Abstract
Pannus formation, in both rheumatoid arthritis (RA) and collagen-induced arthritis (CIA), is angiogenesis-dependent. PPI-2458 [(1R)-1-carbamoyl-2-methyl]-carbamic acid-(3R,3S,5S, 6R)-5-methoxy-4-[(2R,3R)-2-methyl-3-(3-methyl-but-2-enyl)oxiranyl]-1-oxaspiro(2*5)oct-6-yl ester], a new fumagillin derivative known to inhibit methionine aminopeptidase 2 (MetAP-2) and endothelial proliferation at the late G1 phase, was evaluated in CIA rats to study its potential to involute synovitis. Arthritic syngeneic LOU rats received either a vehicle control or various dosages of oral, intravenous, or subcutaneous PPI-2458. Plasma samples were analyzed to determine a pharmacokinetic profile of PPI-2458, and whole blood was evaluated by flow cytometry to assess the effect on lymphocyte subsets. At 15 mg/kg i.v., 30 mg/kg s.c., or 100 mg/kg p.o., there was a significant reduction in clinical severity scores (p < 0.001) and blinded radiographic scores (p < 0.001) compared with vehicle control groups. Structural damage was virtually eliminated with PPI-2458. Continuous inhibition of MetAP-2 was needed to maintain benefits, although pannus involution could be achieved with the inhibitor when escape flares occurred. Pharmacokinetic analysis after a single p.o. dose showed a rapid Tmax value of 15 min followed by biphasic elimination (t½, ∼20 min and t½, ∼5 h) and an estimated oral bioavailability of ∼15%. Flow cytometry revealed a dose-dependent decrease in white blood cells and lymphocytes manifested as decreases in circulating CD3+ T cells and natural killer cells. PPI-2458, however, did not seem to be immunosuppressive, as determined by delayed-type hypersensitivity or IgG antibody assays. These studies indicate that the MetAP-2 inhibitor PPI-2458 can regress established CIA and that angiogenic mechanisms might be important targets in the treatment of other pannus-mediated diseases such as RA.
Angiogenesis, or new blood vessel formation, is limited in nondisease states to embryogenesis/growth, wound healing, and menstrual cycles. Pathologic angiogenesis is observed in more than 70 diseases, including cancer, proliferative retinopathy, and pannus formation in rheumatoid arthritis (RA) (Carmeliet, 2005; Lainer and Brahn, 2005; Lainer-Carr and Brahn, 2007).
The synovium in healthy joints is relatively thin. It contains macrophage-like type A cells that produce degradative enzymes and fibroblast-like type B cells that are responsible for synovial fluid production. The synovium normally does not encroach upon the articular surface of cartilage. The subsynovial tissue is distinct from the synovial lining layer and contains lymphatics and blood vessels as well as fibroblasts and adipocytes.
During the early stages of RA, synovial cell hyperplasia (notably fibroblast-like synovial cells) occurs along with cellular infiltration by lymphocytes and monocytes of the synovial layer. Later stages of RA are also associated with these findings as well as fibrin deposition on the synovial surface and increased plasma cells. The synovial layer thickens considerably to form the pannus, which becomes destructive at the articular cartilage surface and causes bone erosion. Both early and late stages of RA are dependent upon angiogenesis to sustain the cellular expansion (Walsh, 1999).
Fumagillin and structural analogs such as TNP-470 (previously known as AGM-1470) irreversibly inhibit methionine aminopeptidase-2 (MetAP-2), a protein that removes initiating methionine residues during protein translation (Griffith et al., 1997; Sin et al., 1997; Bernier et al., 2005; Yeh et al., 2006; Zhang et al., 2006; Benny et al., 2008). These molecules inhibit angiogenesis by blocking endothelial cell proliferation and migration (Ingber et al., 1990). Rat models of induced arthritis have shown that administration of TNP-470 before the onset of arthritis prevents disease (Peacock et al., 1992, 1995). TNP-470 also suppressed the development of spontaneous arthritis in the KRN/NOD T-cell receptor transgenic mouse model (de Bandt et al., 2000). Adverse neurotoxicity, however, has been reported with TNP-470, and further human trials have been suspended (Logothetis et al., 2001). This suggests that studies with less toxic MetAP-2 inhibitors are indicated.
PPI-2458 is also a fumagillin analog (Fig. 1) that targets MetAP-2 and inhibits endothelial cell proliferation (Bernier et al., 2005). This compound has been shown to reduce swelling in a peptidoglycan-polysaccharide-induced model when administered subcutaneously or orally (Bernier et al., 2004; Hannig et al., 2007) and to suppress murine collagen-induced arthritis (CIA) (Bainbridge et al., 2007). The current study evaluates PPI-2458 in the rat CIA RA model of autoimmune chronic inflammatory arthritis (Trentham et al., 1977). Pharmacokinetics, bioavailability, route of administration, clinical/radiographic/histologic effects, and immune responses were investigated.
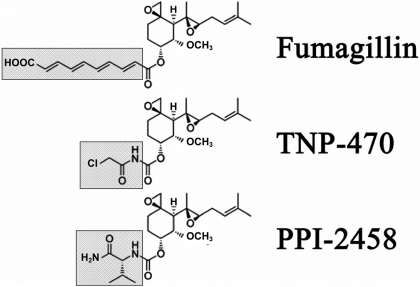
Fig. 1.
Structure of parent molecule, fumagillin, and modifications of side chain (gray box) for TNP-470 and PPI-2458 (Bernier et al., 2004).
