Abstract
Carisoprodol is a frequently prescribed muscle relaxant. In recent years, this drug has been increasingly abused. The effects of carisoprodol have been attributed to its metabolite, meprobamate, a controlled substance that produces sedation via GABAA receptors (GABAARs). Given the structural similarities between carisoprodol and meprobamate, we used electrophysiological and behavioral approaches to investigate whether carisoprodol directly affects GABAAR function. In whole-cell patch-clamp studies, carisoprodol allosterically modulated and directly activated human α1β2γ2 GABAAR function in a barbiturate-like manner. At millimolar concentrations, inhibitory effects were apparent. Similar allosteric effects were not observed for homomeric ρ1 GABA or glycine α1 receptors. In the absence of GABA, carisoprodol produced picrotoxin-sensitive, inward currents that were significantly larger than those produced by meprobamate, suggesting carisoprodol may directly produce GABAergic effects in vivo. When administered to mice via intraperitoneal or oral routes, carisoprodol elicited locomotor depression within 8 to 12 min after injection. Intraperitoneal administration of meprobamate depressed locomotor activity in the same time frame. In drug discrimination studies with carisoprodol-trained rats, the GABAergic ligands pentobarbital, chlordiazepoxide, and meprobamate each substituted for carisoprodol in a dose-dependent manner. In accordance with findings in vitro, the discriminative stimulus effects of carisoprodol were antagonized by a barbiturate antagonist, bemegride, but not by the benzodiazepine site antagonist, flumazenil. The results of our studies in vivo and in vitro collectively suggest the barbiturate-like effects of carisoprodol may not be due solely to its metabolite, meprobamate. Furthermore, the functional traits we have identified probably contribute to the abuse potential of carisoprodol.
Carisoprodol (N-isopropylmeprobamate, Soma) is a centrally acting skeletal muscle relaxant frequently prescribed for the alleviation of lower back pain (Elenbaas, 1980). In 2000, carisoprodol was the second most frequently prescribed muscle relaxant, accounting for greater than 20% of all skeletal muscle relaxant prescriptions in the United States (Luo et al., 2004). Although evidence of carisoprodol abuse has been reported for several years (Morse and Chua, 1978; Elder, 1991; Rust et al., 1993; Reeves et al., 1997), its abuse is on the rise. A report by Elder (1991) ranked carisoprodol 54th among 234 drugs with abuse potential. Only 9 years later, the Drug Abuse Warning Network (2000) identified carisoprodol as the 20th most abused drug, ranking higher than oxycodone, methadone, and d-lysergic acid diethylamide.
Once ingested, carisoprodol is metabolized to hydroxycarisoprodol, hydroxymeprobamate, and meprobamate (Olsen et al., 1994; Dalén et al., 1996). Meprobamate (Miltown, Equanil) is a sedative-hypnotic that was commonly used in the treatment of anxiety and is currently classified as a schedule IV controlled substance at the federal level. Although the central actions of meprobamate have not been fully elucidated, one target of its effects appears to be GABAA receptors (GABAARs), the predominant inhibitory neurotransmitter receptor in the brain. Rho et al. (1997) demonstrated meprobamate potentiates GABA-gated currents by prolonging burst duration of single-channel currents, and it directly activates GABAARs at millimolar concentrations.
It is believed generally that both the sedative and adverse effects of carisoprodol are due to its metabolic conversion to meprobamate. The known ability of meprobamate to modulate GABAAR function does provide a reasonable explanation for the depressant effects attributed to carisoprodol. However, there is a distinction between carisoprodol toxicity and meprobamate toxicity, with the former being characterized by agitation and bizarre movement and the latter involving mainly CNS depression (Goldberg, 1969; Ellenhorn and Barceloux, 1988; Roth et al., 1998). Moreover, these signs of toxicity are observed early in overdose, before carisoprodol is significantly dealkylated to meprobamate (Roth et al., 1998). These findings suggest the actions of carisoprodol are dangerous in their own right and can be distinguished from those of meprobamate.
In light of these observations, we sought to determine whether carisoprodol, independently of its conversion to meprobamate, can modulate GABAARs. We assessed the actions of carisoprodol at both the molecular pharmacologic and behavioral pharmacologic level. Results from our in vitro studies demonstrate carisoprodol allosterically modulates and directly activates GABAARs, with an efficacy and potency greater than that of meprobamate. Moreover, in vivo behavioral experiments demonstrate that carisoprodol has GABAergic activity, with a pharmacologic profile consistent with that observed in our in vitro studies. Our results collectively provide strong evidence that carisoprodol can directly produce notable CNS depressant activity, and this activity may contribute to its abuse potential.
Materials and Methods
In Vitro Studies
Cloned Receptors. Both stably and transiently transfected cells were used in the present study. Because the α1β2γ2 configuration of the GABAA receptor is the predominant configuration expressed in the brain (Huang et al., 2006) and because it is known to be associated with effects of GABAergic agents on locomotor activity (Rudolph et al., 1999; McKernan et al., 2000), it was the focus of the in vitro studies. Human embryonic kidney (HEK) 293 cells stably expressing human α1β2γ2 (short isoform of γ2) GABAARs or homomeric V5-His-tagged α1 glycine receptors (below) were used in the current investigation. A complete description of the preparation and maintenance of the human α1β2γ2 (short isoform) cell line has been published previously (Hawkinson et al., 1996).
A cell line stably expressing human glycine α1 receptors was generated in our laboratory. In brief, the α1 subunit was subcloned into the vector pcDNA3.1/V5-His B (Invitrogen, Carlsbad, CA) using BamHI and EcoRI restriction sites. The glycine α1 cDNA was linearized with PvuI, and the linearized cDNA was transfected into HEK293 cells using the modified calcium phosphate transfection method. Forty-eight hours after transfection, cells were transferred to a medium containing minimum essential media, 10% fetal bovine serum, l-glutamine (200 mM), penicillin and streptomycin (10,000 U/ml), and the selection agent G-418 (500–1000 mg/ml). Cells were maintained in the selection media for 2 weeks. Resistant cells were split at a high dilution and plated in multiwell plates. Single-cell clones were selected and grown in selective media for another week. Each of these clones was tested for expression of functional glycine receptors using whole-cell patch clamp. Clones that responded robustly to the application of a saturating concentration of glycine were selected and maintained in media containing 500 mg/ml G-418. This cell line, established to stably express human glycine α1 receptors, was used to conduct subsequent experiments. To study both GABAA and glycine receptors, cells stably expressing the respective receptors were plated on glass coverslips coated with poly-l-lysine (Sigma-Aldrich, St. Louis, MO) in 35-mm culture dishes and used for electrophysiological analysis 24 to 48 h after plating.
The wild-type human GABA ρ1 subunit was generously provided by David Weiss (University of Texas Health Science Center, San Antonio, TX). To generate barbiturate-sensitive ρ1 (W328M) subunits, tryptophan 328 of the wild-type ρ1 subunit was mutated to methionine using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). Incorporation of the mutation was verified by DNA sequencing. For studies involving GABA ρ1 receptors, HEK293 cells were transiently transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's specifications. In brief, HEK293 cells were plated onto coverslips and transfected using 0.5 μg of wild-type or mutant GABA ρ1 cDNA. Cells were washed and placed in fresh culture medium after incubation (6 h) at 37°C in a humidified incubator with an atmosphere of 5% CO2. Cells were used in electrophysiological studies 24 to 48 h after transfection.
