Abstract
The nonpigmented epithelium (NPE) of the ciliary body represents an important component of the blood-aqueous barrier of the eye. Many therapeutic drugs penetrate poorly across the NPE into the aqueous humor of the eye interior. Several of these therapeutic drugs, such as methotrexate, vincristine, and etoposide, are substrates of the multidrug resistance-associated protein 2 (MRP2). Abundant MRP2 protein was detected by Western blot in homogenates of human ciliary body and freshly dissected porcine NPE. In cultured porcine NPE, the intracellular accumulation of the MRP2 substrates calcein (1.8-fold), 5-(and-6)-carboxy-2′,7′-dichlorofluorescein (22.1-fold), and doxorubicin (1.9-fold) was significantly increased in the presence of 50 μM MK571 ((E)-3-[[[3-[2-(7-chloro-2-quinolinyl)-ethenyl]phenyl]-[[3-dimethylamino)-3-oxopropyl]thio]methyl]thio]-propanoic acid), an MRP inhibitor. In addition, the intracellular accumulation of the MRP2 substrate glutathione methylfluorescein was increased by 50 μM MK571 (4.3-fold), 500 μM indomethacin (2.6-fold), and 50 μM cyclosporin A (2.1-fold) but not by 500 μM sulfinpyrazone. These data are consistent with MRP2-mediated transport activity in cultured NPE, and MRP2 mRNA (reverse transcriptase-polymerase chain reaction) and protein (Western blot) were detected in the cultured cells. Immunolocalization studies in native human and porcine eyes showed MRP2 protein at the apical interface of the NPE and pigmented cell layers. Close examination of MRP2 immunoreactivity supported the conclusion that MRP2 is localized in the apical membrane of the NPE. MRP2 at the apical membrane of NPE cells may be involved in protecting intraocular tissues from exposure to potentially harmful toxins.
The ciliary body epithelium, which secretes aqueous humor into the posterior chamber, represents a significant barrier to the movement of substances from the blood into the eye (Cunha-Vaz, 1979; Freddo, 2001). The epithelium is a bilayer consisting of two developmentally distinct epithelial monolayers whose apical membranes are in contact with one another. The basolateral membrane of the pigmented layer faces the stroma, whereas the basolateral membrane of the nonpigmented epithelium (NPE) faces the aqueous humor. The vasculature perfusing the ciliary body is highly fenestrated, and large molecular weight tracers such as horseradish peroxidase readily leak out into the surrounding interstitium after intravenous administration (Freddo et al., 1990). There are no tight junctions between the pigmented cells; thus, the aforementioned tracers have been shown to infiltrate the paracellular space between the pigmented and NPE cell layers (Smith and Rudt, 1975). However, tight junctions located at the apical aspect of the NPE prevent macromolecules (e.g., protein) from accessing the anterior chamber (Cunha-Vaz, 1979; Freddo, 2001). The NPE, along with the endothelium of the iris blood vessels, constitute the blood-aqueous humor barrier.
Many of the therapeutic drugs used to treat ocular diseases are substrates of the multidrug resistance-associated protein 2 (MRP2). These include vincristine, etoposide, cisplatin, and doxorubicin, used to treat retinoblastoma, and methotrexate and piroxicam, used to treat uveitis (Hooijberg et al., 1999; El-Sheikh et al., 2007; Folmer et al., 2007). MRP2 is an ATP-dependent efflux transporter that shifts, in addition to many therapeutic drugs, endogenous metabolites, such as estradiol 17β-d-glucuronide, and environmental toxins, such as ochratoxin A (van Aubel et al., 1998; Leier et al., 2000). MRP2 occurs in the renal proximal tubule, where it has at least two functions: 1) to secrete potentially harmful toxins into the tubule lumen and 2) to prevent the re-entry of toxins from the tubular lumen back into the bloodstream (van de Water et al., 2005). To our knowledge, this is the first study to localize a xenobiotic efflux transport protein to the NPE and the first report showing apical expression of a solute transporter of any kind in this epithelium. The findings presented are discussed in relation to ocular drug delivery and maintenance of the overall health of intraocular tissues.
Materials and Methods
Ocular Tissues. Porcine eyes were obtained from the University of Arizona Meat Sciences Laboratory (Tucson, AZ) and used within 2 h of enucleation. Human cadaver eyes were obtained from the National Disease Research Interchange (Philadelphia, PA) within 24 h after enucleation. For the human eye, the ciliary processes, retina, retinal pigmented epithelium (RPE), iris, and cornea were dissected under a microscope and stored in liquid nitrogen until further analysis. The use of human cadaver eyes was approved by the Human Subjects Committee of Yale University and, as far as it applies, followed the tenets of the Declaration of Helsinki.
Isolation and Culture of Porcine NPE. Porcine NPE was established in culture using a modification of our previous technique (Shahidullah et al., 2007). Porcine eyes were dissected to obtain the entire NPE attached to the vitreous, leaving the pigmented epithelium layer attached to the ciliary body. The NPE was separated from the vitreous using fine scissors, then cut into 1- to 2-mm pieces. NPE from five to seven eyes was pooled and transferred to a 90-mm Petri dish containing 15 ml of 0.015% collagenase A and 500 U/ml hyaluronidase (Sigma-Aldrich, St. Louis, MO) in a collagenase buffer containing 66.7 mM NaCl, 13.4 mM KCl, 3.8 mM HEPES, and 4.8 mM CaCl2, pH 7.4. The Petri dish was placed for 5 to 7 min on a rotary shaker in a 37°C incubator. The collagenase and hyaluronidase were neutralized with 7 ml of a 1:1 mixture of newborn calf serum and fetal bovine serum. NPE cells were dispersed by gentle trituration using a round-tipped Pasteur pipette and pelleted by centrifugation at 670g for 10 min at 4°C. The pellet was dispersed and the cells incubated without changing the medium for 3 to 4 days in a small volume of Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum at 37°C in 5% CO2/95% air. Thereafter, the medium was changed every other day. The cells became confluent in 7 to 10 days, then were trypsinized and passaged. Cells from passages 3 to 6 were used in the studies described here.
