Abstract
Substance P is known to play a key role in the pathogenesis of acute pancreatitis. Src family kinases (SFKs) are known to be involved in cytokine signaling. However, the involvement of SFKs in substance P-induced chemokine production and its role in acute pancreatitis have not been investigated yet. To that end, we have used primary preparations of mouse pancreatic acinar cells as our model to show that substance P/neurokinin 1 receptor (NK1R) induced activation of SFKs. SFKs mediated the activation of mitogen-activated protein kinases [extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK)], transcription factors [signal transducer and activator of transcription (STAT) 3, nuclear factor (NF) κB, activator protein-1 (AP-1)], and production of chemokines in pancreatic acinar cells. We further tested the significance of the SFK signaling pathway in acute pancreatitis. Our results show, for the first time, that treatment of mice with the potent and selective SFK inhibitor PP2 [4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo [3,4-d] pyrimidine], but not its negative inhibitor PP3 (4-amino-7-phenylpyrazol [3,4-d] pyrimidine), reduced the severity of pancreatitis. This was proven by significant attenuation of hyperamylasemia, pancreatic myeloperoxidase activity, chemokines, and water content. Histological evidence of diminished pancreatic injury also confirmed the protective effect of the inhibition of SFKs. Moreover, treatment with the substance P receptor antagonist CP96345 [(2S,3S)-cis-2-(diphenylmethyl)-N-((2-methoxyphenyl)-methyl)-1-azabicyclo(2.2.2.)-octan-3-amine] attenuated acute pancreatitis-induced activation of SFKs, ERK, JNK, STAT3, NFκB, and AP-1. The proposed signaling pathway through which substance P mediates acute pancreatitis is through substance P/NK1R-SFKs-(ERK, JNK)-(STAT3, NFκB, AP-1) chemokines. In light of our study, we propose that drugs targeting the substance P-mediated signaling pathways could prove beneficial in improving treatment efficacy in acute pancreatitis.
Acute pancreatitis is increasing in incidence and is often a fatal human disease, in which the pancreas digests itself and its surroundings (Bhatia et al., 2000, 2001; Bhatia, 2002; Bhatia and Moochhala, 2004). Accumulating experimental evidence has suggested that substance P/NK1R and chemokines play critical roles in the pathogenesis of acute pancreatitis. We have shown previously that substance P by itself caused an increased synthesis in CC and CXC chemokines in pancreatic acinar cells (Ramnath and Bhatia, 2006). Moreover, blockade of substance P receptor, NK1R, attenuated chemokine production in pancreatic acinar cells and protected mice from acute pancreatitis (Lau et al., 2005; Ramnath et al., 2007; Sun and Bhatia, 2007).
SFKs, specifically Src, have been widely studied in tumorigenesis. However, recent evidence has revealed that SFKs are among the most important families for the intracellular signal transduction related to acute inflammatory responses (Yuan, 2002; Armstrong, 2004; Lowell, 2004). Several animal studies have shown that inhibition of SFKs with small chemical inhibitors prevented ischemia-reperfusion-induced injury in the brain and heart. Moreover, blockade of SFKs attenuated sepsis, acute lung injury, and other organ damage (Paul et al., 2001; Akiyama et al., 2004; Khadaroo et al., 2004; Kusaka et al., 2004; Lennmyr et al., 2004; Weis et al., 2004; Severgnini et al., 2005). SFKs are activated in response to the stimulation of a variety of cell surface receptors (Thomas and Brugge, 1997). One such receptor type is the G protein-coupled receptor. Once activated, Src is then capable of interacting with and activating the transcription factor STAT3 (Brown, 1996). The STAT family of proteins has been implicated in the functions of a wide range of cells (Akira, 2000; Bromberg and Darnell, 2000), and it is known to activate various key inflammatory mediators; for example, the cytokine signaling pathway (Severgnini et al., 2004).
Limited knowledge is available on the role of SFKs in chemokine production in acute pancreatitis. Therefore, the aim was to investigate the role of SFKs in mediating substance P-induced chemokine production in pancreatic acinar cells and also the underlying signal transduction mechanisms involved. Moreover, we sought to test the significance of our in vitro findings in an in vivo model of acute pancreatitis.
Materials and Methods
Animal Welfare. All animal experiments were approved by the Animal Ethics Committee of National University of Singapore and carried out in accordance with established International Guiding Principles for Animal Research. Male Swiss albino mouse (25-30 g) were maintained in the Animal Housing Unit of the National University of Singapore in an environment with controlled temperature (21-24°C) and lighting (12-/12-h light/dark cycle). Standard laboratory chow and drinking water were provided ad libitum. A period of 2 days was allowed for animals to acclimatize before any experimental manipulations were undertaken.
Test System Used. Pancreatic acinar cells were obtained from mouse pancreas by collagenase treatment as described previously (Bhatia et al., 2002; Ramnath et al., 2008). In brief, pancreases from three Swiss mice (20-25 g) were removed, infused with buffer A (140 mM NaCl, 4.7 mM KCl, 1.13 mM MgCl2, 1 mM CaCl2, 10 mM glucose, 10 mM HEPES, pH 7.2) containing 200 IU/ml collagenase (Worthington Biochemicals, Freehold, NJ) and 0.5 mg/ml soybean trypsin inhibitor (Sigma-Aldrich, St. Louis, MO), and incubated in a shaking water bath for 10 min at 37°C. The digested tissue was centrifuged through 50 mg/ml bovine serum albumin and washed twice with buffer A for further experiments. A cell suspension (in buffer A) consisting of only small clumps, around three to five acinar cells, was used to carry out the following experiments.
Viability of Mouse Pancreatic Acinar Cells. Viability of the pancreatic acinar cells was determined by trypan blue dye exclusion assay. One drop of 0.4% trypan blue dye was added to one drop of the isolated acinar cells. The viability was determined under the light microscope (Carl Zeiss GmbH, Jena, Germany). The number of unstained cells/clumps was expressed as a percentage of the total number of cells/clumps. This process was repeated for different fields, and the average was then calculated. In all experiments, cell viability was greater than 95%.