Electrophysiology. Whole-cell patch-clamp electrophysiology was used to assess GABA-, meprobamate-, carisoprodol-, or glycine-activated Cl- currents. All electrophysiology experiments were conducted at room temperature (22–25°C) with the membrane potential clamped at -60 mV. Patch pipettes of borosilicate glass (1B150F; World Precision Instruments, Inc., Sarasota, FL) were pulled (Flaming/Brown, P-87/PC; Sutter Instrument Company, Novato, CA) to a tip resistance of 4 to 6 MΩ. Patch pipettes were filled with a solution consisting of 140 mM CsCl, 10 mM EGTA-Na+, 10 mM HEPES-Na+, and 4 mM Mg2+-ATP, pH 7.2. Coverslips containing cultured cells were placed in the recording chamber on the stage of an inverted light microscope (Olympus IX71; Olympus, Tokyo, Japan) and superfused continuously with an external solution consisting of 125 mM NaCl, 20 mM HEPES, 3 mM CaCl2, 5.5 mM KCl, 0.8 mM MgCl2, and 10 mM glucose, pH 7.3. Agonist-induced Cl- currents were obtained with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA) equipped with a CV-203BU head stage. Currents were low-pass filtered at 5 kHz, monitored simultaneously on an oscilloscope and a chart recorder (Gould TA240; Gould Instrument Systems Inc., Cleveland, OH), and stored on a computer using an on-line data acquisition system (pCLAMP 6.0; Axon Instruments) for subsequent off-line analysis.
Experimental Protocol. The modulatory effects of carisoprodol on GABA-gated currents were assessed using an EC20 concentration of GABA (Huang et al., 2001). GABA (with or without carisoprodol or other GABAA receptor agonists) was prepared in external solution and applied to each cell by gravity flow using a Y-shaped tube positioned adjacent to the cell. For studies investigating direct activation by carisoprodol, carisoprodol (with or without GABAA receptor antagonists) was dissolved in external solution and applied in the manner described above. In the bemegride studies, cells were incubated in external solution containing bemegride at the indicated concentration for 2 min. Control responses were established by observing two consecutive agonist-activated currents that varied in amplitude by no more than ±10%. After establishing the control response, effects of the test drug were determined.
Data Analysis. Concentration-response profiles for the positive modulatory actions of carisoprodol were generated (Origin 5.0; OriginLab Corp., Northampton, MA) using the equation I/Imax = [carisoprodol]n/([carisoprodol]n + EC50n), where I is the normalized current amplitude at a given concentration of carisoprodol, Imax is the maximum GABA current induced by carisoprodol, EC50 is the half-maximal effective concentration of carisoprodol, and n is the Hill coefficient. All data are presented as mean values ± S.E.M. Statistical significance (p < 0.05) between control and test conditions was determined using Student's t test (paired or unpaired) and one-way analysis of variance. Dunnett's post hoc test was performed as needed.
In Vivo Studies
Animals. Male Sprague-Dawley rats were obtained from Harlan (Indianapolis, IN). All rats were housed individually and were maintained on a 12-/12-h light/dark cycle (lights on at 7:00 AM). Body weights were maintained at 320 to 350 g by limiting food to 20 g/day, which included the food received during operant sessions. Water was freely available. Male Swiss-Webster mice were obtained from Harlan at approximately 8 weeks of age and tested at approximately 10 weeks of age. Mice were group-housed in cages on a 12-/12-h light/dark cycle and were allowed free access to food and water. All in vivo testing of rats and mice was done during the light portion of the cycle. All housing and procedures were in accordance with the guidelines of the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Discrimination Training. Standard operant chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC-compatible computers via LVB interfaces (MED Associates, St. Albans, VT). The computers were programmed in MED-PC IV (MED Associates) for the operation of the chambers and collection of data. Rats were trained to discriminate carisoprodol (100 mg/kg p.o.) from vehicle (2% methylcellulose) using a two-lever choice methodology. Food (45-mg food pellets; Bio-Serv, Frenchtown, NJ) was available as a reinforcer under a fixed-ratio 10 schedule when responding occurred on the injection-appropriate lever. There was no consequence for responses on the incorrect lever. The rats received approximately 60 training sessions before they were used in substitution or antagonism experiments. Animals were selected for use in experiments when they had met the criteria of emitting 85% of responses on the injection-correct lever for both the first fixed ratio and for the remainder of the session during their last 10 training sessions. Training sessions occurred in a double alternating fashion (D-D-S-S-D, etc.), and tests were conducted between pairs of identical training sessions (i.e., between either two vehicle or two carisoprodol training sessions). Rats were tested only if they had achieved 85% drug-lever responding for both first fixed-ratio and total session on the two prior training sessions. Before each session, the rats received an injection of either vehicle or carisoprodol. Ten minutes later, the rats were placed in an operant chamber. Each training session lasted a maximum of 10 min, and the rats could earn up to 20 food pellets.
Discrimination Test Procedures. In contrast with training sessions, both levers were active during the discrimination test sessions, such that 10 consecutive responses on either lever led to reinforcement. Data were collected until the first reinforcer was obtained or for a maximum of 20 min. At least 3 days elapsed between test sessions. Groups of nine or 10 rats were tested with each test compound. A repeated measures design was used, such that each rat was tested at all doses. During substitution experiments, oral administration of carisoprodol (by gavage, 1 ml/kg) or its vehicle (2% methylcellulose) occurred 20 min before the start of the test session, and a dose range of 10 to 100 mg/kg was examined. Meprobamate (10–175 mg/kg), pentobarbital (1–25 mg/kg), and chlordiazepoxide (1–10 mg/kg) were administered via intraperitoneal injections (1 ml/kg). Administration of meprobamate or its vehicle (2% methylcellulose) occurred 30 min before the start of the test session. Administration of pentobarbital, chlordiazepoxide, or their vehicle (0.9% saline) occurred 15 min before the start of the test session. During antagonism experiments, intraperitoneal injections (1 ml/kg) of bemegride (1–5 mg/kg), flumazenil (0.5 to 25 mg/kg), or their vehicles occurred 30 min before the start of the test session. Oral administration of carisoprodol (by gavage, 1 ml/kg) occurred 20 min before the start of the test session.
Locomotor Activity. Studies of locomotor activity were conducted using a Digiscan apparatus (model RXYZCM-16; Omnitech Electronics, Columbus, OH) and clear acrylic locomotor activity testing chambers (40.5 × 40.5 × 30.5 cm) housed in sets of two, within sound-attenuating chambers. A panel of infrared beams (16 beams) and corresponding photodetectors were located in the horizontal direction along the sides of each activity chamber. A 7.5-W incandescent light above each chamber provided dim illumination. Fans provided an 80-dB ambient noise level within the chamber.
In studies of the time course of locomotor depression elicited by meprobamate, separate groups of eight mice received either vehicle (2% methylcellulose) or meprobamate (10, 30, 100, or 300 mg/kg) by intraperitoneal injection immediately before locomotor activity testing. Time-course studies of carisoprodol involved separate groups of 16 mice that received either vehicle (2% methylcellulose) or carisoprodol (10, 30, 100, 300, or 560 mg/kg p.o.) (by gavage) immediately before locomotor activity testing. In the time-course studies, photo-cell interruptions (ambulation counts) within the horizontal plane of the testing chambers were recorded within 10-min epochs for a period of 8 h.
Additional studies were performed using separate groups of eight mice to compare the times of onset of locomotor depression after 300 mg/kg carisoprodol (administered orally versus intraperitoneally) or meprobamate (administered intraperitoneally). In these studies, locomotor activity was recorded in 4-min epochs for a period of 24 min after administration.
Data Analysis. Drug discrimination data were expressed as the mean percentage of responses made on the carisoprodol-appropriate lever. Percentage carisoprodol-appropriate responding and response rate were plotted as a function of the dose of the test compound (log scale). Percentage carisoprodol-appropriate responding was calculated for a given dose only if at least three rats completed the fixed ratio. Full substitution was defined as ≥80% carisoprodol-appropriate responding and full antagonism as ≤20% carisoprodol-appropriate responding. Rates of responding were expressed as a function of the number of responses made divided by the total session time. The potencies of carisoprodol, meprobamate, chlordiazepoxide, pentobarbital, and bemegride were calculated by fitting straight lines to the individual dose-response data for each compound by means of Table-Curve 2D (SPSS, Inc., Chicago, IL). Straight lines were fitted to the linear portion of dose-effect curves, defined by doses producing 20 to 80% of the maximal effect, including not more than one dose producing <20% of the maximal effect and not more than one dose producing >80% of the maximal effect. Other doses were excluded from the analyses. The slopes of the dose-effect curves were compared using parallel line procedures (Kenakin, 1997). Comparison of the ED50 values was performed by one-way analysis of variance. Individual comparisons were made using Bonferroni-adjusted F tests. Criterion for significance was set a priori at p < 0.05. Response rate data were analyzed by one-way repeated measures analyses of variance. In the context of a significant overall effect, individual doses were compared with the appropriate control value using individual F tests.