SDS-PAGE and Western Blotting. Human ocular tissues and renal cortex (human and porcine) were homogenized in ice-cold buffer containing 320 mM sucrose, 1 mM EDTA, and 0.1 mM phenylmethanesulfonyl fluoride, pH 7.0 with NaOH. Native porcine NPE and cultured NPE were solubulized in ice-cold radioimmunoprecipitation assay buffer containing 50 mM HEPES, 150 mM NaCl, 1 mM EDTA, 0.1 mM phenylmethanesulfonyl fluoride, 10% glycerol, 1% Triton X-100, and 1% sodium deoxycholate, pH 7.4 with NaOH. Protein concentration was determined using the bicinchoninic acid method (BCA; Pierce Chemical, Rockford, IL). The homogenates were diluted in an equal volume of 2× Laemmli buffer; the final protein concentration did not exceed 2 μg/μl. For breast cancer-resistant protein (BCRP), 2-mercaptoethanol was added to a final concentration of 2.5%, and the samples were heated to 100°C for 5 min. The samples used to probe for MRP1, MRP2, MRP4, and P-glycoprotein (PgP) were maintained under nonreducing conditions (i.e., no 2-mercaptoethanol or heating). Proteins were separated on either 10% (BCRP) or 6% (MRP1, MRP2, MRP4, and PgP) SDS-PAGE gels and electrophoretically transferred to a polyvinylidene difluoride membrane. The membrane was blocked for 1 h in LI-COR blocking buffer (LI-COR Biotechnology, Lincoln, NE) at room temperature, followed by overnight incubation (4°C) with primary antibodies diluted in blocking buffer. The primary antibodies included rat anti-human MRP4 (clone M4I-10, diluted 1:50; GeneTex, Inc., Irvine, CA), mouse anti-human PgP (clone C219, diluted 1:40; Gene-Tex, Inc.), rat anti-human MRP1 (clone MRPr1, diluted 1:40; Gene-Tex, Inc.), and mouse anti-human BCRP (clone BXP21, diluted 1:50; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) antibodies. The antibody used for MRP2 was a generous gift from Dr. Bruno Stieger (Division of Clinical Pharmacology and Toxicology, Department of Medicine, University Hospital, Zurich, Switzerland) and was used at a dilution of 1:20,000 (Madon et al., 2000). The membranes were washed extensively in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8.0 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.3) containing 0.05% Tween 20 before incubation in secondary antibodies diluted 1:5000 in blocking buffer. The secondary antibodies included IRDye 680 goat anti-mouse (LI-COR Biotechnology), IRDye 800 goat anti-rabbit (LI-COR Biotechnology,) and AlexaFluor 680 goat anti-rat (Invitrogen) antibodies. Immunoreactivity corresponding to the transporters was detected using an Odyssey Infrared Imaging System (LI-COR Biotechnology).
RNA Isolation and Reverse Transcriptase-PCR. Total RNA from porcine NPE cultures and porcine renal cortex was isolated using RNA-Bee (Tel-Test Inc., Friendswood, TX) according to the manufacturer's instructions. One microgram of total RNA was reverse transcribed using the QuantiTect Reverse Transcription kit according to the manufacturer's protocol (QIAGEN, Valencia, CA). The reverse transcription protocol included a step to remove genomic DNA. One hundred fifty nanograms of cDNA was used in the PCR reactions. The oligonucleotide probes used for amplifying the porcine ortholog of MRP2 (GenBank accession number DQ530510) were sense strand 5′-CTCTCTGCAGTGTCTATGGTTG-3′ and antisense strand 5′-CTCTGTCCTATGCTCAGGTTGT-3′. The PCR components were assembled followed by a single denaturing step for 2 min at 94°C. This was followed by 35 cycles of: 94°C for 30 s, 55°C for 30 s, and 72°C for 2 min. A final elongation step of 7 min (72°C) was included after the last cycle. PCR products were separated on 1% agarose gels and visualized with ethidium bromide. DNA sequencing with an Applied Biosystems 3730xl DNA analyzer (Applied Biosystems, Foster City, CA) at the University of Arizona sequencing facility confirmed amplification of porcine MRP2 from the porcine NPE cultures and porcine renal cortex.
Immunolocalization. The anterior segment of human eyes and the ciliary body of porcine eyes were fixed overnight at 4°C in a periodate-lysine-paraformaldehyde solution containing 75 mM l-lysine, 10 mM sodium periodate, and 37 mM Na2HPO4, pH 7.4, and 2% paraformaldehyde. After equilibration in 70% ethanol, the tissues were embedded in paraffin, and 5-μm sections were cut and placed on microscope slides. Specimens were incubated in 10% normal goat serum (Zymed Laboratories, South San Francisco, CA) for 1 h at room temperature. The specimens were then incubated overnight at 4°C with anti-MRP2 antibody diluted 1:500 in 10% normal goat serum. After extensive washing, the specimens were incubated for 1 h at room temperature with an AlexaFluor 488 goat anti-rabbit antibody (Invitrogen) diluted 1:500 in 10% normal goat serum. The specimens were then washed with PBS and incubated (room temperature for 10 min) in PBS containing 5 μg/ml propidium iodide to stain nuclei. The specimens were washed and immunoreactivity was examined using a confocal microscope (Nikon PCM 2000 scan head fitted to a Nikon E800 microscope; Nikon, Tokyo, Japan) at the Arizona Cancer Center.
