assessed in both cell lines using a Ras activation assay kit (Millipore Bioscience Research Reagents, Temecula, CA). In brief, the activated p21ras was immunoprecipitated by Raf-1 RBD agarose (glutathione agarose beads). The beads were then boiled in reducing loading buffer, resolved by electrophoresis, transferred to nitrocellulose membrane, and probed with a monoclonal anti-Ras antibody. The S-glutathionylated protein bands (21 kDa) were quantified via a Kodak Image Station 2000 mm (Eastman Kodak, Rochester, NY).
Statistical Analysis
All data are presented as mean ± S.D. unless stated otherwise. For comparison of two groups, Student's t test was used. Comparisons among different pretreatments were made using one-way ANOVA. Statistical significance was then determined by Tukey's post hoc test (SPSS 11.x; SPSS, Inc., Chicago, IL). Correlation between EC50 values and PSSG in rat aorta was examined by Pearson correlation test (SPSS 11.x). Dose-responses of cGMP after different treatments were compared by two-way ANOVA with post hoc analysis (MINITAB 14.x; Minitab, Inc., State College, PA). Differences with p < 0.05 were denoted as statistically significant.
Results
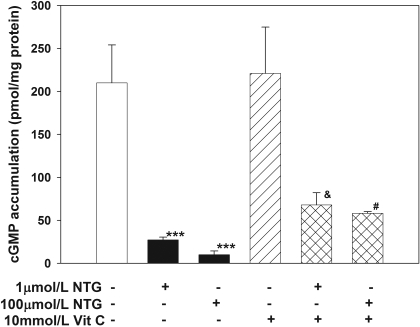
Tolerance Induction by NTG in LLC-PK1 Cells. Pretreatment of LLC-PK1 cells with either 1 or 100 μM NTG for 5 h decreased NTG-induced cGMP production significantly in a dose-dependent manner (p < 0.001, one-way ANOVA; Fig. 1), confirming the induction of cellular nitrate tolerance. In contrast, preincubation of S-nitroso-N-acetyl-l-penicillamine (SNAP), a spontaneous NO donor, did not decrease the responsiveness of these cells toward NTG (control: 27.3 ± 5.0 versus SNAP: 24.6 ± 2.8 pmol/mg protein; p > 0.05). Preincubation of 10 mM vitamin C alone had no effect on cGMP accumulation after NTG induction, but it attenuated NTG-induced tolerance at both NTG concentrations (p < 0.001, one-way ANOVA; Fig. 1).
Fig. 1.
Effects of 1 or 100 μM NTG pretreatment (with or without 10 mM vitamin C) on NTG-stimulated cGMP accumulation in LLC-PK1 cells. Pretreating the cells with 1 or 100 μM NTG significantly reduced NTG-stimulated cGMP production, suggesting development of tolerance (one-way ANOVA, p < 0.001; post hoc tests: ***, p < 0.001 versus control), whereas coincubation with vitamin C partially but significantly attenuated the degree of tolerance (one-way ANOVA, p < 0.001; post hoc tests: &, p < 0.001 versus 1 μM NTG alone; #, p = 0.001 versus 100 μM NTG). Values are expressed as mean ± S.D., n = 4 for each group.
Effect of NTG Tolerance on Protein S-Glutathionylation. Pretreatment of 100 μMNTG for 5 h in the presence of [35S]cysteine resulted in approximately 2-fold increase in total cellular uptake of 35S compared with vehicle control (control versus NTG, 1.81 ± 0.2 × 105 versus 3.63 ± 1.1 × 105 cpm/mg protein; p < 0.001) and enhanced [35S]cysteine incorporation into cellular proteins (control versus NTG, 6.01 ± 1.5 × 104 versus 9.01 ± 0.9 × 104 cpm/mg protein; p < 0.05). Vitamin C alone had no effect on protein [35S]cysteine incorporation, but its coincubation with NTG significantly reduced [35S]cysteine incorporation into proteins, compared with the NTG treatment group (p < 0.001, one-way ANOVA; data not shown). Autoradiography and densitometric analyses indicated (Supplemental Fig. 1A) that multiple proteins incorporated [35S]cysteine under tolerance conditions, and statistical significance was noted for proteins with approximate molecular masses of 11, 13, 17, 20, 23, 25, 30, 33, and 35 kDa (p < 0.05; Supplemental Fig. 1B). As measured by PSSG ELISA, cells treated with NTG showed significantly higher levels of total S-glutathionylated proteins (control: 0.09 ± 0.04 versus 0.14 ± 0.07 Abs/min; p < 0.05). Western blotting showed with a glutathione-specific antibody that multiple proteins in LLC-PK1 cells were S-glutathionylated after pretreatment with NTG (Supplemental Fig. 1C), which was significantly reduced in the presence of vitamin C. Several proteins (with approximate molecular masses of 20, 25, 33, and 40 kDa; p < 0.05 versus control; Fig. 1D) were S-glutathionylated when tolerance was induced, some of which (those with molecular masses of 25, 33, and 105 kDa) were reduced upon coincubation of vitamin C (p < 0.01 versus NTG, one-way ANOVA).
Basal cellular GSH and GSSG levels in LLC-PK1 cells were found to be 0.056 ± 0.009 and 0.0045 ± 0.0004 nmol/μg protein, respectively, compared with literature values of approximately 0.4 and 0.001 nmol/μg protein, respectively (Ayala-Fierro and Carter, 2000). These discrepancies might arise from differences in the cell culture conditions. NTG treatment did not alter intracellular GSH content in LLC-PK1 cells (control: 0.091 ± 0.031 versus NTG: 0.101 ± 0.018 nmol/μg protein), consistent with a report by Boesgaard et al. (1994). In addition, cellular GSSG levels were not significantly altered (0.0058 ± 0.0041 versus 0.0037 ± 0.0004 nmol/μg protein).