For assessment of time-course effects, ambulation counts within each time sampling interval were considered in a two-way analysis of variance, with treatment and time (repeated) as the factors. Only the first 5 h of the test session was considered because no treatment effects were evident over the last 3 h of testing. The 10- to 20-min time period was selected for analysis of dose-response data because this was the earliest period in which maximal locomotor depression first appeared as a function of dose for both compounds. An ED50 (dose producing one half-maximal depressant activity, where maximal depression = 0 counts/10 min), was calculated based on a linear fit to the descending portion of the dose-response curve. A one-way analysis of variance was conducted on ambulation counts for the 10- to 20-min time period for each compound, and individual comparisons of each dose with vehicle control were considered using F tests. For studies of the onset of depression after carisoprodol and meprobamate, data were considered in a two-way analysis of variance, with treatment and time (repeated) as the factors.
Drugs. Carisoprodol (C12H24N2O4), meprobamate (C9H18N2O4), diazepam, chlordiazepoxide, flumazenil, picrotoxin, and pentobarbital sodium were purchased from Sigma-Aldrich. Bemegride was obtained from Pfaltz and Bauer Ltd. (Waterbury, CT). For the electrophysiology studies, stock solutions of these compounds were made by dissolving the compounds in dimethyl sulfoxide. Drugs were diluted in normal saline, so that the final dimethyl sulfoxide concentration (v/v) of the test solutions was ≤0.3%. GABA and bemegride stock solutions were prepared using H2O. For the in vivo studies, carisoprodol, meprobamate, and flumazenil were suspended in 2% methylcellulose. Chlordiazepoxide, bemegride, and pentobarbital were dissolved in 0.9% saline. Carisoprodol was administered by oral gavage in a volume of 1 ml/kg. All remaining drugs were administered intraperitoneally in a volume of 1 ml/kg.
Results
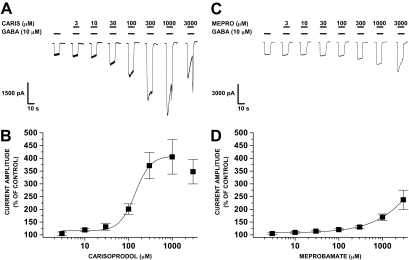
Carisoprodol Allosterically Modulates GABA-Activated Currents. Using whole-cell patch-clamp electrophysiology, we investigated carisoprodol-mediated effects on HEK293 cells stably expressing human α1β2γ2 GABAARs. This approach circumvents the metabolism of carisoprodol to meprobamate, allowing us to focus solely on the effects of the parent drug. When coapplied with GABA, carisoprodol potentiated GABA-gated currents in a concentration-dependent manner (Fig. 1). The actions of carisoprodol were rapid and reversible, suggesting carisoprodol-mediated effects are due to direct interaction with the receptor. The EC50 value for carisoprodol was estimated at 142 ± 13 μM, with a Hill coefficient of 2.46 ± 0.42, although the direct-gating effects of carisoprodol (below) may contribute to recorded current amplitude. Potentiation of GABA-gated currents is characteristic of central nervous system depressants such as benzodiazepines, barbiturates, neurosteroids, and anesthetics (see Huang et al., 2006). At millimolar concentrations, we observed “rebound currents” upon termination of drug application, and we also observed inhibitory actions of carisoprodol on GABA-gated currents. This phenomenon is observed with some other GABA modulators, including barbiturates (Rho et al., 1996) and lactones (Gonzales et al., 2004). In a previous study, meprobamate was shown to allosterically modulate GABA-activated currents (Rho et al., 1997). In the present study, we also found that meprobamate could allosterically enhance GABA-gated currents (Fig. 1, C and D), although both the potency and the efficacy were less than that observed with carisoprodol.
Fig. 1.
Potentiation of GABA-gated currents by carisoprodol and meprobamate. A, representative traces demonstrating potentiation of GABA-gated currents by carisoprodol in a concentration-dependent manner. At millimolar concentrations, offshoot currents were observed upon termination of drug application, and currents were inhibited at carisoprodol concentrations above 1 mM. B, concentration-response curve for the allosteric effects of carisoprodol on GABA-gated currents. Relatively large variance at high concentrations is due to onset of inhibition of some cells. C, representative traces demonstrating potentiation of GABA-gated currents by meprobamate. D, concentration-response curve for the allosteric effects of meprobamate on GABA-gated currents. For both data sets, each point represents the mean ± S.E. of data collected from three to 17 cells.
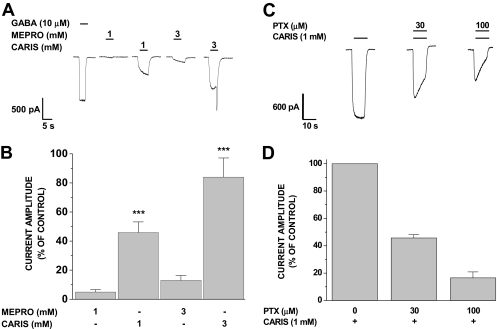
Carisoprodol Activates Inward Currents in the Absence of GABA. Several agents that potentiate GABA-gated currents can directly activate GABAARs in the absence of GABA (see Huang et al., 2006); meprobamate is among these (Rho et al., 1997). Thus, we assessed whether carisoprodol can directly activate GABAARs. As shown in Fig. 2, application of carisoprodol in the absence of GABA elicited a concentration-dependent inward current activation. As was seen with allosterically enhanced GABA currents, we observed rebound currents in response to millimolar carisoprodol (Fig. 2, A and B). In addition, we confirmed the results of Rho et al. (1997) demonstrating direct activation of GABA currents by meprobamate. Although both drugs directly activated GABAARs, carisoprodol was more potent and efficacious than its metabolite (Fig. 2, A and B). Our studies collectively suggest that carisoprodol has the potential to produce sedative effects similar to those of meprobamate, and it can do so without being metabolized to meprobamate.
Fig. 2.
Direct activation of GABAA receptors by carisoprodol and meprobamate. A, representative traces demonstrating inward currents evoked by carisoprodol and meprobamate in the absence of GABA. B, current amplitude of carisoprodol- or meprobamate-evoked currents relative to currents evoked by 10 μM GABA. Each bar represents the mean ± S.E. of a minimum of three cells; ***, significant difference relative to an equal concentration of meprobamate (p < 0.001). C, representative traces of carisoprodol-mediated currents in the presence of various concentrations of PTX. Current amplitude was measured at the end of the 10-s coapplication period. D, summary of results illustrated in C; carisoprodol-activated currents were reduced to 45.7 ± 2.5% and 16.6 ± 4.3% of control in the presence of 30 and 100 μM PTX, respectively. Recovery from PTX was 78.7 ± 9.0% of control (data not shown). Antagonism by PTX suggests the carisoprodol-activated current was conducted via GABAA receptors. Each bar represents the mean ± S.E. of four cells.
To confirm that carisoprodol-activated inward currents were, in fact, mediated by GABAARs, we investigated carisoprodol's effects in the presence of picrotoxin (PTX), a widely utilized GABAAR antagonist. Carisoprodol-activated current amplitude was antagonized by PTX in a concentration-dependent manner (Fig. 2, C and D), indicating that the inward currents are carried by GABAARs.