Transport Studies. Cultured NPE from passages 3 to 6 were used for transport experiments. Each treatment was performed a total of six times on two different passages (three observations per passage). NPE cells were plated at a density of 12,500 cells/well of a 96-well plate. Once confluent (2-3 days after plating), the media was aspirated, and the cells were rinsed 1× with 200 μl of serum-free DMEM. The DMEM was aspirated and replaced with 100 μl of serum-free DMEM containing 5 μM calcein-AM, 5 μM 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate (CDFDA), 100 μM doxorubicin, or 2 μM 5-chloromethylfluorescein diacetate (CMFDA). Calcein-AM, CDFDA, and CMFDA were from Invitrogen, and doxorubicin was from Sigma-Aldrich. The cells were then placed in an incubator at 37°C (5% CO2) for 30 min to allow calcein, doxorubicin, 5-(and-6)-carboxy-2′,7′-dichlorofluorescein (CDF), or glutathione methylfluorescein to accumulate inside the cells. Where indicated, MK571, cyclosporin A, indomethacin, or sulfinpyrazone was present during the loading period. Calcein-AM, CDFDA, and CMFDA are nonfluorescent and membrane permeant but become fluorescent and relatively membrane impermeant after esterase cleavage of their acetoxymethyl ester or diacetate moieties. Chloromethylfluorescein reacts with intracellular glutathione to form glutathione methylfluorescein. Calcein, doxorubicin, CDF, and glutathione methylfluorescein are substrates of MRP2 (Evers et al., 2000; Tian et al., 2004; Folmer et al., 2007; Förster et al., 2008). After the loading period, the transport buffer was aspirated, and the cells were rinsed 3× (200 μl each) with ice-cold PBS. The cells were lysed in 50 μl of lysis buffer containing 50 mM mannitol and 1 mM Tris base, pH 7.4, with HEPES, 1% Triton X-100, and 0.5% SDS. Fluorescence corresponding to calcein (495/525 nm), doxorubicin (470/585 nm), CDF (495/525 nm), and glutathione methylfluorescein (485/518 nm) was measured with a Varioskan Flash fluorescence plate reader. Protein content in each of the wells was determined with the BCA assay. Calcein, doxorubicin, and CDF concentration was extrapolated from standard curves in which the drugs were diluted serially in the aforementioned lysis buffer. The standard curve for calcein was linear between a concentration range of 50 and 1000 nM calcein. The standard curve for doxorubicin was linear between a concentration range of 500 nM and 10 μM. The standard curve for CDF was linear between 5 and 250 nM. The experimental samples were all within the linear portion of the curves. Intracellular calcein, CDF, and doxorubicin accumulation is expressed as nanomoles per milligram of protein. Intracellular glutathione methylfluorescein is reported as fluorescence intensity units after correcting for protein concentration.
Statistics. All data are expressed as means ± S.E.s. One-way analysis of variance was used to test the effect of multiple treatments and was followed by the Student-Newman-Keuls test for pairwise comparisons. Statistical analyses were performed with ProStat 3.81c (Poly Software International, Pearl River, NY) and deemed significant when p < 0.05.
Results
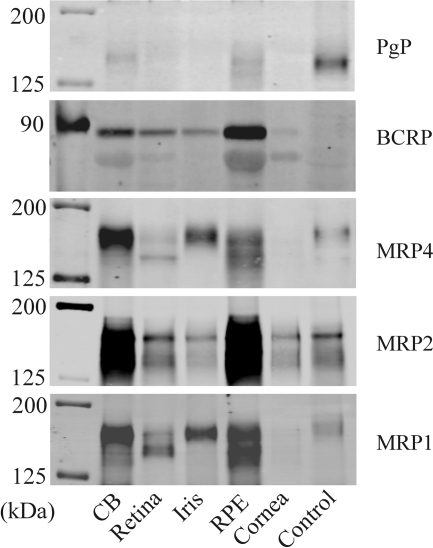
Expression of several ATP-dependent xenobiotic efflux transporters (MRP1, MRP2, MRP4, PgP, and BCRP) was examined by Western blot analysis of microdissected human ocular tissues that contain barrier epithelia, i.e., ciliary body (blood-aqueous humor barrier), retina (blood-retinal barrier), iris (blood-aqueous humor barrier), RPE, and cornea. Immunoreactive band density for each of the transport proteins was highest in the ciliary body and the RPE, although protein for BCRP, MRP4, MRP2, and MRP1 was also detected in other ocular tissues (Fig. 1). The present study focused on MRP2 and the NPE because 1) the NPE of the ciliary body represents an important component of the blood-aqueous humor barrier, 2) many of the therapeutic drugs that do not penetrate across the NPE are MRP2 substrates, and 3) a relatively high level of MRP2 protein was evident in the human ciliary body.
Fig. 1.
