Abstract
Hypermethylation of 5′-cytosine-guanosine islands of tumor suppressor genes resulting in their silencing has been proposed to be a hallmark of various tumors. Modulation of DNA methylation with DNA methylation inhibitors has been shown to result in cancer cell differentiation or apoptosis and represents a novel strategy for chemotherapy. Currently, effective DNA methylation inhibitors are mainly limited to decitabine and 5-azacytidine, which still show unfavorable toxicity profiles in the clinical setting. Thus, discovery and development of novel hypomethylating agents, with a more favorable toxicity profile, is essential to broaden the spectrum of epigenetic therapy. Parthenolide, the principal bioactive sesquiterpene lactone of feverfew, has been shown to alkylate Cys38 of p65 to inhibit nuclear factor-κB activation and exhibit anti-tumor activity in human malignancies. In this article, we report that parthenolide 1) inhibits DNA methyltransferase 1 (DNMT1) with an IC50 of 3.5 μM, possibly through alkylation of the proximal thiolate of Cys1226 of the catalytic domain by its γ-methylene lactone, and 2) down-regulates DNMT1 expression possibly associated with its SubG1 cell-cycle arrest or the interruption of transcriptional factor Sp1 binding to the promoter of DNMT1. These dual functions of parthenolide result in the observed in vitro and in vivo global DNA hypomethylation. Furthermore, parthenolide has been shown to reactivate tumor suppressor HIN-1 gene in vitro possibly associated with its promoter hypomethylation. Hence, our study established parthenolide as an effective DNA methylation inhibitor, representing a novel prototype for DNMT1 inhibitor discovery and development from natural structural-diversified sesquiterpene lactones.
DNA methylation of cytosine residues in the context of the sequence 5′-cytosine-guanosine (CpG) in gene promoter regions is an epigenetic mechanism that controls gene transcription, genome stability, and genetic imprinting (Robertson, 2005). This process is regulated by DNA methyltransferases (DNMT1, DNMT3a, and DNMT3b) in the presence of S-adenosyl-methionine (SAM) that serves as a methyl donor for methylation of cytosine residues at the C-5 position to yield 5-methylcytosine (Robertson, 2005). Aberrant hypermethylation of promoter CpG-rich regions (>55% CG content, the so-called CpG islands) of tumor suppressor genes (TSGs) results in transcriptional silencing in a variety of solid tumors and blood cancers (Yoo and Jones, 2006). In vitro and in vivo treatment with DNA methylation inhibitors has proven to be effective in restoring gene expression and normal patterns of differentiation and apoptosis in malignant cells (Yoo and Jones, 2006).
Two nucleoside analogs (azanucleosides) with hypomethylating activity, i.e., 5-aza-2′-deoxycytidine (decitabine) and 5-azacytidine, have recently been approved by the United States Food and Drug Administration for the treatment of myelodysplastic syndrome, clonal myeloid disorders, and acute myeloid leukemia (Silverman et al., 2002; Kantarjian et al., 2006). Clinical trials with these agents are ongoing in other type of cancers, with encouraging results (Lyko and Brown, 2005). However, toxicities (i.e., myelosuppression) inherent to the cell-cycle phase specificity of nucleoside analogs pose significant limitations to the use of these drugs, especially in patients with solid tumors. Thus, the discovery and development of novel, effective DNA methylation inhibitors that have a more favorable toxicity profile are essential to broaden the spectrum of epigenetic targeting therapeutic strategies (Cheng et al., 2003; Fang et al., 2003; Segura-Pacheco et al., 2003; Villar-Garea et al., 2003; Lee et al., 2005; Mund et al., 2006; Winquist et al., 2006). To date, however, among these evaluated hypomethylating non-nucleoside agents, e.g., procaine, procainamide, RG-108, none shows pharmacologic activity comparable with that of azanucleosides (Chuang et al., 2005). Nevertheless, the recent discovery of the hypomethylation activity of EGCG (Fang et al., 2003), a major polyphenol in green tea and other dietary polyphenols, has paved the way aiming to identify novel and efficient DNA methylation inhibitors from plant-derived natural products (phytochemicals).
Parthenolide, the principal bioactive sesquiterpene lactone component of feverfew, has been used for the treatment of fever, migraine, and arthritis (Kwok et al., 2001). Because of inhibition activity to nuclear transcription factor (NF-κB), this compound has been tested for anti-cancer activities, with encouraging results both in in vitro and in vivo (Wiedhopf et al., 1973; Woynarowski and Konopa, 1981; Patel et al., 2000; García-Piñeres et al., 2001, 2004; Nakshatri et al., 2004; Won et al., 2004; Guzman et al., 2005; Steele et al., 2006). Mechanistic studies demonstrated that parthenolide covalently blocks the thiol group of cysteine at the active site of p65 NF-κB through its highly electrophilic γ-methylene lactone ring. This effect may play a pivotal role in apoptosis (García-Piñeres et al., 2001, 2004). Therefore, we postulated here that a similar interaction could occur between parthenolide and DNMT1. In fact, in DNA methylation, it has been shown that, before the process, cytosine is “flipped out” of the DNA double helix to form a transition state, with a catalytic functional thiol of Cys1226 in the catalytic site of DNMT1 resulting in a reactive 4,5-enamine followed by β-elimination, which releases DNMT1 and the product 5-methylcytosine (Santi et al., 1983). It is possible that parthenolide covalently binds to the Cys1226 thiol group and inhibits the activities of this enzyme without the need to be incorporated into DNA, as is the case for azanucleosides, although DNMT1 turnover is also increased in the presence of azanucleosides (Ghoshal et al., 2005). Recently, bortezomib, a United States Food & Drug Administration-approved proteasomal inhibitor that inhibits NF-κB translocation to the nucleus, was found to be a novel DNA methylation inhibitor, possibly through modulation of DNA methyltransferase activity by disruption of Sp1/NF-κB complex in leukemia cells (Liu et al., 2008). Therefore, we hypothesized that, in addition to chemical inhibition of DNMT1, parthenolide acts as a NF-κB inhibitor and also modulates the DNMT1 expression in leukemia cells.
In this study, we validate these hypotheses by demonstrating for the first time that parthenolide indeed inhibits DNMT activity and down-regulates DNMT1, resulting in significant DNA hypomethylation and gene re-expression both in vitro and in vivo. Our study emphasizes the importance of testing parthenolide as an alternative treatment for abnormal epigenetic patients unresponsive to current DNA methylation inhibitor therapy and, perhaps, for other solid tumors and nonproliferating cancers characterized by aberrant DNA methyltransferase activities.
Materials and Methods
Materials. The high-performance liquid chromatography-grade water (>18 mΩ) was obtained from an E-pure water purification system (Barnstead, Dubuque, IA). Parthenolide, methanol, acetonitrile (high-performance liquid chromatography grade), ammonium formate, ammonium acetate, ammonium bicarbonate, decitabine, 5-methyl-2-deoxycytidine (5mdC), 2dG, nucleophosphatase (NP1), snake venom phosphatase and alkaline phosphatase, deoxynucleotide triphosphate (2.5 mM), AmpliTaqGold polymerase, and 10× PCR buffer were all purchased from Sigma-Aldrich (St. Louis, MO). The primers for amplification of HIN-1 tumor suppressor gene and its bisulfite-converted promoter region, DNMT1, and its binding promoter region (available upon request) and the substrate, a double strand oligonucleotide, the biotinylated oligonucleotide 5′-AGT TAG ATA AAG CCC CGA AAA CCG GCT TTT ATA CTC GA-3′-biotin, as Oligo A, and the digoxigenin NHS ester oligonucleotide, 5′-CTG AGT ATA AAA GCC GGT TTT CGG GGC TTT ATC TAA CT-DigoxN/-3′, as Oligo B for M.SssI assay were purchased from Integrated DNA Technology (Coralville, IA). M.SssI methylase, SAM (3.2 mM), and 10× incubation buffer was purchased from New England Biolab Inc. (Beverly, MA). ParthenolideA was prepared from parthenolide according to the reported procedure, and its structure was confirmed by mass spectrometry and NMR showing consistent mass and 1H NMR spectra (Hwang et al., 2006).