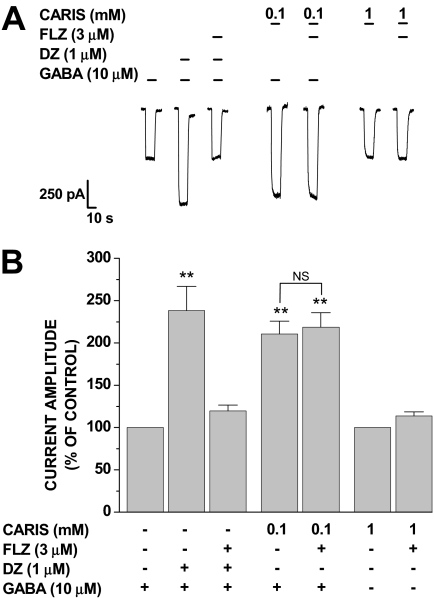
Carisoprodol Does Not Mediate Its Effects via the Benzodiazepine Site of the GABAA Receptor. Aside from the GABA binding site, these receptors have distinct binding sites for several clinically important drugs, including barbiturates and benzodiazepines, among others (see Huang et al., 2006). To assess the potential involvement of the benzodiazepine site in the actions of carisoprodol, we tested its ability to modulate GABA-gated currents in the presence of the benzodiazepine antagonist flumazenil. As shown in Fig. 3, flumazenil blocked the ability of diazepam to augment GABA-gated currents, yet it had no significant effect on the allosteric effects of carisoprodol. Likewise, the presence of flumazenil did not attenuate the amplitude of carisoprodol-mediated currents. These data demonstrate that neither the direct nor allosteric potentiating effects of carisoprodol are mediated via the benzodiazepine site of GABAARs.
Fig. 3.
Effects of flumazenil on carisoprodol activity at GABAA receptors. A, representative traces demonstrating carisoprodol-mediated currents and the potentiation of GABA-gated currents by diazepam and carisoprodol in the presence and absence of flumazenil. B, coapplication of diazepam potentiated GABA-gated currents to 238.3 ± 28.7% of control values. The actions of diazepam were blocked by a saturating concentration of flumazenil. Coapplication with carisoprodol potentiated GABA-gated currents to 210.7 ± 14.9% of control values. The allosteric actions of carisoprodol were not significantly different in the presence of flumazenil (218.6 ± 17.1% of control). Likewise, carisoprodol-mediated currents were 113.8 ± 4.9% of control values in the presence of flumazenil (N.S., p > 0.05). Each bar represents the mean ± S.E. of data collected from four cells.
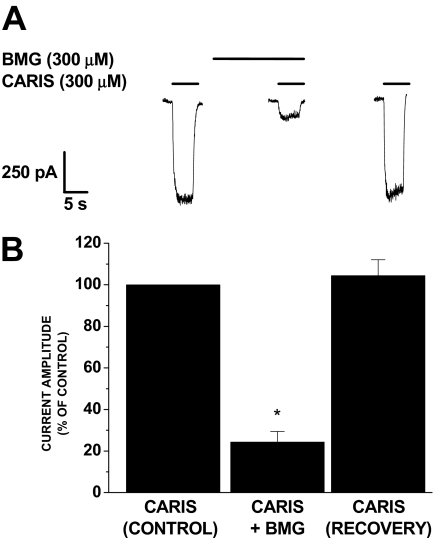
Carisoprodol-Mediated Currents Are Blocked by Bemegride. The actions of meprobamate, the metabolite of carisoprodol, have been characterized as “barbiturate-like” (Rho et al., 1997). Moreover, because the effects of carisoprodol reported here are in several ways also reminiscent of barbiturates, we considered whether the barbiturate site is involved in mediating the effects of carisoprodol. Although not necessarily considered a pure barbiturate antagonist, bemegride has been shown to antagonize the stimulus effects of pentobarbital (Krimmer et al., 1978; Schechter, 1984). Thus, antagonism of carisoprodol's effects by bemegride might provide some insight into whether carisoprodol and barbiturates share a site or mechanism of action. Because high concentrations of bemegride also inhibit GABA-gated currents, we did not assess its effects on allosteric modulation by carisoprodol. Instead, we examined whether bemegride incubation affects carisoprodol-mediated currents. As shown in Fig. 4, carisoprodol-activated currents were significantly and reversibly attenuated in the presence of bemegride. Although not definitive, these findings are consistent with the possibility that the barbiturate site may influence the direct-gating effects of carisoprodol.
Fig. 4.
Antagonism of carisoprodol-mediated currents by bemegride. A, representative traces demonstrating carisoprodol-activated currents are reduced after bemegride incubation. These experiments were conducted using the stable human α1β2γ2 cell line. B, subsequent to incubation with bemegride for 2 min, peak current amplitude was reduced to 24.4 ± 5.0% of control (n = 4; *, p < 0.001). Recovery was not significantly different from control (p > 0.05).
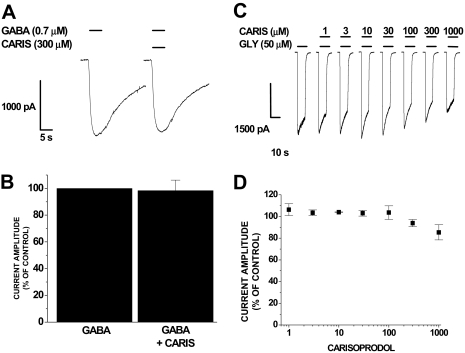
Carisoprodol Does Not Modulate Homomeric ρ1 or Glycine α1 Receptors. To assess the extent to which carisoprodol might selectively potentiate GABAARs, we evaluated its ability to modulate homomeric ρ1 GABA and homomeric α1 glycine receptors, two other anion-selective members of the Cys-loop family of LGICs with pharmacology distinct from that of the GABAAR. Transient transfection of ρ1 cDNA into HEK293 cells resulted in GABA-gated currents that displayed GABA sensitivity (EC50, 0.9 ± 0.08 μM) and activation and deactivation kinetics consistent with those previously reported (Amin and Weiss, 1994). In these homomeric ρ1 receptors, 300 μM carisoprodol had no effect on the GABA-activated current (Fig. 5, A and B). In α1 glycine receptors, concentrations of carisoprodol up to 1 mM had no stimulatory effect on the current amplitude of glycine-gated currents; at 1 mM, modest attenuation of the glycine-gated current was observed (Fig. 5, C and D). Thus, both homomeric ρ1 GABA receptors and homomeric α1 glycine receptors show no potentiation in response to carisoprodol, in contrast to the severalfold potentiation observed in α1β2γ2 GABAARs (Fig. 1 above).
Fig. 5.
Effects of carisoprodol on homomeric ρ1 GABA and homomeric α1 glycine receptors. A, representative traces demonstrating inward currents evoked by GABA alone or in the presence of 300 μM carisoprodol. B, summary graph of experiments described in A. Peak amplitude of GABA-gated current in ρ1 receptors was unaffected by 300 μM carisoprodol. Each bar represents the mean ± S.E. of three cells tested. C, representative traces demonstrating inward currents evoked by glycine in the absence or presence of carisoprodol. D, summary concentration-response curve of studies depicted in C. Carisoprodol did not have a stimulatory effect on glycinegated currents, whereas modest inhibitory effects were observed at 1 mM carisoprodol. Each data point represents the mean ± S.E. of a minimum of three cells tested.
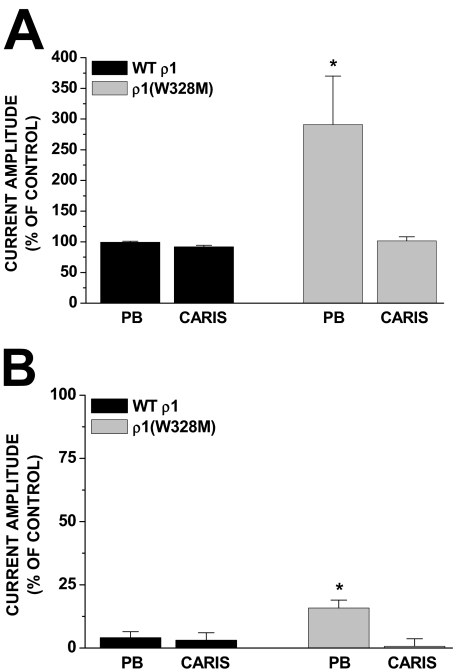
W328M Mutation in ρ1 Receptors Confers Sensitivity to Pentobarbital but Not Carisoprodol. Amin (1999) has shown that introduction of a methionine residue at position 328 in homomeric ρ1 receptors confers sensitivity to both direct-gating and allosteric effects of pentobarbital. Thus, we generated this mutant and assessed whether it could similarly confer sensitivity to carisoprodol. HEK293 cells transfected with the ρ1 subunit cDNA yielded functional receptors with GABA sensitivity and channel kinetics consistent with those previously reported (Amin and Weiss, 1994). As reported by Amin (1999), the W328M mutation did confer sensitivity to both the allosteric modulating and direct gating effects of pentobarbital (Fig. 6, A and B). In contrast, this mutation did not confer to carisoprodol the ability to either allosterically potentiate or directly gate the ρ1 receptor (Fig. 6, A and B). Thus, although our data, in general, demonstrate the actions of carisoprodol are barbiturate-like, these experiments indicate the binding and/or functional domains for the two ligands are not equivalent.