Effect of NTG Tolerance on p21ras Activation. Treatment of EA.hy926 endothelial cells and LLC-PK1 cells with NTG resulted in significant increases in p21ras activity compared with controls. As measured by S-glutathionylation, p21ras activation was increased to 136 ± 28 and 128 ± 49% of normalized controls by NTG, in EA.hy926 and LLC-PK1 cells, respectively (p < 0.05 in each case).
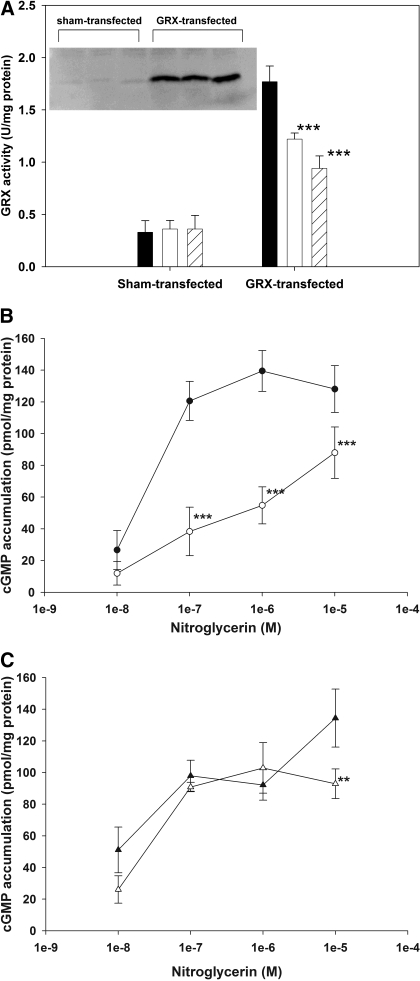
Effect of GRX Expression on NTG Tolerance. Successful overexpression of GRX-1 was validated by Western blot and GRX activity assay (Fig. 2A, inset), and an increase in GRX activity of approximately 5-fold was achieved (Fig. 2A). We found the GSH/GSSG ratio in LLC-PK1 cells to be unaltered from its basal value; therefore, GRX catalysis of protein S-glutathionylation (Beer et al., 2004) was unlikely to occur in our system. Indeed, using the PSSG ELISA assay we developed, we found the extent of PSSG in sham-transfected cells to be approximately 2-fold higher than that in GRX-overexpressed cells (0.375 ± 0.09 versus 0.208 ± 0.06 Abs/min; p < 0.05, Student's t test). NTG dose-dependently inactivated the overexpressed GRX activity (p < 0.001; Fig. 2A); however, under our experimental conditions, GRX activity was still approximately 2- to 3-fold higher than their corresponding controls. Because NTG inactivated GRX activity, a lower NTG dose and a shorter incubation time were used to induce tolerance. Sham-transfected cells pretreated with 0.1 μM NTG for 3 h showed a significant rightward shift of the NTG dose-cGMP response curves versus control, consistent with cellular tolerance development (p < 0.001, two-way ANOVA; Fig. 2B). In the GRX-overexpressed cells, the NTG-response curves of control and NTG-pretreated cells were significantly different from each other (p < 0.001, two-way ANOVA; Fig. 2C). However post hoc analysis showed the significance occurred only at the 10 μM dose, suggesting lack of tolerance at the lower concentrations.
Fig. 2.
A, GRX activity in transfected LLC-PK1 cells over 5 h after exposure to vehicle control (filled bars), 0.01 μM NTG (unfilled bars), and 0.1 μM NTG (striped bars). Data are expressed as mean ± S.D., n = 3. Overexpression of GRX-1 was achieved as indicated by the significant increase in both protein expression and activity. NTG concentration-dependently inactivated the overexpressed GRX activity (one-way ANOVA, ***, p < 0.001). Inset, Western blot of GRX-1 expression in sham-transfected and in GRX-transfected LLC-PK1 cells. B, concentration-response curves toward NTG in sham-transfected cells. Closed symbols denote cell response of vehicle treated controls, whereas open symbols denote those of 0.1 μM NTG-pretreated cells. C, concentration-response curves toward NTG in GRX-overexpressed cells. Closed symbols represent control, and open symbols denote NTG-pretreated cells. Data are expressed as mean ± S.D., n = 4.
GRX-1 siRNAs were then used to suppress endogenous GRX-1 activity in LLC-PK1 cells. We observed the best reduction of GRX activity (approximately 50%) when cells were transfected with 750 pmol of GRX siRNAs for 24 h (sham: 4.57 ± 0.1 × 10-2, siRNA: 2.39 ± 0.1 × 10-2 U/mg protein; p < 0.05). GRX-silenced cells exhibited a higher degree of tolerance after incubation with 0.1 μM NTG for 3 h. In the sham-transfected group, cGMP levels stimulated by NTG were 31.2 ± 2.7 and 19.6 ± 3.7 pmol/mg protein for vehicle and NTG-pretreated cells, resulting in approximately 40% reduction in cGMP response after NTG tolerance. In GRX-silenced cells, the cGMP levels in vehicle and NTG-treated cells were 33.5 ± 2.2 and 13.7 ± 1.4 pmol/mg protein, respectively, resulting in 60% reduction after NTG treatment. Two-way ANOVA indicated that a significant difference was observed between type of transfection (sham- or GRX siRNA-transfected groups) and type of treatment (vehicle or NTG preincubation), suggesting the extent of tolerance in control and GRX siRNA-treated cells were different.