In Vitro Experimental Design. Pancreatic acinar cells (500 μl of cell suspension) were treated with substance P (Sigma-Aldrich) at a concentration of 1 μM for 0, 3, 5, 10, 15, 30, and 45 min at 37°C. After treatment with substance P, the cells were lysed to detect for SFK activation by Western blot analysis. In some experiments, cells were either pretreated with a potent and selective inhibitor of the Src family of protein tyrosine kinases, PP2 (Calbiochem, San Diego, CA) at 1 and 10 μM, or a negative control for the Src family protein tyrosine kinase inhibitor, PP3 (Calbiochem) at 1 μM, for 30 min followed by stimulation with 1 μM substance P or vehicle for 10 or 45 min at 37°C. The supernatant was used subsequently for chemokine detection, and the pellet was used for either nuclear extract to detect STAT3, NFκB (p65), and AP-1(c-Jun) activation or cell lysis for Western blot analysis to detect SFKs, ERK, and JNK. In another experiment, isolated pancreatic acinar cells were preincubated with the selective NK1R antagonist, CP96345, at 1 μM (Pfizer Central Research, Groton, CT) for 30 min followed by treatment with 1 μM substance P or vehicle for 10 min at 37°C. The cells were lysed subsequently and used for Western blot analysis to detect SFKs.
Preparation of Total Cell Lysates for Western Blot Analysis. After treatment, pancreatic acinar cells or pancreatic tissue were homogenized on ice in radioimmunoprecipitation assay buffer supplemented with 1 mM phenylmethylsulfonyl fluoride and the protease inhibitor cocktail (Sigma-Aldrich) containing pepstatin, leupeptin, chymostatin, antipain, and aprotinin (5 μg/ml each) and centrifuged at 4°C for 15 min at 14,000g. The supernatants were collected and stored at -80°C until use. Protein concentrations were determined by using the Bio-Rad (Hercules, CA) protein assay. Five microliters of sample was added to 250 μl of Bradford reagent (Bio-Rad) and read at 595 nm after a short incubation (5 min) at room temperature.
Western Blot Analysis. Cell lysates (50-μg protein) were separated on 12% SDS-polyacrylamide gel and electrophoretically transferred to nitrocellulose membranes (Bio-Rad). Nonspecific binding was blocked by 1-h incubation of the membranes, at room temperature, in 5% nonfat dry milk in phosphate-buffered saline Tween 20 (PBST) (0.05% Tween 20 in phosphate-buffered saline, pH 7.4). The blots were then incubated overnight at 4°C with the primary antibodies (at 1:1000 dilutions in the buffer containing 2.5% nonfat dry milk in PBST), phospho-Src family, phospho-ERK1/2, phosphostress-activated protein kinase/JNK (Cell Signaling Technology Inc., Danvers, MA), and HPRT, purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). HPRT was used as the housekeeping protein. The phospho-Src family (Tyr416) antibody detects endogenous levels of Src only when phosphorylated at Tyr416. The antibody may cross-react with other Src family members (Lyn, Fyn, Lck, Yes, and Hck) when phosphorylated at equivalent sites. It does not crossreact with Src phosphorylated at Tyr527. Afterward, the membranes were washed four times with PBST and finally incubated for 1 h at room temperature with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Inc.) at 1:2000 dilutions in the buffer containing 2.5% nonfat dry milk in PBST. The blots were developed for visualization using an enhanced chemiluminescence detection kit (Pierce Chemical, Rockford, IL). The densities of the bands were quantified using a UVP GelDoc-It Imaging Systems.
Nuclear Extract Preparation. Nuclear extracts were prepared by employing a kit (Active Motif Inc., Carlsbad, CA), as described previously (Ramnath et al., 2008). Nuclear extract was prepared from both isolated pancreatic acinar cells and pancreatic tissue after caerulein-induced acute pancreatitis.
STAT3/NFκB/AP-1 DNA-Binding Activity. The binding of STAT3/NFκB/AP-1 to DNA was measured in nuclear extracts with ELISA-based TransAM STAT3/NFκB p65/AP-1 c-jun assay kits (Active Motif, SciMed, Asia). This assay uses multiwell plates coated with an unlabeled oligonucleotide containing the consensus binding site for STAT [5′-TTCCCGGAA-3′)/NFκB (5′-GGGACTTTCC-3′)/AP-1 (5′-TGAGTCA-3′]. Nuclear proteins (5 μg) were added to each well and incubated for 1 h at room temperature to allow STAT3/NFκB/AP-1 DNA binding. Subsequently, by using an antibody that is directed against STAT3/NFκB p65/AP-1 c-jun subunit, the STAT3/NFκB/AP-1 complex bound to the oligonucleotide is detected. Addition of the secondary antibody conjugated to horseradish peroxidase provides a sensitive colorimetric readout that is easily quantified by spectrophotometry. Absorbance was read at 450 nm within 5 min by using a 96-well microplate reader (Tecan, Durham, NC). The wild-type consensus oligonucleotide is provided as a competitor for STAT3/NFκB/AP-1 binding to monitor the specificity of the assay. Results were expressed as -fold increase over the control group.
Chemokine Detection. Pancreatic acinar cell supernatant and pancreatic homogenate were assayed for MCP-1, MIP-1α, and MIP-2 using a sandwich ELISA, according to the manufacturer's instructions (Duoset kit; R&D Systems, Minneapolis, MN). For the MCP-1 assay, the anti-MCP-1 primary antibody was aliquoted onto ELISA plates and incubated at room temperature overnight. Samples (100 μl of cell supernatant) and standards were incubated at room temperature for 2 h, the plates were washed, and a biotinylated anti-MCP-1 antibody was added and left at room temperature for 2 h. Plates were washed again, and streptavidin bound to horseradish peroxidase was added for 20 min at room temperature. After a further wash, tetramethylbenzidine was added for color development, and the reaction was terminated with 2 M H2SO4. Absorbance was read at 450 nm within 5 min by using a 96-well microplate reader (Tecan). The same procedure was followed for the detection of the remaining chemokines MIP-1α and MIP-2.