Western blot analysis of PgP, BCRP, MRP1, MRP2, and MRP4 in the human ciliary body (CB), retina, iris, RPE, and cornea. Crude homogenate of human kidney cortex served as controls for MRP1, MRP2, and MRP4. Crude homogenate of human liver served as a control for PgP. No apparent protein expression corresponding to BCRP was detected in either the liver or kidney. In most cases, 50 μg of crude homogenate from each of the tissues was separated on 6% (MRP1, MRP2, MRP4, and PgP) or 10% (BCRP) SDS-PAGE gels before electrophoretic transfer of the proteins to polyvinylidene difluoride membranes. Only 6 μg of corneal crude homogenate was used because of the low protein concentration in the sample. Only 5 μg of renal cortex crude homogenate was used for MRP4 because of the rich expression of this transporter in the human kidney.
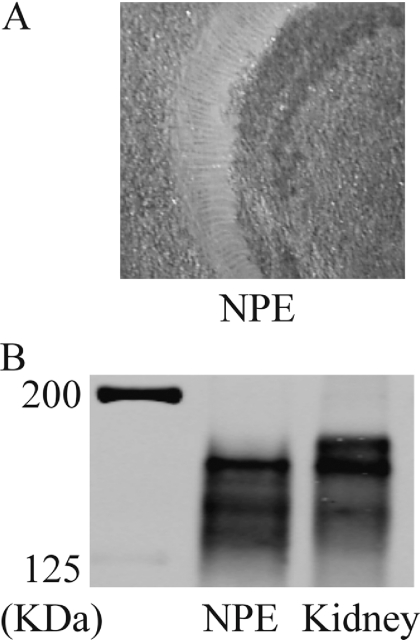
Because the ciliary body consists of several different cell types, it is impossible to determine which cells gave rise to the MRP2 immunoreactive band detected by Western blot (Fig. 1). To focus specifically on the NPE, porcine eyes were used because the porcine NPE can be separated from the stroma and pigmented cell layer (Shahidullah et al., 2007). The porcine NPE, which seemed to be largely devoid of vascular tissue and pigmented cells (Fig. 2A), was subjected to Western blot analysis. A dense MRP2 immunoreactive band was detected, and immunoreactivity was similar to that observed in the porcine renal cortex (Fig. 2B).
Fig. 2.
A, image showing the freshly dissected porcine NPE. The tissue is opaque in color, whereas the background is tan. Pigmented cells and vascular tissue are not discernable. B, Western blot showing expression of MRP2 in the native porcine NPE and porcine renal cortex. Twenty-five micrograms of NPE lysate and renal cortex (kidney) crude homogenate was used for electrophoresis.
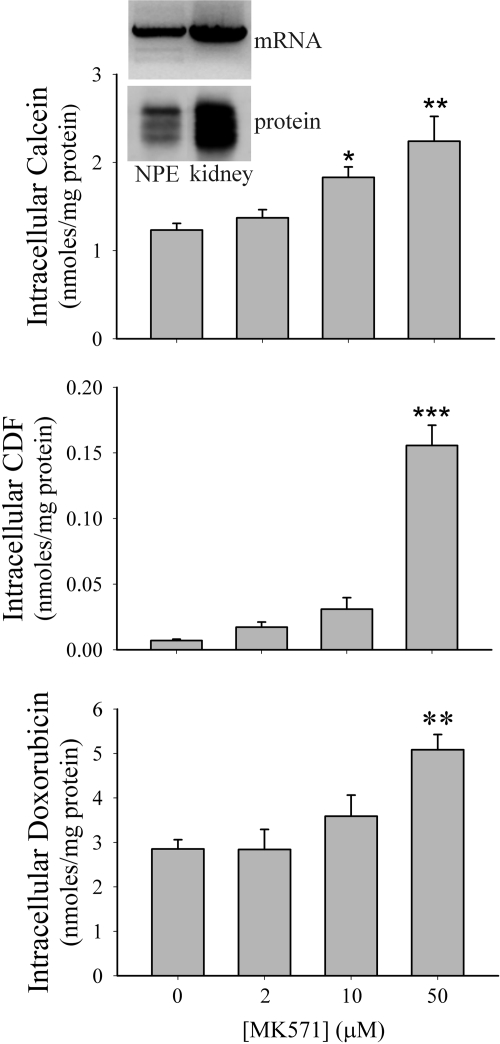
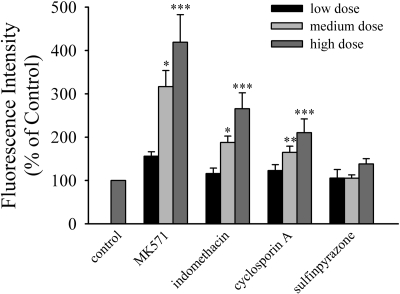
Porcine NPE cells can be grown in culture (Shahidullah et al., 2007), and these cells were found to retain MRP2 mRNA and protein expression (Fig. 3, inset). The intracellular accumulation of the MRP2 substrates calcein, CDF, and doxorubicin was examined in cultured NPE cells in the presence of increasing concentrations of the MRP inhibitor MK571 (van Aubel et al., 1998) (Fig. 3). The intracellular accumulation of calcein (1.8-fold), CDF (22-fold), and doxorubicin (1.8-fold) was significantly elevated by 50 μM MK571 (Fig. 3). Förster et al. (2008) recently has examined the effect of known MRP modulators on the transport of glutathione methylfluorescein by MRP2 heterologously expressed in MDCK cells. The modulators were classified into several groups based on their ability to inhibit MRP2-mediated transport of glutathione methylfluorescein; strong inhibitors, moderate inhibitors, weak inhibitors, and those with no interaction. Based on these findings, the effect of MK571 and cyclosporin A (strong inhibitors), indomethacin (moderate inhibitor), and sulfinpyrazone (no interaction) on the intracellular accumulation of glutathione methylfluorescein in cultured NPE cells was examined (Fig. 4). MK571 (50 μM) caused a 4.3-fold increase in intracellular glutathione methylfluorescein levels. Although not as pronounced as MK571, both 500 μM indomethacin (2.6-fold) and 50 μM cyclosporin A (2.1-fold) also increased intracellular glutathione methylfluorescein. In contrast, sulfinpyrazone had no effect on the intracellular accumulation of glutathione methylfluorescein.