Fig. 6.
W328M confers sensitivity to pentobarbital but not carisoprodol. A, in wild-type homomeric ρ1 receptors, neither pentobarbital (300 μM) nor carisoprodol (1 mM) enhanced GABA (EC20)-gated current. In W328M mutant receptors, pentobarbital, but not carisoprodol, could enhance GABA-activated currents. The GABA EC20 is denoted as control current amplitude. B, similar phenomenon existed with regard to direct-gating effects. In the W328M mutant receptors, pentobarbital could directly gate the channel to approximately 15% of the maximal current amplitude gated by GABA. In contrast, carisoprodol was ineffective in direct gating in either wild-type or W328M mutant receptors. Each data point represents the mean ± S.E. of four cells. Maximal GABA-gated current is denoted as 100%. *, significantly different from the wild-type response (p < 0.05).
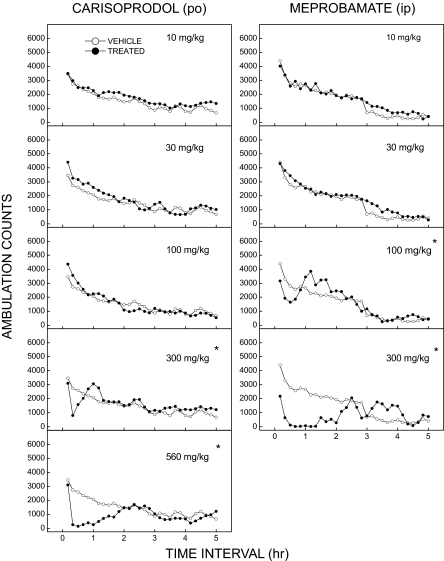
Carisoprodol Produces Time-Dependent Depression of Locomotor Activity. Behavioral studies were carried out to assess the in vivo actions of carisoprodol. First, we investigated the ability of carisoprodol to depress the locomotor activity of nonhabituated, male Swiss-Webster mice. As depicted in Fig. 7, left, treatment with carisoprodol resulted in maximal depression of locomotor activity (lasting 40 min to 2 h) after doses of 300 or 560 mg/kg, respectively. For both doses, maximal depressant effects were first evident within the time period from 10 to 20 min after treatment, and an ED50 of 240 mg/kg was estimated based on dose-response data for this period. A significant interaction of treatment and time period supported the overall observation of dose- and time-dependent effects [F(145,2610) = 3.47, p < 0.001], and individual comparisons with the vehicle group for the 10- to 20-min time period confirmed a significant depressant effect for 300 and 560 mg/kg (all p < 0.001).
Fig. 7.
Time course of carisoprodol- and meprobamate-induced locomotor depression. Each panel shows mean ambulation counts per 10-min interval for one dose of the test compound in comparison with the saline control in mice. Treatment with carisoprodol (left, n = 16) resulted in depression of locomotor activity after 300 or 560 mg/kg p.o. Maximal depressant effects for these doses were evident after 10 min and ended 40 or 110 min, respectively, after administration. Treatment with meprobamate (right, n = 8) resulted in dose-dependent depression of locomotor activity after 100 or 300 mg/kg i.p. Depressant effects of these doses were evident within 10 min and ended 50 or 150 min, respectively, after injection. *, doses significantly different (p < 0.05) from vehicle for the period 10 to 20 min after injection.
Although the above results are consistent with previous reports that carisoprodol produces sedation, loss of balance, and increased reaction time (Robertson and Marinetti, 2003), they did not address whether the effects are due to carisoprodol or its metabolite, meprobamate. To address this question, experiments were also conducted with meprobamate to determine whether the extent of locomotor depression and its time course would be consistent with that observed after oral carisoprodol. Intraperitoneal treatment with meprobamate resulted in partial depression of locomotor activity (lasting approximately 40 min) after 100 mg/kg and maximal depression (lasting 2.5 h) after 300 mg/kg (Fig. 7, right). Maximal depressant effects of meprobamate were first evident 10 to 20 min after 300 mg/kg, and an ED50 of 135 mg/kg was estimated based on dose-response data for this time period. A significant interaction of treatment and time period [F(116,1015) = 4.89, p < 0.001] supported the overall observation of time- and dose-dependent effects, and individual comparisons with the vehicle group for the 10- to 20-min time period confirmed significant depression for the 100 and 300 mg/kg doses (all p < 0.001). It is noteworthy that after 300 mg/kg, maximum depression relative to vehicle control was evident for both compounds within 20 min after injection, yet the time course of depression was dramatically longer for meprobamate. This observation would be consistent with reports suggesting a shorter plasma half-life of carisoprodol compared with meprobamate (e.g., Bramness et al., 2004).
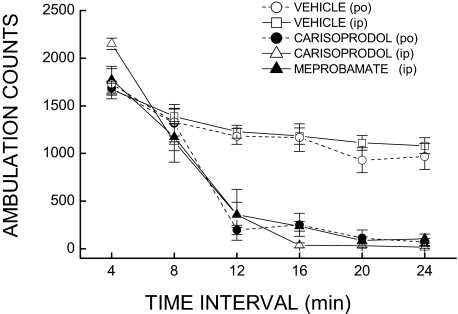
To determine the influence of the oral route of administration on the rate of onset of locomotor depression after carisoprodol relative to the intraperitoneal administration of meprobamate, we compared the effects of those treatments when locomotor activity was monitored within 4-min epochs for 24 min. As suggested by the results shown in Fig. 8, administration of 300 mg/kg i.p. carisoprodol failed to significantly accelerate (or delay) the onset of locomotor depression relative to the same dose administered orally. Moreover, carisoprodol by either route produced locomotor depression that was equivalent in magnitude and rate of onset to meprobamate injected intraperitoneally. However, carisoprodol administered intraperitoneally resulted in stimulation of locomotor activity during the first 4 min after injection, an effect not evident after the other treatments. Analysis of these data yielded a significant treatment × time period interaction [F(20,175) = 9.7, p < 0.001], in accordance with the decrease in locomotor activity after all drug treatments that began 8 min after administration of carisoprodol or meprobamate. Individual comparisons at each time period confirmed a significant difference from control for all treatments during periods 3 to 6 and a significant difference between intraperitoneal carisoprodol and the intraperitoneal vehicle control during the first 4 min of testing (p < 0.01).
Fig. 8.
Rate of onset for behavioral depression after carisoprodol or meprobamate. Mean ambulation counts as a function of 4-min time periods for separate groups of eight mice receiving carisoprodol orally, carisoprodol intraperitoneally, meprobamate intraperitoneally, or the vehicle administered intraperitoneally or orally. The dose administered to each drug group was 300 mg/kg. No difference in the rate of onset of behavioral depression was evident after any of the drug treatments.
Discriminative Stimulus Effects of Carisoprodol. Our functional studies performed in vitro strongly suggested carisoprodol has the potential to mediate GABAergic effects in vivo. Thus, we sought to determine whether the stimulus effects of carisoprodol generalized to those of other compounds known to modulate GABAARs. The purpose of these experiments was 2-fold: 1) to train carisoprodol as a discriminative stimulus and 2) to test whether the mechanism for the discriminative stimulus effects of carisoprodol is GABA-like by testing for substitution with a number of GABAergic compounds.