Induction of Acute Pancreatitis. Swiss mice (20-25 g) were randomly assigned to control or experimental groups using 10 or more animals for each group. Animals were given hourly intraperitoneal injections of normal saline or saline containing caerulein (50 μg/kg) for 10 h. PP2 at doses of 0.5, 1.0, and 1.5 mg/kg, PP3 at a dose of 1.0 mg/kg, and CP96345 at a dose of 2.5 mg/kg were administered to mice intraperitoneally either 1 h before or 1 h after the first caerulein injection. One hour after the last caerulein injection, animals were sacrificed by an intraperitoneal injection of a lethal dose of pentobarbitone. Harvested heparinized blood was centrifuged, and the plasma was removed and stored at -80°C. Random cross-sections of the head, body, and tail of the pancreas were fixed in 4% neutral phosphate-buffered formalin, then embedded in paraffin wax. Freshly harvested samples of pancreas were weighed and then dried and reweighed to determine pancreatic water content (Bhatia et al., 1998). Samples of pancreas were stored at -80°C for subsequent measurement of tissue myeloperoxidase (MPO) activity and for ELISA and Western blot analysis.
Amylase Estimation. Amylase activity was measured using a kinetic spectrophotometric assay. Plasma samples were incubated with the substrate, 4,6-ethylidene (G7)-p-nitrophenyl (G1)-1-d-maltoheptoside (Sigma-Aldrich), for 2 min at 37°C and absorbance measured every minute for the subsequent 2 min at 405 nm (Pierre et al., 1976; Bhatia et al., 1998, 2000). The change in absorbance was used to calculate the amylase activity.
Myeloperoxidase Estimation. Neutrophil sequestration in pancreas was quantified by measuring tissue MPO activity (Bhatia et al., 1998, 2000). Tissue samples were thawed, homogenized in 20 mM phosphate buffer, pH 7.4, centrifuged (10,000g, 10 min, 4°C), and the resulting pellet was resuspended in 50 mM phosphate buffer, pH 6.0, containing 0.5% hexadecyltrimethylammonium bromide (Sigma-Aldrich). The suspension was subjected to four cycles of freezing and thawing and further disrupted by sonication (40 s). The sample was then centrifuged (10,000g, 5 min, 4°C), and the supernatant was used for the MPO assay. The reaction mixture consisted of the supernatant, 1.6 mM tetramethylbenzidine (Sigma-Aldrich), 80 mM sodium phosphate buffer, pH 5.4, and 0.3 mM hydrogen peroxide. This mixture was incubated for 120 s, the reaction was terminated with 2 M H2SO4, and the absorbance was measured at 450 nm. This absorbance was then corrected for the protein content of the tissue sample.
Morphological Examination. Paraffin-embedded pancreas samples were sectioned (5 μm), stained with hematoxylin/eosin, and examined under light microscopy at ×400 magnifications. For these studies, eight randomly chosen microscopic fields (×125) were examined for each tissue sample. Pancreatic damage was assessed based on the presence of acinar cell ghosts, vacuolization, swelling of acinar cells, edema, and infiltration of neutrophils.
Statistics. Data are expressed as the mean + S.E.M. The significance of changes was evaluated by using analysis of variance. If analysis of variance indicated a significant difference, the data were analyzed by using Tukey's method as a post hoc test for the difference between groups. A P value of <0.05 was considered to indicate a significant difference.
Results
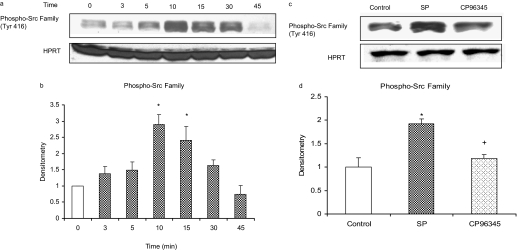
Substance P/NK1R Induces a Rapid and Transient Increase in Phosphorylation of Src Family (Tyr416) in Mouse Pancreatic Acinar Cells. Pancreatic acinar cells were stimulated with 1 μM substance P or vehicle (saline) for 0, 3, 5, 10, 15, 30, and 45 min at 37°C. Cells were then lysed, and cell proteins were subjected to Western blot analysis. As shown in Fig. 1, a and b, substance P induced phosphorylation of the Src family (Tyr416) in a time-dependent manner up to 10 min, followed by a time-dependent decrease. Densitometric analysis of Western blot experiments revealed a significant increase in phosphorylation Src family (Tyr416) at 10 and 15 min compared with 0-min control. No significant change was observed when pancreatic acinar cells were treated with the vehicle at different time points. Pancreatic acini were also pretreated with 1 μM CP96345, a selective NK1R antagonist, for 30 min followed by stimulation with 1 μM substance P for 10 min. Cells were then lysed, and cell proteins were subjected to Western blot analysis. As demonstrated in Fig. 1, c and d, CP96345 significantly reduced substance P-induced phosphorylation of Src family (Tyr416) in pancreatic acinar cells. No significant change was detected in the housekeeping proteins HPRT. This shows that substance P-induced phosphorylation of Src family is mediated through substance P/NK1R in pancreatic acinar cells.
Fig. 1.
Substance P (SP)/NK1R induces a time-dependent increase and decrease in phosphorylation of Src family (Tyr416) in mouse pancreatic acinar cells. Freshly isolated pancreatic acini were incubated with either 1 μM SP/vehicle (saline) for 0, 3, 5, 10, 15, 30, and 45 min at 37°C or preincubated with 1 μM CP96345 for 30 min followed by stimulation with 1 μM SP for 10 min. The cells were lysed, and cell proteins were subjected to Western blot analysis using antibodies against phospho-Src family (Tyr416) and HPRT (a and c). Densitometric analysis of Western blot experiments were performed, and the group data from three independent preparations (n = 3) are presented in b and d. The results are representative of three independent (n = 3) experiments. Results shown are the means + S.E. *, P ≤ 0.05 compared with 0-min control; +, P ≤ 0.05 compared with SP.
