Abstract
Nicotinic receptor activation is inextricably linked to desensitization. This duality affects our ability to develop useful therapeutics targeting nicotinic acetylcholine receptor (nAChR). Nicotine and some α7-selective experimental partial agonists produce a transient activation of α7 receptors followed by a period of prolonged residual inhibition or desensitization (RID). The object of the present study was to determine whether RID was primarily due to prolonged desensitization or due to channel block. To make this determination, we used agents that varied significantly in their production of RID and two α7-selective positive allosteric modulators (PAMs): 5-hydroxyindole (5HI), a type 1 PAM that does not prevent desensitization; and 1-(5-chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxanol-3-yl)-urea (PNU-120596), a type 2 PAM that reactivates desensitized receptors. The RID-producing compounds nicotine and 3-(2,4-dimethoxybenzylidene)anabaseine (diMeOBA) could obscure the potentiating effects of 5HI. However, through the use of nicotine, diMeOBA, and the RID-negative compound 3-(2,4-dihydroxybenzylidene)anabaseine (diOHBA) in combination with PNU-120596, we confirmed that diMeOBA produces short-lived channel block of α7 but that RID is because of the induction of a desensitized state that is stable in the absence of PNU-120596 and activated in the presence of PNU-120596. In contrast, diOHBA produced channel block but only readily reversible desensitization, whereas nicotine produced desensitization that could be converted into activation by PNU-120596 but no demonstrable channel block. Steady-state currents through receptors that would otherwise be desensitized could also be produced by the application of PNU-120596 in the presence of a physiologically relevant concentration of choline (60 μM), which may be significant for the therapeutic development of type 2 PAMs.
The challenges associated with targeting neuronal nicotinic acetylcholine receptors (nAChR) for therapeutics are compounded by both the complex diversity of nAChR subtypes and the fact that agents briefly applied will activate the receptors yet with prolonged application will desensitize the receptors. This conundrum affects our efforts to understand the use and addictive properties of nicotine (Picciotto et al., 2008) and will need to be considered if we are to successfully develop new selective agents to target specific nAChR subtypes for conditions such as schizophrenia (Olincy et al., 2006; Freedman et al., 2008) and Alzheimer's disease (Kem, 2000; Kitagawa et al., 2003).
All nAChR are pentameric ligand-gated ion channels, members of a superfamily of receptors that includes GABA, glycine, and serotonin-gated channels. The nAChR of the neuromuscular junction, autonomic ganglia, and many subtypes in the brain are heteromeric receptors, containing at least two different kinds of subunits, with at least one subunit being classified as an α subunit. Ten α subunits (α1–α10) have been identified and, with the exception of α5, have the structural specializations to form the primary face of an acetylcholine (ACh) binding domain. In heteromeric nAChR, non-α subunits provide a complementary face to the ACh binding domain. These specializations of α and non-α subunits limit the number of agonist binding sites in heteromeric receptors to two and may be associated with the ability of heteromeric receptors to retain high affinity for agonist upon conversion to a desensitized state.
The second main class of nAChR are homomeric, most commonly consisting of α7 subunits, and are found in the brain, autonomic ganglia, and some non-neuronal tissues (Gahring and Rogers, 2005). They have high-calcium permeability and have been implicated in controlling essential signal transduction pathways and in modulating synaptic function.
Many experimental agents have been identified that selectively activate α7 nAChR (Horenstein et al., 2008). The structural diversity of these agents is matched by a diversity in their functional properties, for example, in regard to whether they act as antagonists of other nAChR subtypes and/or 5-hydroxytryptamine3 (5HT3) receptors. Select agents may not only fail to activate heteromeric nAChR, but to varying degrees may also be functional antagonists of non-α7 nAChR. As a result, the potential development of any nAChR-targeting drugs must consider the entire spectrum of effects for any given agent, with especially complex profiles that may encompass activation of one class of receptor and inhibition of others.
We have previously characterized benzylidene anabaseines (BAs), compounds that selectively activate homomeric α7 nAChR, with their potency and efficacy for activation of α7 as determined by their specific chemical compositions (Papke et al., 2004a). Another important quality of these α7 agonists, which varies greatly, is the degree to which they promote and maintain the receptor in desensitized or other nonfunctional (e.g., blocked) states after an initial phase of receptor activation. Lacking the structural specializations of heteromeric receptors, upon desensitization α7 nAChR do not convert to a state with high affinity for ACh. Once the ACh has been removed or metabolized, the receptor rapidly resensitizes and can be activated again by ACh. However, α7 receptors do not readily return to a functional state after the application of nicotine or the α7-selective agonist 3-(2,4-dimethoxybenzylidene)anabaseine [diMeOBA; also DMXBA or GTS-21)]. It has remained a question whether the failure of the receptors to resensitize after exposure to nicotine or diMeOBA is because these drugs, unlike ACh, can promote and stabilize a desensitized state for α7 or whether they have secondary inhibitory effects. In deference to this question, we refer to the failure of α7 receptors to fully recover after a nicotine or diMeOBA application as the manifestation of residual inhibition or desensitization (RID). RID is therefore defined relative to the reversibility of ACh-induced desensitization. Whereas receptors desensitized by ACh application recover fully in a 5-min period, receptors experiencing RID do not. A goal of the present study is to clarify whether RID occurs because drugs such as nicotine and diMeOBA stay bound to the agonist binding site of the receptors in a desensitized state, or whether, alternatively, RID is because there is channel block by the agonist molecules. We focus on the contrast between diMeOBA and 3-(2,4-dihydroxybenzylidene)anabaseine (diOHBA), a BA that, like ACh, activates the receptors and allows them to resensitize fully after washout from the bath solution. Specifically, we use positive allosteric modulators (PAMs) of α7 nAChR to probe the functional and nonfunctional states of the receptors after activation by ACh, nicotine, or each of the two contrasting BA compounds.
Materials and Methods
Synthetic Chemistry. BA compounds were synthesized by reaction of anabaseine dihydrochloride with the appropriate benzaldehyde as reported previously (Kem, 1971; Zoltewicz et al., 1993; Kem et al., 2004). They were purified by silica gel chromatography, and their purity was established by NMR. Details of their synthesis will be described elsewhere (W. R. Kem, F. Soti, and S. LeFrancois, unpublished data). Calculations of estimated log P for the BA compounds were made with Chem 3D Ultra (CamSci, Cambridge, MA).
Note that the prototypical agent of the family of BAs, 3-(2,4-dimethoxybenzylidene)anabaseine, has been introduced previously to the literature variously as GTS-21, DMXB, or DMXBA. Because this work examines the properties of a large number of related compounds, we have adopted what we believe to be a more understandable structure-based abbreviation for 3-(2,4-dimethoxybenzylidene)anabaseine, “diMeOBA,” to refer to this compound and other BAs in a consistent manner.
cDNA Clones. The human α7 nAChR receptor clone was obtained from Dr. Jon Lindstrom (The University of Pennsylvania, Philadelphia, PA). The RIC-3 clone was obtained from Dr. Millet Treinin (Hebrew University, Jerusalem, Israel).
Preparation of RNA. Subsequent to linearization and purification of cloned cDNAs, RNA transcripts were prepared in vitro using the appropriate mMessage mMachine kit from Ambion (Austin, TX).
Expression in Xenopus laevis Oocytes. Mature (>9 cm) female X. laevis African frogs (Nasco, Ft. Atkinson, WI) were used as a source of oocytes. Before surgery the frogs were anesthetized by placing them in a 1.5 g/l solution of tricaine methanesulfonate for 30 min. Oocytes were removed from an incision made in the abdomen.
Harvested oocytes were treated with 1.25 mg/ml collagenase (Worthington Biochemicals, Freehold, NJ) for 2 h at room temperature in calcium-free Barth's solution (88 mM NaCl, 1 mM KCl, 2.38 mM NaHCO3, 0.82 mM MgSO4, 15 mM HEPES, pH 7.6, and 12 mg/l tetracycline) to remove the follicular layer. Stage-5 oocytes were isolated and injected with 50 nl (5–20 ng) of α7 cRNA, usually in combination with human RIC-3 to accelerate and increase the level of nAChR expression (Halevi et al., 2003). Recordings were normally conducted 2 to 5 days after injection, except in the case of PNU-120596 experiments because the large PNU-120596 effect on ordinary levels of expression resulted in currents too large to be recorded in voltage clamp. Cells used for PNU-120596 experiments were typically used on the first day after injections.
Electrophysiology. Experiments were conducted using OpusXpress6000A (Molecular Devices, Sunnyvale, CA). OpusXpress is an integrated system that provides automated impalement and voltage clamp of up to eight oocytes in parallel. Both the voltage and current electrodes were filled with 3 M KCl. The oocytes were clamped at a holding potential of -60 mV.
Data were collected at 50 Hz and filtered at 20 Hz. The oocytes were bath-perfused with Ringer's solution. Agonist solutions were delivered from a 96-well plate using disposable tips. Flow rates were set at 2 ml/min. Drug applications usually alternated between ACh controls and test solutions of ACh or other experimental agonists at varying concentrations. PAMs were applied in the bath solution and then coapplied with agonist during the evoked responses.