Materials and Methods
Animals. The animal experiments were performed in accordance with the Institutional Animal Care and Use Committee at UCLA. Pathogen-free syngeneic LOU rats (breeders originally obtained from the National Institutes of Health Small Animal Facility, Bethesda, MD) were maintained and bred in an in house colony. Animals were provided with ad libitum food and water and weighed at the beginning and end of the study. Female rats, weighing 125 to 150 g (8-10 weeks old), were used in all experiments.
Induction of CIA. To induce arthritis, anesthetized rats (inhaled isoflurane, 4-5% for induction and 1.5-2.5% for maintenance) were injected intradermally on day 0 with 0.5 mg of native chick type II collagen (Elastin Products Co., Owensville, MO), solubilized in 0.1 M acetic acid, and emulsified in equal volume of IFA (Difco, Detroit, MI) (Brahn and Trentham, 1984b). Disease onset of one or both hind limbs typically developed in 90 to 100% of animals on day 10. Only rats that showed evidence of arthritis on day 10 were entered into the studies.
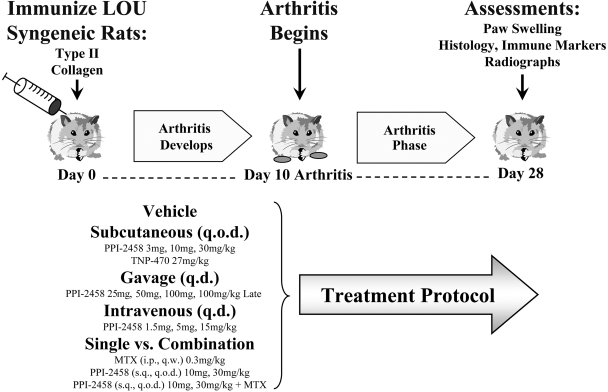
Protocols. LOU rats were immunized with native type II collagen in IFA on day 0 and were randomized to treatment groups of 12 to 14 rats for each of the 19 protocols investigated (total n = 250), after the establishment of clinically evident arthritis (day 10 after immunization except where noted). Synovial hyperplasia and synovitis is histologically evident at this time point (Caulfield et al., 1982). They received a control vehicle or various dosages of PPI-2458 (Praecis Pharmaceuticals, Waltham, MA), methotrexate (Lederle Laboratories, Pearl River, NY), or TNP-470 (Fig. 2). Subcutaneous PPI-2458 (in 5% dextrose; 5 ml/kg) was administered q.o.d. at 3, 10, or 30 mg/kg and compared with TNP-470 at 27 mg/kg. Gavage (oral) PPI-2458 (in 11% hydroxypropyl β-cyclodextrin) was given q.d. at 25, 50, and 100 mg/kg in a volume of 5 ml/kg. An additional gavage group was given 100 mg/kg late, beginning 1 week after clinical disease onset (day 16). Intravenous PPI-2458 (in 5% dextrose; 5 ml/kg) was injected q.d. at 1.5, 5, and 15 mg/kg via the lateral tail vein. Single agent PPI-2458 (subcutaneous, q.o.d.) at 10 or 30 mg/kg was compared with single agent methotrexate (intraperitoneal, every week) at 0.3 mg/kg (in normal saline at 0.1 mg/ml) or in combination with PPI-2458 at 10 and 30 mg/kg. The severity of arthritis was scored daily, and radiographs of limbs were obtained at the end of each protocol (day 28). Serum samples were analyzed to determine antibody levels to type II collagen and delayed type hypersensitivity to type II collagen was measured in vivo. Flow cytometry was used to assess the effect of PPI-2458 treatment on lymphocyte subsets.
Fig. 2.
In vivo studies. Experimental protocols of PPI-2458, TNP-470, and methotrexate are noted. Rats were immunized with type II collagen on day 0, developed clinical arthritis on day 10, and sacrificed on day 28. The route and frequency are summarized.
In separate studies to evaluate disease flare and therapeutic recapture, subcutaneous q.o.d. PPI-2458 was begun at 15 or 30 mg/kg on day 10 and discontinued on days 18 to 28. In the 15 mg/kg group, PPI-2458 was then restarted for days 28 to 47 to determine whether therapeutic benefit could be re-established.
Pharmacokinetics and Bioavailability of PPI-2458. PPI-2458 was administered in polyethylene glycol 200 (5 ml/kg) to naive LOU rats intravenously (10 mg/kg), subcutaneously (30 mg/kg), or by oral gavage (100 mg/kg). Serial serum samples were obtained from whole blood collected from 5 min to 6 h after dose from four rats per each route of administration group. The serum samples were stored at or below -20°C until quantitative analysis of PP-2458 concentration. For this analysis, the serum samples were thawed, and protein was precipitated by the addition of a 2-fold volume of acetonitrile containing a deuterated internal standard (d8-PPI-2458). The supernatants obtained after protein precipitation and centrifugation were then dried, and the residual was reconstituted in high-performance liquid chromatography mobile phase before injection on an API4000 triple quadrupole mass spectrometer (PerkinElmerSciex Instruments, Boston, MA) equipped with binary high-performance liquid chromatography pumps (PerkinElmer Series 200; PerkinElmerSciex Instruments) and a LEAP HTS-PAL autosampler (Leap Technologies, Carrboro, NC). PPI-2458 and its internal standard were eluted off of a Haisil CS Clipeus C8 column (2.1 × 50 mm; The Nest Group, Inc., Southborough, MA) with a linear gradient of water and increasing amounts of acetonitrile each containing 0.1% formic acid. Analyte detection was achieved using positive electrospray ionization and multiple reaction monitoring of transitions specific for PPI-2458 and its internal standard. Data acquisition and peak integration were performed using Analyst 1.4.1 software (PerkinElmerSciex Instruments). A linear regression of standards (0.1-1000 ng/ml) was determined with a weighting factor of 1/x2. This equation was then used to calculate the concentrations in the serum samples. Pharmacokinetic analysis was conducted using WinNolin (Pharsight, Mountain View, CA). The maximal plasma concentration (Cmax) and the time at which maximal concentration occurred (Tmax) are the observed values. Areas under the curve (AUCs) were calculated using the linear/log linear trapezoidal rule. Bioavailability was calculated from the dose-adjusted AUC ratios with the intravenous AUC representing 100% bioavailability.