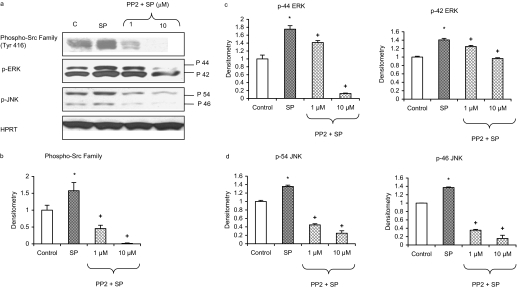
Involvement of SFKs in Substance P-Induced MAP Kinases in Pancreatic Acinar Cells. Stimulation of pancreatic acinar cells with 1 μM substance P significantly upregulated phosphorylation of the Src family (Tyr416), ERK, and JNK. Pancreatic acinar cells were pretreated for 30 min with the selective inhibitor of the Src family of protein tyrosine kinases, PP2, at 1 and 10 μM, followed by stimulation with 1 μM substance P for 10 min. Cells were then lysed, and cell proteins were subjected to Western blot analysis. As shown in Fig. 2, PP2 significantly decreased substance P-induced phosphorylation of the Src family (Tyr416), hence confirming its inhibitory effect. Moreover, PP2 dose-dependently attenuated substance P-induced phosphorylation of ERK and JNK in pancreatic acinar cells (Fig. 2, c and d). No significant change was detected in the housekeeping proteins HPRT.
Fig. 2.
Substance P (SP)-induced activation of ERK and JNK is mediated by SFKs in pancreatic acinar cells. Freshly isolated pancreatic acini were preincubated with PP2 at 1 or 10 μM or vehicle (dimethyl sulfoxide) for 30 min at 37°C followed by stimulation with 1 μM SP for 10 min at 37°C. Cells were subsequently lysed, and cell proteins were subjected to Western blot analysis using antibodies against phospho-Src family (Tyr416), phospho-ERK, phospho-JNK, and HPRT (a). The phosphorylated subunits, such as the p-Src family, p-44 ERK, p-42 ERK, p-54 JNK, and p-46 JNK, have been quantified. Densitometric analyses of Western blot experiments were performed, and the group data from three independent preparations (n = 3) are presented in b to d. Results shown are the means + S.E. *, P ≤ 0.05 compared with control; +, P ≤ 0.05 compared with SP.
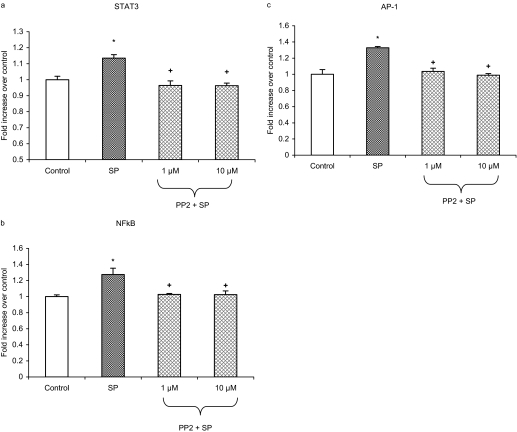
Substance P-Induced SFK Is Involved in Activation of STAT3, NFκB, and AP-1 in Pancreatic Acinar Cells. Pancreatic acinar cells were preincubated for 30 min with PP2 at 1 and 10 μM followed by stimulation with 1 μM substance P for 45 min. This time point was established from earlier data (Ramnath and Bhatia, 2006). STAT3, NFκB, and AP-1 DNA-binding assay revealed that treatment with substance P led to a notable increase in the activity of STAT3, NFκB, and AP-1 in pancreatic acinar cell. As shown in Fig. 3, a to c, pretreatment with PP2 attenuated substance P-induced DNA-binding activity of STAT3, NFκB, and AP-1.
Fig. 3.
SFKs are involved in SP-induced STAT3, NFκB, and AP-1 activation in pancreatic acinar cells. Freshly isolated pancreatic acini were preincubated with PP2 at 1 and 10 μM or vehicle (dimethyl sulfoxide) followed by stimulation with 1 μM SP for 45 min. The cells were separated from incubation medium by centrifugation. The pellet (cells) was used for STAT3 (a), NFκB (b), and AP-1 (c) nuclear extraction. STAT3, NFκB, and AP-1 DNA-binding assays were then carried out as described under Materials and Methods. The results are representative of three independent (n = 3) experiments. Results shown are the means + SE. *, P ≤ 0.05 compared with control; +, P ≤ 0.05 compared with SP.
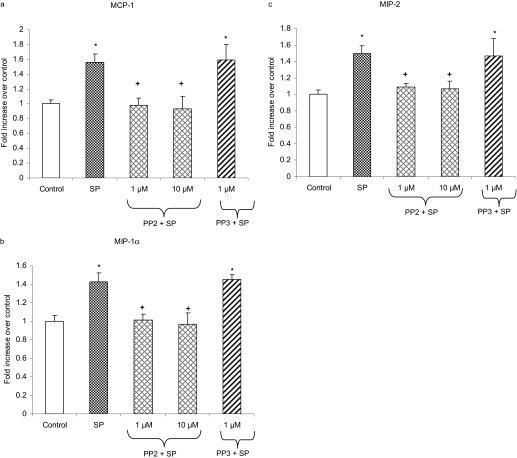
SFKs Mediate Substance P-Induced Production of CC and CXC Chemokines in Pancreatic Acinar Cells. Treatment of pancreatic acini with 1 μM substance P resulted in increased production of CC chemokine MCP-1, MIP-1α, and CXC chemokine MIP-2 (Fig. 4). Pancreatic acinar cells were pretreated with either PP2 at 1 and 10 μMorits negative control PP3 at 1 μM for 30 min followed by stimulation with 1 μM substance P for 45 min. The protein levels of these chemokines were determined by ELISA. Figure 4, a to c, shows that pretreatment with PP2 markedly decreased MCP-1, MIP-1α, and MIP-2 production. However, treatment with the negative control PP3 had no significant effect on chemokine production compared with substance P-only treated cells.
Fig. 4.
SFKs are involved in the secretion of CC and CXC chemokines in pancreatic acinar cells. Freshly isolated pancreatic acini were preincubated with either PP2 at 1 and 10 μM or PP3 at 1 μM for 30 min followed by stimulation with 1 μM SP for 45 min. The supernatant was used to measure MCP-1 (a), MIP-1α (b), and MIP-2 (c) levels by ELISA as described under Materials and Methods. The results are representative of three independent (n = 3) experiments. Results shown are the means + S.E. *, P ≤ 0.05 compared with control; +, P ≤ 0.05 compared with SP.