Experimental Protocols and Data Analysis. Responses of α7 receptors were calculated as net charge (Papke and Papke, 2002), except in PNU-120596 experiments, as noted. Each oocyte received two initial control applications of ACh, then experimental drug applications, and follow-up control applications of ACh. For most experiments, the control ACh concentration was 300 μM, a concentration that is sufficient to evoke a maximal net charge response (Papke and Papke, 2002). For experiments with the PAMs, the ACh control concentration was 60 μM, the EC50 value for stimulation of the receptor as measured by net charge. In most experiments, alternating applications of ACh and other ligands were made to establish whether cells retained their initial responsiveness to ACh throughout the course of the experiment or whether there were cumulative changes in responsiveness. For experiments where ACh responses remained relatively stable, data related to experimental drug applications were calculated relative to the preceding ACh control responses. For experiments in which ACh control responses varied through the course of the experiment, because of either RID or allosteric modulation, the initial ACh control responses from each cell were used to normalize the data for all subsequent responses. These normalization procedures had the effect of compensating for differences in the level of channel expression among the oocytes. Mean values and standard errors (S.E.M.) were calculated from the normalized responses of at least four oocytes for each experimental concentration, except where otherwise noted. For concentration-response relations, data were plotted using Kaleidagraph 3.0.2 (Abelbeck/Synergy, Reading, PA), and curves were generated from the Hill equation as follows: Response = Imax [agonist]n/[agonist]n + (EC50)n, where Imax denotes the maximal response for a particular agonist/subunit combination, and n represents the Hill coefficient. Imax, n, and the EC50 were all unconstrained for the fitting procedures.
Results
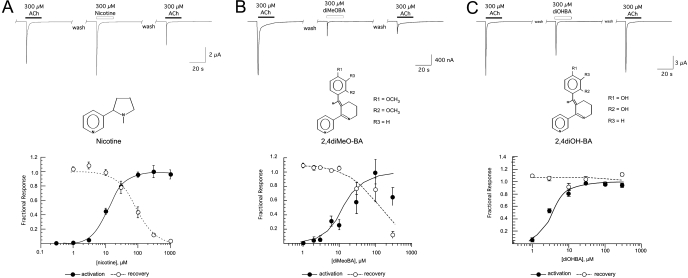
Activation and RID of α7 nAChR by (-)-Nicotine and Select BAs. Nicotine-evoked net charge responses of cells expressing human α7 receptors were compared with ACh responses in the same oocytes. Nicotine was found to be approximately 80% as efficacious as ACh (data not shown). The EC50 value for the activation of human α7 by nicotine was 12 ± 1 μM (Fig. 1A). As shown in Fig. 1A, after the application of nicotine at concentrations greater than 30 μM, subsequent ACh control applications were significantly decreased in amplitude. The IC50 value for the nicotine-evoked RID (defined as residual inhibition or desensitization that is not fully reversible after a 5-min washout period) was 80 ± 9 μM.
Fig. 1.
Activation and RID of α7 nAChR by (-)-nicotine and BAs. A, representative raw data traces showing the stimulation of α7 receptors by a high concentration of nicotine compared with a prior ACh control response in the same cell are shown at the top. The right-most trace shows an ACh response obtained subsequent to the nicotine-evoked response. Below, the activation and inhibition data (net charge) obtained from multiple cells. The progressive decrease in recovery values is indicative of RID. B, partial agonist activity and strong RID produced by diMeOBA. Representative raw data traces are shown at the top, illustrating the RID of α7 nAChR produced after transient activation by diMeOBA (GTS-21 or DMXBA). The concentration dependence data obtained from multiple cells are plotted below, as described for A. C, partial agonist activity and absence of RID produced by diOHBA. A to C, to visualize the respective concentration dependence of activation and RID, activation data (net charge) are plotted relative to the maximal activation produced by the specific drugs, which was 80, 10, and 47% that of maximal ACh-evoked (net charge) responses for nicotine, diMeOBA, and diOHBA, respectively. Recovery was calculated as the ratio of a 300 μM ACh-evoked response obtained after an application of nicotine or the BA compounds to that of the 300 μM ACh-evoked response obtained before the application of nicotine or a BA compound. Each point is the average (± S.E.M.) of at least four oocytes. Note that for the concentrations of nicotine and diMeOBA >30 μM, determination of activation and subsequent recovery had to be made on separate sets of oocytes, normalized to their own internal ACh controls, because cells did not recover adequately after the application of nicotine or diMeOBA at those concentrations for further repeated measurements to be made on the same cells.
Multiple BAs were tested for their ability to activate α7 receptors and also for the production of RID. These compounds differed in the specific functional groups of the benzylidene moiety (Fig. 1; Table 1). Previously characterized compounds included diMeOBA (also known as GTS-21 and DMXBA), as well as its hydroxy metabolites (Papke and Papke, 2002; Kem et al., 2004). Published data for these compounds are given in Table 1, along with new data on other disubstituted and monosubstituted BA compounds.
TABLE 1.
Activation and inhibition parameters for benzylidene anabaseines
| Name | R1 | R2 | R3 | Imax | EC50 | IC50 | Ratio |
|---|---|---|---|---|---|---|---|
| BAa | H | H | H | 0.11 | 40 | 108 | 2.7 |
| 2,4DiMeOBAb | OCH3 | OCH3 | H | 0.10 | 11 | 25 | 2.3 |
| 2,4DiOHBAc | OH | OH | H | 0.47 | 2.9 | >2000 | >1000 |
| 4OH2MeOBAb | OH | OCH3 | H | 0.37 | 4.5 | 1140 | 253 |
| 2OH4MeOBAc | OCH3 | OH | H | 0.55 | 2.4 | 971 | 404 |
| 4MeS-BAa | SCH3 | H | H | 0.11 | 85 | 342 | 4.0 |
| 4OH-BAa | OH | H | H | 0.29 | 3.5 | 171 | 49 |
| 4AminoBA | NH2 | H | H | 0.5 | 3.7 | 334 | 90 |
| 4MeOBA | OCH3 | H | H | 0.34 | 15 | 71 | 4.7 |
| 4MeBA | CH3 | H | H | 0.13 | 76 | 216 | 2.8 |
| 2MeBA | H | CH3 | H | 0.07 | 32 | >1000 | >100 |
| 3MeBA | H | H | CH3 | 0.17 | 240 | 103 | 0.43 |
| 3CNBA | H | H | CN | 0.06 | >100 | 1400 | ≈5 |
| 3NitroBA | H | H | NO2 | 0.1 | >100 | 750 | ≈2.5 |
| 3OHBA | H | H | OH | 0.66 | 6.3 | 146 | 23 |
| 3MeOBA | H | H | OCH3 | 0.19 | 50 | 125 | 2.5 |
| 2MeOBA | H | OCH3 | H | 0.15 | 26 | 360 | 14 |
Correlational analysis was conducted on the data in Table 1 (data not shown), and no apparent relationship was discernible between the EC50 and IC50 values of the BA compounds. However, it may be noted that, in general, compounds with hydroxyl groups, especially at the 2-and 4-positions, produced less RID than compounds with methoxy groups at the same positions. It was also the case that 3OHBA was both more efficacious than 3MeOBA and less potent for producing RID.
We evaluated whether the EC50, Imax, or IC50 values for the various BA compounds in Table 1 would correlate to either their estimated log P values (Chem 3D Ultra) or the previously reported pKa values (LeFrancois, 2004). Although some of these analyses suggested modest trends (data not shown), none of the r values generated from linear regressions were very high, ranging from r = 0.331 for log P on log IC50 to r = 0.639 for log P on Imax (Imax = 0.979 - 0.271 log P).
Therefore, our data suggest that the complete activity profiles for BA compounds are regulated by both the nature of the substitutions and the position of the substitution on the benzylidene group. Note, for example, that although the physical properties of the three compounds with single methyl groups at the 2-, 3-, or 4-positions are relatively similar, the biological activities of these compounds are very different. This suggests that point-to-point intermolecular interactions between the ligand and specific residues in the receptor ligand binding site are likely to be important determinants of function.
To illustrate the range of α7 activities associated with the various BA compounds, raw data and concentration-response curves for diMeOBA and diOHBA are shown in Fig. 1, B and C, respectively. Note that although diMeOBA produced a transient activation followed by RID, diOHBA (Kem et al., 2004) was not only more efficacious than diMeOBA but also did not produce significant RID.
Based on our earlier studies of diMeOBA and its hydroxy metabolites (Meyer et al., 1998; Kem et al., 2004), we hypothesized that the presence of hydrophobic side groups would promote more stable binding of BA compounds to the α7 receptor and therefore more RID. It was proposed that the large hydrophobic groups present on α7-selective agonists such as the BA compounds are accommodated within a comparably large hydrophobic pocket in the α7 agonist binding site (Horenstein et al., 2008). This would be consistent with the hypothesis that these agonists may remain bound to the receptor even after being washed out of the bath. The tightness of binding would logically be modulated by the nature and the positions of the side groups on the benzylidene ring.
The responses of α7 receptors can be sustained at a low level for relatively long periods if the agonist concentration is at the EC50 for net charge or below (Papke, 2006), but if the agonist concentration rises further, channel activations synchronize, resulting in larger peak current but no further increase in net charge. Nicotine and the BA compounds show similar concentration-dependent effects on α7 response waveforms. Because the agonist concentration dependence of fast desensitization is similar for all three compounds, the induction of this form of desensitization is not likely to underlie the difference between these compounds in their production of RID.
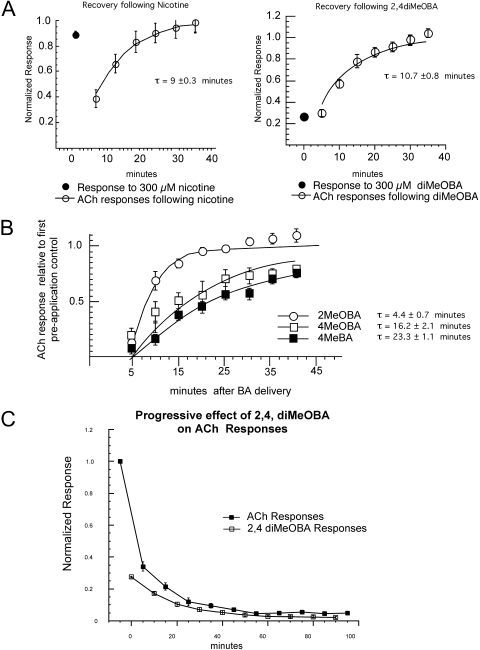
Variations in RID Recovery Time Course for Nicotine and BA Compounds. Nicotine and the various BA compounds induce RID to different degrees, and, as noted above, one hypothesis is that the compounds that produce RID may remain bound to the receptor in the desensitized state at or near the ACh binding site. An alternative hypothesis is that the binding of the ligands induces a stable desensitized state that persists even after the ligands have dissociated from the receptor. If the latter hypothesis is the case, then although ligands might vary in their effectiveness for inducing RID, the reverse process of recovery from RID would be an intrinsic property of the receptor and not dependent on the ligand used to induce RID. In contrast, if RID arises from prolonged binding of the ligands to the receptor, recovery would be related to the respective dissociation rates of the drugs and would vary for the different agents.