Clinical Assessment of Arthritis. The severity of arthritis was evaluated by standard methods as described previously (Brahn and Trentham, 1984b). Clinical severity was quantified by blinded daily scoring of each hind paw from 0 to 4 (0, normal; 4, maximum) based on increasing levels of swelling and periarticular erythema (0, no erythema or swelling; 1+, isolated ankle swelling; 2+, swelling/erythema of ankle and proximal half of tarsal joints; 3+, swelling/erythema of ankle and all tarsal joints up to metatarsal phalangeal joints; and 4+, swelling/erythema of entire paw including digits). The arthritic index of a rat was defined as the sum of its four limb scores. Because rat CIA typically involves only the hind limbs, an arthritic index of 6 to 8 was considered severe arthritis.
Radiographic Assessment of Arthritis. Severity of CIA was assessed blindly. High-resolution digital radiographs of both hind limbs were performed for day 28-sacrificed rats (45 kV at 10 milli-ampere second and imaged on high-speed Kodachrome; Eastman Kodak, Rochester, NY). Each limb was scored 0 to 3 with a summated maximal score of 6 (Brahn and Trentham, 1984b) based on the extent of soft tissue swelling, joint space narrowing, bone destruction, and periosteal new bone formation (0, normal; 1+, soft tissue swelling only; 2+, soft tissue swelling and early erosions; and 3+, severe erosions) (Brahn and Trentham, 1984b).
Histology of Joints. For light microscopy, hind limbs were harvested, fixed in phosphate-buffered 10% formaldehyde, decalcified, and stained. Paraffin blocks were sectioned at approximately 5 μm. The tarsus and digits were cut in a sagittal plane, and sections were stained with hematoxylin and eosin or safranin O. Selected sections were scored (0-17 scale) by a single blinded observer using a Mankin method (Mankin et al., 1971) adapted for rat tissue. Digital images were captured with a BX51 light and fluorescent microscope (Olympus, Tokyo, Japan) equipped with a DP70 digital camera (Olympus) and Microsuite TM5 imaging software (Olympus Soft Imaging Solutions Corp., Lakewood, CO). Final images were prepared using Adobe Photoshop (Adobe Systems, Mountain View, CA).
Antibodies to Collagen. Serum antibody titers to type II collagen were assayed by ELISA using peroxidase-conjugated goat anti-rat IgG antibodies according to the manufacturer's instructions (Accurate Chemical & Scientific, Westbury, NY) (Brahn and Trentham, 1984b). In brief, type II collagen was solubilized at a concentration of 2.5 mg/ml in 0.1 M acetic acid overnight and then dialyzed with 0.2 M NaCl/0.05 M Tris, pH 7.2. Plates were coated with collagen solution at a concentration of 12.5 μg/ml and incubated for at least 72 h at 4°C. After incubation, the plates were washed three times with buffer, and quadruplicate 200 μl of test serum, along with appropriate controls, were added at a dilution of 1:2500 based on previous titrations. Plates were incubated for 18 h at 4°C, washed, and 200 μl of the peroxidase-conjugated goat anti-rat IgG antibody at a dilution of 1:20,000 was added to each well. Plates were incubated in the dark for 30 min at room temperature, washed, and 200 μl of O-phenyl-enediamine was added and incubated for 1 h at room temperature in the dark. The reaction was stopped with 25 μl of 0.25 N sulfuric acid, and the plates were read at 490 nm.
Delayed Type Hypersensitivity to Collagen. DTH to native type II collagen was quantitated in vivo by a radiometric ear assay (Brahn and Trentham, 1984b). In brief, rats are pulsed with tracer thymidine and challenged with type II collagen intradermal in the right ear and buffer in the left ear, with subsequent tracer measurement 48 h later. Radiometric ear indices ≥1.4 (right ear/left ear), on day 28, represent more than 2 S.D. above the naive control mean and are a significant response to collagen.
Flow Cytometry. Using standard protocols, day 28 peripheral blood samples were evaluated by flow cytometry on a Cytomation MoFlo (Dako North America, Inc., Carpinteria, CA). Fluorescence-conjugated anti-rat antibodies could identify CD3 (BD Pharmingen, San Diego, CA), CD2 (BD Pharmingen), CD4 (BD Pharmingen), CD8 (BD Pharmingen), CD161a (BD Pharmingen), and CD45 (BD Pharmingen). Stained cells from vehicle control CIA rats were compared with naive rats and rats administered intravenous PPI-2458 q.d. at 1.5, 5, or 15 mg/kg beginning at the time of arthritis onset, day 10.
Statistics. Differences in clinical disease activity between control and treatment groups were compared using Student's t test for parametric continuous variables. Group data were presented as the mean ± S.E.M. except where indicated. The control groups were also compared with each treatment group using repeated measures regression modeling techniques for differences in clinical joint counts from days 10 to 28. Several covariance structures were tested across the treatment groups, including autoregressive, autoregressive heterogeneous, and autoregressive moving average. In previous experiments, the heterogeneous autoregressive covariance structures provided the best fit and were used in the regression models to evaluate differences in clinical joint counts between groups over time. SAS Proc Mixed (version 8.2; SAS Institute, Cary, NC) was used for regression modeling. For the radiographic and flow cytometry analyses, treatment groups were compared using Student's t test for parametric continuous variables and the Wilcoxon rank-sum test for nonparametric continuous variables. The chi-square test for association was used for categorical variables; Fisher's exact test was used for small sample sizes. The significance level was prespecified at p < 0.05.