Human DNMT1 Homology Modeling. The homology model of human DNMT1 (hDNMT1) catalytic domain was built with the known crystal structure of the bacterial DNA methyltransferase (Hhal) catalytic domain (Protein Data Bank code 5MHT) as the modeling template. Their catalytic domains were aligned by a combination of ClustalW and Smith-Waterman methods. The two aligned regions were modeled using Modeller version 8 (Sali and Blundell, 1993). Together with the cofactor SAM and flipped cytosine substrate plus 15-Å truncated octahedron TIP3P (transferable intermolecular potential, three position) water box, the resulting structure was energy-minimized.
Docking of Parthenolide. AutoDock version 4.0.0 (Huey et al., 2007) was used for the docking simulation. We selected the Lamarckian genetic algorithm for ligand conformational searching, because it has enhanced performance relative to the simulated annealing or the simple genetic algorithm (Friesner et al., 2004). For parthenolide, all hydrogen atoms were added, and Gasteiger charges (Morris et al., 1996) were assigned; nonpolar hydrogen atoms then were merged. Three-dimensional affinity grids (80 × 100 × 70) centered on the empty binding site with 0.375 Å spacing were calculated for each of the following atom types: a) protein: A (aromatic C), C, HD, N, NA, OA, SA; and b) ligand: C, A, OA, HD, e (electrostatic) and d (desolvation) using Autogrid4. The translation, rotation, and internal torsions of the ligand are defined as its state variables, and each gene represents a state variable. Lamarckian genetic algorithm adds local minimization to the genetic algorithm, enabling modification of the gene population. The docking parameters were as follows: trials of 100 dockings, population size of 250, random starting position and conformation, translation step ranges of 2.0 Å, rotation step ranges of 50°, elitism of 1, mutation rate of 0.02, crossover rate of 0.8, local search rate of 0.06, and 50 million energy evaluations. Final docked conformations were clustered using a tolerance of 1.5-Å root-meansquare deviations. We define E-binding as the difference between the potential energy of the ligand-enzyme (E-complex) and the sum of potential energies of the ligand (E-ligand) and enzyme (E-enzyme); thus, E-binding = E-complex - (E-ligand + E-enzyme). A favorable (more negative) binding energy is taken as evidence in which the ligand possesses high affinity for DNMT1.
In vitro Inhibition Effect of Parthenolide and ParthenolideA on M.SssI and DNMT1. Oligo A and Oligo B with the sequence described under Materials and Methods were hybridized by heating a solution containing 1 μM Oligo A and Oligo B dissolved in hybridization buffer (1.0 M sodium chloride, 60 mM sodium phosphate, pH 7.4) at 95°C for 10 min, and then chilling on ice for 30 min followed by incubating at 37°C for 2.5 h to yield a sequence complementary double strand oligonucleotide. The double strand oligonucleotide (10 nM) then was methylated with 2.0 U of M.SssI in the presence of SAM (320 μM) in the buffer containing 5.0 mM sodium chloride, 1.0 mM MgCl2, 0.1 mM dithiothreitol without the following concentration of parthenolide or parthenolideA (0, 0.3, 1.0, 3.0, 10, 30, and 100 μM) in a total volume of 50 μl at 37°C for 1 h. The reaction mixture was diluted 10 times with the hybridization buffer, and a 100-μl aliquot of the diluted solution was transferred to a 96-well streptavidin-coated plate to allow the attachment of the ds-oligonucleotide at room temperature for 30 min. The restriction endonuclease HpaII was then added, and the mixture was incubated at 37°C for 12 h, followed by five times of washing with washing buffer to remove cut-off fragments of oligonucleotide, endonuclease HpaII, and reaction buffer. Anti-digoxigenin-alkaline phosphatase with super bovine serum albumin block buffer then was added into the wells, followed by incubation for 30 min at room temperature. After washing, an appropriate volume of Attophos (Promega Madison, WI) substrate solution in diethanolamine buffer was added into each well, and the plate was incubated at 37°C for 30 min. Attosphos substrate solution with low fluorescence was cleaved by the conjugated alkaline phosphatase to form a highly fluorescent product. The generated fluorescence was measured at excitation 430/emission 560 (filter = 550 nm) using a Gemini XS fluorescence microtiter plate reader (Molecular Devices, Sunnyvale, CA), and the inhibition effect of parthenolide was expressed as a decrease in fluorescence intensity. The inhibition effect of parthenolide on DNMT1 was evaluated using the DNMT1 activity assay kit (Epigentek, New York, NY) according to the protocol provided by the manufacturer.
Cytoxicity, Cell Cycle and Apoptosis Analysis of Parthenolide-Treated Cells. K562, Kasumi-1, MV4-11 leukemia cell lines were cultured in RPMI 1640 medium (VWR International, Inc., West Chester, PA), and the breast cancer cell line MCF-7 was maintained in Dulbecco's modified Eagle's medium (Mediatech, Herndon, VA) supplemented with 10 or 15% (Kasumi-1) fetal bovine serum (Invitrogen, Carlsbad, CA) and 1% (v/v) penicillin/streptomycin (Invitrogen) antibiotic solution at 37°C in an incubator under 5% CO2 atmosphere. Cell lines were treated with the indicated concentrations of parthenolide or decitabine (as control). The cytotoxicity was measured using MTS assay parallel with cell counting. The cell cycle and apoptosis of MV4-11 cells were analyzed by treating 1 to 2 × 105 cells/ml with parthenolide at 0, 3, and 10 μM for 24 h on a Becton Dickinson BD-LSR flow cytometer (BD Biosciences, San Jose, CA), according to the standard protocol provided by the manufacturer.
Quantification of Global DNA Methylation. The genomic DNA isolated from the above cell lines using DNeasy Tissue kit (Qiagen, Minneapolis, MN) was hydrolyzed according to the manufacturer's instruction, and the resulting hydrolysate was analyzed for DNA methylation level by the LC-MS/MS method under conditions as described previously (Liu et al., 2007a).
Immunoblotting. Fresh cells (106-108 cells) in culture medium or frozen cell pellets, thawed on ice and re-suspended in 1 ml of ice-cold PBS, were centrifuged (<1000 g) at 4°C for 5 min, and the supernatant was removed and discarded. The pellet was re-suspended in 100 to 200 μl of ice-cold lysis buffer (20 mM, pH 7.0 HEPES, 150 mM NaCl, 0.1% Nonidet P-40 supplemented with 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM NaF, 1 mM benzamidine, and 1 mM phenylmethylsulfonyl fluoride) with protease inhibitors (Protease Inhibitor cocktail Set III) (Calbiochem-Novabiochem Corporation, La Jolla, CA) and incubated on ice for 40 min. The lysate was centrifuged at 1000g for 15 min at 4°C. The supernatants were frozen in liquid nitrogen and stored at -80°C. Equal amounts of protein for each sample were then separated on 5 to 15% SDS-polyacrylamide gradient gels as confirmed by probing with β-actin. The DNMT1 and β-actin levels were determined using appropriate antibodies. The blots were blocked in Tris-buffered saline Tween 20 (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% Tween 20) containing 5% nonfat milk and subsequently incubated with anti-DNMT1 (New England Biolabs; 1:2000). Protein recognized by the antibody was detected using the chemiluminescent detection kit (Pierce, Rockford, IL).
Electrophoretic Mobility-Shift Assays and Antibody-Supershift Assays. Three pairs of oligonucleotides derived from hDNMT1 promoter regions that contain putative Sp1 binding sites were chemically synthesized, annealed to complementary oligos, and labeled with [32P]dCTP and Klenow. All reactions were processed on ice, with the exception of specific indication. The oligo sequences used were: DNMT1/Sp1-1F, 5′-GGGCTCCGCGTGGGGGGGGTGTGTGCCCGCCTTGCGC-3′; DNMT1/Sp1-1R, 5′-GCGCAAGGCGGGCACACACCCCCCCCACGCGGAG-3′; DNMT1/Sp1-2F, 5′-GGGCATGGCCGGCTCCGTTCCATCCTTC-3′; DNMT1/Sp1-2R, 5′-GAAGGATGGAACGGAGCCGGCCATG-5′; DNMT1/Sp1-3F, 5′-CCCGGACTGGGGTGGTAGA CGCCG-3′; and DNMT1/Sp1-3R, 5′-GGGCGGCGTCTACCACCCCAGTCCGGG-3′. Total protein extracts were isolated from MV4-11 cells using M-PER (mammalian protein extraction reagent; Pierce), and nuclear extracts were prepared using NE-PER (nuclear and cytoplasmic extraction reagent; Pierce). EMSA with total extracts or nuclear extracts and 32P-labeled DNMNT1 promoter oligomers were performed as described previously (Liu et al., 2008). For antibody-supershift assays, a 2-μg aliquot of antibodies (Sp1, NF-κB/p65) or control rabbit IgG was added after the binding reactions had proceeded for 10 min and further incubated for another 20 min before gel loading. Alternatively, nuclear extracts were preincubated with individual antibodies at 4°C for 16 h before adding to the binding reactions.