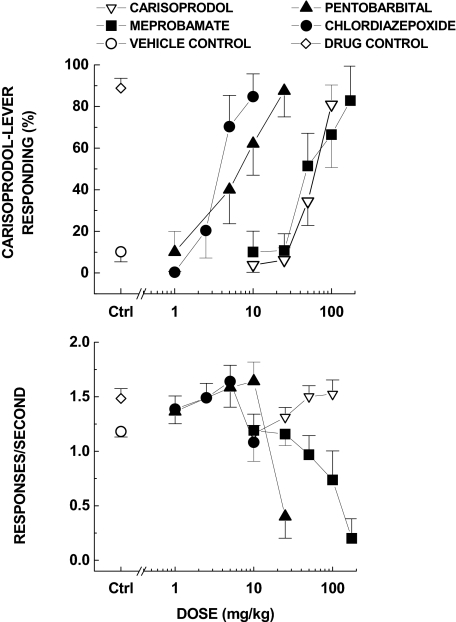
After training of the discrimination for 100 mg/kg carisoprodol, different doses (10, 25, 50, 100 mg/kg p.o.) produced dose-dependent increases in carisoprodol-appropriate responding (Fig. 9). Intraperitoneal injections of pentobarbital (1, 5, 10, 25 mg/kg), meprobamate (10, 25, 50, 100, 175 mg/kg), and chlordiazepoxide (1, 2.5, 5, 10 mg/kg) each produced dose-dependent substitution for the discriminative stimulus effects of carisoprodol (Fig. 9). ED50 values are shown in Table 1. Vehicles for the test compounds produced predominantly vehicle-appropriate responding. Pentobarbital and chlordiazepoxide were more potent than carisoprodol or meprobamate [F(3,27) = 9.23, p < 0.001]. Pentobarbital and chlordiazepoxide did not differ in potency, nor did carisoprodol and meprobamate.
Fig. 9.
Substitution for the discriminative stimulus effects of carisoprodol. Top, percentage of total responses made on the carisoprodol-appropriate lever. Bottom, rate of responding in responses per second (r/s). Carisoprodol, pentobarbital, meprobamate, and chlordiazepoxide fully substituted for the discriminative stimulus effects of 100 mg/kg carisoprodol. Carisoprodol produced a modest increase in response rate, whereas chlordiazepoxide produced no effect, and pentobarbital markedly reduced rates at the highest doses tested (n = 10 rats).
TABLE 1.
ED50 values of substitution for carisoprodol
| Compound | ED50 | 95% Confidence Interval |
|---|---|---|
| mg/kg | ||
| Carisoprodol | 46.71 | 34.08–59.34 |
| Meprobamate | 60.08 | 22.36–97.80 |
| Pentobarbital | 4.46 | 2.39–6.53 |
| Chlordiazepoxide | 3.32 | 2.41–4.23 |
Carisoprodol dose-dependently increased response rate [F(4,64) = 9.13, p < 0.001], and chlordiazepoxide increased response rate after 2.5 and 5 mg/kg [F(4,36) = 5.46, p = 0.002]. In contrast, pentobarbital increased response rate after 5 and 10 mg/kg and decreased response rate after 25 mg/kg [F(4,36) = 12.35, p < 0.001]. Meprobamate dose-dependently decreased response rate [F(5,45) = 6.65, p < 0.001].
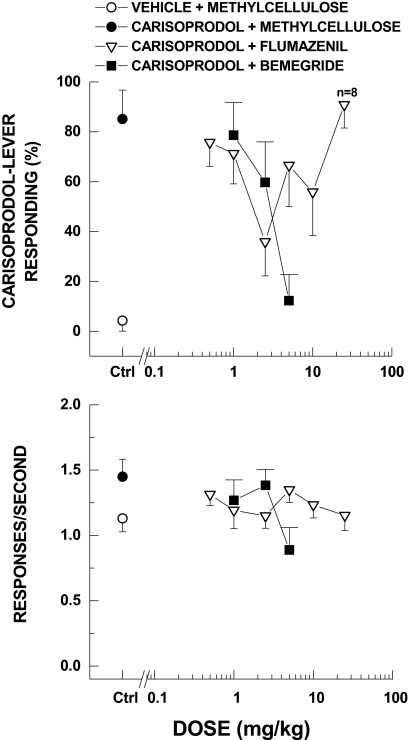
Next, we examined whether the discriminative stimulus effects of carisoprodol could be antagonized in vivo. To determine whether carisoprodol may be acting at barbiturate- or benzodiazepine-sensitive sites, carisoprodol testing was performed in combination with antagonists for those sites on the receptor. Bemegride (1, 2.5, 5 mg/kg) dose-dependently attenuated the discriminative stimulus effects of the training dose of carisoprodol (Fig. 10). The ED50 value was 2.83 mg/kg (95% confidence interval = 2.27–3.36 mg/kg). In contrast, flumazenil failed to fully antagonize the discriminative stimulus effects of carisoprodol (defined as less than or equal to 20% drug-appropriate responding). An intermediate dose (2.5 mg/kg) reduced carisoprodol-appropriate responding to 36%, but a higher dose (25 mg/kg) resulted in 91% carisoprodol-appropriate responding. Response rate was decreased to 79% carisoprodol control after 5 mg/kg bemegride [F(3,27) = 4.45, p = 0.012], whereas flumazenil did not affect response rate [F(6,425) = 0.903, p = 0.504]. These results are consistent with the in vitro data in which the benzodiazepine site antagonist flumazenil had no significant effect on either the allosteric or the direct activity of carisoprodol, whereas bemegride significantly reduced carisoprodol-mediated currents.
Fig. 10.
Blockade of the discriminative stimulus effects of the training dose of carisoprodol (100 mg/kg p.o.). Top, percentage carisoprodol-lever responding. Bottom, rate of responding (r/s). The barbiturate antagonist bemegride fully antagonized the discriminative stimulus effects of carisoprodol, whereas the benzodiazepine antagonist flumazenil had little or no effect. Response rates were not significantly affected by either compound (n = 10 rats for bemegride and n = 9 for flumazenil, except where shown).
Discussion
The mechanism of action of carisoprodol is unclear. The general consensus has been that the therapeutic and addictive properties associated with carisoprodol are due to its metabolism to meprobamate. This assertion is supported by the findings of Rho et al. (1996, 1997), which demonstrated the propanediol dicarbamates meprobamate and felbamate act in a barbiturate-like manner at GABAARs. Given that carisoprodol is also a dicarbamate, the current study examined whether carisoprodol acts in a similar manner in vitro and in vivo.
Whole-cell patch-clamp studies demonstrated potentiation of GABA-gated currents at micromolar concentrations. High concentrations of carisoprodol in the presence of GABA produced inhibition, followed by rebound currents upon termination of drug application. This phenomenon is consistent with the proposed channel block observed with some GABAergic compounds at high concentrations (Rho et al., 1996; Williams et al., 1997). In the absence of GABA, micromolar concentrations of carisoprodol produced rapid and reversible inward currents that were blocked by picrotoxin, indicating that the currents were mediated by GABAARs.
As reported previously (Rho et al., 1997), we found the metabolite meprobamate also had direct-gating and allosteric effects at GABAA receptors. For both actions, our studies demonstrate carisoprodol is more potent and efficacious (up to the 3 mM concentration assessed) than its metabolite. After therapeutic use in humans, the effects of carisoprodol begin within 30 min of ingestion, with peak plasma concentrations reaching 4 to 7 μg/ml (Littrell et al., 1993). This translates to a concentration of approximately 27 μM carisoprodol, suggesting that even the therapeutic use of this drug can result in allosteric and direct effects.