Fig. 3.
Intracellular accumulation of calcein, CDF, or doxorubicin in cultured porcine NPE treated with increasing concentrations of MK571. Cells were loaded with 5 μM calcein-AM, 5 μM 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate, or 100 μM doxorubicin for 30 min at 37°C in the presence of increasing concentrations of MK571. Significantly different from control (no MK571 present), *, p < 0.05; **, p < 0.01; ***, p < 0.001. Inset, agarose gel (mRNA) and Western blot (protein) showing MRP2 expression in cultured porcine NPE and porcine kidney.
Fig. 4.
Intracellular accumulation of glutathione methylfluorescein in cultured porcine NPE treated with increasing concentrations of the MRP modulators MK571 (2, 10, or 50 μM), indomethacin (20, 100, or 500 μM), cyclosporin A (2, 10, or 50 μM), or sulfinpyrazone (20, 100, or 500 μM). Cells were loaded with 2 μM 5-chloromethylfluorescein diacetate for 30 min at 37°C in the presence of the MRP modulators. Significantly different from control (no modulator present). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
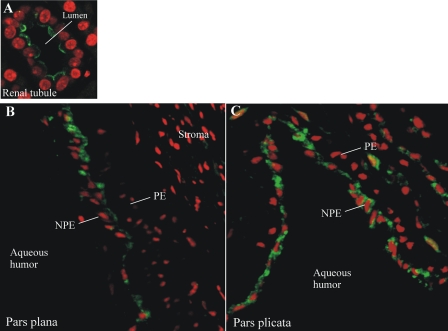
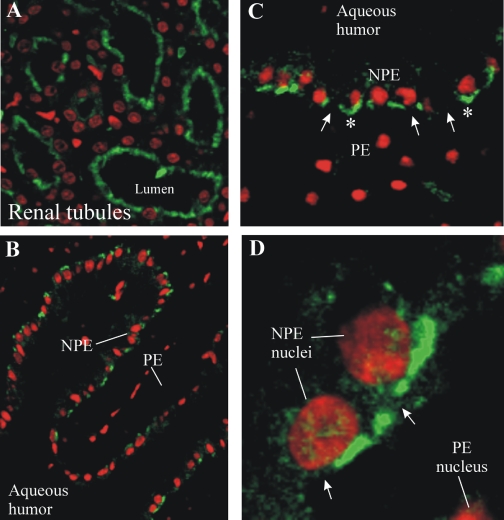
MRP2 in the ciliary epithelium was examined by immunohistochemistry (Figs. 5 and 6). Localization of MRP2 to the brush border membranes of human renal tubule cells indicated that the MRP2 antibody used in this study was adequate for use in immunohistochemistry (Fig. 5A). MRP2 immunoreactivity was present in NPE cells of the pars plana (Fig. 5B) and pars plicata (Fig. 5C) of the human ciliary body. MRP2 seemed to be localized to the apical aspect of NPE cells, with perinuclear staining also observed in some cells. Little to no MRP2 immunoreactivity was noted in the pigmented cell layer or the ciliary body stroma. MRP2 expression was also examined in the porcine ciliary body and kidney (Fig. 6). Similar to the human kidney, MRP2 was localized to brush border membranes of porcine renal tubule cells (Fig. 6A). Within the porcine ciliary body (pars plicata), MRP2 was clearly restricted to the interface of the pigmented and the NPE cells, with no staining apparent in the stroma (Fig. 6B). Because the porcine tissue could be fixed immediately after dissection, allowing for better preservation of tissue morphology (compared with the human tissue), high magnification examination was possible. The pattern of MRP2 localization was discontinuous in some places (Fig. 6, C and D). Closer examination of MRP2 immunoreactivity strongly suggested that the protein was localized in the apical membrane of NPE cells (Fig. 6, C and D).
Fig. 5.
Immunolocalization of MRP2 to the human kidney (A) and pars plana (B) and pars plicata (C) of the human ciliary body. MRP2 is in green and nuclei are stained red with propidium iodide. PE, pigmented cell layer. No immunoreactivity was detected when the primary antibody was omitted (data not shown).
Fig. 6.
Immunolocalization of MRP2 to the porcine kidney (A) and pars plicata (B-D) of the porcine ciliary body. Magnification is increasing from B to C to D. MRP2 is in green, and nuclei are stained red with propidium iodide. PE, pigmented cell layer. Arrows, discontinuous pattern of MRP2 immunoreactivity. *, MRP2 immunoreactivity that was concave with respect to the NPE. No immunoreactivity was detected when the primary antibody was omitted (data not shown).
Discussion
The limited ability of many drugs to penetrate into the eye has led to the development of invasive therapies for the treatment of intraocular diseases. Despite the importance to ocular drug delivery and efficacy, it is not clear why some therapeutics do not readily accumulate inside the eye. Many of the therapeutics used to treat ocular diseases, including cisplatin and methotrexate, are substrates of MRP2. MRP2 occurs in several barrier epithelia that play important roles in protecting sanctuary sites (e.g., central nervous system) from exposure to xenobiotics (Dallas et al., 2006; Deeley et al., 2006). Consistent with this barrier function, protein for MRP2 was abundant in the human ciliary body and the RPE. The NPE of the ciliary body was the main focus of the present study because the NPE represents a significant barrier to the movement of substances from the fenestrated capillaries of the ciliary body into the aqueous humor (Cunha-Vaz, 1979; Freddo, 2001).