As shown in Fig. 2A, when receptors were stimulated repeatedly with ACh after the induction of RID by nicotine or diMeOBA, the time constants for recovery were significantly longer than the interstimulus interval of 5 min used to detect RID. RID recovery rates were determined for additional BA compounds that induced RID when applied at a concentration of 100 μM (Fig. 2B). The variation seen in the recovery rates would be consistent with these ligands varying in their dissociation rates.
Fig. 2.
Evaluation of recovery rates with repeated ACh application after RID produced by experimental agonists. A, concentrations of either nicotine or diMeOBA sufficient to produce ≥50% residual inhibition were made to oocytes expressing α7. Cells were washed and repeatedly stimulated with ACh to evaluate the rates of recovery. B, recovery time constants varied for the RID produced by various BAs, based on the chemical character and position of the benzylidene side groups. C, because of the slow recovery from the diMeOBA-induced residual inhibition/desensitization, inhibition of both 60 μM ACh- and 100 μM diMeOBA-evoked responses can progressively accumulate with repeated applications of diMeOBA. To show the change in both ACh and diMeOBA responses over time, all data were normalized to the initial ACh response from each oocyte.
For further investigation of RID, we chose to focus on three agents, chosen in part because of their clinical importance: nicotine, diMeOBA, and diOHBA. Nicotine produces RID and is of course broadly used both in vitro and in vivo. One of the most effective BA compounds at producing RID is diMeOBA (GTS-21), which remains a prototypical α7-selective agent and has been brought forward in clinical trials for both Alzheimer's disease and schizophrenia. The diMeOBA metabolite, diOHBA, was also chosen to be studied in further detail, because it is an efficacious α7-selective drug but essentially lacks RID activity.
Because the time constant for the reversibility of diMeOBA RID was longer than our interstimulus intervals, repeated application of diMeOBA would be expected to produce progressively greater RID. As shown in Fig. 2C, alternating applications of 60 μM ACh and 100 μM diMeOBA generated progressively smaller currents, such that after seven diMeOBA applications, RID was approximately 10-fold greater than after a single application. Once RID reached a level of approximately 95%, it was not significantly increased by further diMeOBA applications.
PAMs of α7 nAChR as Potential Tools to Investigate RID. Recently, several PAMs have been identified that selectively increase the amplitude and/or duration of α7 nAChR responses (Grønlien et al., 2007), apparently by binding within the transmembrane domains (Bertrand et al., 2008; Young et al., 2008). PAMs have been identified that fall into two different classes: type 1 modulators, which increase currents during the initial phases of activation but do not perturb the ultimate desensitization of the receptors; and type 2 modulators, which amplify all phases of the response and, moreover, seem to prevent desensitization. Furthermore, type 2 modulators not only limit the conversion of activated receptors into the desensitized state but also can convert receptors that have been desensitized back into a form that conducts current. It is unclear whether the effect of a type 2 modulator is to destabilize desensitized states, and so promote conversion back to the active states, or alternatively to convert the desensitized state into a conducting state. Because these two mechanisms are functionally indistinguishable, the term “modulator sensitized” is used to refer to the α7 receptors in the presence of a type 2 modulator, which are unlikely to convert to a nonconducting desensitized state in the presence of agonist. Although type 2 modulators typically produce greater effects than type 1 modulators, both types of modulators require that agonists also be bound to the receptors for activation to be observed.
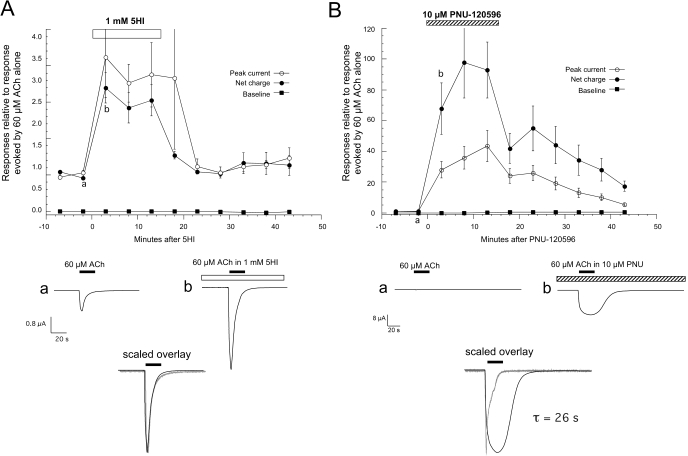
The prototype for type 1 modulators is 5-hydroxyindole (5HI) (Gurley et al., 2000; Zwart et al., 2002). The effects of 1 mM 5HI on the current evoked by 60 μM ACh are shown in Fig. 3A. The modulator was added to the bath solution and then also coapplied with ACh. A large and readily reversible increase was seen in the ACh-evoked responses during the 5HI application. The potentiation was maximal upon the first ACh coapplication with 5HI and returned nearly to baseline immediately upon washout. As shown in Fig. 5B, the normal kinetics of the ACh-evoked responses was not perturbed by the 5HI potentiation. This would suggest that after a transient phase of activation, channels were predominantly desensitized before the agonist application was over.
Fig. 3.
Enhancement of ACh-evoked α7-mediated currents by PAMs. A, the rapid and transient increase in ACh-evoked responses produced by the type 1 modulator 5HI. After two initial control applications of 60 μM ACh, the bath solution was switched to one containing 1 mM 5HI, and ACh was then coapplied with 5HI three times before the bath solution was switched back to control Ringer's solution. Responses (net charge) were normalized to the average of the two pre-5HI control responses in each cell. Each point is the average (± S.E.M.) of at least four oocytes. Responses obtained at points a and b from a single oocyte in the experiment are shown below. The black bars represent the timing and duration of agonist application. Note that the responses were increased in amplitude but not duration, as shown in the overlay of the two currents, scaled to the same peak amplitude. B, multiple responses of α7-expressing oocytes obtained in the presence of the type 2 modulator PNU-120596 (PNU). To normalize the data from multiple oocytes (n = 3), the peak current and baseline data were calculated relative to the peak amplitude of initial ACh control responses for each cell (correcting for the small holding current before the initial control application of ACh). Net charge measures were normalized based on the net charge of the initial ACh controls. Shown below are representative responses evoked by the application of 60 μM ACh in the absence and presence of PNU-120596 (traces obtained at points a and b in B, respectively). Also shown is the response to ACh alone (gray) increased in scale in the lower traces and overlaid with an ACh response obtained in the presence of PNU-120596 to show how, characteristically for a type 2 PAM, ACh responses in the presence of PNU-120596 were increased in both amplitude and duration.
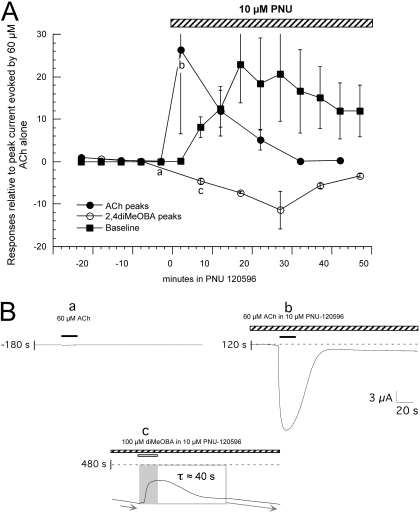
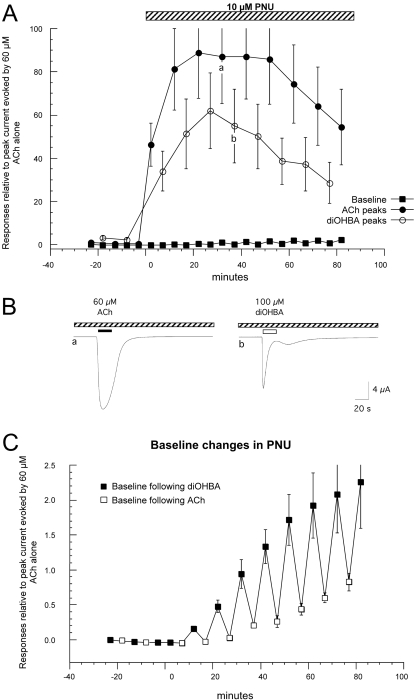
Fig. 5.
PNU-120596 in combination with diMeOBA and ACh. A, average values (± S.E.M.) from three cells treated with 60 μM ACh and 100 μM diMeOBA applied in alternation before and after the addition of 10 μM PNU-120596 (PNU) to the bath. To normalize the data, the peak current and baseline data were calculated relative to the peak amplitude of the initial ACh control for each cell (correcting for the small holding current before the initial control application of ACh). B, the topmost traces shown are ACh-evoked responses just before (a) and 3 min after (b) the switch to the PNU-containing bath solution. Note that the ACh-evoked response before PNU-120596 is particularly small because it has been decreased because of the residual inhibition/desensitization produced by the prior applications of diMeOBA. As indicated in A, there were large increases in holding current after the applications of diMeOBA in the presence of PNU-120596, indicative of steady-state activation of the α7 receptors. In addition to this effect on holding current, applications of 100 μM diMeOBA produced transient decreases in the progressively increasing steady-state current (arrows), as illustrated in the bottom trace. Shown is a diMeOBA response obtained 480 s after application of PNU-120596 (c in A). The decreases in the inward current were of approximately the same duration as the drug application and reversed on the time scale of solution washout (Papke and Thinschmidt, 1998).