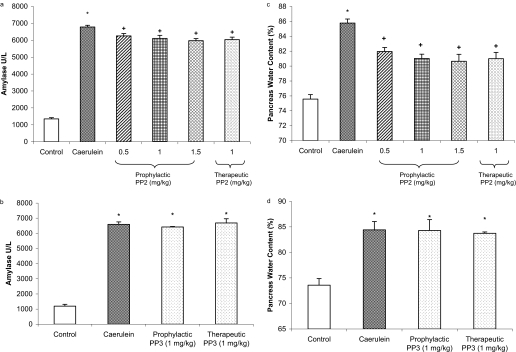
Effect of Prophylactic and Therapeutic Treatment with PP2 on the Severity of Caerulein-Induced Acute Pancreatitis. Acute pancreatitis was induced by intraperitoneal administration of caerulein at a dose of 50 μg/kg hourly for 10 h. Evidence of pancreatic injury in acute pancreatitis mice was confirmed by a rise in plasma amylase, as shown in Fig. 5, a and b. There was also an increase in pancreatic edema as evidenced by elevated pancreatic water content, as shown in Fig. 5, c and d. Animals were administered either PP2 or PP3 1 h before or after starting caerulein injection. Both prophylactic and therapeutic treatment with PP2 significantly attenuated plasma amylase levels and pancreatic water content compared with animals treated with caerulein alone (Fig. 5, a and c). Qualitative histological examination of pancreas sections confirmed a protection by PP2 treatment on acute pancreatitis in terms of acinar cell injury/necrosis, neutrophil infiltration, and edema (Fig. 6). However, prophylactic and therapeutic treatment with PP3 had no protective effect on pancreatic injury in acute pancreatitis compared with mice treated with caerulein alone.
Fig. 5.
Effects of prophylactic and therapeutic PP2 administration on the severity of acute pancreatitis. Mice (n = 10 in each group) were given 10 hourly injections of caerulein (50 μg/kg i.p.). PP2 or PP3 was administered to mice intraperitoneally either prophylactically (1 h before) or therapeutically 1 h after the first caerulein injection. One hour after the last caerulein injection, mice were sacrificed by an intraperitoneal injection of a lethal dose of pentobarbitone. Plasma amylase activity (a and b) and pancreatic edema (water content; c and d) were determined as described under Materials and Methods. Results shown are the means + S.E. *, P ≤ 0.05 when caerulein- or PP3-treated animals were compared with placebo-treated animals. +, P ≤ 0.05 when PP2-treated animals were compared with caerulein-treated animals.
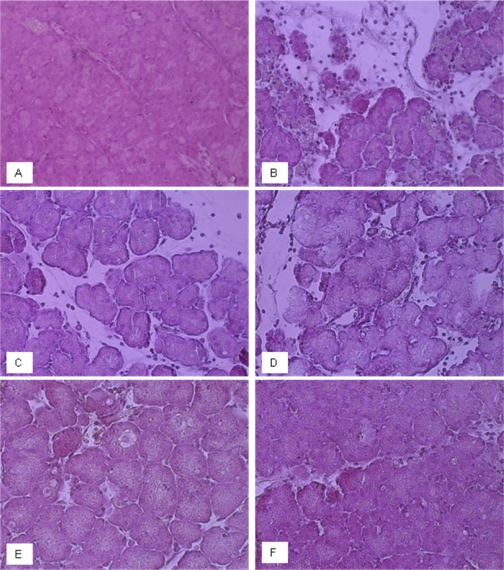
Fig. 6.
Morphological changes in mouse pancreas on induction of acute pancreatitis with/without prophylactic and therapeutic treatment with PP2 or PP3. Representative micrographs from each group were shown (×400 magnifications). A, control, no pancreatitis. B, caerulein-induced acute pancreatitis. C, caerulein-induced acute pancreatitis in mice administered 1 mg/kg PP3 (prophylactic). D, caerulein-induced acute pancreatitis in mice administered 1 mg/kg PP3 (therapeutic). E, caerulein-induced acute pancreatitis in mice administered 1 mg/kg PP2 (prophylactic). F, caerulein-induced acute pancreatitis in mice administered 1 mg/kg PP2 (therapeutic).
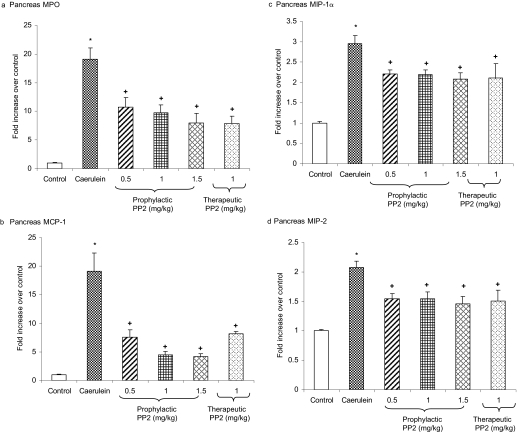
Involvement of SFKs in the Mobilization of Neutrophils and Chemokines in Acute Pancreatitis. Induction of acute pancreatitis by caerulein hyperstimulation resulted in a heightened pancreatic MPO, a measure of neutrophil infiltration (Fig. 7). Acute pancreatitis also resulted in increased levels of pancreatic CC chemokine MCP-1, MIP-1α, and CXC chemokine MIP-2 compared with placebo-treated animals. Prophylactic treatment with PP2 resulted in a decrease in MPO activity, MCP-1, MIP-1α, and MIP-2 levels in the pancreas compared with caerulein-treated animals. Moreover, mice administered with PP2 therapeutically had a significant reduction in pancreatic MPO activity and chemokines MCP-1, MIP-1α, and MIP-2 levels compared with caerulein-treated mice (Fig. 7).
Fig. 7.
Involvement of SFKs in the mobilization of pancreatic neutrophils and chemokine in acute pancreatitis. Mice (n = 10 in each group) were given 10 hourly injections of caerulein (50 μg/kg i.p.). PP2 was administered in mice at doses of 0.5, 1, and 1.5 mg/kg i.p. 1 h before or at a dose of 1 mg/kg 1 h after the first caerulein injection. One hour after the last caerulein injection, mice were sacrificed by an intraperitoneal injection of a lethal dose of pentobarbitone, and pancreatic MPO (a), MCP-1 (b), MIP-1α (c), and MIP-2 (d) levels were measured as described under Materials and Methods. Results shown are the means + S.E. *, P ≤ 0.05 when caerulein-treated animals were compared with placebo-treated animals. +, P ≤ 0.05 when PP2-treated animals were compared with caerulein-treated animals.