Chromatin Immunoprecipitation. Chromatin immunoprecipitation (ChIP) was performed using the chromatin immunoprecipitation assay kit (Millipore, Billerica, MA) according to the manufacturer's protocol. In brief, MV4-11 cells, untreated or treated with parthenolide for 24 h, were cross-linked for 20 min at room temperature by adding 270 μl of formaldehyde directly to 10 ml of culture medium. Formaldehyde was then quenched with glycine at 0.125 M final concentration. Cells were washed twice with ice-cold PBS containing protease inhibitors (Protease Inhibitor Cocktail Set III; Calbiochem) and were harvested and placed in SDS lysis buffer containing protease inhibitors and left on ice for 15 min. Chromatin was then fragmented to an average size of 0.2 to 1 kilobases by sonication. After centrifugation, the supernatant was equally divided and diluted 10-fold in ChIP dilution buffer. Approximately 5 to 10% of the solution was then saved for control (input DNA). After preclearing with 80 μl of salmon sperm DNA/protein A/agarose-50% slurry (Millipore) for 30 min at 4°C, antibodies (see below) were added and incubated overnight with gentle rotation at 4°C. Another 80-μl aliquot of salmon sperm DNA/protein A/agarose-50% slurry was then added to the solution, which was incubated for an additional4hat 4°C with agitation. The beads were then washed once each with low-salt immune complex buffer, high-salt immune complex buffer, and LiCI immune complex buffer and twice with Tris-EDTA buffer (Millipore). The immunoprecipitated protein-DNA complex was eluted with elution buffer (1% SDS and 0.1 M NaHCO3). The input and immunoprecipitated chromatins were then incubated at 65°C for 6 h to reverse the formaldehyde cross-links and digested with proteinase K (RNA grade, 20 μg/ml; Invitrogen) for 4 h at 50°C to remove proteins. The DNA was extracted with pheno-chloroform, precipitated with ethanol, and dissolved in water. The antibodies used for immunoprecipitation were anti-Sp1 and anti-p65 (Millipore). Immunoprecipitated chromatin was analyzed by PCR with primers: specific DNMT1 gene promoter, forward 5′-TCTAAAACTCCTGGGCTCAA-3′, and reverse 5′-TATAGAGGCCCATGCCACTT-3′. Syber Green quantitative PCR was performed. Each reaction used 2 μl of DNA template and 12.5 μl of SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) and was normalized to input DNA. The specificity of PCR products was analyzed by the addition of a melting curve cycle, which consisted of 1 cycle each of 60°C for 15 s, 95°C for 20 s, and 60°C for 15 s, followed by analysis on Dissociation Curve Analysis software, version 1.0 (Applied Biosystems).
Hypomethylation of Parthenolide on HIN-1 Promoter in MCF-7 Cells. Genomic DNA isolated from MCF-7 cells was treated with bisulfite using the Epi methylation Kit (Qiagen) according to the instruction from the manufacturer followed by PCR amplification as follows. The sequences of the primers used were: for HIN-1, forward 5′-GTA GGG ATT AGG GAG TTA GGA ATT G-3′, and reverse 5′-TAA AAC CCT CTA AAA ACA AAC AAA C-3′. PCR amplifications were performed as follows: 95°C for 5.5 min, 55°C for 30s for 5 cycles, 52°C for 30 s for 40 cycles, and 72°C for 30s for 45 cycles, with a final step at 72°C for 4 min. The PCR product size and melting temperature of the promoter of HIN-1 were 182 base pairs and 71.2°C, respectively. PCRs were carried out in a 100-μl volume containing 10 μl of buffer (10×), 2 μl of each primer (10 μM), 2 μl of (10 mM) dNTPs, 2 U of Platinum Taq DNA polymerase (Invitrogen), 70.6 μl of doubly distilled water and 10 μl of bisulfite-treated DNA. PCR amplifications were performed in a GeneAmp 9700 thermal cycler (PerkinElmer Life and Analytical Sciences, Norwalk, CT). PCR products were purified using QIAquick columns and eluted in water. A 500-ng aliquot of the PCR product was incubated with 25 U of M.SssI and 320 μM SAM solutions, pH 7.9, containing 50 mM NaCl, 10 mM Tris-HCl, 10 mM MgCl2, 1 mM dithiothreitol, and 5 μl of nuclear extract buffer and H2O to make up a final volume of 50 μl for 90 min. Fully methylated PCR products were purified using the QIAquick PCR Purification kit (Qiagen), according to the manufacturer's instructions; 200 ng of the purified DNA was hydrolyzed, and the concentrations of 5mdC and 2dC in the hydrolysate were measured by LC-MS/MS using the conditions as described above. The methylation level of some samples was also analyzed using bisulfite-sequencing as described previously (Rush et al., 2004). Primers and PCR conditions were the same as those used for gene-specific LC-MS/MS. Clones (10-12) were randomly selected for sequencing from each transfection.
Xenograft Animal Model Study. Female athymic nu/nu mice (4-6 weeks old, 18-22 g) were obtained from Charles River Laboratory (Wilmington, MA) and acclimated for 1 week in a pathogen-free enclosure before start of study. Animals were given sterile rodent chow and water ad libitum and were housed in sterile filter-top cages with 12-h light/dark cycles. All experiments were conducted in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). MV4-11 cells (5 × 106 cells per mouse) were suspended with 50% Matrigel mixture (BD Biosciences) and subcutaneously implanted into the right flank of the athymic nu/nu mice. When tumors were grown between 100 and 200 mm3, treatments were initiated. Mice were randomly assigned into two cohorts for pharmacodynamic studies, with three mice per group and two cohorts for the anti-tumor growth activity studies, with six mice per group. For pharmacodynamic studies, parthenolide was given as a solution via the tail vein at the dose of 10 mg/kg. After 48 h, the animals were sacrificed in accordance with institutional guidelines, and the tumors were removed. The genomic DNA was extracted from these tumor tissues using QIAquick columns according to the manufacturer's instruction. Global DNA methylation was measured by the LC-MS/MS method (Liu et al., 2007a). For its anti-tumor growth activity study, parthenolide was administered intraperitoneally to MV4-11-engrafted nude mice at the dose of 4 mg/kg daily × 5 for 7 days. All of these doses were chosen based on the pilot toxicity studies of parthenolide in mouse.
Real Time RT-PCR Assays. Quantitative real-time RT-PCR was used to quantify the expression of HIN-1 and DNMT1. Untreated cells were used as the negative control. RT-PCR was performed using 2 μg of total RNA extracted with TRIzol reagent (Invitrogen) and reverse-transcribed by Moloney murine leukemia virus reverse transcriptase (Invitrogen). Real-time RT-PCR reactions were performed with an ABI Prism 7700 sequence detector (TaqMan; Applied Biosystems), and data were analyzed with the Sequence Detector version 1.6 software to establish the PCR cycle at which the fluorescence exceeded a set critical threshold for each sample. Data were analyzed according to the comparative critical threshold method using the internal control (18S RNA) transcript levels to normalize differences in sample loading and preparation. Results representing the n-fold difference of transcript levels between different samples were expressed as the mean ± S.D. from triplicate determinations.