PNU-120596 is a prototypical type 2 PAM of α7 nAChR. Multiple ACh-evoked responses obtained in the presence of PNU-120596 (Fig. 3B) showed a large increase in peak current and an even larger increase in net charge. No more than two applications were necessary to obtain maximum potentiation. It has been proposed that the prolonged duration of the ACh-evoked currents in the presence of PNU-120596 may be because of a destabilization of the normal desensitized state of the receptor (Grønlien et al., 2007), resulting in “modulator sensitized” receptors. As shown in the inset of Fig. 3B, in the presence of PNU-120596, channels remained active throughout the ACh application. However, after each ACh application in the presence of PNU-120596, currents returned to the original baseline once the ACh was washed out, even though PNU-120596 remained in the bath. This confirms that even though PNU-120596 is a strong α7 potentiator, its activity requires the presence of agonist on the receptor. Note that the ACh-evoked current progressively increased for the full 20 s of the agonist application and then decayed as the agonist was washed from the chamber, with a time constant of approximately 26 s. The effects of PNU-120596 reversed more slowly than the effects of 5HI (Fig. 3A). This slow reversibility suggests that there is prolonged binding of PNU-120596 to its site on the receptor.
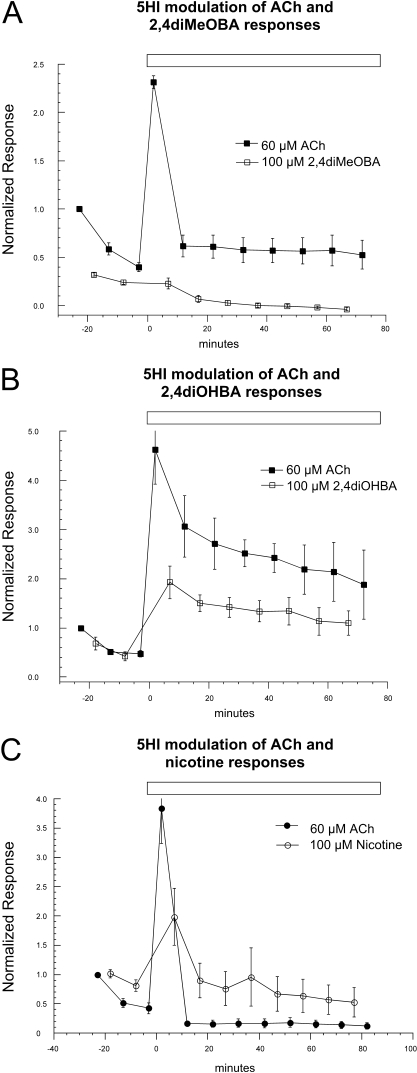
The Enhancement of α7 Function with a Type 1 PAM Does Not Prevent RID. Because type 1 PAMs do not perturb desensitization, we hypothesized that the component of RID that might be associated with enhanced desensitization would be maintained in the presence of 5HI. Cells expressing human α7 were stimulated by 60 μM ACh and 100 μM diMeOBA twice in alternation, producing a large decrease in the ACh-evoked responses (Fig. 4A). Cells were then switched to a bath solution containing 1 mM 5HI, and alternating ACh and diMeOBA applications, also containing 1 mM 5HI, were continued. After the bath application of 5HI, only the very first coapplication of ACh and 5HI showed significant potentiation over the initial ACh control response. Subsequently, despite the continued presence of the PAM, the ACh responses fell to a level that was less than the initial ACh control. The subsequent diMeOBA responses were not potentiated by 5HI and, in fact, still showed a slow decline in the continued presence of 5HI. Similar negative modulation of α7-mediated BA responses of hippocampal interneurons by 5HI was obtained in brain slice experiments (López-Hernández et al., 2009).
Fig. 4.
Interactions between a type 1 PAM and compounds producing varying amounts of RID. Alternating applications of ACh and either diMeOBA, diOHBA, or nicotine (A, B, and C, respectively) were made before and after switching to a bath solution containing 1 mM 5HI. Responses were normalized to the first control ACh responses of each cell. The data represent the average responses of five cells ± S.E.M. at each time point.
When 5HI was used with alternating applications of ACh and diOHBA (Fig. 4B), there was a potentiation of both the ACh- and diOHBA-evoked responses. For ACh, the initial potentiation was not significantly different from that obtained when just ACh was applied repeatedly in the presence of 5HI (Fig. 3A). However, full potentiation was not maintained after the applications of diOHBA, and both the ACh and diOHBA responses reached relatively stable levels of submaximal potentiation that were nonetheless greater than the initial control responses.
Interestingly, when 5HI was used with alternating applications of ACh and nicotine, there was only a transient potentiation of the ACh applications (Fig. 4C). In the continued presence of the PAM, the ACh responses fell to a level that was much lower than the initial ACh control. Although the nicotine-evoked responses were ultimately not potentiated above their original levels, they remained large and were better sustained than the ACh responses.
The Modulation of α7 Function with a Type 2 PAM Converts RID to Steady-State Current. When PNU-120596 was used in combination with alternating ACh and diMeOBA applications (Fig. 5), there was transient potentiation of the ACh-evoked currents but not the immediate diMeOBA-evoked responses. However with repeated applications of diMeOBA, large steady-state currents developed, as evidenced by a large increase in the baseline offset. Before the addition of PNU-120596, the baseline current was stable, and there were small declines in peak current amplitudes, as expected (Fig. 2). Once the cells treated alternately with ACh and diMeOBA were switched to the PNU-120596 solution, a steady-state current was produced that was not seen when PNU-120596 and ACh were combined without intervening applications of diMeOBA (Fig. 5). As the steady-state current increased, the PNU-120596 potentiation of ACh-evoked responses decreased in parallel, suggesting that the steady-state current plateaued at a maximal level of Popen. Although the diMeOBA applications had the effect of producing a steady-state current over time, they also had the effect of transiently inhibiting that steady-state current. This inhibition had a superficial resemblance to outward currents unless considered relative to the original baseline (Fig. 5), which suggested that the effect was, in fact, a decrease in steady inward current. To test this hypothesis, we conducted similar experiments at varying holding potentials (data not shown). The results indicated that the steady-state current had a reversal potential that was consistent with nAChR-mediated currents and the transient decreases in inward current produced by diMeOBA applications were similarly diminished when the driving force on the steady-state current was decreased. Note that if diMeOBA was activating a nAChR-independent outward current, depolarization would have had the effect of increasing, rather than decreasing, that current.
These data suggest that diMeOBA can produce both prolonged desensitization (reversible on a time scale of >10 min) and a transient block of open channels. Because PNU-120596 induces a form of modulator-sensitized receptors, it is able to change the accumulated desensitization, which would have been promoted by repeated diMeOBA applications alone, into a steady-state current. Hypothetically, this could be because of prolonged binding of diMeOBA to the activation site (normally associated with the production of desensitization), binding that persists after diMeOBA has been largely washed out of the bath. However, diMeOBA has also been reported to produce use-dependent channel block, most commonly detected with heteromeric nAChR such as α4β2 (de Fiebre et al., 1995). Although desensitizing effects are prolonged, the use-dependent channel block of α7 is apparently more rapidly reversible and may account for the transient decreases in the outward current produced by the application of diMeOBA in the presence of PNU-120596 (Fig. 5).
When PNU-120596 was used in combination with ACh and diOHBA, large potentiation of both diOHBA- and ACh-evoked responses was observed (Fig. 6). Before the addition of PNU-120596, the baseline current was stable, and, as expected, there were no significant changes in peak current amplitudes with repeated applications of ACh and diOHBA. Once the cells were switched to the PNU-120596 solution, the evoked currents increased, reaching a maximal level of potentiation after two to three applications of each drug. Large inward currents were measured during the diOHBA applications. However, diOHBA responses were typically briefer than the drug application and were followed by small rebound currents (Fig. 6B). This observation suggests that some use-dependent block probably did also occur during the diOHBA applications. Baseline currents were relatively steady when PNU-120596 was used in combination with ACh and diOHBA but showed some oscillations that were associated with which agonist had most recently been applied. Although overall there was only a small increase in steady-state current over the entire period of PNU-120596 application, compared with the steady-state current stimulated by 2,4,diMeOBA, or to the PNU-120596 potentiation of ACh- and diOHBA-evoked responses, the steady-state currents were consistently increased after diOHBA application and decreased after ACh applications (Fig. 6C).
Fig. 6.
PNU-120596 in combination with ACh and diOHBA. A, average values (± S.E.M.) from four cells treated with 60 μM ACh and 100 μM diOHBA applied in alternation, before and after the addition of 10 μM PNU-120596 to the bath. To normalize the data, the peak current and baseline data were calculated relative to the peak amplitude of the initial ACh control for each cell (correcting for the small holding current before the initial control application of ACh). B, shown are the fourth ACh and diOHBA responses obtained after application of PNU-120596 (from points a and b in A, respectively). C, baseline current levels expressed relative to initial ACh-evoked responses are plotted at an enlarged scale and show that there were oscillations associated with which agonist had most recently been applied. The baseline currents were measured just before the application of either ACh or diOHBA, so that changes in baseline current were the result of the preceding drug applications.