NTG Tolerance Inactivated Thiol-Sensitive Enzymes. The cellular activity of CK, a sulfhydryl-sensitive enzyme, was reduced significantly by 1 and 100 μM NTG under tolerance conditions (control: 0.45 ± 0.01 U/mg protein, 1 μM NTG: 0.41 ± 0.02 U/mg protein, 100 μM NTG: 0.37 ± 0.01 U/mg protein; p < 0.05 versus control). SNAP incubation did not affect CK activity in these cells (control: 0.31 ± 0.04 U/mg protein versus SNAP: 0.43 ± 0.15 U/mg protein). Under nitrate tolerant conditions, XOR activity was significantly decreased as well (1.54 ± 0.16 U/mg protein for control versus 1.03 ± 0.24 U/mg protein for NTG; p < 0.05).
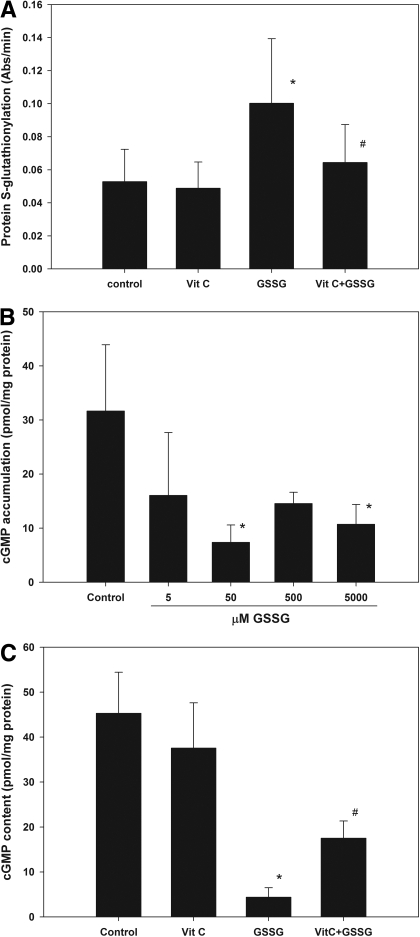
Effect of GSSG on Protein S-Glutathionylation and NTG-Stimulated cGMP. Intracellular GSSG in LLC-PK1 cells elevated approximately 95-fold when extracellular GSSG concentration was increased from 50 μM to 25 mM. This is possibly mediated by the abundant expression of γ-glutamyl transpeptidase on the apical membrane of these cells when confluent (Rabito et al., 1984). This enzyme plays a crucial role in the γ-glutamyl cycle and catabolizes GSH and GSSG into intermediate peptides or amino acids that can be taken up by cells (Jones et al., 1979; Tate et al., 1979). Once in the cells, these peptides and amino acids can be used to synthesize GSH and GSSG (Griffith and Meister, 1979), thus leading to an increase in our system. Pretreatment of LLC-PK1 cells with 50 μM GSSG for 1 h also led to significant S-glutathionylation of multiple cellular proteins (with molecular masses of approximately 20, 23, 33, and 40 kDa), which was reduced by coincubation with vitamin C (Supplemental Fig. 2, A and B). Total S-glutathionylated proteins in the lysate were further quantified by ELISA (Fig. 3A). PSSG was significantly higher in GSSG-treated cells compared with control (p < 0.01), and addition of vitamin C significantly reduced it (p < 0.05). LLC-PK1 cells incubated with GSSG ranging from 5 μM to 5 mM showed significant lower cGMP response from NTG stimulation at 50 μM and 5 mM (p < 0.05 versus control, one-way ANOVA; Fig. 3B). In a follow-up study, pretreating the cells with 50 μM GSSG significantly reduced the sensitivity to NTG (p < 0.001 versus control, one-way ANOVA; Fig. 3C), whereas coincubation with vitamin C partially and significantly improved the responsiveness of these cells toward NTG (p < 0.001 versus GSSG, one-way ANOVA).
Fig. 3.
Effects of GSSG pretreatment (with or without vitamin C) on PSSG- and NTG-stimulated cGMP accumulation in LLC-PK1 cells. A, GSSG (50 μM) significantly increased total PSSG production cells (one-way ANOVA, p < 0.05; post hoc test: *, p < 0.01 versus control), and addition of vitamin C significantly reduced it (one-way ANOVA, p < 0.05; post hoc test: #, p < 0.05 versus GSSG). B, pretreating the cells with 50 μM or 5 mM GSSG significantly reduced NTG-stimulated cGMP production, suggesting that GSSG reduced the sensitivity to nitrate in these cells (one-way ANOVA, p < 0.05; post hoc test: *, p < 0.05 versus control). C, pretreatment of LLC-PK1 cells with 50 μM GSSG significantly reduced the sensitivity toward NTG (one-way ANOVA, p < 0.001; post hoc test: *, p < 0.001 versus control), whereas the coincubation with vitamin C partially but significantly attenuated the degree of tolerance (one-way ANOVA, p < 0.001; post hoc test: #, p < 0.001 versus GSSG). Values are expressed as mean ± S.D., n = 3 to 5 for each group.