Results
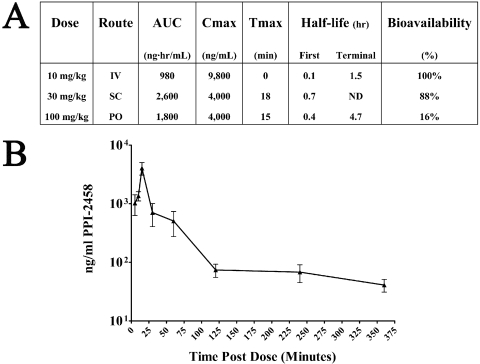
Pharmacokinetics and Bioavailability of PPI-2458. The PPI-2458 pharmacokinetics is summarized in Fig. 3A, and serum levels over time, after 100 mg/kg p.o., are shown in Fig. 3B. In female LOU rats, PPI-2458 was demonstrated to be active after intravenous, subcutaneous, or oral dosing and was characterized by a short terminal half-life. Tmax occurred early after oral or subcutaneous dosing. Dose-adjusted bioavailabilities were moderate for oral dosing at 16% and near complete for subcutaneous dosing at 88% relative to intravenous dosing. Because PPI-2458 is known to be a covalent inhibitor of its intended target. MetAP-2, pharmacodynamic effects would be expected to extend the duration beyond significant plasma concentrations.
Fig. 3.
Pharmacokinetics of PPI-2458. A, AUC, Cmax, Tmax, half-life, and dose-adjusted bioavailability are shown for intravenous, subcutaneous, and oral routes of administration. B, mean serum levels of PPI-2458 (±S.E.M.) are shown after a single dose of 100 mg/kg p.o. in normal LOU rats.
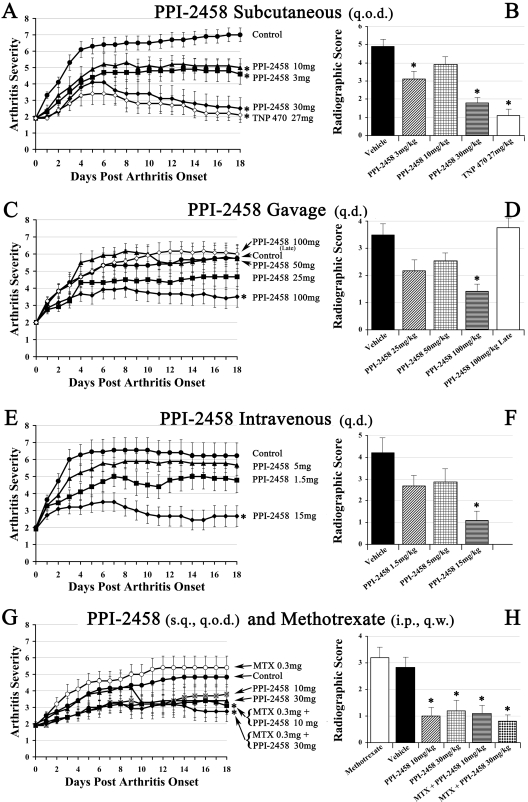
PPI-2458 Regresses Clinical Arthritis in CIA. Female LOU rats developed clinical arthritis that began 10 days after immunization with collagen type II in incomplete Freund's adjuvant. Vehicle control (untreated) rats rapidly progressed to severe arthritis within 5 to 7 days after onset that persisted until the end of the study. The highest mean arthritic score of any vehicle group at the end of the study, on day 18 after arthritis onset, was 7.0 ± 0.04. This is evident in Fig. 4 in which 10 days after immunization is designated day 0 after arthritis onset. The levels of clinical arthritis in the vehicle control groups were statistically comparable, and a representative hind paw is shown in Fig. 5A.
Fig. 4.
Clinical and radiographic scores (±S.E.M.). The left column (A, C, E, and G) shows longitudinal clinical scores for each dose (on a per kilogram basis) and route of administration evaluated. The right column (B, D, F, and H) depicts mean radiographic scores (±S.E.M.). *, statistically different results compared with the respective control group.
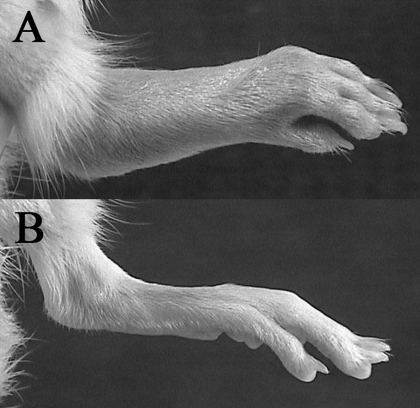
Fig. 5.
Clinical arthritis. A, hind limb of a vehicle control rat on day 28 with an arthritis score of 4 (maximum). Erythema and soft tissue swelling is evident at the proximal and distal foot. B, hind limb of a PPI-2458-treated rat on day 28 with an arthritis score of 0.