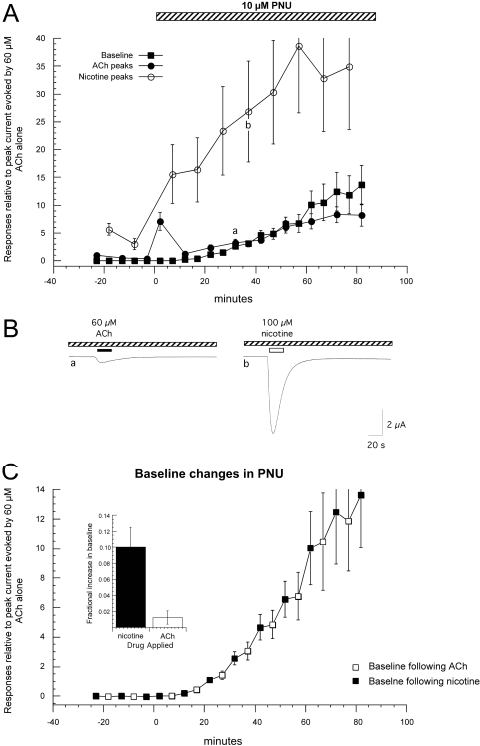
When PNU-120596 was used in combination with nicotine and ACh, there was large potentiation of nicotine-evoked responses and only modest potentiation of ACh-evoked responses (Fig. 7A). Before the addition of PNU-120596, the baseline current was stable, and, as expected, there were progressive decreases in both the ACh and nicotine-evoked peak current amplitudes. Once the cells were switched to the PNU-120596 solution, there was an initial transient potentiation of ACh-evoked responses and progressively increasing potentiation of nicotine-evoked currents. With continued applications, there were further increases in ACh-evoked responses and parallel increases in steady-state current. Although the ACh and nicotine responses differed in the degree to which they were potentiated by PNU-120596, there was no indication of channel block by either drug (Fig. 7B). That is, when these drugs were applied in the presence of PNU-120596, the currents increased and decayed in a normal manner, without a peak and rebound indicative of channel block, as was seen when diOHBA was applied in the presence of PNU-120596 (Fig. 6B). There were oscillations in the baseline (i.e., steady-state) current associated with which agonist had most recently been applied. Overall, the increase in steady-state current over the entire period of PNU-120596 application was small compared with that evoked by diMeOBA but large compared with ACh alone or ACh in alternation with diOHBA (Fig. 7C). The steady-state currents were more consistently increased after nicotine application than after ACh application. As shown in the inset of Fig. 7B, approximately 10% of the total increase in steady-state current occurred after each nicotine application, with a 10-fold smaller change occurring after ACh applications.
Fig. 7.
PNU-120596 in combination with nicotine and ACh. A, average values (±S.E.M.) from six cells treated with 60 μM ACh and 100 μM nicotine applied in alternation, before and after the addition of 10 μM PNU-120596 to the bath. To normalize the data, the peak current and baseline data were calculated relative to the peak amplitude of the initial ACh control for each cell (correcting for the small holding current before the initial control application of ACh). B, shown are the fourth ACh and nicotine responses obtained after application of PNU-120596 (from points a and b in A, respectively). C, baseline current levels expressed relative to initial ACh-evoked responses are plotted at an enlarged scale and show that there were oscillations associated with which agonist had most recently been applied. The inset shows a plot of the percentage of the total change in baseline currents associated with the applications of ACh or nicotine. On average, the increase in baseline current after the applications of nicotine were 10-fold higher than after the applications of ACh.
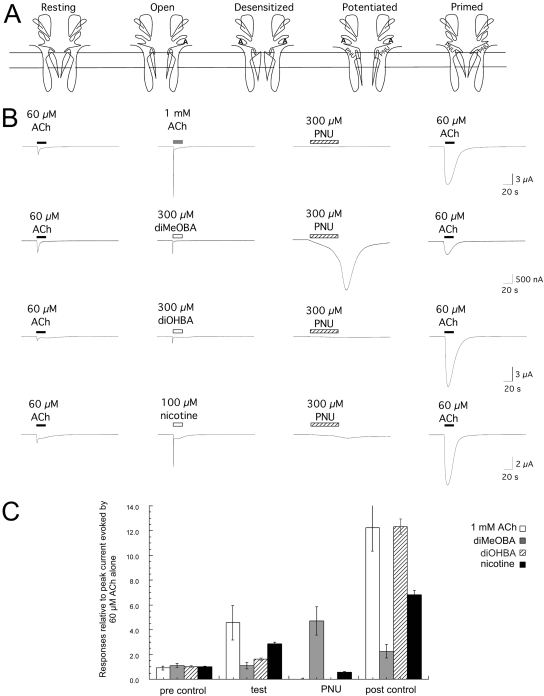
PNU-120596 Applied Alone Converts RID into Activation. The multiple functional states of α7 nAChR are schematically represented in Fig. 8A. Currents are most likely to be generated when channels are either in a condition of low partial agonist occupancy or when both agonist and potentiator are bound. Prolonged binding of agonist alone promotes stable desensitization, and binding of a type 2 modulator alone likewise does not cause channels to open but rather primes the channels so that activation can occur with reduced desensitization if agonist is also bound. It does not seem to matter if agonist is bound before or after the type 2 modulator is bound for channels to manifest potentiated currents. Therefore, we hypothesized that the application of PNU-120596 alone would unmask pre-existing desensitization that might be associated with RID.
Fig. 8.
A, schematic representation of the conducting and nonconducting states of α7 nAChR: the unbound resting state; the open activated state, which is transiently promoted by low to intermediate levels of agonist binding; the desensitized closed state, which predominates once multiple ligand binding sites have been occupied by agonist; the potentiated (desensitization resistant) conducting state, which requires both the binding of agonist and a type 2 allosteric modulator; and the allosterically primed state, which will readily convert to the potentiated conducting state upon the binding of agonist. B, PNU-120596 applied alone reverses RID. Shown are representative responses from single oocytes to 60 μM ACh and then either ACh at 1 mM (a concentration that produces essentially instantaneous desensitization) or the experimental agonists at the indicated concentrations. Cells were then washed with control buffer, and after 5 min 300 μM PNU-120596 (PNU) was applied for 60 s. The high concentration of PNU-120596 was used to achieve a rapid onset of potentiation. Five minutes after application of PNU alone, ACh was again applied at a concentration of 60 μM. C, average values (± S.E.M.) from at least six cells under each of the application conditions shown in B. To normalize the data, the peak current data were calculated relative to the peak amplitude of an initial ACh control response for each cell (obtained 5 min before the 60 μM ACh responses shown in A).
To further test the hypothesis that α7 RID represents the persistence of ligand-dependent desensitization, we stimulated oocytes with a high concentration of ACh, diMeOBA, diOHBA, or nicotine, and then we used our normal 5-min washout procedure before the application of 300 μM PNU-120596 alone for 60 s. Because previous experiments indicated that 10 μM PNU-120596 required several minutes to fully potentiate receptors (Fig. 3), the higher concentration of PNU-120596 was used to achieve a more rapid effect and improve our ability to detect any potential reversal of desensitization. As shown in Fig. 8, application of 300 μM PNU-120596 alone had no immediate effect after the treatment of cells with either 1 mM ACh or 300 μM diOHBA, although as expected, subsequent ACh responses were increased. However, cells previously treated with 300 μM diMeOBA and, to a lesser degree, those previously treated with 100 μM nicotine responded to the application of PNU-120596 alone. These data indicate that the application of the RID-producing compounds nicotine and diMeOBA had an effect on the receptors that persisted after washout of the compounds from the bath. One likely hypothesis is that the RID-producing compounds remained bound to the receptors, keeping the receptors in the desensitized state until they were exposed to PNU-120596, which caused reactivation.
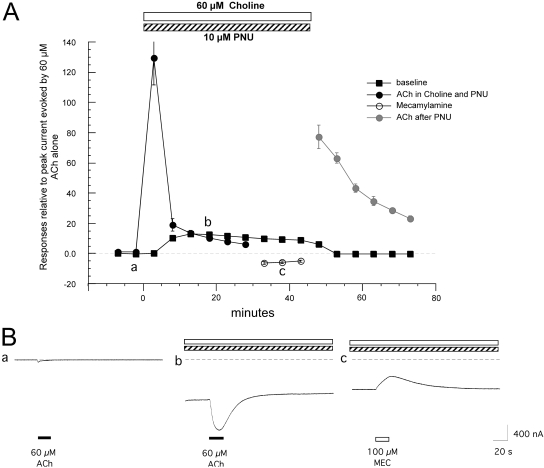
Choline and Mecamylamine Effects in the Presence of PNU-120596. Our data suggest that in the presence of PNU-120596, diMeOBA had the dual effects of producing desensitization that could be converted to steady-state current by the sensitizing effects of PNU-120596 and also of producing transient channel block. We tested the hypothesis that these two effects could be dissociated by using an agonist at a concentration that would normally produce a predesensitization of ACh-evoked responses in combination with a noncompetitive antagonist. Choline is an endogenous factor that is a low-potency α7 agonist, and the steady-state presence of choline can predesensitize α7 receptors to ACh applications either in oocytes (data not shown), hippocampal slices (Frazier et al., 2003), or in acutely dissociated neurons (Uteshev et al., 2003). After obtaining control responses to 60 μM ACh from oocytes expressing α7, we applied 10 μM PNU-120596 and 60 μM choline to the bath, and we made a series of 60 μM ACh applications at 5-min intervals. Although there was a very large potentiation of the first ACh-evoked response after the switch to the choline-PNU-120596-containing bath solution, subsequent ACh responses declined, while a large steady-state current developed (Fig. 9). After the third ACh-evoked response, peak currents were less than the sustained current. Once a large shift in baseline was obtained, a sequence of mecamylamine (100 μM) applications was made to confirm that the sustained current was receptor-mediated. Each mecamylamine application had the transient effect of decreasing steady-state current in a manner similar to the transient effects of diMeOBA. Similar transient decreases in steady-state current could be obtained with applications of 50 nM of the α7-selective antagonist methyllycaconitine (MLA) during the coapplication of 60 μM choline and 10 μM PNU-120596 (data not shown). Once the bath application of choline and PNU-120596 was discontinued, the choline effects on steady-state current and the subsequent suppression of ACh-evoked responses were rapidly reversed. However, PNU-120596 effects on the potentiation of ACh responses returned once choline was removed, and loss of the PNU-120596-potentiating effects on ACh responses occurred slowly (similar to the data shown in Fig. 3).
Fig. 9.