In addition to the human ciliary body, MRP2 was detected in native and cultured porcine NPE. The MRP2 substrates calcein, CDF, and doxorubicin were used, along with the MRP inhibitor MK571, to probe for MRP2-mediated transport activity in cultured NPE. MK571 caused increased intracellular accumulation of each of the substrates tested. To further probe for evidence of MRP2 transport activity, we examined the effect of MK571, indomethacin, cyclosporin A, and sulfinpyrazone on the intracellular accumulation of the MRP2 substrate glutathione methylfluorescein. MK571, indomethacin, cyclosporin A, and sulfinpyrazone were examined because their interaction with glutathione methylfluorescein transport mediated by MRP2 heterologously expressed in MDCK cells has been tested rigorously (Förster et al., 2008). Specifically, Förster et al. (2008) found that MK571 and cyclosporin A are strong inhibitors, indomethacin is a moderate inhibitor, and sulfinpyrazone has no effect on MRP2-mediated glutathione methylfluorescein transport. Consistent with the expression of MRP2 in cultured NPE cells, MK571, cyclosporin A, and indomethacin all caused increased accumulation of glutathione methylfluoroscein, whereas sulfinpyrazone was without effect.
MRP2 was localized to the apical interface of the NPE and pigmented cell layer of both the human and porcine ciliary body. The following lines of evidence strongly suggest that MRP2 is located in the apical membrane of NPE cells: 1) as exemplified in Fig. 6C, the pattern of staining was often concave with respect to the NPE; 2) there was some diffuse staining in the NPE cells (Figs. 5 and 6); 3) there was rich expression of MRP2 in the freshly dissected native porcine NPE (Fig. 2); and 4) cultured cells derived from the native porcine NPE retained MRP2 expression and transport activity consistent with MRP2 (Figs. 3 and 4). However, we cannot exclude the possibility that MRP2 may also be present in the pigmented cell layer because the pigment in these cells may mask immunoreactivity. The nearly undetectable level of MRP2 immunoreactivity in the choroid implies that the functional barrier to xenobiotic entry into the eye (at the level of the ciliary body) does not exist in the fenestrated capillary endothelium. It is important that MRP2 immunoreactivity was present in both the pars plana and pars plicata, both regions of the ciliary body that are perfused with a fenestrated capillary endothelium. The apical location of MRP2 in the NPE is consistent with reports that MRP2 is localized to apical membranes in other barrier epithelia (Deeley et al., 2006). In the currently accepted model of solute transport across the ciliary epithelium, solute transporters and ion channels are basolaterally located in the pigmented cell layer and the NPE (Do and Civan, 2004). To our knowledge, this is the first report of a transporter at the NPE apical surface.
The pattern of MRP2 immunoreactivity at the NPE apical surface was discontinuous in some areas. This differed from MRP2 immunoreactivity in the renal tubule cells. The NPE and pigmented cells are coupled at their apical membranes via gap junctions, and immunoreactivity for connexin isoforms that comprise these gap junctions is punctate (Coffey et al., 2002). We speculate that MRP2 may be excluded from junctional plaques that form between the two cell types.
Several other proteins associated with xenobiotic metabolism have been identified previously in NPE cells. A number of organic anion-transporting polypeptide (OATP; SLC21A family) isoforms occur in the basolateral membrane of NPE cells (Gao et al., 2005). In liver, OATPs mediate the sodium-independent uptake of xenobiotics across the basolateral membrane of hepatocytes, and MRP2 at the canalicular (apical) membrane is involved in the final excretion of many of these xenobiotics into the bile (Takikawa, 2002). The sodium-dicarboxylate cotransporter 3 (NaDC3; SLC13A3) has been identified in NPE cells using in situ hybridization (George et al., 2004). In the kidney, NaDC3 is localized in the basolateral membrane of renal proximal tubule cells and is responsible for maintaining an outwardly directed α-ketoglutarate gradient that drives organic anion uptake (organic anion/α-ketoglutarate exchange) by OAT1 (SLC22A6) and OAT3 (SLC22A8) (Dantzler and Wright, 2003). The expression of several cytochrome P450 enzymes in NPE cells makes these cells well suited for metabolizing xenobiotics (Zhao and Shichi, 1995; Doshi et al., 2006). MRP2 is probably one of several different transport proteins that contributes to xenobiotic transport by the NPE.
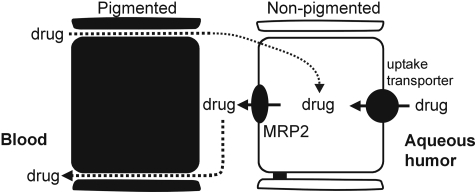
Based on the current findings and the work of others, we hypothesize that the NPE has two likely roles with respect to ocular transport of xenobiotics, “barrier” and “absorptive” roles (Fig. 7). MRP2 (and possibly other ATP-dependent xenobiotic transporters) at the apical membrane may rapidly export substrates that enter NPE cells from the blood, thus serving an active barrier role with respect to drug penetration (blood-to-aqueous humor). This scheme would be most relevant to hydrophobic drugs that are present in the circulation, such as those administered intravenously. This could explain why no measurable amount of the chemotherapeutic etoposide, an MRP2 substrate, was found in the aqueous or vitreous of primates (Macaca fascicularis) after intravenous administration, despite its relatively high octanol-to-water partition coefficient (LogP = 0.6) (Mendelsohn et al., 1998; Folmer et al., 2007). Doxorubicin is another lipophilic (LogP = 1.27) chemotherapeutic that is a substrate of MRP2 (Folmer et al., 2007), and it is currently being used in phase II and phase III clinical trials (National Cancer Institute NCT00004006 and NCT00186888) aimed at treating retinoblastoma in children. In the present study, MRP-mediated transport activity was effective in preventing doxorubicin from accumulating in the intracellular compartment of cultured NPE. The barrier role of MRP2 and the detoxifying role of cytochrome P450 enzymes may explain why there are no apparent defects in NPE function, i.e., the tissue is well protected. That is, the NPE is involved in aqueous humor formation, and the rate of its formation changes very little with age or disease (Brubaker et al., 1981).