Effect of GSSG on
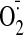 Production and Thiol-Sensitive Enzyme Activities. Cellular
Production and Thiol-Sensitive Enzyme Activities. Cellular
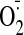 generation by GSSG was monitored by dihydroethidium fluorescence [fluorescence
unit (FU)]. Incubation of LLC-PK1 cells with 50 μM GSSG for 1 h led to
significantly enhanced
generation by GSSG was monitored by dihydroethidium fluorescence [fluorescence
unit (FU)]. Incubation of LLC-PK1 cells with 50 μM GSSG for 1 h led to
significantly enhanced
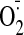 accumulation (154 ± 24 FU) compared with control 52.9 ± 12 FU
(p < 0.01). Addition of 10 mM tiron significantly reduced
GSSG-stimulated
accumulation (154 ± 24 FU) compared with control 52.9 ± 12 FU
(p < 0.01). Addition of 10 mM tiron significantly reduced
GSSG-stimulated
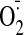 accumulation in control (45.4 ± 13 FU) and in GSSG-treated cells (31.7
± 2.6 FU; p < 0.01 between these two groups). Coincubation
of GSSG and 100 μM oxypurinol significantly reduced GSSG-stimulated
accumulation in control (45.4 ± 13 FU) and in GSSG-treated cells (31.7
± 2.6 FU; p < 0.01 between these two groups). Coincubation
of GSSG and 100 μM oxypurinol significantly reduced GSSG-stimulated
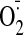 production (control: 42.1 ± 5.0 FU, GSSG: 82.5 ± 4.8 FU;
p < 0.001).
production (control: 42.1 ± 5.0 FU, GSSG: 82.5 ± 4.8 FU;
p < 0.001).
We examined whether GSSG incubation affected the activity of enzymes that contained critical cysteine groups at their active sites. At 5 mM, GSSG significantly reduced ALDH and CK activities (p < 0.05, Student's t test; Table 1). XOR activity was not altered by GSSG preincubation at this concentration.
TABLE 1.
Effect of GSSG on aldehyde dehydrogenase, creatine kinase, and xanthine oxidoreductase activities in LLC-PK1 cells Data expressed as mean ± S.D., n = 3. At 5 mM, GSSG significantly inhibited both aldehyde dehydrogenase and creatine kinase activities.
| Enzyme Activity | Control | 50 μM GSSG | 5 mM GSSG |
|---|---|---|---|
| Aldehyde dehydrogenase × 104 (U/mg protein) | 5.25 ± 0.26 | 5.67 ± 0.70 | 3.57 ± 0.67* |
| Creatine kinas × 103 (U/mg protein) | 3.56 ± 0.20 | 3.97 ± 0.49 | 1.41 ± 0.24* |
| Xanthine oxidoreductase (U/mg protein) | 1.11 ± 0.20 | 1.05 ± 0.24 | N.D. |
p < 0.05 vs. control, Student's t test
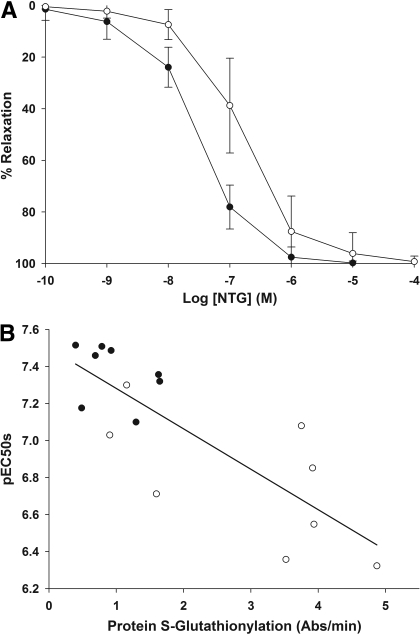
Effect of in Vivo NTG Tolerance on Protein S-Glutathionylation. Figure 4A shows the ex vivo NTG concentration-relaxation curves of isolated aorta toward NTG in rats after in vivo tolerance induction over 3 days of subcutaneous drug delivery of NTG. Vascular tolerance was evident, as indicated by the rightward shift of the NTG-relaxation curve compared with treatment with vehicle control. The pEC50s for control- and NTG-treated animals were 7.38 ± 0.15 and 6.82 ± 0.36 (p < 0.01, Student's t test). The levels of S-glutathionylated proteins were 0.98 ± 0.49 and 2.96 ± 1.5 Abs/min for control and tolerant animals (p < 0.01, Student's t test). The correlation between the pEC50 values and extent of PSSG in the aorta was statistically significant, with a Pearson correlation coefficient (r) of 0.806 (p = 0.01; Fig. 4B).
Fig. 4.
Extent of protein S-glutathionylation versus in vivo tolerance development in rats. Data are expressed as mean ± S.D., n = 9. A, ex vivo concentration versus relaxation curves of isolated rat aorta toward NTG in rats after treatment with control (filled symbols) versus NTG (3 days 50 μg/h via osmotic pump; unfilled symbols). The concentration-relaxation response curve was right-shifted after NTG treatment, consistent with vascular tolerance development. The pEC50 values were significantly different: control (7.38 ± 0.15) versus NTG-treated (6.82 ± 0.36, p < 0.01, Student's t test). B, correlation between pEC50 values of NTG dose-response curve and their corresponding protein S-glutathionylation levels in rat aorta: vehicle control (closed symbols) and NTG-treated (open symbols). The line represents the regression line for all the samples. The Pearson correlation coefficient (r) is 0.805 and significant at 0.01 level, suggesting there is a correlation between the extent of vascular response and vascular protein S-glutathionylation.