PNU-120596 in combination with choline and ACh. A, average values (±S.E.M.) from five cells treated with 60 μM ACh, before and after the addition of 10 μM PNU-120596 plus 60 μM choline to the bath. To normalize the data, the peak current and baseline data were calculated relative to the peak amplitude of the initial ACh control response for each cell (correcting for the small holding current before the initial control application of ACh). After evoking six responses to 60 μM ACh in the presence of PNU-120596 and choline, three applications of 100 μM mecamylamine (open circles) were made to confirm that the steady-state current was receptor-mediated. Each application of mecamylamine produced a transient decrease in the steady-state current (shown as negative peaks). B, shown are representative ACh-evoked responses from a single oocyte obtained before the addition of PNU-120596 and choline (point a), a PNU-120596-potentiated response recorded on top of a large steady-state current (point b), and a mecamylamine-induced reduction in baseline current.
In separate experiments, we noted that MLA was only able to produce complete inhibition of steady-state current generated by 60 μM choline and 10 μM PNU-120596 at concentrations of MLA >1 μM. This suggests that a previously undocumented effect of PNU-120596 may be to decrease sensitivity of α7 receptors to the competitive antagonist MLA. Likewise, there was only a partial block produced by 100 μM mecamylamine. It may be that in addition to having relatively low potency for inhibition of α7, mecamylamine, which is also use-dependent, may have relatively rapid reversibility. Although there is a significant amount of steady-state current with the experimental paradigm, the actual single-molecule Popen is likely to be much less than 0.10 during the period of choline/PNU-120596 coapplication (based on the augmentation of ACh-evoked peak current responses after the choline was removed; Fig. 9).
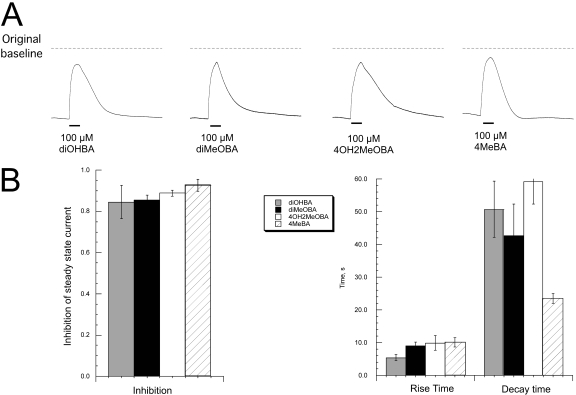
Probing Stable Steady-State Currents for Channel Block by BA Compounds. In addition to producing agonist concentration-dependent fast desensitization, some BA compounds have been reported to also produce transient channel block when applied at high concentration (Uteshev et al., 2002). Evidence for the transient nature of this form of inhibition is provided by the appearance of rebound currents, unmasking ongoing channel activation as agonist concentrations fall below the levels that produce channel block (Horenstein et al., 2007). The application of diMeOBA in the presence of PNU-120596 had two clear effects. One effect of the drug (Fig. 5) was to produce a large PNU-120596-dependent steady-state current. The second effect was to produce a short-term block of the steady-state current during the period of time when the drug was being applied and the BA concentration was highest. Rebound currents produced by diOHBA in both the absence (data not shown) and presence of PNU-120596 (Fig. 6) suggested that this BA also produced some sort of short-term block. However, the complex nature of responses involving both activation and inhibition by the same agent makes comparative analysis of the blocking properties of the various BAs difficult. Therefore, we adapted the protocol shown in Fig. 9 to generate large and stable steady-state currents that could be used to probe for the blocking ability of BA compounds. We made two control applications of ACh and then switched to a bath containing 60 μM choline and PNU-120596. We then gave intermittent applications of 60 μM ACh until the transient ACh responses were equal to the steady-state current (equivalent to point b in Fig. 9A). We then gave 20-s applications of BA compounds. We tested diOHBA, diMeOBA, and two additional compounds that differ markedly in RID production, 4OH,2MeOBA (4OH-GTS-21) and 4MeBA (see Table 1 for structures). As shown in Fig. 10, when applied at 100 μM, all four of these BAs produced very similar block of the choline-PNU-120596-generated steady-state current. This supports the interpretation that the transient blocking properties of the BA compounds are not correlated to their RID-producing activity. In fact, 4MeBA, a compound that produces very slowly reversing RID (Fig. 2), produced the briefest period of block (Fig. 10) in the steady-state current.
Fig. 10.
Block of steady-state currents by BA compounds. Cells expressing α7 were exposed to bath solution containing 60 μM choline and 10 μM PNU-120596 and stimulated at 5-min intervals with 60 μM ACh until the transient ACh-evoked currents were roughly equal to the steady-state current (point b in Fig. 9). They were then given 12-s applications of the BA compounds. A, averaged traces obtained from six oocytes stimulated in parallel. The traces are shown relative to the average steady-state current and the original current baselines (dashed lines). B, magnitude and kinetics of the block of steady-state currents by BA compounds. As indicated by the graph on the left, each BA was able to block 80 to 90% of the steady-state currents. The rise time of the block was measured from the 20 to 90% points on the deflection from the steady-state current to the peak of the block. The decay times were measured from the 90 to 20% points on the return to the steady-state condition.
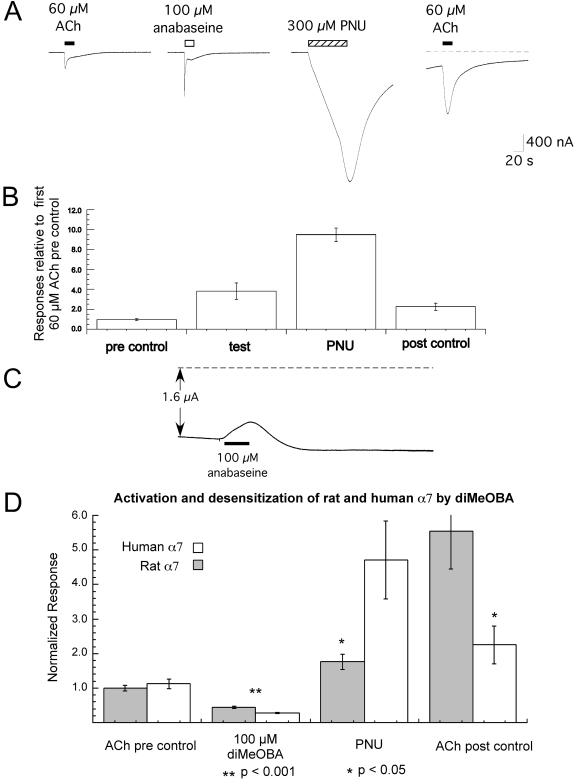
Desensitizing and Blocking Properties of Anabaseine. Having confirmed that BA compounds have both blocking and, in some cases, stable desensitizing effects, we investigated the properties of the core agonist anabaseine. As shown in Fig. 11, A and B, this nonselective agonist is very effective at producing stable desensitization of α7 nAChR, because an application of PNU-120596 after the washout of anabaseine from the chamber produced a very large modulator-resensitized response. Anabaseine was severalfold more effective than nicotine at putting receptors into a modulator-sensitive state. This is consistent with the previous finding that anabaseine was approximately 30-fold more potent than nicotine at producing RID of rat α7 receptors (Kem et al., 1997). The blocking effects of anabaseine were also investigated by applying 100 μM anabaseine to cells that had large steady-state currents induced in the presence of 60 μM choline and PNU-120596. Anabaseine application produced a clear reduction in the steady-state currents of approximately 32 ± 5% (Fig. 11C), significantly less than what was produced by the BA compounds (p < 0.01).
Fig. 11.
A to C, desensitizing and blocking activity of anabaseine. A, shown are representative responses from a single oocyte to 60 μM ACh and then to 100 μM anabaseine. The cell was then washed with control buffer, and after 5 min 300 μM PNU-120596 (PNU) was applied for 60 s. Five minutes after application of PNU alone, ACh was again applied at a concentration of 60 μM. B, average values (± S.E.M.) from at least six cells under each of the application conditions shown in A. To normalize the data, the peak-current data were calculated relative to the peak amplitude of an initial ACh control response for each cell (obtained 5 min before the 60 μM ACh responses shown in A). C, cells expressing α7 were exposed to bath solution containing 60 μM choline and 10 μM PNU and stimulated at 5-min intervals with 60 μM ACh until the transient ACh-evoked currents were roughly equal to the steady-state current (point b in Fig. 9). Cells were then given 20-s applications of anabaseine. Shown is a representative blocking response of the steady-state current, relative to the original current baselines (dashed line). On average, 100 μM anabaseine only blocked 32 ± 5% of the steady-state current (n = 3), and the blockade was relatively transient (90–20% fall time of 18 ± 1 s; n = 3). D, stable desensitization of human α7 by diMeOBA associated with reduced efficacy compared with rat α7. Net charge responses of rat and human α7 to the application of 100 μM diMeOBA were measured relative to a preapplication 60 μM ACh control response, and after washout cells were treated with 300 μM PNU-120596. Five minutes after application of PNU alone, ACh was again applied at a concentration of 60 μM. Plotted are the average responses of six or more cells under each condition (± S.E.M.).
Differences in diMeOBA Efficacy between Rat and Human α7 Related to Differences in PNU-120596-Sensitive Desensitization. We aimed to test the hypothesis that the production of RID depends on both the chemical properties of the agent as well as specific sequence elements of the receptor, such that the combination of these factors could relate to the efficacy of a given BA compound for a particular α7 receptor subtype or mutant. It has been reported previously that diMeOBA is much less efficacious for human α7 nAChR than for rat α7 (Briggs et al., 1997; Meyer et al., 1998). This has been related to sequence differences in the ligand-binding domain of the two receptors (Stokes et al., 2004). We hypothesized that the efficient induction of RID-associated, PNU-120596-sensitive desensitization might be an important factor limiting the activation of human α7 by diMeOBA. As shown in Fig. 11D, diMeOBA produced greater activation of rat α7 than human α7(p < 0.001) and induced significantly less PNU-120596-sensitive desensitization (p < 0.05). The subsequent ACh control responses were also greater in rat α7 than human α7. These data are consistent with the hypothesis that RID-associated, PNU-120596-sensitive desensitization is regulated by structural elements of both the BA compounds and of the receptor.