The clinical benefits of subcutaneous q.o.d. PPI-2458, beginning therapy at arthritis onset, are graphically depicted in Fig. 4A. A representative limb from a PPI-2458-treated rat at the conclusion of the study is shown in Fig. 5B. The 3 and 10 mg/kg PPI-2458 treatment groups demonstrated similar clinical improvements, with final mean scores of 4.6 ± 0.6 and 5.0 ± 0.5, respectively. The clinical differences between both the 3 and 10 mg/kg groups and the vehicle control group became statistically significant (p < 0.05) within 3 to 5 days after treatment initiation. These clinical differences persisted throughout the remainder of the study. PPI-2458 at 30 mg/kg demonstrated significant improvement in clinical scores within 1 day and achieved a final score of 2.6 ± 0.6 by the completion of the study (p < 0.00001 versus controls). Thirty percent of the rats had no clinically detectable disease. This was similar to the improvement observed with TNP-470 at 27 mg/kg.
Daily oral (gavage) therapy with PPI-2458 (Fig. 4C) required more than 50 mg/kg to achieve statistical clinical benefit. The 100 mg/kg PPI-2458 group had an arthritis score of 3.5 ± 0.6 (p < 0.01 versus controls) at the end of the protocol. Delaying 100 mg/kg PPI-2458 therapy initiation until a week after arthritis onset (an advanced stage of the disease with structural damage) did not regress CIA in the time period studied.
Intravenous q.d. PPI-2458 was superior to oral. At 15 mg/kg, beginning at arthritis onset, clinical scores were significantly reduced within 1 day (an effect similar to subcutaneous q.o.d. 30 mg/kg) (Fig. 4E). Clinical arthritis scores 18 days later were 2.7 ± 0.6 (p < 0.001 versus controls).
Single agent methotrexate and PPI-2458, administered subcutaneously, are shown compared with combination therapy in Fig. 4G. As a single agent, at 0.3 mg/kg weekly, methotrexate had no effect on CIA. By the study endpoint, however, combination PPI-2458 at either 10 mg/kg or 30 mg/kg significantly reduced CIA compared with vehicle controls or methotrexate alone (arthritic scores of 3.1 ± 0.5 and 2.7 ± 0.6, respectively, for each dose).
PPI-2458 Withdrawal and Therapeutic Recapture. Additional studies administered subcutaneous PPI-2458 q.o.d. at 15 or 30 mg/kg from arthritis onset (day 10 after immunization) until day 18. After complete discontinuation of agent, a clinical flare was evident in both groups within 3 days. PPI-2458 was restarted in the 15 mg/kg group on day 28 (maximal flare score 3.8 ± 0.5) and continued until day 47. Within 3 days of reinstitution of therapy, clinical scores began to improve and progressively decreased to 2.5 ± 0.7.
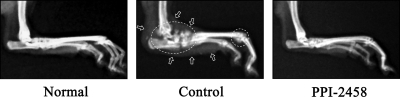
Suppression of Radiographic Damage with PPI-2458. Hind paw radiographs taken at the conclusion of the study were scored by a blinded reader, and group means, for each treatment arm, are shown in the right column of Fig. 4. Because CIA may be unilateral, a mean summated score of between 4 and 5 represents a typical level of structural damage in control rats. A control limb radiograph, from the end of the study, is shown in Fig. 6 (middle).
Fig. 6.
Radiographic damage. Left, a normal limb. Middle, an arthritic control limb on day 28. Periarticular soft tissue swelling (arrows) and severe periosteal reactions/osteolysis/erosions (within the dashed circles) are evident at the tarsus, tarsals, and metatarsophalangeal joints [radiographic score is 3 (maximum)]. Right, a hind limb, day 28 (18 days after arthritis onset on day 10), treated with PPI-2458 (30 mg/kg s.c. q.o.d). No soft tissue swelling or bone destruction is evident, and it seems similar to the normal limb (radiographic score is 0).
In general, radiographic scores paralleled and confirmed clinical evaluations. Subcutaneous PPI-2458 at 30 mg/kg q.o.d. (Fig. 4B) resulted in a radiographic score of 1.8 ± 0.4 (p < 0.0001 versus controls) that was similar to the parent molecule TNP-470. All limbs had reduced severity, and 55% had no detectable erosions (Fig. 6, right). Oral PPI-2458 at 100 mg/kg q.d. had a radiographic score of 1.4 ± 0.3 (p < 0.01 versus controls), with 58% devoid of erosions (Fig. 4D). As suggested by the clinical results, late therapy at 100 mg/kg did not prevent structural damage. Intravenous therapy at 15 mg/kg PPI-2458 q.d. (Fig. 4F) reduced the score to 1.1 ± 0.5 (p < 0.003 versus controls), with 83% showing no structural consequence. Combination methotrexate (0.3 mg/week s.c.) with PPI-2458 (30 mg/kg s.c. q.o.d.) (Fig. 4H) significantly reduced radiographic damage (radiographic score, 0.8 ± 0.3; p < 0.005 versus controls) and was superior to PPI-2458 alone. With respect to methotrexate as a single agent, disease severity at this dose was not affected. These data confirm our previous studies (Brahn et al., 1991).
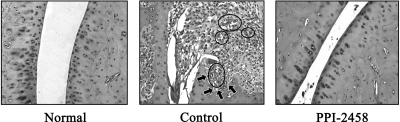
Histology of Joints. Pannus was evident in control synovium as were numerous blood vessels (Fig. 7, middle). Structural damage, manifested as erosions of cartilage and bone, were also present. Therapy with PPI-2458 regressed angiogenesis and pannus with subsequent preservation of the cartilage and bone (Fig. 7, right).
Fig. 7.
Joint histology. Left, a normal tarsus joint stained with hematoxylin and eosin (original magnification, 300×). Middle, from a day 28 arthritic control rat. Pannus with numerous blood vessels (circled) are evident along with an erosion of cartilage and subchondral bone (arrows). Right, from a day 28 rat treated with PPI-2458 30 mg/kg s.c. q.o.d. (beginning on day 10). The cartilage and bone seem normal, and no pannus is evident.