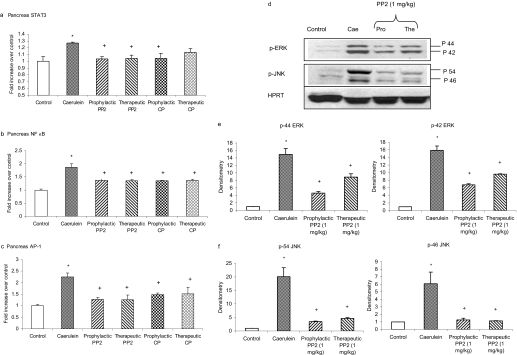
Inhibition of SFKs Attenuated the Activation of Pancreatic STAT3, NFκB, AP-1, and MAP Kinases in Acute Pancreatitis. Caerulein-induced acute pancreatitis resulted in significant activation of transcription factors STAT3, NFκB, and AP-1 in pancreas compared with placebo-treated animals (Fig. 8). Treatment with PP2 both prophylactically and therapeutically significantly attenuated the activation of pancreatic STAT3, NFκB, and AP-1 compared with caerulein-treated animals, as shown in Fig. 8, a to c. To further explore the signaling pathways mediated by SRC tyrosine kinases in acute pancreatitis, we examined the role of MAP kinases. Acute pancreatitis increased phosphorylation of MAP kinases ERK and JNK in the pancreas compared with placebo-treated animals. Both prophylactic and therapeutic treatment with PP2 significantly attenuated activation of ERK and JNK compared with caerulein-treated mice (Fig. 8, d to f). No significant change was observed in the housekeeping protein HPRT in the pancreas.
Fig. 8.
Inhibition of SFKs attenuated the activation of pancreatic STAT3, NFκB, AP-1, and MAP kinases in acute pancreatitis. Mice (n = 10 in each group) were given 10 hourly injections of caerulein (50 μg/kg i.p.). PP2 was administered to mice at a dose of 1 mg/kg i.p. 1 h before or 1 h after the first caerulein injection. One hour after the last caerulein injection, mice were sacrificed by an intraperitoneal injection of a lethal dose of pentobarbitone. The activation of pancreatic STAT3 (a), NFκB (b), AP-1 (c), and MAP kinases (d-f) was quantified as described under Materials and Methods. Results shown are the means + S.E. *, P ≤ 0.05 when caerulein-treated animals were compared with placebo-treated animals. +, P ≤ 0.05 when PP2-treated animals were compared with caerulein-treated animals.
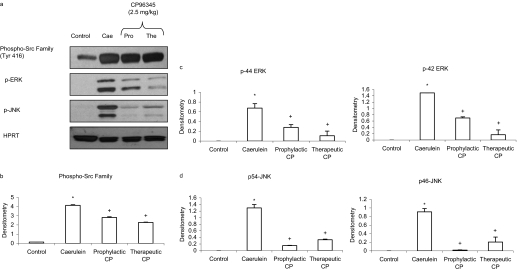
Substance P/NKIR-Mediated Activation of Pancreatic SFKs and Its Downstream Signaling Pathway in Acute Pancreatitis. Treatment with CP96345 both prophylactically and therapeutically attenuated the activation of pancreatic SFKs and MAP kinases ERK and JNK compared with caerulein-treated mice as shown in Fig. 9. No significant change was observed in the housekeeping protein HPRT in the pancreas. Moreover, as shown in Fig. 8, a to c, CP96345 blocked the up-regulation of pancreatic STAT3, NFκB, and AP-1 in acute pancreatitis.
Fig. 9.
Blockade of NK1R reduced acute pancreatitis-induced activation of MAP kinases and transcription factors STAT3, NFκB, and AP-1 in the pancreas. Mice (n = 6 in each group) were given 10 hourly injections of caerulein (50 μg/kg i.p.). CP96345 was administered to mice at a dose of 2.5 mg/kg i.p., 1 h before or 1 h after the first caerulein injection. One hour after the last caerulein injection, mice were sacrificed by an intraperitoneal injection of a lethal dose of pentobarbitone. The activation of pancreatic STAT3 and MAP kinases (a) and the densitometry analysis (c and d) were described under Materials and Methods. Results shown are the means + S.E. *, P ≤ 0.05 when caerulein-treated animals were compared with placebo-treated animals. +, P ≤ 0.05 when CP96345-treated animals were compared with caerulein-treated animals.
Discussion
SFKs have been shown to play a key role in cytokine signaling and inflammatory responses (Chaturvedi et al., 1998; Song et al., 2003). However, the involvement of SFKs in substance P-induced chemokine production and also its role in acute pancreatitis have not been investigated yet.
To that end, we have demonstrated that substance P/NK1R induced activation of the Src family in pancreatic acinar cells. SFKs also mediated substance P-induced activation of MAP kinases ERK and JNK. We also illustrated that substance P-induced phosphorylation of Src tyrosine kinases was involved in the activation of STAT3, NFκB, and AP-1 and production of chemokines MCP-1, MIP-1α, and MIP-2 in pancreatic acinar cells. Our results are in agreement with several other studies, whereby substance P was found to induce Src activation, which was also implicated in the activation of MAP kinases (Della Rocca et al., 1997; DeFea et al., 2000; Yamaguchi et al., 2005). Moreover, MAP kinases were found to modulate the transcriptional activity of STAT3 (Chung et al., 1997; Turkson et al., 1999). We have demonstrated previously the involvement of ERK, JNK, NFκB, and AP-1 in substance P induced chemokine synthesis (Ramnath et al., 2007). Substance P-induced activation of SFKs most likely is mediating chemokine production through the ERK- and JNK-induced transcription factors NFκB and AP-1. However, whether substance P-induced STAT3 activation is directly involved in the production of chemokines remained to be investigated. On the one hand, studies have suggested that STAT3 plays a pivotal role in orchestrating inflammatory responses by increasing the expression of inflammatory mediators, cytokines, and chemokines (Schumann et al., 1996; Severgnini et al., 2004). However, on the other hand, studies have revealed that STAT3 modulates anti-inflammatory responses (Takeda et al., 1999; Matsukawa et al., 2003). Hence, more work needs to be carried out to elucidate the complexity of STAT3 signaling mechanisms in acute pancreatitis.