Discussion
The identification of α7 nAChR as a potential target for indications, such as Alzheimer's disease, schizophrenia, and chronic inflammation, has motivated the development of α7-selective drugs. The drugs that selectively activate α7 receptors achieve selectivity through various structural motifs. BAs were the first class of molecules identified as α7-selective agonists (de Fiebre et al., 1995). The essential elements of the benzylidene motif (Horenstein et al., 2008) are a core agonist such as quinuclidine or anabaseine and a large hydrophobic substitution that is accommodated in the α7 agonist binding site in such a way as to still permit channel activation. Such compounds may bind to other nAChR but fail to produce activation.
To conduct systematic analyses of α7-selective compounds, we have focused on the benzylidene scaffold. We have shown previously that although the addition of an unsubstituted benzylidene group to a core agonist is sufficient to limit agonist activity to α7 nAChR, efficacy and potency are also determined by specific substituents on the benzylidene group, whether the core agonist is anabaseine (Papke et al., 1996; Kem et al., 2004; Papke et al., 2004a) or quinuclidine (Horenstein et al., 2008). One factor that seems to limit the efficacy, and perhaps the therapeutic utility, of some α7 agonists such as diMeOBA is the potential for generating RID. In summary, our data support the hypothesis that compounds that produce RID may be retained at the agonist binding site on the receptor and continue to keep receptors desensitized as long as they remain bound so that α7 RID is primarily because of prolonged ligand-dependent desensitization.
Although the current study has focused on α7 RID, it is well documented that the applications of BA compounds to heteromeric nAChR can also produce relatively long-lived inhibitory effects. However, for non-α7 receptors, there is more evidence that such effects are because of noncompetitive use-dependent inhibition. For example diMeOBA was 10-fold more potent for inhibiting α4β2 receptors when coapplied with ACh than when applied alone (de Fiebre et al., 1995). It was also shown that inhibition of α4β2 receptors by 4OH,2MeOBA (Meyer et al., 1998) only occurred when the BA was coapplied with ACh. In studies of BA effects on α4β2 receptors, it was easy to determine use dependence because the BAs have little or no intrinsic agonist activity. However, such a determination is more difficult to make if the drug, such as nicotine, has both agonist and antagonist activity. Although nicotine has also been reported to produce RID of α4β2 nAChR, it produces only a transient channel block of α3β4 nAChR. Currents through α3β4 receptors activated by nicotine reach a peak rapidly, and that peak current is much less than what might result from stimulation by ACh. Once α3β4 receptors have been activated by nicotine and the nicotine is being washed from the chamber, channels are apparently released from inhibition and a rebound current is generated (Papke et al., 2000b). However the transient inhibition of α3β4 receptors by nicotine can be converted to long-lived inhibition when two mutations are made in the pore-forming second transmembrane domain (Webster et al., 1999). Another case for clear separation between the agonist and antagonist activity of a single molecule was obtained with α4Y190Fβ2 mutant receptors. For these receptors, 4OH,2MeOBA is a potent agonist, 4-fold more efficacious than ACh. Although relatively low concentrations of 4OH,2MeOBA were very effective at activating the mutant receptors, higher concentrations produced a transient channel block and rebound current (Horenstein et al., 2007).
Although 4OH,2MeOBA can produce potent use-dependent inhibition of α4β2 receptors, it has been reported previously that like diOHBA, it produces relatively little RID of α7. However, at concentrations greater than 30 μM, 4OH,2MeOBA did produce a readily reversible voltage-independent channel block of currents obtained from dissociated neurons (Uteshev et al., 2002). In the present experiments, we saw evidence for reversible use-dependent inhibition and rebound currents in the diMeOBA-stimulated responses recorded under control conditions and in the diOHBA-stimulated currents in the presence of PNU-120596 (Fig. 6). Most remarkably, we saw a separation between the effects of diMeOBA on generating a steady-state activation of PNU-120596 modulator-sensitized receptors and a transient use-dependent inhibition of the same currents.
Under normal conditions, α7 receptors are able to open at low levels of agonist occupancy (Papke et al., 2000a) and desensitize rapidly if agonist remains bound. The slow reversibility of the RID produced by nicotine and some BA compounds would be consistent with the retention of those agonists at one or more binding sites, stabilizing the receptors in desensitized states. Consistent with our data, this activity could not be reversed by a type 1 PAM. However, the type 2 PAM, PNU-120596, which activates receptors that would otherwise be desensitized, was able to convert RID into steady-state current, supporting the interpretation that the limiting factor in the RID of α7 receptors is desensitization. Anabaseine, the core agonist of all the BA compounds, also is very effective at producing RID, based on stable desensitization reversible by PNU-120596. Therefore, the benzylidene of BA compounds accounts for selective activation of α7 and also provides a structural scaffold upon which specific side-group substitutions can modulate the activating and desensitizing properties of specific compounds. In addition, the differing effects of diMeOBA on rat and human α7 receptors in regard to efficacy and RID support the hypothesis that there are key point-to-point molecular interactions between the ligands and the receptor that determine these properties.
Our experiments also draw attention to a potentially important consideration regarding the development of PAMs for therapeutics. The α7 nAChR have a very high-calcium permeability, and high levels of calcium influx through other ligand-gated ion channels, such as the N-methyl-d-aspartate-type glutamate receptors, can have dangerous excitotoxic effects, resulting in neuronal cell death. However, under normal conditions, the intrinsically low Popen of α7 receptors prevents them from generating dangerously high calcium signals. A type 2 PAM may remove this safety factor. Choline can be considered an endogenous regulator of α7 receptor function. Although brief applications of choline at high concentration can produce transient activation of α7, lower steady-state levels of choline induce predesensitization and down-regulate responses to ACh. Because PNU-120596 can convert that predesensitization to steady-state activation, in a therapeutic setting type 2 PAMs may produce dangerously high levels of receptor activation. The concentration of choline used in our experiment (60 μM) was at the high end of the physiological range for choline in the brain, and higher (100 μM) levels may be achieved under conditions of stress, trauma, or disease (Jope and Gu, 1991; Scremin and Jenden, 1991; Farooqui and Horrocks, 1994; Klein et al., 1997). Because of potential interaction with endogenous choline, the presence of a type 2 α7 PAM could therefore be most dangerous at times when the brain would be most vulnerable to long-term damage from stroke or seizure disorders. Our data also suggest that a type 2 α7 PAM could produce a potentially dangerous dysregulation/overactivation of α7 receptors in smokers or individuals receiving nicotine replacement therapies.
In conclusion, the future therapeutic development of agents targeting α7 receptors will benefit from a comprehensive consideration of multiple factors. By no means should all α7-selective agonists and partial agonist be considered equivalent, distinguished by only differences in efficacy and potency. Some agents such as BA compounds, while activating α7 receptors, inhibit non-α7 nAChR, whereas other compounds, such as AR-R17779, activate α7 without affecting non-α7 receptors (Papke et al., 2004b). Because α7 and non-α7 receptors can have opposing effects within a single brain system (Mansvelder et al., 2002), a BA compound might better tune a system for α7 effects than an agent such as AR-R17779. Moreover, important factors such as the degree to which specific agents induce prolonged desensitization will vary even among a single class of compounds such as the BAs and should be considered as part of the complete pharmacological profile. Likewise, although the development of α7 PAMs seems an attractive alternative pathway for drug development, our data suggest that type 2 PAMs should be used with caution. Type 1 PAMs should be safer than type 2 PAMs and might even be used in combinations with direct-acting agonists. However, the effectiveness of such a combination therapy would be limited by the desensitizing effects of the specific α7 agonist.
Acknowledgments
We thank Clare Stokes, Lynda Cortes, Sara Braley, and Shehd Al Rubaiy for technical assistance and Dr. Steve Baker for helpful discussions.
This study was supported by the National Institutes of Health [Grants GM57481, T32-AG00196, P01-AG10485].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.150151.
ABBREVIATIONS: nAChR, nicotinic acetylcholine receptor(s); ACh, acetylcholine; BA, benzylidene anabaseine; diMeOBA, 3-(2,4-dimethoxybenzylidene) anabaseine (also DMXBA or GTS-21); RID, residual inhibition or desensitization; diOHBA, 3-(2,4-dihydroxybenzylidene) anabaseine; PAM, positive allosteric modulators; PNU-120596, 1-(5-chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxanol-3-yl)-urea; 2,4DiMeOBA, (E)-3-(2,4-dimethoxybenzylidene)-anabaseine; 2,4DiOHBA, (E)-3-(2,4-dihydroxybenzylidene)-anabaseine; 4OH2MeOBA, (E)-3-(4-hydroxy-2-methoxybenzylidene)-anabaseine; 2OH4MeOBA, (E)-3-(2-hydroxy-4-methoxybenzylidene)-anabaseine; Popen, open probability; 4MeS-BA, (E)-3-(4-methylthiobenzylidene)-anabaseine; 4OH-BA, (E)-3-(4-hydroxybenzylidene)-anabaseine; 4AminoBA, (E)-3-(4-aminobenzylidene)-anabaseine; 4MeOBA, (E)-3-(4-methoxybenzylidene)-anabaseine; 4MeBA, (E)-3-(4-methylbenzylidene)-anabaseine; 2MeBA, (E)-3-(2-methylbenzylidene)-anabaseine; 3MeBA, (E)-3-(3-methylbenzylidene)-anabaseine; 3CNBA, (E)-3-(3-cyanobenzylidene)-anabaseine; 3NitroBA, (E)-3-(3-nitrobenzylidene)-anabaseine; 3OHBA, (E)-3-(3-hydroxybenzylidene)-anabaseine; 3MeOBA, (E)-3-(3-methoxybenzylidene)-anabaseine; 2MeOBA, (E)-3-(2-methoxybenzylidene)-anabaseine; 5HI, 5-hydroxyindole; MLA, methyllycaconitin; AR-R17779, spiro(1-azabicyclo(2.2.2)octane-3,5′-oxazolidin-2′-one).