Fig. 7.
Model depicting the proposed barrier and absorptive roles for MRP2 in xenobiotic transport by the NPE of the ciliary body. The proposed barrier role played by MRP2 is as follows. Xenobiotics (hydrophobic) diffuse out of the fenestrated ciliary body capillaries and between (or across) the pigmented epithelial cells, which do not contain tight junctions. Apically located MRP2 could prevent these xenobiotics from accumulating inside NPE cells and the aqueous humor by actively pumping the drugs back into the paracellular space between the pigmented and NPE cells. According to the absorptive model, xenobiotics (hydrophilic) originating inside the eye could be taken up across the basolateral membrane by uptake transporters (possibly the OATPs). MRP2 in the apical membrane of the NPE could prevent the intracellular accumulation of these drugs by pumping them into the paracellular space between the pigmented and NPE cells.
A “hepatocyte-like” or “renal tubule-like” absorptive role for MRP2 may affect transport of substrates from aqueous humor to blood as follows. Xenobiotics present in the aqueous humor potentially would be taken up across the basolateral membrane of NPE cells by “uptake” transporters (Fig. 7). These could be the OATPs that have been shown to occur in NPE cells (Gao et al., 2005). Alternatively, uptake could be mediated by the OATs, which are most efficient when coexpressed with NaDC3 (shown to be expressed in NPE cells; George et al., 2004). However, to our knowledge, the actual expression of OATs in the ciliary body has not been demonstrated. Once inside NPE cells, xenobiotics potentially would be transported into the ciliary body stroma by apically located MRP2 (and possibly other ATP-dependent xenobiotic transporters). This mechanism could be most relevant to relatively hydrophilic drugs present in the intraocular compartment. Such an absorptive mechanism may also be important for removal of endogenous substances that originate inside the eye but cannot readily diffuse across lipid bilayers. Of course, outflow through the trabecular meshwork may also be an efficient means of removing potentially toxic substances from the eye interior, as aqueous humor turns over every 90 to 100 min in healthy individuals.
It is important to acknowledge that ocular epithelia other than the NPE and transporters other than MRP2 are also likely to influence the accumulation of xenobiotics in the eye interior. In addition to the NPE (blood-aqueous humor barrier), there are several other barrier epithelia in the eye, including the capillary endothelium of the retina (blood-retinal barrier) and iris (blood-aqueous humor barrier), the RPE (blood-retinal barrier), and the cornea. Several ATP-dependent xenobiotic transporters other than MRP2 were identified by Western blot in human ocular tissues that contain these barrier epithelia. Like the ciliary body, MRP1, MRP2, MRP4, BCRP, and PgP were expressed in the human RPE, and all but PgP were detected in the retina and iris. In addition, MRP2 immunoreactivity was demonstrated in the human cornea. MRP2 and these other transporters may be especially important for protecting intraocular tissues with a low rate of turnover, such as the retina and lens epithelium, from exposure to potentially harmful toxins.
Acknowledgments
We thank Dr. Nathan J. Cherrington for providing the human liver sample used for Western blot analysis of P-glycoprotein.
This work was supported by the National Institutes of Health National Eye Institute [Grant EY006915]; and by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK074022].
doi:10.1124/jpet.108.149625.
ABBREVIATIONS: NPE, nonpigmented epithelium; MRP, multidrug resistance-associated protein; RPE, retinal pigmented epithelium; DMEM, Dulbecco's modified Eagle's medium; PAGE, polyacrylamide gel electrophoresis; BCRP, breast cancer-resistant protein; PgP, P-glycoprotein; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; AM, acetoxymethyl ester; CDFDA, 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate; CMFDA, 5-chloromethylfluorescein diacetate; CDF, 5-(and-6)-carboxy-2′,7′-dichlorofluorescein; MK571, ((E)-3-[[[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-[[3-dimethylamino)-3-oxopropyl]thio]methyl]thio]-propanoic acid; OATP, organic anion-transporting polypeptide; NaDC3, sodium-dicarboxylate cotransporter 3.