Results
The Interaction of Parthenolide with hDNMT1 Suggests that Parthenolide May Inhibit DNMT1 through Covalent Block Catalytic Cysteine (Cys1226) by Its γ-Methylene Lactone. To test the hypothesis that parthenolide may be an effective DNA methylation inhibitor through inhibition of DNMT1, a DNMT1 homology modeling using M.HhaI (Protein Data Bank code 5MHT) as a template protein was used to investigate the potential interaction of this protein with parthenolide, because its crystal structure is available. As shown in Fig. 1, the catalytic domain of 478 amino acid residue (AA, 1139-1616) of hDNMT1 were aligned against 327 AA residue of Hhal methylase catalytic domain. Among these AA, there are 178 AA (37.2% of entire sequence), with 25% identical sequence and 41% similar sequence in the N-terminal, and 46 AA (9.6% of entire sequence), with 36% identical sequence and 58% similar sequence in the C-terminal, of the two proteins, which suggests that the N- and C-terminal sequences align well with the template. However, there was little homology in the middle sequence between these two proteins. Figure 2A shows the simulated bindings of parthenolide, the cofactor SAM, and the “flipped-out” substrate cytosine onto the catalytic site of the DNMT1 homology model. The DNMT1 catalytic site is a deep pocket buttressed by a typical pseudo-Rossmann fold in the bottom and walled by helices and loops. The pocket is largely hydrophobic in nature with polar residues in the binding subpockets at the methionine end of the cofactor and at the side of the pyrimidine ring of the substrate. Parthenolide binding competes with both the methionine and the pyrimidine ring, with its macro-ring interacting with the hydrophobic pocket. The γ-methylene lactone of parthenolide overlaps with the C-5 atom of the cytosine ring in the catalytic space. The distance between the γ-C atom of parthenolide and C-5 of cytosine is only 1.4 Å, and these atoms are 5.0 and 4.3 Å away from the S-atom of the catalytic cysteine, respectively. This model clearly demonstrates that parthenolide has the potential to inhibit the DNMT1 catalytic function through its γ-methylene lactone by covalently linking with its catalytic cysteine (Cys1226).
Fig. 1.
Human DNMT1 homology model. Alignment of the catalytic domain of human DNA methyltransferase I (hDNMT1) against that of the bacterial DNA methyltransferase M.HhaI (5MHT). The figure shows an alignment of 327 AA of M.HhaI with 478 AA of hDNMT1. Conserved amino acids of these two regions are highlighted in green and red, respectively. There is a 25% identical sequence and 41% similar sequence in the N-terminal 178 AA (37.2% of entire sequence) and a 36% identical sequence and 58% similar sequence in the C-terminal 46 AA (9.6% of entire sequence) of the two proteins. The middle sequence has little homology between these two proteins.
Fig. 2.
Structure of the DNMT1 catalytic domain and its docking with several DNMT1 inhibitors. A, docking of parthenolide into the DNMT1 catalytic site. The DNMT1 catalytic domain is represented by the ribbon model. Docked parthenolide and catalytic Cys1226 are shown in ball and stick models. Docked cofactor SAM and flipped cytosine are shown in red and blue lines, respectively. B, superimposed docking of parthenolide, EGCG, and RG-108 into the DNMT1 catalytic site with catalytic cysteine and parthenolide in red, EGCG in green, and RG-108 in blue shown in ball and stick models. DNMT1 active site is represented as a topographic surface is in ball and stick.
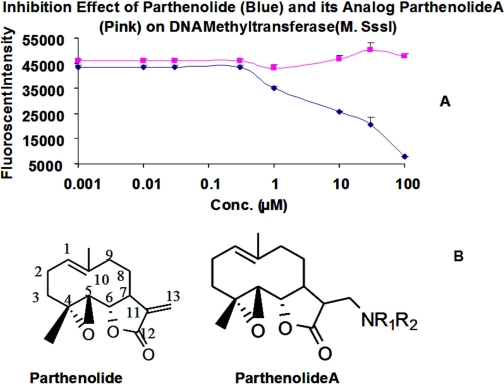
In Vitro EC50 of Parthenolide on M.SssI and DNMT1. To validate the results of the DNMT1 homology modeling study, the inhibition of the enzymatic activity of M.SssI, an analog of DNMT1 from bacteria, by parthenolide was tested in vitro. M.SssI has been reported to have a robust methylation activity, and its catalytic domain is structurally similar to that of DNMT1 (Chuang et al., 2005). Thus, we employed a 38-base pair double strand oligonucleotide labeled with 3′-biotinylation in one strand (Oligo A) and 3′-digoxigenin-NHS ester in its complementary strand (Oligo B) as a substrate for M.SssI in a solution containing SAM and the endonuclease HapII. The ds-oligonucleotide contains a CCGG sequence, which when cleaved by HapII resulted in a loss of the 3′-digoxigenin-NHS ester. When anti-digoxigenin-alkaline phosphatase antibody and the substrate Attophos were added to the solution, no detectable fluorescence signal was detected. In contrast, when the CCGG sequence of the ds-oligonucleotide is methylated to CCmGG, it resulted in resistance to the cleavage activity of HapII, maintenance of the 3′-digoxigenin-NHS ester of the ds-oligonucleotide, and in turn, generation of fluorescence signal under the same condition. The methylation level of the ds-oligonucleotide was found to directly correlate with the intensity of the assay fluorescence signal and, in turn, with the enzymatic activity of M.SssI (data not shown). Exposure to various concentrations (1, 10, 30, and 300 nM and 1, 3, 10, 30, and 100 μM) of parthenolide resulted in a dose-dependent decrease in fluorescence intensity, reflecting inhibition of the M.SssI methylation activity (Fig. 3). The apparent IC50 of parthenolide with respect to M.SssI inhibition was found to be 5 μM. To test the ability of our model in prediction of covalent inhibitors, a parthenolide analog, N,N-dimethylamine parthenolide (parthenolideA) devoid of γ-methylene lactone was prepared according to the published procedure (Hwang et al., 2006), and its inhibitory activity on M.SssI was evaluated using the same assay. As shown in Fig. 3, no dose-dependent decrease in fluorescence intensity from 1 nM to 100 μM was found, indicating that there is no inhibitory activity of parthenolideA on M.SssI. This result suggests that the γ-methylene lactone is critical for the inhibitory activity of parthenolide and that this may be due to the potential formation of a covalent Michael adduct of parthenolide with M.SssI, resulting in blocking the functional thiolate (Cys142) of the enzyme.
Fig. 3.
The DNA methylation inhibition effects of parthenolide (A) and its analog parthenolideA (B) on M.SssI. The double strand oligonucleotide substrate (10 nM) was treated with M.SssI (2 U), SAM without parthenolide or its analog parthenolideA (0, 0.01, 0.03, 0.3, 1, 10, 30, and 100 μM), followed by cutting with HpaII and the addition of Attophos substrate solution to generate fluorescence at excitation 430/emission 560 (filter = 550 nm). The inhibition effect of parthenolide was reflected as decrease in fluorescence intensity and expressed as an IC50.
The inhibitory activity of parthenolide on DNMT1 was also evaluated using DNMT1 activity assay kit (Epigentek). A similar EC50 of 3.5 μM was determined, indicating that our assay of M.SssI activity can reflect the actual inhibitory activities of DNMT1 inhibitors on DNMT1.
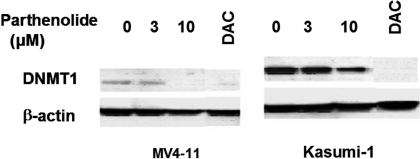
Dose-Dependent Down-Regulation of DNMT1 Proteins by Parthenolide in MV4-11 and Kasumi-1 Cells. We have shown above that parthenolide inhibits a DNMT1 analog M.SssI. To examine whether such activity would also affect the expression of DNMTs, Kasumi-1 and MV4-11 cells were treated with various concentrations of parthenolide for 24 h. DNMTs levels were measured by immunoblotting; the results are shown in Fig. 4. DNMT1 protein level in both cell lines remained unchanged when exposed to 3 μM parthenolide. However, when exposed to 10 μM parthenolide, DNMT1 protein level was totally depleted in MV4-11 cells and decreased by approximately 50% in Kasumi-1 cells as shown in Fig. 4. These results demonstrated that perturbation on DNMT1 protein levels by parthenolide was dose- and cell type-dependent and that its down-regulation requires a relatively high concentration of parthenolide.
Fig. 4.
Parthenolide inhibits protein expressions of DNMT1 in MV4-11 (left) and Kasumi-1 (right) cells for 24 h in a dose-dependent manner. MV4-11 and Kasumi-1 cells were incubated with the indicated concentrations of parthenolide (0, 1, 3, and 10 μM). DNMT1 protein levels were detected by Western blot.