We further sought to investigate the in vivo relevance of the in vitro findings. Our results show that treatment of animals with the potent and selective inhibitor of the Src family of protein tyrosine kinases, PP2, but not its negative inhibitor, PP3 (either prophylactic or therapeutic), reduces the severity of pancreatitis as evidenced by a significant attenuation of hyperamylasemia, pancreatic MPO activity, chemokine production, and water content, which is a measure of edema. Moreover, histological evidence of diminished pancreatic injury, such as acinar cell injury/necrosis, neutrophil infiltration, and edema, also confirmed the protective effect of the inhibition of SFKs on acute pancreatitis. In line with our study, Src tyrosine kinase inhibitors were found to suppress inflammatory responses in vivo by blocking the function of inflammatory cells including neutrophils, monocytes, and macrophages (Okutani et al., 2006).
Using the in vivo model of acute pancreatitis, we further explored the molecular mechanisms through which SFKs protected against acute pancreatitis, and we found that SFKs mediate their protective effects through the same signaling pathway that we have shown in our in vitro model of isolated acinar cells. It has been shown that treatment with the NK1R antagonist CP96345 protected mice against acute pancreatitis by attenuating chemokine production (Lau et al., 2005; Sun and Bhatia, 2007). In the present study, we have further shown that CP96345 attenuated activation of SFKs, ERK, JNK, STAT3, NFκB, and AP-1 in acute pancreatitis. Based on our results, we proposed that elevated level of substance P, which is produced as a result of acute pancreatitis, binds to NK1R to activate several signaling molecules to mediate chemokine production. One such signaling complex is SFK, and its blockade attenuated MAP kinases, STAT3, NFκB, and AP-1, and chemokines, thus resulting in protection against acute pancreatitis. The proposed signaling pathway through which substance P mediates acute pancreatitis is through substance P/NK1R-SFKs-(ERK, JNK)-(STAT3, NFκB, AP-1)-(MCP-1, MIP-1α, MIP-2).
The mechanism of activation of SFKs in acute pancreatitis is most likely to be multifactorial; however, one of the factors involved is the neuropeptide substance P. Using isolated pancreatic acinar cell preparations, we were able to show that substance P/NK1R induces activation of SRC tyrosine kinases. Blockade of SFKs attenuated chemokine production both in vitro and in vivo and protected the mice against acute pancreatitis. This study gives us a deeper insight into the mechanisms by which substance P contributes to acute pancreatitis. Increased understanding of the signal transduction mechanisms involved in acute pancreatitis would facilitate the discovery of novel therapeutic approaches that can target selective pathways to prevent disease progression in acute pancreatitis and/or improve treatment efficacy.
Acknowledgments
We thank Mei Leng Shoon for technical assistance.
This work was supported by the National Medical Research Council [Grant R-184-000-156-213]; and by the Biomedical Research Council [Grant R-184-000-069-305].
doi:10.1124/jpet.108.148684.
ABBREVIATIONS: NK1R, neurokinin 1 receptor; SFK, Src family kinase; STAT, signal transducer and activator of transcription; PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo [3,4-d] pyrimidine; PP3, 4-amino-7-phenylpyrazol [3,4-d] pyrimidine; NF, nuclear factor; AP-1, activator protein-1; ERK, extracellular signal-regulated kinase; JNK, c-Jun NH2-terminal kinase; CP96345, (2S,3S)-cis-2-(diphenylmethyl)-N-((2-methoxyphenyl)-methyl)-1-azabicyclo(2.2.2.)-octan-3-amine; PBST, 0.05% Tween 20 in phosphate-buffered saline; HPRT, hypoxanthine-guanine phosphoribosyl transferase; ELISA, enzyme-linked immunosorbent assay; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; MPO, myeloperoxidase; MAP, mitogen-activated protein; SP, substance P.
References
- Akira S (2000) Roles of STAT3 defined by tissue-specific gene targeting. Oncogene 19 2607-2611. [DOI] [PubMed] [Google Scholar]
- Akiyama C, Yuguchi T, Nishio M, Tomishima T, Fujinaka T, Taniguchi M, Nakajima Y, Kohmura E, and Yoshimine T (2004) Src family kinase inhibitor PP1 reduces secondary damage after spinal cord compression in rats. J Neurotrauma 21 923-931. [DOI] [PubMed] [Google Scholar]
- Armstrong SC (2004) Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc Res 61 427-436. [DOI] [PubMed] [Google Scholar]
- Bhatia M (2002) Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr Drug Targets Inflamm Allergy 1 343-351. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Brady M, Kang YK, Costello E, Newton DJ, Christmas SE, Neoptolemos JP, and Slavin J (2002) MCP-1 but not CINC synthesis is increased in rat pancreatic acini in response to cerulein hyperstimulation. Am J Physiol Gastro-intest Liver Physiol 282 G77-G85. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, and Slavin J (2000) Inflammatory mediators in acute pancreatitis. J Pathol 190 117-125. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Brady M, Zagorski J, Christmas SE, Campbell F, Neoptolemos JP, and Slavin J (2000) Treatment with neutralizing antibody against cytokine induced neutrophil chemoattractant (CINC) protects rats against acute pancreatitis associated lung injury. Gut 47 838-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M and Moochhala S (2004) Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol 202 145-156. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Neoptolemos JP, and Slavin J (2001) Inflammatory mediators as therapeutic targets in acute pancreatitis. Curr Opin Investig Drugs 2 496-501. [PubMed] [Google Scholar]
- Bhatia M, Saluja AK, Hofbauer B, Frossard JL, Lee HS, Castagliuolo I, Wang CC, Gerard N, Pothoulakis C, and Steer ML (1998) Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis associated lung injury. Proc Natl Acad Sci U S A 95 4760-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg J and Darnell JE Jr (2000) The role of STATs in transcriptional control and their impact on cellular function. Oncogene 19 2468-2473. [DOI] [PubMed] [Google Scholar]