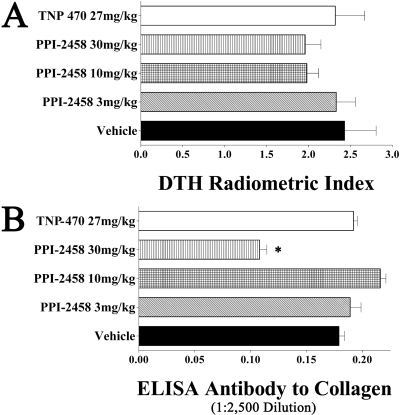
Immunologic Effects. Cell-mediated immune responses to type II collagen, as determined in vivo with a radiometric ear assay, were not significantly lower in rats administered TNP-470 or any tested dose of PPI-2458 compared with vehicle controls (Fig. 8A).
Fig. 8.
DTH and antibody. A, DTH responses, on day 28, to native type II collagen in rats administered PPI-2458, TNP-470, or vehicle subcutaneously beginning on day 10. B, antibody levels by ELISA, on day 28, to native type II collagen in rats administered PPI-2458, TNP-470, or vehicle subcutaneously beginning on day 10. Serum was assayed at a 1:2500 dilution and reported as optical density at 490 nm. *, statistically different results compared with the vehicle control group.
All rats immunized with collagen had robust IgG antibody responses as determined by an ELISA performed at a 1:2500 serum dilution (Fig. 8B). The 30 mg/kg s.c. PPI-2458-treated rats had a statistically lower titer than vehicle controls, although such reductions in high titers are frequently seen (Brahn and Trentham, 1984a,b; Brahn et al., 1991, 1994; Peacock et al., 1992) with interventions that prevent structural damage and subsequent resensitization to native type II collagen exposed in eroded cartilage. The biologic importance of these reductions is unclear.
Peripheral WBC at Study Completion. The induction of CIA, compared with naive rats, had no significant impact on total WBC (9.3 versus 8.9), percentage of neutrophils (18 versus 19%), absolute neutrophil counts (1.6 versus 1.3 × 10-3), percentage of lymphocytes (80 versus 77%), or absolute lymphocyte counts (7.5 versus 7.2 × 10-3), respectively (Fig. 9, A and B).
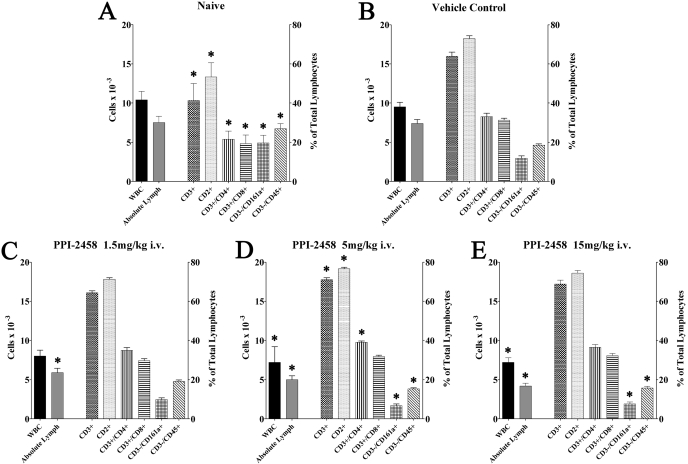
Fig. 9.
Flow cytometry of day 28 peripheral blood. A, naive rats. B, vehicle control rats. C to E, rats administered the indicated dose of PPI-2458 intravenously beginning on day 10, the onset of clinical arthritis. *, statistically different results compared with the vehicle control group.
In all tested doses of intravenous PPI-2458 (Fig. 9, C-E), compared with CIA vehicle controls, the percentage of lymphocytes (63-68 versus 80%) and absolute lymphocyte counts (4.3-5.9 versus 7.5 × 10-3) were significantly lower, whereas the percentage of neutrophils (29-34 versus 18%) and absolute neutrophil counts (2.3-2.6 × 10-3 versus 1.6 × 10-3) were higher, respectively. The total WBC was reduced by the 5 mg/kg dose (6.8 × 10-3) and 15 mg/kg dose (7.7 × 10-3) but not by the lower 1.5 mg/kg dose (8.8 × 10-3).
Flow Cytometry of Lymphocytes at Study Completion. CIA vehicle control rats, compared with naive rats (Fig. 9, A and B), had higher levels of T cells (CD3+ or CD2+), helper T cells (CD3+/CD4+), and cytotoxic T cells (CD3+/CD8+). In contrast, NK cells (CD3-/CD161a+) and B cells (CD3-/CD45+) were significantly lower. Rats administered PPI-2458 intravenously at either 5 or 15 mg/kg had a significantly lower percentage of NK and B cells.
Discussion
In experimental models, antiangiogenic compounds can effectively curb pannus formation and growth by blocking neovascularization that supports synovial hyperplasia (Lainer and Brahn, 2005; Lainer-Carr and Brahn, 2007). Other studies have also shown that inhibiting angiogenesis by administration of angiostatin, endostatin, paclitaxel, and TNP-470 improves outcomes in RA models. PPI-2458 is a novel angiogenesis inhibitor that irreversibly forms covalent bonds with MetAP-2, an enzyme that selectively removes the N-terminal methionine from proteins during synthesis (Bernier et al., 2004, 2005). By binding to MetAP-2, PPI-2458 inhibits endothelial cell proliferation, a crucial mechanism in angiogenesis (Griffith et al., 1997; Sin et al., 1997).