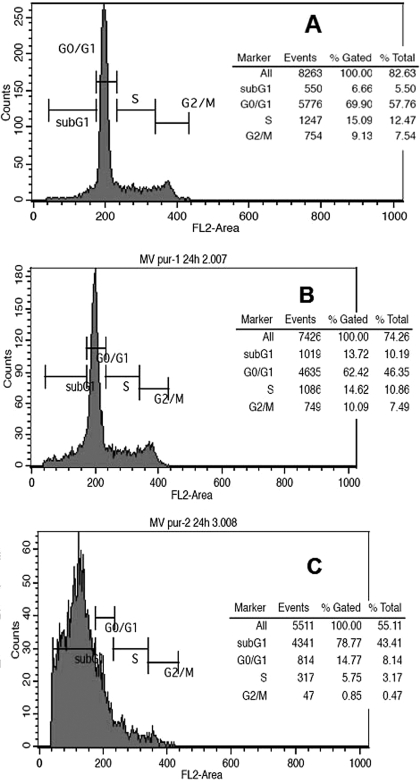
Down-Regulation of DNMT1 of Parthenolide May Be Associated with Its Cell-Cycle Arrest and Apoptosis Induction. Previous study demonstrated that DNMT1 levels varies in cell cycles (Robertson et al., 2000) and are extremely low in the SubG1 phase and overexpressed in the S phase. To understand whether the down-regulation of DNMT1 protein level is cell-cycle-dependent, cell-cycle distribution of MV4-11 cells treated with 0, 3, and 10 μM parthenolide at 24 h, at which, DNMT1 protein level was not known to change or be depleted, was analyzed by flow cytometry. As shown in Fig. 5A, more than half of untreated MV4-11 cells (69.9%) were in the G0/G1 phase, and approximately 6.66, 15.09, and 9.1% of cells were in the subG1, S, and G2/M phases, respectively. As shown in Fig. 5B, there was no significant change in the cell population distribution of G0/G1, S, and G2/M phases, with values of 62.42, 14.61, and 10.09%, respectively, but with more than double (14.61) in the subG1 phase treated with 3 μM parthenolide compared with the control. The cell-cycle distribution of 10 μM parthenolide-treated MV4-11 cells is completely different from the control (Fig. 5C). Approximately 80% of cells are arrested in the SubG1 phase, however, and the cell population in the G0/G1, S, and G2/M phases decreased significantly to 14.77, 5.75, and 0.85%, respectively. These data suggested that parthenolide arrests MV4-11 cells in the subG1 phase and decreases the S phase population at 10 μM, which might be responsible for DNMT1 protein level depletion in parthenolide-treated MV4-11 cells (Fig. 4). Therefore, parthenolide-induced cell-cycle arrest may at least partially account for the alteration of DNMT1 protein levels in MV4-11 cells. Consistent with the cell-cycle arrest, a significant apoptotic effect (approximately 73%) was observed in 10 μM parthenolide-treated MV4-11 cells compared with 7.5% in the control.
Fig. 5.
Effect of parthenolide on cell-cycle distribution in MV4-11 cells. MV4-11 cells were placed in serum-free medium to synchronize the cell cycle for 24 h. Cells were then treated with parthenolide (0, 3, and 10 μM) for 24 h. Cell-cycle distribution was determined by flow cytometry. A, representative sets of histogram for MV4-11 cells (A) and 3 μM (B) and 10 μM (C) parthenolide-treated cells. Percentages of cells in SubG1, G0/G1, S, and G2/M phase are shown as insets for each experiment.
Down-Regulation of DNMT1 Expression May be Associated with Its Disruption of the Physical Interaction of Sp1 and DNMT1 Promoter. In addition to the potential association of alteration of DNMT1 protein levels with the cell-cycle arrest in MV4-11 cells induced by parthenolide, we have recently found that the Sp1-NF-κB/p65 complex is a novel modulator of DNMT1 expression by physical interaction with the promoter of DNMT1 (Liu et al., 2008). Disruption of Sp1-NF-κB/p65 complex by a proteasomal inhibitor bortezomib in MV4-11 cells can down-regulate DNMT1 transcriptional level and protein level (Liu et al., 2008). In addition, parthenolide has been found to inhibit the activation of NF-κB, possibly through alkylation of Cys38 of p65 in various cancer cells (García-Piñeres et al., 2001, 2004). Therefore, it is hypothesized that the down-regulation of DNMT1 might also be associated with disruption of complex of Sp1-NF-κB/p65. To understand this mechanism for down-regulation of DNMT1 in MV4-11 cells induced by parthenolide, the Sp1 expression of 10 μM parthenolide-treated MV4-11 cells was examined, and it was found that parthenolide treatment induced a significant decrease in Sp1 protein level in MV4-11 cells (Fig. 6A). However, there is no apparent change in Sp1 proteins in decitabine-treated MV4-11 cells (Fig. 6A). EMSAs were then performed with whole lysates or nuclear extracts from MV4-11 cells treated with parthenolide (10 μM) and decitabine (2.5 μM) for 24 h to determine the effect of parthenolide on Sp1 binding to the DNMT1 promoter. As shown in Fig. 6B, parthenolide decreased Sp1 protein binding to the DNMT1 promoter in the nuclear extract but to a lesser extent in the whole lysates. In parallel, Sp1 antibody ChIP experiment demonstrated that the promoter level of DNMT1 decreased approximately 50% in 10 μM parthenolide-treated MV4-11 cells relative to the untreated control (Fig. 6C), similar to the EMSA result. Notably, the transcriptional level of DNMT1 in MV4-11 cells treated with 10 μM parthenolide was significantly down-regulated to 20% of its control level (Fig. 6D). It is noteworthy that the transcriptional level of DNMT1 was also down-regulated in decitabine-treated MV4-11 cells. Taken together, we concluded that DNMT1 down-regulation in MV4-11 cells may be partially associated with its depletion of Sp1, resulting in disruption of its physical interaction with DNMT1 promoter and sequentially down-regulation of DNMT1 in MV4-11 cells.
Fig. 6.
Down-regulation of DNMT1 is associated with depletion of Sp1 in parthenolide-treated MV4-11 cells. A, reduced Sp1 protein expression in MV4-11 cells treated with the indicated concentrations of parthenolide for 24 h. B, parthenolide abolishes Sp1 binding to DNMT1 promoter. EMSA was performed with total cell lysate (left) and nuclear extracts (right) prepared from MV4-11 cells treated without parthenolide or decitabine. C, ChIP of DNMT1 gene promoter with Sp1 shows the dissociation of the transcriptional activator Sp1 from the DNMT1 gene promoter. D, parthenolide decreases RNA levels of DNMT1, DNMT3a, and DNMT3b in MV4-11 cells for 24 h. Ct, untreated; DAC, decitabine (2.5 μM); Par, parthenolide (10 μM).
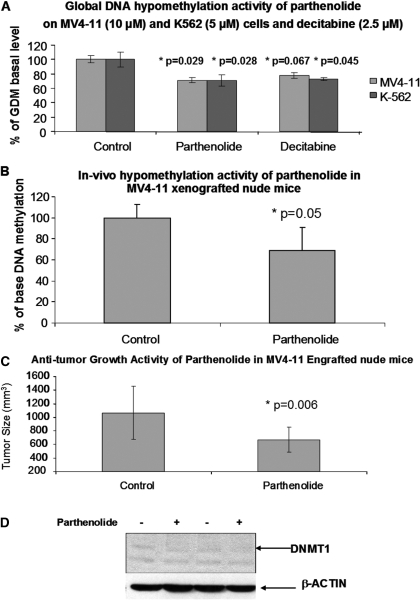
Alteration of in Vitro and in Vivo Global DNA Methylation Levels by Parthenolide and the in Vivo Anti-Tumor Growth Activity of Parthenolide. Because parthenolide was found to possess a dual DNMT1 inhibitory effect, down-regulation and inhibition of DNMT1, we investigated whether exposure to parthenolide at its effective concentration for (5-10 μM) could also result in global DNA hypomethylation. First, MV4-11 cells were exposed to 10 μM parthenolide and 2.5 μM decitabine (control). The cell pellets were collected at 24, 48, and 72 h. Global DNA methylation analysis of these genomic DNA extracted from these MV4-11 cells using a recently established LC-MS/MS method (Liu et al., 2007a) showed that parthenolide at the dose induced a 25% decrease (p = 0.029, n = 3, two sample t test) in global DNA methylation relative to the untreated baseline (Fig. 7A) in 24 h and to the same degree of hypomethylation induced by decitabine; however, no significant changes in global DNA methylation for 48 and 72 h of incubation were found (data not shown). Likewise, the global DNA methylation also decreased approximately 25% in K562 cells exposed to 5 μM parthenolide in 24 h, and there was no significant change for 48- and 72-h incubations as shown in Fig. 7A. This result suggested that parthenolide is an effective DNA methylation inhibitor.