Our functional studies performed in vitro strongly suggested carisoprodol has the potential to mediate barbiturate-like effects in vivo. Carisoprodol produced maximal depression of locomotor activity in mice within 8 min when administered via oral or intraperitoneal routes of administration. Behavioral depression elicited by 300 mg/kg carisoprodol followed a relatively short time course, with full recovery after 40 min. This pattern matches that reported for the time course of its plasma concentrations in mice reported in the literature (Bossoni et al., 1979; Chan, 2000). In a National Toxicology Program study, Chan (2000) reported that a single oral dose of 300 mg/kg carisoprodol yielded a peak plasma concentration of 15.7 μg/ml at 15 min after treatment and a decline to 4.5 μg/ml by 60 min, after which carisoprodol was not detectable. After a 600 mg/kg dose, the plasma concentration peaked and fell to 5 μg/ml within 2 h. Compared with the current studies of locomotor activity employing the same doses, it seems that falling plasma concentrations of carisoprodol closely parallel the offset of behavioral depression.
Hepatic conversion of carisoprodol to meprobamate in mice is relatively rapid (van der Kleijn, 1969), and in the current study, this metabolite administered by itself could indeed elicit behavioral depression with potency comparable to or greater than carisoprodol. However, it seems unlikely that hepatic conversion to meprobamate could fully account for the rapid time course of behavioral depression elicited by carisoprodol. The accumulation of meprobamate in the brain is markedly slower than carisoprodol (van der Kleijn, 1969), attributable to a greater lipid solubility of carisoprodol. In addition, in accordance with its markedly longer duration of depressant action demonstrated in the current study, the plasma half-life of meprobamate is nearly 8-fold longer than that of carisoprodol (Olsen et al., 1994; Bramness et al., 2004). If depressant effects of carisoprodol are fully attributable to formation of meprobamate, it is not clear why recovery of depression should parallel the disappearance of carisoprodol from plasma, a period when concentrations of meprobamate should persist.
Given that carisoprodol, itself, also has a barbiturate-like action in vitro, it would seem reasonable to consider that carisoprodol, itself, or perhaps a product of carisoprodol and meprobamate, may be largely responsible for the initial behavioral depression after its oral administration. Additional results reported in the literature are consistent with this view. The induction of hepatic microsomal enzymes that increase metabolism of carisoprodol (and presumably accelerate the appearance of meprobamate) has been reported to result in a shortening of the duration of carisoprodol-induced behavioral depression (Kato and Takanaka, 1968). In human studies, high plasma concentrations of carisoprodol, but not meprobamate, have been linked to impaired driving ability (Bramness et al., 2004).
Drug discrimination studies are often used to identify similarities in the stimulus effects of drugs and are likely to indicate a drug's potential for abuse. Thus, we investigated whether the stimulus effects of GABAergic compounds substituted for those of carisoprodol. Meprobamate, pentobarbital, and chlordiazepoxide all substituted for the discriminative stimulus effects of carisoprodol. These findings indicate carisoprodol produces at least part of its discriminative stimulus effects through actions at GABAARs and provide further support for the barbiturate-like actions of carisoprodol. It is interesting that we anticipated generalization would occur at a lower dose of pentobarbital (∼5 mg/kg); however, we did not observe full substitution in this range. We hypothesize this disparity may be due to cross-tolerance between these two drugs. Although these studies are promising, they do not allow us to definitively conclude the generalization of pentobarbital is due to carisoprodol-mediated events rather than metabolism of carisoprodol to meprobamate. In addition, non-GABAergic compounds have not been tested in the carisoprodol-trained rats, so it is possible other receptors may also contribute to the mechanism of action of carisoprodol. For example, meprobamate has been shown to inhibit NMDA-activated currents (Rho et al., 1997), and carisoprodol toxicity has been described as having characteristics similar to those of serotonin syndrome (Bramness et al., 2005).
Although subjects trained to discriminate barbiturates do not generally distinguish between benzodiazepines and barbiturates in substitution studies (De Vry and Slangen, 1986; Ator and Griffiths, 1989; Woolverton and Nader, 1995), antagonists relatively selective for these sites will selectively block the discriminative stimulus effects of test compounds. That is, bemegride, a barbiturate antagonist, blocks the discriminative stimulus effects of pentobarbital but not benzodiazepines, whereas flumazenil, a benzodiazepine site antagonist, blocks the discriminative stimulus effects of benzodiazepines but not pentobarbital (Herling and Shannon, 1982; Schechter, 1984; De Vry and Slangen, 1986). Furthermore, the antagonists also block cross-substitution; for example, bemegride blocks both the discriminative stimulus effects of pentobarbital and the ability of pentobarbital to substitute for chlordiazepoxide (Schechter, 1984). This is important because it provides a method for distinguishing the effects of benzodiazepines and barbiturates in rats trained to discriminate pentobarbital.
In the present study, bemegride fully antagonized the discriminative stimulus effects of carisoprodol whereas flumazenil failed to produce a consistent, dose-dependent blockade. These findings are in agreement with the electrophysiological studies and suggest the behavioral effects of carisoprodol are barbiturate-like, but not benzodiazepine-like. It is important to note that Roberge et al. (2000) reported the use of flumazenil to reverse carisoprodol intoxication. Flumazenil was considered a benzodiazepine-specific antagonist known to block the actions of benzodiazepines at GABAARs. This seems contradictory to our findings; however, it has also been demonstrated that this drug can reverse the actions of nonbenzodiazepine drugs, such as ethanol, tetrahydrocannabinol, and meprobamate in vivo (Roberge et al., 2000).
Although both our in vitro and in vivo studies are consistent with barbiturate-like effects of carisoprodol, we are not concluding that carisoprodol is acting at the barbiturate site of the receptor. As noted, other GABAAR modulators, such as the lactones, have the ability to allosterically modulate, directly gate, and antagonize the receptor (Williams et al., 1997; Gonzales et al., 2004). In addition, we found that homomeric ρ1 GABA receptors and homomeric α1 glycine receptors, which are insensitive to barbiturates, are also insensitive to carisoprodol. However, although we confirmed the W328M mutation in ρ1 GABA receptors confers sensitivity to barbiturates (Amin, 1999), this mutation did not confer sensitivity to carisoprodol. Thus, although the characteristics of carisoprodol can be described as barbiturate-like at the receptor and whole-animal levels, the distinct effects of the two ligands on the mutant receptor suggest the functional domain(s) for these ligands are distinct.
In recent years, there has been increasing concern regarding carisoprodol's potential as a drug of abuse. In light of our findings, it seems highly likely that the barbiturate-like activity of carisoprodol may underlie its capacity to enhance the sedative effects of CNS depressants, contributing to its potential for abuse. In our hands, carisoprodol was more potent and efficacious than its metabolite, suggesting carisoprodol is equally as dangerous as meprobamate. Thus, the question remains: Why is carisoprodol, the parent drug, a noncontrolled substance? Despite its emerging role as a drug of abuse, there is currently no standard treatment for carisoprodol dependence and withdrawal. Given the present and potential dangers posed by carisoprodol abuse, it is of crucial importance to determine the mechanism of action of this drug. This knowledge may provide insight into effectively treating carisoprodol tolerance, dependence, and withdrawal.
Acknowledgments
We thank Quynh Nguyen for providing technical assistance.
This work was supported in part by the National Institutes of Health National Institute on Drug Abuse [Contract N01DA-2-8822 through the Addiction Treatment Discovery Program]; and the National Institutes of Health National Institute on Aging [Grant T32-AG020494].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.151142.
ABBREVIATIONS: GABAAR, GABAA receptor; CNS, central nervous system; PTX, picrotoxin.