Discussion
Here, we showed that 1) NTG increased nonspecific sulfur uptake and PSSG
formation in LLC-PK1 cells; 2) a biomarker of cellular signaling,
p21ras, was significantly S-glutathionylated by NTG
exposure in both LLC-PK1 and Ea.hy926 endothelial cells; 3) under tolerance
condition, the activities of several thiol-sensitive enzymes were reduced; 4)
manipulation of cellular GRX expression indicated that cellular nitrate
tolerance was related to the extent of PSSG; 5) GSSG, which promotes PSSG,
produced several effects that were observed in nitrate tolerance, namely:
diminution of NTG-triggered cGMP response, reduction in thiol-sensitive enzyme
activities, and increase in
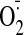 accumulation; 6) the tolerance-sparing effect of vitamin C was observed after
both nitrate and GSSG preincubation; and 7) increased PSSG was observed in rat
aorta when tolerance was induced in vivo, and a moderate correlation was
observed between the degrees of tolerance and S-glutathionylation.
These results, taken together, are highly supportive of a crucial role of
protein S-glutathionylation in cellular nitrate tolerance.
accumulation; 6) the tolerance-sparing effect of vitamin C was observed after
both nitrate and GSSG preincubation; and 7) increased PSSG was observed in rat
aorta when tolerance was induced in vivo, and a moderate correlation was
observed between the degrees of tolerance and S-glutathionylation.
These results, taken together, are highly supportive of a crucial role of
protein S-glutathionylation in cellular nitrate tolerance.
A possible concern of the applicability of this work may arise because we used LLC-PK1 cells, a porcine kidney epithelial cell line, to examine the cellular mechanisms of nitrate tolerance. However, these cells have been used for studying the pathways of nitrate-induced activation and desensitization of cGMP response in several studies (Hinz and Schröder, 1998, 1999; Tsou et al., 2008) and have shown many parallel hallmarks of vascular nitrate tolerance, including the lack of tolerance and cross-tolerance toward strict NO donors such as SNAP, dose-dependent tolerance induction, and tolerance attenuation by vitamin C. We have attempted to examine cellular NTG tolerance in cultured human vascular smooth muscle cells. However, consistent with literature reports (Bennett et al., 1989), these cells, when cultured, were poorly responsive to NTG.
Our results indicated that NTG, under tolerance-inducing conditions and in the presence of a GSH source, caused nonspecific sulfur incorporation and S-glutathionylation in multiple cellular proteins. Continuous NTG treatment in rats resulted in tolerance and a significant elevation of vascular PSSG. A modest but significant correlation (p < 0.01) between rat aorta pEC50 value and PSSG was observed. These results indicate that increased PSSG was associated with nitrate tolerance, both in vitro and in vivo.
We hypothesized that cells overexpressing GRX, an enzyme that regulates PSSG, will be more resistant to tolerance development, whereas those deficient of this enzyme will be more prone to tolerance. We chose GRX-1 because of its specificity in cleaving S-glutathionylated bonds. This enzyme possesses the active motif (Cys-X-X-Cys) for deglutathionylation and has higher activity compared with GRX-3 (Fernandes and Holmgren, 2004). Figure 2 shows that manipulation of cellular PSSG levels through GRX expression affected the extent of nitrate tolerance in the anticipated direction; thus, increased GRX expression led to less tolerance, whereas suppressed GRX presence enhanced the degree of tolerance.
GSSG is a strong oxidant that promotes PSSG formation
(Beer et al., 2004). We showed
that GSSG mimicked several major phenomena of nitrate tolerance, namely,
increased cellular PSSG, decreased nitrate sensitivity, inactivation of
several thiol-sensitive enzymes, and increased
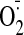 production. In addition, coincubation with vitamin C attenuated the extent of
GSSG-induced PSSG as well as nitrate desensitization. Thiol-oxidizing agents
such as GSSG rapidly inactivated NO-stimulated guanylyl cyclase activity
(Braughler, 1983), suggesting
the involvement of sulfhydryl groups in the regulation of its activity.
Guanylyl cyclase desensitization might also be a mechanism of NTG tolerance
(Waldman et al., 1986), but
this mechanism probably occurs only at very high NTG exposure conditions.
production. In addition, coincubation with vitamin C attenuated the extent of
GSSG-induced PSSG as well as nitrate desensitization. Thiol-oxidizing agents
such as GSSG rapidly inactivated NO-stimulated guanylyl cyclase activity
(Braughler, 1983), suggesting
the involvement of sulfhydryl groups in the regulation of its activity.
Guanylyl cyclase desensitization might also be a mechanism of NTG tolerance
(Waldman et al., 1986), but
this mechanism probably occurs only at very high NTG exposure conditions.
Our results are consistent with findings that vitamin C attenuated or reversed nitrate tolerance in animals (Fink et al., 1999; Mülsch et al., 2001; Wada et al., 2002). We demonstrated here that vitamin C coincubation also significantly reduced NTG-stimulated [35S]cysteine incorporation and PSSG, indicating that the vitamin C effects on nitrate tolerance might be mediated, at least partially, by alteration of the oxidation status of PSH.