- Brown MT and Cooper JA (1996) Regulation, substrates and functions of Src. Biochim Biophys Acta 1287 121-149. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Reddy MV, and Reddy EP (1998) Src kinases and not JAKs activate STATs during IL-3 induced myeloid cell proliferation. Oncogene 16 1749-1758. [DOI] [PubMed] [Google Scholar]
- Chung J, Uchida E, Grammer TC, and Blenis J (1997) STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol 17 6508-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea KA, Vaughn ZD, O'Bryan EM, Nishijima D, Déry O, and Bunnett NW (2000) The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta-arrestin-dependent scaffolding complex. Proc Natl Acad Sci U S A 97 11086-11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Rocca GJ, van Biesen T, Daaka Y, Luttrell DK, Luttrell LM, and Lefkowitz RJ (1997) Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors: convergence of Gi- and Gq-mediated pathways on calcium/calmodulin, Pyk2, and Src kinase. J Biol Chem 272 19125-19132. [DOI] [PubMed] [Google Scholar]
- Khadaroo RG, He R, Parodo J, Powers KA, Marshall JC, Kapus A, and Rotstein OD (2004) The role of the Src family of tyrosine kinases after oxidant-induced lung injury in vivo. Surgery 136 483-488. [DOI] [PubMed] [Google Scholar]
- Kusaka G, Ishikawa M, Nanda A, Granger DN, and Zhang JH (2004) Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 24 916-925. [DOI] [PubMed] [Google Scholar]
- Lau HY, Wong FL, and Bhatia M (2005) A key role of neurokinin 1 receptors in acute pancreatitis and associated lung injury. Biochem Biophys Res Commun 327 509-515. [DOI] [PubMed] [Google Scholar]
- Lennmyr F, Ericsson A, Gerwins P, Akterin S, Ahlström H, and Terént A (2004) Src family kinase-inhibitor PP2 reduces focal ischemic brain injury. Acta Neurol Scand 110 175-179. [DOI] [PubMed] [Google Scholar]
- Lowell CA (2004) Src-family kinases: rheostats of immune cell signaling. Mol Immunol 41 631-643. [DOI] [PubMed] [Google Scholar]
- Matsukawa A, Takeda K, Kudo S, Maeda T, Kagayama M, and Akira S (2003) Aberrant inflammation and lethality to septic peritonitis in mice lacking STAT3 in macrophages and neutrophils. J Immunol 171 6198-6205. [DOI] [PubMed] [Google Scholar]
- Okutani D, Lodyga M, Han B, and Liu M (2006) Src protein tyrosine kinase family and acute inflammatory responses. Am J Physiol Lung Cell Mol Physiol 29 L129-L141. [DOI] [PubMed] [Google Scholar]
- Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RL, Chopp M, and Cheresh DA (2001) Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat Med 7 222-227. [DOI] [PubMed] [Google Scholar]
- Pierre KJ, Tung KK, and Nadj H (1976) A new enzymatic kinetic method for the determination of serum and urine amylase. Clin Chem 22 1219. [Google Scholar]
- Ramnath RD and Bhatia M (2006) Substance P treatment stimulates chemokine synthesis in pancreatic acinar cells via the activation of NF-kappaB. Am J Physiol Gastrointest Liver Physiol 291 G1113-G1119. [DOI] [PubMed] [Google Scholar]
- Ramnath RD, Sun J, Adhikari S, and Bhatia M (2007) Effect of MAP kinases on chemokine synthesis induced by substance P in mouse pancreatic acinar cells. J Cell Mol Med 11 1326-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnath RD, Sun J, and Bhatia M (2008) Role of calcium in substance P-induced chemokine synthesis in mouse pancreatic acinar cells. Br J Pharmacol 154 1339-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann RR, Kirschning CJ, Unbehaun A, Aberle HP, Knope HP, Lamping N, Ulevitch RJ, and Herrmann F (1996) The lipopolysaccharide-binding protein is a secretory class 1 acute-phase protein whose gene is transcriptionally activated by APRF/STAT/3 and other cytokine-inducible nuclear proteins. Mol Cell Biol 16 3490-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severgnini M, Takahashi S, Rozo LM, Homer RJ, Kuhn C, Jhung JW, Perides G, Steer M, Hassoun PM, Fanburg BL, et al. (2004) Activation of the STAT pathway in acute lung injury. Am J Physiol Lung Cell Mol Physiol 286 L1282-L1292. [DOI] [PubMed] [Google Scholar]
- Severgnini M, Takahashi S, Tu P, Perides G, Homer RJ, Jhung JW, Bhavsar D, Cochran BH, and Simon AR (2005) Inhibition of the Src and Jak kinases protects against lipopolysaccharide-induced acute lung injury. Am J Respir Crit Care Med 171 858-867. [DOI] [PubMed] [Google Scholar]
- Song L, Turkson J, Karras JG, Jove R, and Haura EB (2003) Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene 22 4150-4165. [DOI] [PubMed] [Google Scholar]
- Sun J and Bhatia M (2007) Blockade of neurokinin 1 receptor attenuates CC and CXC chemokine production in experimental acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol 292 G143-G153. [DOI] [PubMed] [Google Scholar]
- Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Förster I, and Akira S (1999) Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10 39-49. [DOI] [PubMed] [Google Scholar]
- Thomas SM and Brugge JS (1997) Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13 513-609. [DOI] [PubMed] [Google Scholar]
- Turkson J, Bowman T, Adnane J, Zhang Y, Djeu JY, Sekharam M, Frank DA, Holzman LB, Wu J, Sebti S, et al. (1999) Requirement for Ras/Rac1-mediated p38 and c-Jun N-terminal kinase signaling in Stat3 transcriptional activity induced by the Src oncoprotein. Mol Cell Biol 19 7519-7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Shintani S, Weber A, Kirchmair R, Wood M, Cravens A, McSharry H, Iwakura A, Yoon YS, Himes N, et al. (2004) Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J Clin Invest 113 885-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Kugimiya T, and Miyazaki T (2005) Substance P receptor in U373 MG human astrocytoma cells activates mitogen-activated protein kinases ERK1/2 through Src. Brain Tumor Pathol 22 1-8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Richardson MD, Bigner DD, and Kwatra MM (2005) Signal transduction through substance P receptor in human glioblastoma cells: roles for Src and PKCdelta. Cancer Chemother Pharmacol 56 585-593. [DOI] [PubMed] [Google Scholar]
- Yuan SY (2002) Protein kinase signaling in the modulation of microvascular permeability. Vascul Pharmacol 39 213-223. [DOI] [PubMed] [Google Scholar]