References
- Amin J (1999) A single hydrophobic residue confers barbiturate sensitivity to gamma-aminobutryic acid type C receptor. Mol Pharmacol 55 411-423. [PubMed] [Google Scholar]
- Amin J and Weiss DS (1994) Homomeric rho 1 GABA channels: activation properties and domains. Receptors Channels 2: 227-236. [PubMed] [Google Scholar]
- Ator NA and Griffiths RR (1989) Differential generalization to pentobarbital in rats trained to discriminate lorazepam, chlordiazepoxide, diazepam, or triazolam. Psychopharmacology 98 20-30. [DOI] [PubMed] [Google Scholar]
- Bossoni G, Colasanti P, Bianchi S, Riva M, and Usardi MM (1979) Influence of species specificity on gastric emptying rate and blood levels of carisoprodol. Pharmacol Res Commun 11 693-702. [DOI] [PubMed] [Google Scholar]
- Bramness JG, Mørland J, Sørlid HK, Rudberg N, and Jacobsen D (2005) Carisoprodol intoxications and serotonergic features. Clin Toxicol (Phila) 43 39-45. [DOI] [PubMed] [Google Scholar]
- Bramness JG, Skurtveit S, and Mørland J (2004) Impairment due to intake of carisoprodol. Drug Alcohol Depend 74 311-318. [DOI] [PubMed] [Google Scholar]
- Chan PC (2000) NTP toxicity studies of carisoprodol (CAS No. 78-44-4) administered by Gavage to F344/N rats and B6C3F1 mice. Toxic Rep Ser 56 1-G14. [PubMed] [Google Scholar]
- Dalén P, Alvan G, Wakelkamp M, and Olsen H (1996) Formation of meprobamate from carisoprodol is catalysed by CYP2C19. Pharmacogenetics 6: 387-394. [DOI] [PubMed] [Google Scholar]
- De Vry J and Slangen JL (1986) Differential interactions between chlordiazepoxide, pentobarbital and benzodiazepine antagonists Ro 15–1788 and CGS 8216 in a drug discrimination procedure. Pharmacol Biochem Behav 24 999-1005. [DOI] [PubMed] [Google Scholar]
- Drug Abuse Warning Network (2000) Emergency Room Data. DAWN Series D-20, DHHS Publication No. (SMA) 02-3634, Rockville, MD.
- Elder NC (1991) Abuse of skeletal muscle relaxants. Am Fam Physician 44 1223-1226. [PubMed] [Google Scholar]
- Elenbaas JK (1980) Centrally acting oral skeletal muscle relaxants. Am J Hosp Pharm 37: 1313-1323. [PubMed] [Google Scholar]
- Ellenhorn MJ and Barceloux DG (1988) Medical Toxicology: Diagnosis and Treatment of Human Poisoning, Elsevier Science Publishing, New York.
- Goldberg D (1969) Carisoprodol toxicity. Mil Med 134 597-601. [PubMed] [Google Scholar]
- Gonzales EB, Bell-Horner CL, de la Cruz MA, Ferrendelli JA, Covey DF, and Dillon GH (2004) (2004) Enantiomeric selectivity of the interaction of α-benzyl-α-methyl-γ-butyrolactone-mediated modulation of anticonvulsant activity and GABAA receptor function. J Pharmacol Exp Ther 309 677-683. [DOI] [PubMed] [Google Scholar]
- Hawkinson JE, Drewe JA, Kimbrough CL, Chen JS, Hogenkamp DJ, Lan NC, Gee KW, Shen KZ, Whittemore ER, and Woodward RM (1996) 3 alpha-Hydroxy-3 beta-trifluoromethyl-5 alpha-pregnan-20-one (Co 2–1970): a partial agonist at the neuroactive steroid site of the gamma-aminobutyric acid A receptor. Mol Pharmacol 49 897-906. [PubMed] [Google Scholar]
- Herling S and Shannon HE (1982) Ro 15-1788 antagonizes the discriminative stimulus effects of diazepam in rats but not similar effects of pentobarbital. Life Sci 31 2105-2112. [DOI] [PubMed] [Google Scholar]
- Huang RQ, Bell-Horner CL, Dibas MI, Covey DF, Drewe JA, and Dillon GH (2001) Pentylenetetrazole-induced inhibition of recombinant GABAA receptors: mechanism and site of action. J Pharmacol Exp Ther 298 986-995. [PubMed] [Google Scholar]
- Huang RQ, Gonzales EB, and Dillon GH (2006) GABAA receptors: structure, function and modulation, in Biological and Biophysical Aspects of Ligand-Gated Ion Channel Receptor Superfamilies (Arias H ed), pp 171-198, Research Signpost, Kerala, India.
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC.
- Kato R and Takanaka A (1968) Metabolism of drugs in old rats. II. Metabolism in vivo and effect of drugs in old rats. Jpn J Pharmacol 18 389-396. [DOI] [PubMed] [Google Scholar]
- Kenakin T (1997) Pharmacologic Analysis of Drug-Receptor Interaction, Lippincott Williams & Wilkins, Philadelphia, PA.
- Krimmer EC, Barry H III, and Coltrin D (1978) Antagonism of pentobarbital discriminative stimulus by bemegride in immobilized rats, in Stimulus Properties of Drugs: Ten Years of Progress (Colpaert FC and Rosecrans JA eds) p 167, Elsevier/North Holland Biomedical Press, Amsterdam.
- Littrell RA, Hayes LR, and Stillner V (1993) Carisoprodol (Soma): a new and cautious perspective on an old agent. South Med J 86: 753-756. [DOI] [PubMed] [Google Scholar]
- Luo X, Pietrobon R, Curtis LH, and Hey LA (2004) Prescription of nonsteroidal anti-inflammatory drugs and muscle relaxants for back pain in the united states. Spine 29 E531-E537. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, et al. (2000) Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci 3 587-592. [DOI] [PubMed] [Google Scholar]
- Morse RM and Chua L (1978) Carisoprodol dependence: a case report. Am J Drug Alcohol Abuse 5 527-530. [DOI] [PubMed] [Google Scholar]
- Olsen H, Koppang E, Alvan G, and Mørland J (1994) Carisoprodol elimination in humans. Ther Drug Monit 16: 337-340. [DOI] [PubMed] [Google Scholar]
- Reeves RR, Pinkofsky HB, and Carter OS (1997) Carisoprodol: a drug of continuing abuse. J Am Osteopath Assoc 97 723-724. [DOI] [PubMed] [Google Scholar]
- Rho JM, Donevan SD, and Rogawski MA (1996) Direct activation of GABAA receptors by barbiturates in cultured rat hippocampal neurons. J Physiol 497: 509-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho JM, Donevan SD, and Rogawski MA (1997) Barbiturate-like actions of the propanediol dicarbamates felbamate and meprobamate. J Pharmacol Exp Ther 280: 1383-1391. [PubMed] [Google Scholar]
- Roberge RJ, Lin E, and Krenzelok EP (2000) Flumazenil reversal of carisoprodol (Soma) intoxication. J Emerg Med 18 61-64. [DOI] [PubMed] [Google Scholar]
- Robertson MD and Marinetti LJ (2003) Carisoprodol: effects on human performance and behavior. Forensic Sci Rev 15 1-9. [PubMed] [Google Scholar]
- Roth BA, Vinson DR, and Kim S (1998) Carisoprodol-induced myoclonic encephalopathy. J Toxicol Clin Toxicol 36: 609-612. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, and Möhler H (1999) Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature 401: 796-800. [DOI] [PubMed] [Google Scholar]
- Rust GS, Hatch R, and Gums JG (1993) Carisoprodol as a drug of abuse. Arch Fam Med 2 429-432. [DOI] [PubMed] [Google Scholar]
- Schechter MD (1984) Specific antagonism of the behavioral effects of chlordiazepoxide and pentobarbital in the rat. Prog Neuropsychopharmacol Biol Psychiatry 8 359-364. [PubMed] [Google Scholar]
- van der Kleijn E (1969) Kinetics of distribution and metabolism of ataractics of the meprobamate-group in mice. Arch Int Pharmacodyn Ther 178 457-480. [PubMed] [Google Scholar]
- Williams KL, Tucker JB, White G, Weiss DS, Ferrendelli JA, Covey DF, Krause JE, and Rothman SM (1997) Lactone modulation of the gamma-aminobutyric acid A receptor: evidence for a positive modulatory site. Mol Pharmacol 52 114-119. [DOI] [PubMed] [Google Scholar]
- Woolverton WL and Nader MA (1995) Effects of several benzodiazepines, alone and in combination with flumazenil, in rhesus monkeys trained to discriminate pentobarbital from saline. Psychopharmacology 122 230-236. [DOI] [PubMed] [Google Scholar]