Increased
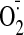 production was observed in both NTG-tolerant-
(Tsou et al., 2008) and
GSSG-treated cells. In both cases,
production was observed in both NTG-tolerant-
(Tsou et al., 2008) and
GSSG-treated cells. In both cases,
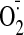 production was partially reversed by oxypurinol, a XO inhibitor, suggesting
that this enzyme played a contributory role. XO can be converted from xanthine
dehydrogenase through SH oxidation (Borges
et al., 2002). In addition, it has been reported that
S-glutathionylation of mitochondrial complex I under oxidative stress
increased the production of
production was partially reversed by oxypurinol, a XO inhibitor, suggesting
that this enzyme played a contributory role. XO can be converted from xanthine
dehydrogenase through SH oxidation (Borges
et al., 2002). In addition, it has been reported that
S-glutathionylation of mitochondrial complex I under oxidative stress
increased the production of
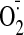 (Taylor et al., 2003). Thus,
increased protein SH oxidation and S-glutathionylation, brought about
by either NTG or GSSG, can lead to increased intracellular
(Taylor et al., 2003). Thus,
increased protein SH oxidation and S-glutathionylation, brought about
by either NTG or GSSG, can lead to increased intracellular
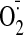 production without the obligatory participation of the angiotensin II (Ang
II)/NOX pathway (Tsou et al.,
2008).
production without the obligatory participation of the angiotensin II (Ang
II)/NOX pathway (Tsou et al.,
2008).
PSSG formation may proceed through several mechanisms (Shelton et al., 2005), namely, 1) thiol-disulfide exchange between cysteine groups in proteins with GSSG; 2) sulfenic acid intermediates; 3) S-nitrosothiol intermediates; and 4) thiyl radical intermediates, e.g., PS· or GS·. Direct interaction between PSH and GSSG only occurs when GSH/GSSG ratios were low. In our experiments, the GSH/GSSG ratios were comparatively high; therefore, thiol-disulfide exchange is unlikely to be operative. However, in the GSSG-treated cells, cellular GSH/GSSG ratios were 20.6 ± 4.4 and 2.04 ± 0.21 when cells were exposed to 0.05 and 5 mM GSSG, thus significant thiol-disulfide exchange might exist under these conditions. In other studies in our laboratory that used mass spectroscopy (A. S. Krishnatry and T. Kamei, unpublished data), we have shown that NTG can induce PSOH formation in proteins and peptides. Sulfenic acid derivatives in proteins can be further oxidized to sulfinic acids and sulfonic acids, which cannot be readily reversed to the reduced PSH by thiol reducing agents (Giustarini et al., 2004). This phenomenon can provide an explanation why sulfhydryl donors had limited success on reversing nitrate tolerance (Packer et al., 1987; Boesgaard et al., 1994). Preliminary studies in our laboratory (A. S. Krishnatry and T. Kamei, unpublished data) indeed confirmed the formation of sulfinic and sulfonic acid derivatives when NTG was incubated with several peptides and proteins. The formation of these irreversible oxidation products is consistent with our previous study that showed NTG to be a suicide substrate for purified glutathione transferase from rabbit liver (Lee and Fung, 2003). These results therefore suggest that NTG-induced PSSG may proceed through sulfenyl intermediates.
We showed here that NTG incubation significantly increased the S-glutathionylation, and thereby activation, of p21ras in both Ea.hy926 endothelial cells and LLC-PK1 cells. p21ras is a G protein that plays an integral role in several transduction pathways. Activation of p21ras by peroxynitrite was shown to be mediated through S-glutathionylation, resulting in its interaction with the downstream targets, Raf-1 kinase and phosphatidylinositol 3-kinase followed by increases in extracellular signal-regulated kinase 1/2 and Akt phosphorylation (Clavreul et al., 2006b). Increased extracellular signal-regulated kinase phosphorylation mediates cellular proliferation and inflammation and has been implicated in cardiomyocyte and vascular smooth muscle cell proliferation (Muslin, 2008). In the vasculature, Akt is responsible for phosphorylation and endothelial NO synthase activation, leading to vasodilatation (Bertrand et al., 2008). In cultured rat vascular smooth muscle cells, Ang II was shown to stimulate the mitogen-activated protein kinase pathway by activating p21ras (Eguchi et al., 1996). Later studies suggested the activation was mediated through the stimulation of NOX (Adachi et al., 2004). The increased oxidative stress led to increased production of S-glutathionylated p21ras and subsequently the downstream signaling events. The role of Ang II-NOX-induced endothelial dysfunction was proposed in several studies to explain the mechanism of nitrate tolerance (Münzel et al., 1995; Mollnau et al., 2002). We showed in both in vitro and in vivo conditions the Ang II-NOX pathway is not critically involved in nitrate tolerance (Tsou et al., 2008). In addition, we showed in this study NTG is capable of activating p21ras through direct S-glutathionylation. Our findings therefore provide a possible link between NTG tolerance and the downstream signaling pathways without the obligatory involvement of the Ang II pathway.
In conclusion, the present study demonstrated that protein
S-glutathionylation plays a crucial role in the mechanism of nitrate
tolerance. We hypothesize that NTG is bioactivated through its interaction
with multiple PSH, producing NO or NO-related species for pharmacological
action on the one hand and an oxidized protein with altered function on the
other hand. In addition to causing enzyme inactivation and reduced
bioactivation (e.g., for ALDH2), NTG produces S-glutathionylated
proteins such as p21ras that potentially contribute to the
downstream effects of nitrate tolerance. This mechanism therefore has the
potential to explain all the major observations in nitrate tolerance,
including reduced pharmacological response, sulfhydryl dependence, impaired
biotransformation (through SH oxidation of NTG-metabolizing enzymes such as
ALDH2 and CK), oxidative stress
(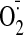 production mediated by XO), altered gene expression, and withdrawal rebound
(through redox regulation of S-glutathionylated proteins such as
p21ras). We believe that this hypothesis can provide a potentially
unifying biochemical mechanism for initiation of nitrate tolerance.
production mediated by XO), altered gene expression, and withdrawal rebound
(through redox regulation of S-glutathionylated proteins such as
p21ras). We believe that this hypothesis can provide a potentially
unifying biochemical mechanism for initiation of nitrate tolerance.