References
- Bertrand D, Bertrand S, Cassar S, Gubbins E, Li J, and Gopalakrishnan M (2008) Positive allosteric modulation of the alpha7 nicotinic acetylcholine receptor: ligand interactions with distinct binding sites and evidence for a prominent role of the M2–M3 segment. Mol Pharmacol 74 1407-1416. [DOI] [PubMed] [Google Scholar]
- Briggs CA, Anderson DJ, Brioni JD, Buccafusco JJ, Buckley MJ, Campbell JE, Decker MW, Donnelly-Roberts D, Elliott RL, Gopalakrishnan M, et al. (1997) Functional characterization of the novel nicotinic receptor ligand GTS-21 in vitro and in vivo. Pharmacol Biochem Behav 57 231-241. [DOI] [PubMed] [Google Scholar]
- de Fiebre CM, Meyer EM, Henry JC, Muraskin SI, Kem WR, and Papke RL (1995) Characterization of a family of anabaseine-derived compounds reveals that the 3-(4)-dimethylaminocinnamylidine derivative (DMAC) is a selective agonist at neuronal nicotinic α7/[125I]α-bungarotoxin receptor subtypes. Mol Pharmacol 47 164-171. [PubMed] [Google Scholar]
- Farooqui AA and Horrocks LA (1994) Excitotoxicity and neurological disorders: involvement of membrane phospholipids. Int Rev Neurobiol 36 267-323. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Strowbridge BW, and Papke RL (2003) Nicotinic acetylcholine receptors on local circuit neurons in the dentate gyrus: a potential role in the regulation of granule cell excitability. J Neurophysiol 89 3018-3028. [DOI] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, Allensworth D, Guzman-Bonilla A, Clement B, Ball MP, et al. (2008) Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry 165 1040-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahring LC and Rogers SW (2005) Neuronal nicotinic acetylcholine receptor expression and function on nonneuronal cells. AAPS J 7 E885-E894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønlien JH, Håkerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, and Malysz J (2007) Distinct profiles of {alpha}7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol 72 715-724. [DOI] [PubMed] [Google Scholar]
- Gurley D, Harris EW, Li C, Johnson EC, and Lanthorn T (2000) 5-Hydroxyindole potentiates the nicotinic acetylcholine receptor alpha7 subtype. Soc Neurosci Abstr 26 827. [Google Scholar]
- Halevi S, Yassin L, Eshel M, Sala F, Sala S, Criado M, and Treinin M (2003) Conservation within the RIC-3 gene family. Effectors of mammalian nicotinic acetylcholine receptor expression. J Biol Chem 278 34411-34417. [DOI] [PubMed] [Google Scholar]
- Horenstein NA, Leonik FM, and Papke RL (2008) Multiple pharmacophores for the selective activation of nicotinic {alpha}7-type acetylcholine receptors. Mol Pharmacol 74 1496-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein NA, McCormack TJ, Stokes C, Ren K, and Papke RL (2007) Reversal of agonist selectivity by mutations of conserved amino acids in the binding site of nicotinic acetylcholine receptors. J Biol Chem 282 5899-5909. [DOI] [PubMed] [Google Scholar]
- Jope RS and Gu X (1991) Seizures increase acetylcholine and choline concentrations in rat brain regions. Neurochem Res 16 1219-1226. [DOI] [PubMed] [Google Scholar]
- Kem WR (1971) A study of the occurrence of anabaseine in Paranemertes and other nemertines. Toxicon 9 23-32. [DOI] [PubMed] [Google Scholar]
- Kem WR (2000) The brain alpha7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer's disease: studies with DMXBA (GTS-21). Behav Brain Res 113 169-181. [DOI] [PubMed] [Google Scholar]
- Kem WR, Mahnir VM, Papke RL, and Lingle CJ (1997) Anabaseine is a potent agonist upon muscle and neuronal α-bungarotoxin sensitive nicotinic receptors. J Pharmacol Exp Ther 283 979-992. [PubMed] [Google Scholar]
- Kem WR, Mahnir VM, Prokai L, Papke RL, Cao X, LeFrancois S, Wildeboer K, Prokai-Tatrai K, Porter-Papke J, and Soti F (2004) Hydroxy metabolites of the Alzheimer's drug candidate DMXBA (GTS-21): their molecular properties, interactions with brain nicotinic receptors and brain penetration. Mol Pharmacol 65 56-67. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Takenouchi T, Azuma R, Wesnes KA, Kramer WG, Clody DE, and Burnett AL (2003) Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology 28 542-551. [DOI] [PubMed] [Google Scholar]
- Klein J, Chatterjee SS, and Löffelholz K (1997) Phospholipid breakdown and choline release under hypoxic conditions: inhibition by bilobalide, a constituent of Ginkgo biloba. Brain Res 755 347-350. [DOI] [PubMed] [Google Scholar]
- LeFrancois S (2004) A structural investigation of benzylidene anabaseine interactions with the alpha7 nicotinic acetylcholine receptor, in Pharmacology and Therapeutics, p. 124, University of Florida, Gainesville, FL.
- López-Hernández GY, Thinschmidt JS, Morain P, Trocme-Thibierge C, Kem WR, Soti F and Papke RL (2009) Positive modulation of alpha7 nAChR responses in rat hippocampal interneurons to full agonists and the alpha7-selective partial agonists, 4OH-GTS-21 and S 24795. Neuropharmacology, 52 821-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, and McGehee DS (2002) Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33 905-919. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Kuryatov A, Gerzanich V, Lindstrom J, and Papke RL (1998) Analysis of 4OH-GTS-21 selectivity and activity at human and rat α7 nicotinic receptors. J Pharmacol Exp Ther 287 918-925. [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE, et al. (2006) Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry 63 630-638. [DOI] [PubMed] [Google Scholar]
- Papke RL (2006) Estimation of both the potency and efficacy of alpha7 nAChR agonists from single-concentration responses. Life Sci 78 2812-2819. [DOI] [PubMed] [Google Scholar]
- Papke RL and Porter Papke JK (2002) Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J Pharmacol 137 49-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL and Thinschmidt JS (1998) The correction of alpha7 nicotinic acetylcholine receptor concentration-response relationships in Xenopus oocytes. Neurosci Lett 256 163-166. [DOI] [PubMed] [Google Scholar]
- Papke RL, Meyer E, Nutter T, and Uteshev VV (2000a) Alpha7-selective agonists and modes of alpha7 receptor activation. Eur J Pharmacol 393 179-195. [DOI] [PubMed] [Google Scholar]
- Papke RL, Meyer EM, and de Fiebre CM (1996) Differential discrimination between human and rat α7 nAChR by GTS-21 and its primary metabolite, 4-OH,2 methoxybenzylidene anabaseine., in 26th Annual Meeting of the Society for Neuroscience; 1996 November 16–21; Washington, DC. p. 602.604, Society for Neuroscience, Washington, DC.
- Papke RL, Meyer EM, Lavieri S, Bollampally SR, Papke TA, Horenstein NA, Itoh Y, and Porter Papke JK (2004a) Effects at a distance in alpha7 nAChR selective agonists: benzylidene substitutions regulate potency and efficacy. Neuropharmacology 46 1023-1038. [DOI] [PubMed] [Google Scholar]
- Papke RL, Porter Papke JK, and Rose GM (2004b) Activity of alpha7-selective agonists at nicotinic and serotonin receptors expressed in Xenopus oocytes. Bioorg Med Chem Lett 14 1849-1853. [DOI] [PubMed] [Google Scholar]
- Papke RL, Webster JC, Lippiello PM, Bencherif M, and Francis MM (2000b) The activation and inhibition of human nAChR by RJR-2403 indicate a selectivity for the α4β2 receptor subtype. J Neurochem 75 204-216. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, and Brunzell DH (2008) It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 84 329-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scremin OU and Jenden DJ (1991) Time-dependent changes in cerebral choline and acetylcholine induced by transient global ischemia in rats. Stroke 22 643-647. [DOI] [PubMed] [Google Scholar]
- Stokes C, Papke JK, Horenstein NA, Kem WR, McCormack TJ, and Papke RL (2004) The structural basis for drug selectivity between human and rat nicotinic alpha7 receptors. Mol Pharmacol 66 14-24. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM, and Papke RL (2002) Activation and inhibition of native neuronal alpha-bungarotoxin-sensitive nicotinic ACh receptors. Brain Res 948 33-46. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM, and Papke RL (2003) Regulation of neuronal function by choline and 4OH-GTS-21 through alpha7 nicotinic receptors. J Neurophysiol 89 1797-1806. [DOI] [PubMed] [Google Scholar]
- Webster JC, Francis MM, Porter JK, Robinson G, Stokes C, Horenstein B, and Papke RL (1999) Antagonist activities of mecamylamine and nicotine show reciprocal dependence on beta subunit sequence in the second transmembrane domain. Br J Pharmacol 127 1337-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GT, Zwart R, Walker AS, Sher E, and Millar NS (2008) Potentiation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci U S A 105 14686-14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoltewicz JA, Prokai-Tatrai K, Bloom LB, and Kem WR (1993) Long range transmission of polar effects in cholinergic 3-arylidene anabaseines. Heterocycles 35 171-179. [Google Scholar]
- Zwart R, De Filippi G, Broad LM, McPhie GI, Pearson KH, Baldwinson T, and Sher E (2002) 5-Hydroxyindole potentiates human alpha 7 nicotinic receptor-mediated responses and enhances acetylcholine-induced glutamate release in cerebellar slices. Neuropharmacology 43 374-384. [DOI] [PubMed] [Google Scholar]