The current study was designed to assess the novel angiogenesis inhibitor PPI-2458 in rat CIA. Clinical outcomes in this model of RA were significantly better with PPI-2458 treatment. Subcutaneous administration of PPI-2458 at 3, 10, and 30 mg/kg every 48 h was effective in altering arthritis severity (daily arthritis scores) and joint destruction (radiographic scores). The therapeutic effectiveness of PPI-2458 was apparent within 24 h after its administration. This rapid response suggests that inflammation is intimately linked with angiogenesis and that neovascularization actively participates in the pathogenesis.
Interestingly, PPI-2458 at 30 mg/kg s.c. had efficacy that was similar to TNP-470, an earlier antiangiogenic agent with known toxicities (Ingber et al., 1990; Peacock et al., 1992, 1995; Yanase et al., 1993). TNP-470 is also a fumagillin analog (Fig. 1) that inhibits MetAP-2, but concerns about neurotoxicity have been noted (Kudelka et al., 1997; Bhargava et al., 1999; de Bandt et al., 2000; Logothetis et al., 2001; Herbst et al., 2002). PPI-2458 was designed to maintain antiproliferative and antiangiogenic properties while limiting blood-brain barrier permeability, cerebellar toxicity, and seizures observed with TNP-470. Delivery of PPI-2458 was well tolerated in this study. No drug-related deaths, toxicities, or significant differences in body weight were detected in the treatment protocol. Excellent wound healing, of the collagen/IFA immunization sites, was evident, and no infections occurred. Consequently, PPI-2458 may provide the therapeutic benefits of TNP-470 with fewer adverse events. Recent studies have evaluated an oral TNP-470 (lodamin) in nanopolymeric micelles. It was formulated to have better bioavailability and less neurologic toxicity. Lodamin was an effective inhibitor of tumor angiogenesis and liver metastasis (Benny et al., 2008).
In studies of murine CIA (Bainbridge et al., 2007), PPI-2458 effectively reduced clinical and histologic disease at 1.5 mg/kg i.p. No pharmacokinetic data were reported to directly compare with our rat doses. Interesting effects of PPI-2458 on human cultured cells were observed, including inhibition of endothelial proliferation and angiogenesis. It is noteworthy that the lack of direct effects on inflammatory cytokines and chemokines, although obtained from in vitro studies, was consistent with our in vivo humoral and cellular responses to type II collagen. In addition, their histology scores paralleled our blinded radiographic outcomes.
Combination drug therapies for RA have already been used effectively in clinical rheumatology, particularly with methotrexate. This has been supported by preclinical studies with combination interventions (Brahn et al., 1991; Bendele et al., 1999; Hisadome et al., 2004; Silva et al., 2005). Previous work with PPI-2458 demonstrated that it did not reduce interleukin-6 and vascular endothelial growth factor secretion (Bernier et al., 2004). How MetAP-2-dependent proliferation is regulated in PPI-2458-sensitive cells is not known (Bernier et al., 2004), although this mechanism distinguishes it from other agents used in RA, such as anti-tumor necrosis factor-α therapy whose actions are directed at the proinflammatory cytokine network. These unique aspects of PPI-2458 provide further justification to combine treatment with other drugs that act through different pathways to achieve additive or synergistic benefits. Consequently, we showed that PPI-2458 at 30 mg/kg, in combination with methotrexate, improved clinical and radiographic scores compared with the control group or either agent alone. As we have seen previously in rat CIA (Brahn et al., 1991), methotrexate as a single agent at 0.3 mg/kg weekly was ineffective. This could be because of dose, interval, or disease kinetics.
The current study is also the first to examine the effects of withdrawing PPI-2458 on arthritis. Our experiments indicate that to maintain efficacy, continuous inhibition of MetAP-2 is required. Three days after PPI-2458 was withdrawn, arthritis soon became evident, and this effect was independent of the two doses tested (15 or 30 mg/kg s.c. q.o.d.). When subcutaneous PPI-2458 was resumed, however, clinical disease responded within 3 days. Refractory arthritis was not observed.
It is likely that there is a point beyond which treatment initiation with an antiangiogenic agent, such as PPI-2458, becomes less effective as inflammation and joint destruction progress. Administration of a high oral dose (100 mg/kg), after severe disease had been established, was unable to reduce arthritis. Because a strong response was observed with oral administration of PPI-2458 at an earlier time point, this lack of response is likely because of the severity of disease when treatment was initiated. At later stages in the disease process, cytokine networks intensify and inflammation advances within the joints. This leads to local antigen presentation of type II collagen in the cartilage, formation of secondary lymphoid aggregates, and subsequent activation of enzymes and osteoclasts that result in matrix break down with irreversible joint destruction (Lazarus et al., 2008).
Subcutaneous administration of PPI-2458, as well as oral administration, was effective at improving arthritis. Fumagillin analogs are orally active, and we have shown that bioavailability is moderate for this route of administration. Because PPI-2458 is known to be a covalent inhibitor of MetAP-2, we expected pharmacodynamic effects to continue even beyond significant plasma concentrations. We established that oral administration, at a higher dose of 100 mg/kg at the onset of arthritis, significantly reduced CIA severity. The higher dose was probably more effective than the 25 or 50 mg/kg dose because increased levels in the plasma may correlate with increased effectiveness.