Fig. 7.
Induction of global DNA hypomethylation by parthenolide in vitro and in vivo and its anti-tumor growth activity. A, global DNA methylation was found to be decreased in K562 and MV4-11 cells, each treated with 5, 10 or 30 μM parthenolide for 24 h. B, the in vivo hypomethylation effect of parthenolide in tumors from MV4-11 xenograft mice following treatment with a single intravenous bolus dose (10 mg/kg) of parthenolide formulated in polyethylene glycol 400, ethanol, and PBS. The global DNA methylation level of the treated tumor tissue is approximately 70% of the control group (p = 0.05), as well as the anti-tumor growth activity of parthenolide on MV4-11-engrafted tumor in nu/nu mice at day 7, after five times daily dosing at 4 mg/kg i.p. (C). The tumor size is approximately 63% of that in control group (p = 0.006). D, the in vivo down-regulation of protein levels of DNMT1 in MV4-11-engrafted tumor tissues collected from nude mice in the anti-tumor growth activity study. DNMT1 protein levels were detected by Western blot.
Having found the significant in vitro hypomethylation activity of parthenolide, we proceed to confirm this activity in vivo and that a MV4-11-engrafted nude mouse model (Lopes de Menezes et al., 2005) was used. Levels of global DNA methylation levels in these MV4-11 xenograft tumors were then compared with those of the untreated controls (i.e., vehicle only). Consistent with our in vitro results, we found a significant decrease in DNA methylation level (30%, p = 0.05) (Fig. 7B) at 10 mg/kg parthenolide. Furthermore, a pilot anti-tumor growth activity study with parthenolide in a MV4-11-engrafted nude mice at a five times daily dose of 4 mg/kg i.p. was also carried out, and the results demonstrated that the tumor size decreased 37% relative to the control (p = 0.006) (Fig. 7C). This dose was selected based on a reported preclinical pharmacology study with parthenolide (Cheng et al., 2005). Notably, the protein level of DNMT1 was down-regulated in parthenolide-treated MV4-11-engrafted tissues compared with that of the placebo-treated tissues as shown in Fig. 7D.
Hypomethylation of HIN-1 Promoter Methylation. HIN-1 is a hypermethylation-silenced TSG present in various cancers including breast cancers (Krop et al., 2001). Recently, an LC-MS/MS method for determination of its promoter methylation in MCF-7 cells has been established in our laboratory (Lie et al., 2007b). Our study demonstrated that HIN-1 can be reactivated in MCF-7 cells treated with decitabine, at least partially through its promoter hypomethylation. The result suggests that HIN-1 acts as an effective pharmacodynamic endpoint for DNA methylation inhibitors, and this promoter system represents a useful cell-based model to characterize the modulation effect of DNA methylation by DNA methylation inhibitors. To examine whether parthenolide could also decrease the methylation level of hypermethylation-associated silenced TSGs' promoters, the cell viability of MCF-7 cells exposed to parthenolide was first evaluated. It was found that the EC50 of parthenolide on MCF-7 cells is approximately 24 μM (data not shown). Therefore, MCF-7 cells were treated with 10 or 30 μM parthenolide for 72 h to ensure that approximately 50% of these cells were still alive after treatment with parthenolide; the detected promoter methylation level and mRNA level should reflect the newly replicated cells. Genomic DNA was extracted and bisulfite-converted according to the manufacturer's instructions. The promoter region between -611 and -792 nucleotides of the HIN-1 gene was amplified by PCR, and the amplicon was methylated by M.SssI as detailed under Materials and Methods. Methylation levels were determined using the LC-MS/MS method. As shown in Fig. 8A, there was a slight but insignificant perturbation ∼30% hypomethylation at 10 μM in the promoter DNA methylation. However, at 30 μM, parthenolide caused a 60% (p = 0.034) decrease in the methylation level of the promoter region of HIN-1 compared with the baseline (p = 0.034). This result was also confirmed by bisulfite sequencing (Rush et al., 2004), which showed an insignificant decrease (5%) at 10 μM but a 15% decrease in the promoter methylation level at 30 μM (Fig. 8B), which is similar to that induced by decitabine (0.75 μM; Fig. 8B). Parallel to the hypomethylation effect, the expression level of HIN-1 was increased by approximately 2.5- and 3.0-fold after treatment with 30 μM parthenolide and 0.75 μM decitabine compared with the untreated control (Fig. 8C), respectively. Thus, it seems that the effect of HIN-1 reactivation of parthenolide is possibly due to promoter hypomethylation similar to decitabine (Fig. 8C).
Fig. 8.
Parthenolide induces promoter hypomethylation methylation-silenced genes and their re-expression. A, perturbation of HIN-1 promoter methylation in MCF-7 cells exposed to indicated concentrations of parthenolide. DNA (1 μg) from parthenolide-treated or untreated MCF-7 cells were treated with bisulfite followed by PCR amplification, M.SssI methylation, and digestion to nucleosides. 5mdC and 2dC in the hydrolysates were measured using the LC-MS/MS method, and the ratio of 5mdC to 2dC is used as a DNA methylation indicator. B, hypomethylation activities of parthenolide (10 and 30 μM) and decitabine (0.75 μM) on HIN-1 promoter detected by bisulfite-sequencing. A 15% decrease of the promoter methylation in parthenolide-treated group was found. The solid circles represent methylated CpG loci, and the open circles represent nonmethylated CpG loci. C, parthenolide increased expression of HIN-1 gene in MCF-7 cells treated with parthenolide for the indicated time points and dosages. HIN-1 gene expression was measured by real-time RT-PCR. Conc, untreated; DAC, decitabine (0.75 μM); Par, parthenolide.
Discussion
Identification and development of small molecules that block the active sites of human DNMTs represent a novel strategy in epigenetic modulators discovery. Several categories of DNMT1 inhibitors have been identified. These compounds work mainly through the following mechanisms: 1) covalent-trapping of DNMT1 through incorporation into DNA (i.e., nucleoside analogs decitabine, 5-azacytidine, and zebularine) (Santi et al., 1983); 2) noncovalent blocking of DNMT1 catalytic active site (i.e., EGCG and RG-108) (Fang et al., 2003; Mund et al., 2006); 3) interruption of binding site of DNMT1 to DNA (i.e., procaine and procainamide) (Villar-Garea et al., 2003; Lee et al., 2005); 4) degradation of DNMT1 (i.e., decitabine) (Ghoshal et al., 2005); and 5) suppression of DNMT1 expression (i.e., anti-sense MG-98, miR29b) (Winquist et al., 2006; Fabbri et al., 2007). Among these, DNMT1 covalent-trapping compounds represented by azanucleosides and down-regulation of DNMT1 by its antisense oligonucleotides (Winquist et al., 2006) and microRNAs (Fabbri et al., 2007) appear to be the two most effective classes of agents in inducing global DNA hypomethylation and reactivation of epigenetically silenced TSG in malignant cells. However, the inherent toxicity and potential onset of resistance of azanucleosides and the inefficiency delivery of oligonucleotides to the target site pose considerable limitations for their clinical development, especially in the potentially expanded use in nonproliferating cancers. Despite the potential advantage for using non-nucleoside analogs compounds, only a few have been investigated and show relatively low efficacy compared with azanucleosides and oligonucleotides (Chuang et al., 2005).
We hypothesized that non-nucleosides that could inhibit DNA methyltransferase activity by either covalently blocking the catalytic site or down-regulation of DNMT1 or both without DNA incorporation may result in significant advantage in reduced toxicity or drug delivery compared with azanucleosides and oligonucleotides. Hence, we initially sought to identify lead compounds from plant-derived natural products (phytochemicals), which have been shown to covalently bind to thiol groups of enzymes/transcriptional factors. These phytochemicals in general are considered to have low toxicity and, therefore, could be potentially used as chemopreventive agents.