References
- Brubaker RF, Nagataki S, Townsend DJ, Burns RR, Higgins RG, and Wentworth W (1981) The effect of age on aqueous humor formation in man. Ophthalmology 88 283-288. [DOI] [PubMed] [Google Scholar]
- Coffey KL, Krushinsky A, Green CR, and Donaldson PJ (2002) Molecular profiling and cellular localization of connexin isoforms in the rat ciliary epithelium. Exp Eye Res 75 9-21. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz J (1979) The blood-ocular barriers. Surv Ophthalmol 23 279-296. [DOI] [PubMed] [Google Scholar]
- Dallas S, Miller DS, and Bendayan R (2006) Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev 58 140-161. [DOI] [PubMed] [Google Scholar]
- Dantzler WH and Wright SH (2003) The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules. Biochim Biophys Acta 1618 185-193. [DOI] [PubMed] [Google Scholar]
- Deeley RG, Westlake C, and Cole SP (2006) Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev 86 849-899. [DOI] [PubMed] [Google Scholar]
- Do CW and Civan MM (2004) Basis of chloride transport in ciliary epithelium. J Membr Biol 200 1-13. [DOI] [PubMed] [Google Scholar]
- Doshi M, Marcus C, Bejjani BA, and Edward DP (2006) Immunolocalization of CYP1B1 in normal, human, fetal and adult eyes. Exp Eye Res 82 24-32. [DOI] [PubMed] [Google Scholar]
- El-Sheikh AA, van den Heuvel JJ, Koenderink JB, and Russel FG (2007) Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J Pharmacol Exp Ther 320 229-235. [DOI] [PubMed] [Google Scholar]
- Evers R, Kool M, Smith AJ, van Deemter L, de Haas M, and Borst P (2000) Inhibitory effect of the reversal agents V-104, GF120918 and Pluronic L61 on MDR1 Pgp-, MRP1- and MRP2-mediated transport. Br J Cancer 83 366-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer Y, Schneider M, Blum HE, and Hafkemeyer P (2007) Reversal of drug resistance of hepatocellular carcinoma cells by adenoviral delivery of anti-ABCC2 antisense constructs. Cancer Gene Ther 14 875-884. [DOI] [PubMed] [Google Scholar]
- Förster F, Volz A, and Fricker G (2008) Compound profiling for ABCC2 (MRP2) using a fluorescent microplate assay system. Eur J Pharm Biopharm 69 396-403. [DOI] [PubMed] [Google Scholar]
- Freddo TF (2001) Shifting the paradigm of the blood-aqueous barrier. Exp Eye Res 73 581-592. [DOI] [PubMed] [Google Scholar]
- Freddo TF, Bartels SP, Barsotti MF, and Kamm RD (1990) The source of proteins in the aqueous humor of the normal rabbit. Invest Ophthalmol Vis Sci 31 125-137. [PubMed] [Google Scholar]
- Gao B, Huber RD, Wenzel A, Vavricka SR, Ismair MG, Remé C, and Meier PJ (2005) Localization of organic anion transporting polypeptides in the rat and human ciliary body epithelium. Exp Eye Res 80 61-72. [DOI] [PubMed] [Google Scholar]
- George RL, Huang W, Naggar HA, Smith SB, and Ganapathy V (2004) Transport of N-acetylaspartate via murine sodium/dicarboxylate cotransporter NaDC3 and expression of this transporter and aspartoacylase II in ocular tissues in mouse. Biochim Biophys Acta 1690 63-69. [DOI] [PubMed] [Google Scholar]
- Hooijberg JH, Broxterman HJ, Kool M, Assaraf YG, Peters GJ, Noordhuis P, Scheper RJ, Borst P, Pinedo HM, and Jansen G (1999) Antifolate resistance mediated by the multidrug resistance proteins MRP1 and MRP2. Cancer Res 59 2532-2535. [PubMed] [Google Scholar]
- Leier I, Hummel-Eisenbeiss J, Cui Y, and Keppler D (2000) ATP-dependent para-aminohippurate transport by apical multidrug resistance protein MRP2. Kidney Int 57 1636-1642. [DOI] [PubMed] [Google Scholar]
- Madon J, Hagenbuch B, Landmann L, Meier PJ, and Stieger B (2000) Transport function and hepatocellular localization of mrp6 in rat liver. Mol Pharmacol 57 634-641. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Abramson DH, Madden T, Tong W, Tran HT, and Dunkel IJ (1998) Intraocular concentrations of chemotherapeutic agents after systemic or local administration. Arch Ophthalmol 116 1209-1212. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Tamiya S, and Delamere NA (2007) Primary culture of porcine nonpigmented ciliary epithelium. Curr Eye Res 32 511-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS and Rudt LA (1975) Ocular vascular and epithelial barriers to microperoxidase. Invest Ophthalmol 14 556-560. [PubMed] [Google Scholar]
- Takikawa H (2002) Hepatobiliary transport of bile acids and organic anions. J Hepatobiliary Pancreat Surg 9 443-447. [DOI] [PubMed] [Google Scholar]
- Tian X, Zamek-Gliszczynski MJ, Zhang P, and Brouwer KL (2004) Modulation of multidrug resistance-associated protein 2 (Mrp2) and Mrp3 expression and function with small interfering RNA in sandwich-cultured rat hepatocytes. Mol Pharmacol 66 1004-1010. [DOI] [PubMed] [Google Scholar]
- van Aubel RA, van Kuijck MA, Koenderink JB, Deen PM, van Os CH, and Russel FG (1998) Adenosine triphosphate-dependent transport of anionic conjugates by the rabbit multidrug resistance-associated protein mrp2 expressed in insect cells. Mol Pharmacol 53 1062-1067. [PubMed] [Google Scholar]
- van de Water FM, Masereeuw R, and Russel FG (2005) Function and regulation of multidrug resistance proteins (MRPs) in the renal elimination of organic anions. Drug Metab Rev 37 443-471. [DOI] [PubMed] [Google Scholar]
- Zhao C and Shichi H (1995) Immunocytochemical study of cytochrome P450 (1A1/1A2) induction in murine ocular tissues. Exp Eye Res 60 143-152. [DOI] [PubMed] [Google Scholar]