Supplementary Material
Acknowledgments
We thank Sun Mi Fung, Kate E. Jank, and Steven G. Turowski for technical support.
This study was supported by the National Institutes of Health [Grant HL081580]; a Schering Plough Predoctoral Fellowship (to P.-S.T.); and a Schering Plough-Rho-Chi-American Foundation for Pharmaceutical Education First Year Graduate School Scholarship (to N.P.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.149997.
ABBREVIATIONS: NTG, nitroglycerin; ALDH2, mitochondrial aldehyde
dehydrogenase; NOX, NADPH oxidase; SH, sulfhydryl; PSSG, protein
S-glutathionylation; GSH, glutathione; GRX, glutaredoxin; PSH,
protein sulfhydryls; XOR, xanthine oxidoreductase; XO, xanthine oxidase; GSSG,
oxidized glutathione; FBS, fetal bovine serum; siRNA, small interfering RNA;
ELISA, enzyme-linked immunosorbent assay; Abs, absorbance; CK, creatine
kinase; ANOVA, analysis of variance; SNAP,
S-nitroso-N-acetyl-l-penicillamine;
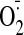 ,
superoxide; FU, fluorescence unit; Ang II, angiotensin II.
,
superoxide; FU, fluorescence unit; Ang II, angiotensin II.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
References
- Adachi T, Pimentel DR, Heibeck T, Hou X, Lee YJ, Jiang B, Ido Y, and Cohen RA (2004) S-Glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem 279 29857-29862. [DOI] [PubMed] [Google Scholar]
- Ayala-Fierro F and Carter DE (2000) LLC-PK1 cells as a model for renal toxicity caused by arsine exposure. J Toxicol Environ Health A 60 67-79. [DOI] [PubMed] [Google Scholar]
- Beer SM, Taylor ER, Brown SE, Dahm CC, Costa NJ, Runswick MJ, and Murphy MP (2004) Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant DEFENSE. J Biol Chem 279 47939-47951. [DOI] [PubMed] [Google Scholar]
- Bennett BM, Leitman DC, Schröder H, Kawamoto JH, Nakatsu K, and Murad F (1989) Relationship between biotransformation of glyceryl trinitrate and cyclic GMP accumulation in various cultured cell lines. J Pharmacol Exp Ther 250 316-323. [PubMed] [Google Scholar]
- Beretta M, Gruber K, Kollau A, Russwurm M, Koesling D, Goessler W, Keung WM, Schmidt K, and Mayer B (2008) Bioactivation of nitroglycerin by purified mitochondrial and cytosolic aldehyde dehydrogenases. J Biol Chem 283 17873-17880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand L, Horman S, Beauloye C, and Vanoverschelde JL (2008) Insulin signalling in the heart. Cardiovasc Res 79 238-248. [DOI] [PubMed] [Google Scholar]
- Boesgaard S, Aldershvile J, Poulsen HE, Loft S, Anderson ME, and Meister A (1994) Nitrate tolerance in vivo is not associated with depletion of arterial or venous thiol levels. Circ Res 74 115-120. [DOI] [PubMed] [Google Scholar]
- Borges F, Fernandes E, and Roleira F (2002) Progress towards the discovery of xanthine oxidase inhibitors. Curr Med Chem 9 195-217. [DOI] [PubMed] [Google Scholar]
- Braughler JM (1983) Soluble guanylate cyclase activation by nitric oxide and its reversal. Involvement of sulfhydryl group oxidation and reduction. Biochem Pharmacol 32 811-818. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhang J, and Stamler JS (2002) Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci U S A 99 8306-8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong S and Fung HL (1989) Kinetic mechanisms for the concentration dependency of in vitro degradation of nitroglycerin and glyceryl dinitrates in human blood: metabolite inhibition or cosubstrate depletion? J Pharm Sci 78 295-302. [DOI] [PubMed] [Google Scholar]
- Clavreul N, Adachi T, Pimental DR, Ido Y, Schöneich C, and Cohen RA (2006a) S-Glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. FASEB J 20 518-520. [DOI] [PubMed] [Google Scholar]
- Clavreul N, Bachschmid MM, Hou X, Shi C, Idrizovic A, Ido Y, Pimentel D, and Cohen RA (2006b) S-Glutathiolation of p21ras by peroxynitrite mediates endothelial insulin resistance caused by oxidized low-density lipoprotein. Arterioscler Thromb Vasc Biol 26 2454-2461. [DOI] [PubMed] [Google Scholar]
- Eguchi S, Matsumoto T, Motley ED, Utsunomiya H, and Inagami T (1996) Identification of an essential signaling cascade for mitogen-activated protein kinase activation by angiotensin II in cultured rat vascular smooth muscle cells. Possible requirement of Gq-mediated p21ras activation coupled to a Ca2+/calmodulin-sensitive tyrosine kinase. J Biol Chem 271 14169-14175. [DOI] [PubMed] [Google Scholar]
- Fernandes AP and Holmgren A (2004) Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal 6 63-74. [DOI] [PubMed] [Google Scholar]
- Fink B, Schwemmer M, Fink N, and Bassenge E (1999) Tolerance to nitrates with enhanced radical formation suppressed by carvedilol. J Cardiovasc Pharmacol 34 800-805. [DOI] [PubMed] [Google Scholar]