Among these, sesquiterpene lactones (SLs) are biologically active and structurally diversified herbal components, and one of the SLs, parthenolide, was previously used as an anti-inflammatory drug. Parthenolide has been shown pharmacologically to inhibit NF-κB and possesses significant anti-cancer effects (Wiedhopf et al., 1973; Woynarowski and Konopa, 1981; Patel et al., 2000; García-Piñeres et al., 2001, 2004; Kwok et al., 2001; Nakshatri et al., 2004; Won et al., 2004; Guzman et al., 2005; Steele et al., 2006). Previous studies on molecular mechanism in parthenolide-treated cells show that there is a covalent blocking of the thiol group of cysteine in the active site of p65, which plays a pivotal role in the apoptotic process (García-Piñeres et al., 2001, 2004).
Recently, a DNMT1 homology modeling (Siedlecki et al., 2003) was built to provide mechanism-based virtual databases of candidate compounds that inhibit human DNA methyltransferases. As proof of principle, we adapted and improved the DNMT1 homology modeling and docked parthenolide. We found that parthenolide might be particularly effective in inhibiting DNMT activity through initial noncovalent binding to the catalytic pocket of DNMT1, possibly in competition with the substrate cytosine and cofactor SAM (Fig. 2A). Subsequently, the γ-methylene lactone moiety may covalently bind with the thiol group of DNMT1, thus inhibiting the enzyme activity. We also docked EGCG (Fang et al., 2003) and RG-108 (Mund et al., 2006), two published DNMT1 inhibitors, with results consistent with previously published data (Fang et al., 2003; Mund et al., 2006). Both compounds bind to the cytosine binding subsite and possibly interfere with the cofactor binding, validating our use of DNMT1 homology modeling in identifying potential DNMT1 inhibitors (Fig. 2B). Although, its noncovalent binding energy is lower than both EGCG and RG-108, the potential subsequent covalent linkage with catalytic cysteine will greatly enhance its efficacy as an inhibitor of DNA methylation as demonstrated by its activity on M.SssI (Fig. 3) and its hypomethylation activity.
To validate this model experimentally, the inhibition activity of parthenolide was initially evaluated in a cell-free in vitro DNA methyltransferase assay. It was found that parthenolide possesses the inhibition activity with an IC50 of 5.0 μM on M.SssI. Furthermore, parthenolide also down-regulates the transcriptional level of DNMT1 and inhibit the protein expression in a dose-dependent manner in one leukemia MV4-11 cell line, possibly through the disruption of the physical interaction of Sp1 with the DNMT1 promoter.
Thus, the dual inhibition activity of parthenolide, i.e., down-regulation of DMNT1 and covalent binding, renders its comparable global DNA hypomethylation in several cancer cells lines, e.g., K562, MV4-11, and MCF-7. It is noteworthy that the global DNA methylation level of MV4-11 cells decreases up to 25% after exposure to 10 μM parthenolide, with a similar in vivo hypomethylation activity (30% decrease) in MV4-11 cell-engrafted tumors in nude mice. Furthermore, parthenolide at 30 μM also induces hypomethylation of the hypermethylated HIN-1 gene promoter and enhances its expression in MCF-7 cells. Hence, we were able to establish parthenolide as a novel, active DNA methylation inhibitor. Thus, parthenolide represents a novel structural scaffold for the inhibition of human DNMT1. The proposed strong dependence of the methylation inhibition on the γ-methylene lactone group of parthenolide based on our DNMT homology model and in vitro cell-free enzymatic assay may suggest a considerable specificity of the compound for DNA methyltransferases, which is also confirmed by a lack of inhibition activity of parthenolideA.
In conclusion, parthenolide has been characterized as an effective DNA methylation inhibitor as shown in global DNA hypomethylation and reactivation of one TSG HIN-1 gene, possibly associated with a decrease of its promoter methylation via its dual functions as potential covalent binding to the active site of DNA methyltransferases or down-regulation of DNMTs protein levels. Parthenolide represents a large family of sesquiterpene lactone natural products and may serve as a lead compound in a novel paradigm for identification of DNMT1 inhibitors from a natural reservoir. However, the relative importance of these dual functions of parthenolide remains unknown. Therefore, further work to dissect the two potential mechanisms for its DNA methylation inhibition activity is ongoing in our laboratory, which will provide insight for discovery and development of other SLs as DNA methylation inhibitors and their epigenetic targeting and antitumor activities in preclinical and early clinical studies.
This work was supported in part by the National Institutes of Health National Cancer Institute [Grants RO1-CA102031, UO1-CA76576]; the Bio-Medical Mass Spectrometry Laboratory, College of Pharmacy; and the Ohio Supercomputer Center, The Ohio State University.
Z.L. and S.L. contributed equally to this work.
This work was presented previously at the following conferences: Cheng D, Xiao JJ, Cheng H, Liu Z, Covey JM, and Chan, KK (2005) at The Annual Meeting of the American Association of Cancer Research; 2005 Apr 16-20; Anaheim, CA; Liu Z, Liu S, Xie Z, Li C, Aimiuwu S, Chen P, Pang J, Marcucci G, and Chan KK (2007) at The Annual Meeting of The American Association of Cancer Research; 2007 April 14-19; Los Angeles, CA; and Liu Z, Xie Z, Wu J, Aimiuwu J, Liu S, Huang H, Plass C, Marcucci G, and Chan KK (2007) at The AAPS Annual Meeting; 2007 Nov 11-15; San Diego, CA. American Association of Pharmaceutical Scientists, Arlington, VA.
doi:10.1124/jpet.108.147934.
ABBREVIATIONS: CpG, 5′-cytosine-guanosine; SLs, sesquiterpene lactones; TSGs, tumor suppressor genes; 5mdC, 5-methyl-2′-deoxycytidine; 2dC, 2′-deoxycytidine; 2dG, 2′-deoxyguanosine; DNMT1, DNA methyltransferase 1; hDNMT1, human DNMT1; ds, double strand; RT, reverse transcription; PCR, polymerase chain reaction; ECGC, (-)-epigallocutechin-3-gallate; AA, amino acid; EMSA, electrophoretic mobility shift assay; ChIP, chromatin immunoprecipitation; LC-MS/MS, liquid chromatography-mass spectrometry/mass spectrometry; SAM, S-adenosyl-methionine; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; NF-κB, nuclear factor κB; RG-108, b2-(1,3-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-3-yl) propanoic acid; MG-98, 5′-UAG CAC CAU UUG AAA UCA GU-3′.
References
- Cheng JC, Matsen CB, Gonzales FA, Ye W, Greer S, Marquez VE, Jones PA, and Selker EU (2003) Inhibition of DNA methylation and reactivation of silenced genes by zebularine. J Natl Cancer Inst 95 399-409. [DOI] [PubMed] [Google Scholar]
- Cheng D, Xiao JJ, Cheng H, Liu Z, Covey JM, and Chan, KK (2005) Analytical method development and pharmacokinetics studies with parthenolide (NSC 157035) and a water-soluble analog (NSC 734325), in The Annual Meeting of the American Association of Cancer Research, Abstract number 4184, American Association of Cancer Research, Philadelphia, PA.