- Fung HL (2004) Biochemical mechanism of nitroglycerin action and tolerance: is this old mystery solved? Annu Rev Pharmacol Toxicol 44 67-85. [DOI] [PubMed] [Google Scholar]
- Giustarini D, Rossi R, Milzani A, Colombo R, and Dalle-Donne I (2004) S-glutathionylation: from redox regulation of protein functions to human diseases. J Cell Mol Med 8 201-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW and Meister A (1979) Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A 76 5606-5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B and Schröder H (1998) Nitrate tolerance is specific for nitric acid esters and its recovery requires an intact protein synthesis. Biochem Biophys Res Commun 252 232-235. [DOI] [PubMed] [Google Scholar]
- Hinz B and Schröder H (1999) The nitric oxide donor SIN-1 is free of tolerance and maintains its cyclic GMP stimulatory potency in nitrate-tolerant LLC-PK1 cells. Pharm Res 16 633-636. [DOI] [PubMed] [Google Scholar]
- Jones DP, Moldfus P, Stead AH, Ormstad K, Jörnvall H, and Orrenius S (1979) Metabolism of glutathione and a glutathione conjugate by isolated kidney cells. J Biol Chem 254 2787-2792. [PubMed] [Google Scholar]
- Klatt P and Lamas S (2000) Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem 267 4928-4944. [DOI] [PubMed] [Google Scholar]
- Lee WI and Fung HL (2003) Mechanism-based partial inactivation of glutathione S-transferases by nitroglycerin: tyrosine nitration vs sulfhydryl oxidation. Nitric Oxide 8 103-110. [DOI] [PubMed] [Google Scholar]
- Mollnau H, Wendt M, Szöcs K, Lassègue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, et al. (2002) Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 90 E58-E65. [DOI] [PubMed] [Google Scholar]
- Mülsch A, Oelze M, Klöss S, Mollnau H, Töpfer A, Smolenski A, Walter U, Stasch JP, Warnholtz A, Hink U, et al. (2001) Effects of in vivo nitroglycerin treatment on activity and expression of the guanylyl cyclase and cGMP-dependent protein kinase and their downstream target vasodilator-stimulated phosphoprotein in aorta. Circulation 103 2188-2194. [DOI] [PubMed] [Google Scholar]
- Münzel T, Daiber A, and Mülsch A (2005) Explaining the phenomenon of nitrate tolerance. Circ Res 97 618-628. [DOI] [PubMed] [Google Scholar]
- Münzel T, Sayegh H, Freeman BA, Tarpey MM, and Harrison DG (1995) Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J Clin Invest 95 187-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslin AJ (2008) MAPK signalling in cardiovascular health and disease: molecular mechanisms and therapeutic targets. Clin Sci (Lond) 115 203-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman P and Johnson EM Jr (1973) Mechanism of tolerance development to organic nitrates. J Pharmacol Exp Ther 184 709-715. [PubMed] [Google Scholar]
- Packer M, Lee WH, Kessler PD, Gottlieb SS, Medina N, and Yushak M (1987) Prevention and reversal of nitrate tolerance in patients with congestive heart failure. N Engl J Med 317 799-804. [DOI] [PubMed] [Google Scholar]
- Rabito CA, Kreisberg JI, and Wight D (1984) Alkaline phosphatase and gamma-glutamyl transpeptidase as polarization markers during the organization of LLC-PK1 cells into an epithelial membrane. J Biol Chem 259 574-582. [PubMed] [Google Scholar]
- Shelton MD, Chock PB, and Mieyal JJ (2005) Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal 7 348-366. [DOI] [PubMed] [Google Scholar]
- Tate SS, Grau EM, and Meister A (1979) Conversion of glutathione to glutathione disulfide by cell membrane-bound oxidase activity. Proc Natl Acad Sci U S A 76 2715-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor ER, Hurrell F, Shannon RJ, Lin TK, Hirst J, and Murphy MP (2003) Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J Biol Chem 278 19603-19610. [DOI] [PubMed] [Google Scholar]
- Tsou PS, Addanki V, and Fung HL (2008) Dissociation between superoxide accumulation and nitroglycerin-induced tolerance. J Pharmacol Exp Ther 327 97-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada A, Ueda S, Masumori-Maemoto S, Kuji N, Sugimoto K, and Umemura S (2002) Angiotensin II attenuates the vasodilating effect of a nitric oxide donor, glyceryl trinitrate: roles of superoxide and angiotensin II type 1 receptors. Clin Pharmacol Ther 71 440-447. [DOI] [PubMed] [Google Scholar]
- Waldman SA, Rapoport RM, Ginsburg R, and Murad F (1986) Desensitization to nitroglycerin in vascular smooth muscle from rat and human. Biochem Pharmacol 35 3525-3531. [DOI] [PubMed] [Google Scholar]
- Wenzel P, Hink U, Oelze M, Schuppan S, Schaeuble K, Schildknecht S, Ho KK, Weiner H, Bachschmid M, Münzel T, et al. (2007) Role of reduced lipoic acid in the redox regulation of mitochondrial aldehyde dehydrogenase (ALDH-2) activity. Implications for mitochondrial oxidative stress and nitrate tolerance. J Biol Chem 282 792-799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.