- Chuang JC, Yoo CB, Kwan JM, Li TW, Liang G, Yang AS, and Jones PA (2005) Comparison of biological effects of non-nucleoside DNA methylation inhibitors versus 5-aza-2′-deoxycytidine. Mol Cancer Ther 4 1515-1520. [DOI] [PubMed] [Google Scholar]
- Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, Liu S, Alder H, Costinean S, Fernandez-Cymering C, et al. (2007) MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A 104 15805-15810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, and Yang CS (2003) Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res 63 7563-7570. [PubMed] [Google Scholar]
- Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, et al. (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47 1739-1749. [DOI] [PubMed] [Google Scholar]
- García-Piñeres AJ, Castro V, Mora G, Schmidt TJ, Strunck E, Pahl HL, and Merfort I (2001) Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J Biol Chem 276 39713-39720. [DOI] [PubMed] [Google Scholar]
- García-Piñeres AJ, Lindenmeyer MT, and Merfort I (2004) Role of cysteine residues of p65/NF-kappaB on the inhibition by the sesquiterpene lactone parthenolide and N-ethyl maleimide, and on its transactivating potential. Life Sci 75 841-856. [DOI] [PubMed] [Google Scholar]
- Ghoshal K, Datta J, Majumder S, Bai S, Kutay H, Motiwala T, and Jacob ST (2005) 5-Aza-deoxycytidine induces selective degradation of DNA methyltransferase 1 by a proteasomal pathway that requires the KEN box, bromo-adjacent homology domain, and nuclear localization signal. Mol Cell Biol 25 4727-4741. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, and Jordan CT (2005) The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 105 4163-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey R, Morris GM, Olson AJ, and Goodsell DS (2007) A semiempirical free energy force field with charge-based desolvation. J Comput Chem 28 1145-1152. [DOI] [PubMed] [Google Scholar]
- Hwang DR, Wu YS, Chang CW, Lien TW, Chen WC, Tan UK, Hsu JT, and Hsieh HP (2006) Synthesis and anti-viral activity of a series of sesquiterpene lactones and analogues in the subgenomic HCV replicon system. Bioorg Med Chem 14 83-91. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, Klimek V, Slack J, de Castro C, Ravandi F, et al. (2006) Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 106 1794-1803. [DOI] [PubMed] [Google Scholar]
- Krop IE, Sgroi D, Porter DA, Lunetta KL, LeVangie R, Seth P, Kaelin CM, Rhei E, Bosenberg M, Schnitt S, et al. (2001) HIN-1, a putative cytokine highly expressed in normal but not cancerous mammary epithelial cells. Proc Natl Acad Sci U S A 98 9796-9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok BH, Koh B, Ndubuisi MI, Elofsson M, and Crews CM (2001) The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol 8 759-766. [DOI] [PubMed] [Google Scholar]
- Lee BH, Yegnasubramanian S, Lin X, and Nelson WG (2005) Procainamide is a specific inhibitor of DNA methyltransferase 1. J Biol Chem 280 40749-40756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Liu Z, Xie Z, Pang J, Yu J, Lehmann E, Huynh L, Vukosavljevic T, Takeki M, Klisovic RB, et al. (2008) Bortezomib induces DNA hypomethylation and silenced gene transcription by interfering with Sp1/NF-kappaB-dependent DNA methyltransferase activity in acute myeloid leukemia. Blood 111 2364-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Liu S, Xie Z, Blum W, Perrotti D, Paschka P, Klisovic R, Byrd J, Chan KK, and Marcucci G (2007a) Characterization of in vitro and in vivo hypomethylating effects of decitabine in acute myeloid leukemia by a rapid, specific and sensitive LC-MS/MS method. Nucleic Acids Res 35 e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Xie Z, Wu J, Aimiuwu J, Liu S, Huang H, Plass C, Marcucci G, and Chan KK (2007b) A specific and sensitive LC-MS/MS method for accurate quantification of gene specific methylation (GSM), in The AAPS Annual Meeting, Abstract number T3010, American Association of Pharmaceutical Scientists, Arlington, VA.
- Lopes de Menezes DE, Peng J, Garrett EN, Louie SG, Lee SH, Wiesmann M, Tang Y, Shephard L, Goldbeck C, Oei Y, et al. (2005) CHIR-258: a potent inhibitor of FLT3 kinase in experimental tumor xenograft models of human acute myelogenous leukemia. Clin Cancer Res 11 5281-5291. [DOI] [PubMed] [Google Scholar]
- Lyko F and Brown R (2005) DNA methyltransferase inhibitors and the development of epigenetic cancer therapies. J Natl Cancer Inst 97 1498-1506. [DOI] [PubMed] [Google Scholar]
- Morris GM, Goodsell DS, Huey R, and Olson AJ (1996) Distributed automated docking of flexible ligands to proteins: parallel applications of AutoDock 2.4. J Comput Aided Mol Des 10 293-304. [DOI] [PubMed] [Google Scholar]
- Mund C, Brueckner B, and Lyko F (2006) Reactivation of epigenetically silenced genes by DNA methyltransferase inhibitors: basic concepts and clinical applications. Epigenetics 1 7-13. [DOI] [PubMed] [Google Scholar]
- Nakshatri H, Rice SE, and Bhat-Nakshatri P (2004) Antitumor agent parthenolide reverses resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene 23 7330-7344. [DOI] [PubMed] [Google Scholar]
- Patel NM, Nozaki S, Shortle NH, Bhat-Nakshatri P, Newton TR, Rice S, Gelfanov V, Boswell SH, Goulet RJ Jr, Sledge GW Jr, et al. (2000) Paclitaxel sensitivity of breast cancer cells with constitutively active NF-kappaB is enhanced by Ikappa-Balpha super-repressor and parthenolide. Oncogene 19 4159-4169. [DOI] [PubMed] [Google Scholar]
- Robertson KD (2005) DNA methylation and human disease. Nat Rev Genet 6 597-610. [DOI] [PubMed] [Google Scholar]
- Robertson KD, Keyomarsi K, Gonzales FA, Velicescu M, and Jones PA (2000) Differential mRNA expression of the human DNA methyltransferases (DNMTs) 1, 3a and 3b during the G(0)/G(1) to S phase transition in normal and tumor cells. Nucleic Acids Res 28 2108-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush LJ, Raval A, Funchain P, Johnson AJ, Smith L, Lucas DM, Bembea M, Liu TH, Heerema NA, Rassenti L, et al. (2004) Epigenetic profiling in chronic lymphocytic leukemia reveals novel methylation targets. Cancer Res 64 2424-2433. [DOI] [PubMed] [Google Scholar]
- Sali A and Blundell TL (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234 779-815. [DOI] [PubMed] [Google Scholar]
- Santi DV, Garrett CE, and Barr PJ (1983) On the mechanism of inhibition of DNA-cytosine methyltransferases by cytosine analogs. Cell 33 9-10. [DOI] [PubMed] [Google Scholar]
- Segura-Pacheco B, Trejo-Becerril C, Perez-Cardenas E, Taja-Chayeb L, Mariscal I, Chavez A, Acuña C, Salazar AM, Lizano M, and Dueñas-Gonzalez A (2003) Reactivation of tumor suppressor genes by the cardiovascular drugs hydralazine and procainamide and their potential use in cancer therapy. Clin Cancer Res 9 1596-1603. [PubMed] [Google Scholar]
- Siedlecki P, Boy RG, Comagic S, Schirrmacher R, Wiessler M, Zielenkiewicz P, Suhai S, and Lyko F (2003) Establishment and functional validation of a structural homology model for human DNA methyltransferase 1. Biochem Biophys Res Commun 306 558-563. [DOI] [PubMed] [Google Scholar]
- Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, Stone RM, Nelson D, Powell BL, DeCastro CM, et al. (2002) Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol 20 2429-2440. [DOI] [PubMed] [Google Scholar]
- Steele AJ, Jones DT, Ganeshaguru K, Duke VM, Yogashangary BC, North JM, Lowdell MW, Kottaridis PD, Mehta AB, Prentice AG, et al. (2006) The sesquiterpene lactone parthenolide induces selective apoptosis of B-chronic lymphocytic leukemia cells in vitro. Leukemia 20 1073-1079. [DOI] [PubMed] [Google Scholar]
- Villar-Garea A, Fraga MF, Espada J, and Esteller M (2003) Procaine is a DNA-demethylating agent with growth-inhibitory effects in human cancer cells. Cancer Res 63 4984-4989. [PubMed] [Google Scholar]
- Wiedhopf RM, Young M, Bianchi E, and Cole JR (1973) Tumor inhibitory agent from Magnolia grandiflora (Magnoliaceae). I. Parthenolide. J Pharm Sci 62 345. [DOI] [PubMed] [Google Scholar]
- Winquist E, Knox J, Ayoub JP, Wood L, Wainman N, Reid GK, Pearce L, Shah A, and Eisenhauer E (2006) Phase II trial of DNA methyltransferase 1 inhibition with the antisense oligonucleotide MG98 in patients with metastatic renal carcinoma: a National Cancer Institute of Canada Clinical Trials Group investigational new drug study. Invest New Drugs 24 159-167. [DOI] [PubMed] [Google Scholar]
- Won YK, Ong CN, Shi X, and Shen HM (2004) Chemopreventive activity of parthenolide against UVB-induced skin cancer and its mechanisms. Carcinogenesis 25 1449-1458. [DOI] [PubMed] [Google Scholar]
- Woynarowski JM and Konopa J (1981) Inhibition of DNA biosynthesis in HeLa cells by cytotoxic and antitumor sesquiterpene lactones. Mol Pharmacol 19 97-102. [PubMed] [Google Scholar]
- Yoo CB and Jones PA (2006) Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov 5 37-50. [DOI] [PubMed] [Google Scholar]