Similar to both subcutaneous and oral administration, daily intravenous administration of PPI-2458 at 15 mg/kg was effective at clinically improving arthritis throughout the longitudinal disease course. The therapeutic efficacy of PPI-2458 began within 24 h after initiation. All routes showed comparable outcomes, although parenteral administration achieved it at lower doses. When different intervals of subcutaneous administration were compared (data not shown), more frequent treatment (every 48 h) lowered arthritis the most. This is consistent with our observation of the need for continued inhibition of MetAP-2 to reduce arthritis. In addition, the improved clinical scores correlated with our histologic findings. This is most likely because of PPI-2458 inhibition of human fibroblast-like synoviocyte proliferation (Bernier et al., 2004) in pannus growth and contributes to migration of inflammatory cells from the periphery (Bernier et al., 2005). Furthermore, previous in vitro work has demonstrated that PPI-2458 inhibits osteoclast precursor differentiation into multinucleated osteoclasts (Hannig et al., 2007; Lazarus et al., 2008), possibly by inhibiting fibroblast-like synoviocytes that release receptor activator of nuclear factor-κB ligand, which promotes osteoclast formation and activation (Teitelbaum, 2000; Bernier et al., 2004; Wu et al., 2005; Hannig et al., 2007). Such an effect would alter the balance between bone formation and bone resorption (Campagnuolo et al., 2002). The decline in pannus formation in rats treated with PPI-2458 and the decline in proliferation of synoviocytes exposed to PPI-2458 are likely to be related (Lazarus et al., 2008).
Compared with naive rats, CIA had no significant influence on WBC (Fig. 9). However, rats treated with higher doses of intravenous PPI-2458 (5 and 15 mg/kg) had significantly decreased WBC compared with vehicle controls. This reduced level may reflect attenuated inflammation compared with control rats with aggressive synovitis. Rats at all tested doses of intravenous PPI-2458 also had significantly decreased absolute lymphocyte counts. In addition, rats at higher doses of intravenous PPI-2458 (5 and 15 mg/kg) had a lower percentage of NK and B cells compared with controls. In all tested doses of intravenous PPI-2458, the percentage and absolute level of neutrophils in the treated rats were higher. What implications these findings might have for potential human trials is unclear, particularly because the peripheral blood of normal rats has approximately 20% neutrophils and 70% lymphocytes, the inverse of humans. A broad effect on the bone marrow was not seen, as evidenced by unchanged platelet, red blood cell, and hemoglobin levels.
All the experimental and control rats had strong immune responses to the relevant antigen native type II collagen. DTH responses to collagen were not statistically different in the treatment and control groups. Humoral immunity was not a primary factor in determining therapeutic outcome in this antigen-specific autoimmune model. All rats had high titer antibody levels, measured at a 1:2500 dilution (Fig. 8), that were not statistically different from the control group except for a reduction in the 30 mg/kg PPI-2458 rats. Consequently, the biologic importance of this attenuated antibody response remains unclear, although agents that prevent structure damage of hyaline cartilage also preclude re-exposure (and resensitization) to type II collagen.
The current study evaluated PPI-2458, a new antiangiogenic and antiproliferative modulator, in the CIA rat model of RA. Our findings demonstrate that PPI-2458, alone or in combination with methotrexate, significantly regressed clinical, radiographic, and histologic disease. PPI-2458 may represent a novel class of angiogenesis inhibitors that warrants additional studies in the management of RA.
This work was supported in part by the National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases [Grant AR42200]; VA Greater Los Angeles Healthcare System (to S.L.); and by Praecis Pharmaceuticals.
doi:10.1124/jpet.108.148478.
ABBREVIATIONS: RA, rheumatoid arthritis; MetAP-2, methionine aminopeptidase 2; CIA, collagen-induced arthritis; IFA, incomplete Freund's adjuvant; AUC, area under the curve; ELISA, enzyme-linked immunosorbent assay; DTH, delayed type hypersensitivity; WBC, white blood cells; NK, natural killer; q.o.d., every other day; q.d., every day.
References
- Bainbridge J, Madden L, Essex D, Binks M, Malhotra R, and Paleolog EM (2007) Methionine aminopeptidase-2 blockade reduces chronic collagen-induced arthritis: potential role for angiogenesis inhibition. Arthritis Res Ther 9 R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendele AM, McComb J, Gould T, Frazier J, Chlipala E, Seely J, Kieft G, and Edwards CK 3rd (1999) Effects of PEGylated soluble tumor necrosis factor receptor type I (PEG sTNF-RI) alone and in combination with methotrexate in adjuvant arthritic rats. Clin Exp Rheumatol 17 553-560. [PubMed] [Google Scholar]
- Benny O, Fainaru O, Adini A, Cassiola F, Bazinet L, Adini I, Pravda E, Nahmias Y, Koirala S, Corfas G, et al. (2008) An orally delivered small-molecule formulation with antiangiogenic and anticancer activity. Nat Biotechnol 26 799-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier SG, Lazarus DD, Clark E, Doyle B, Labenski MT, Thompson CD, Westlin WF, and Hannig G (2004) A methionine aminopeptidase-2 inhibitor, PPI-2458, for the treatment of rheumatoid arthritis. Proc Natl Acad Sci U S A 101 10768-10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier SG, Taghizadeh N, Thompson CD, Westlin WF, and Hannig G (2005) Methionine aminopeptidases type I and type II are essential to control cell proliferation. J Cell Biochem 95 1191-1203. [DOI] [PubMed] [Google Scholar]
- Bhargava P, Marshall JL, Rizvi N, Dahut W, Yoe J, Figuera M, Phipps K, Ong VS, Kato A, and Hawkins MJ (1999) A Phase I and pharmacokinetic study of TNP-470 administered weekly to patients with advanced cancer. Clin Cancer Res 5 1989-1995. [PubMed] [Google Scholar]
- Brahn E, Peacock DJ, and Banquerigo ML (1991) Suppression of collagen-induced arthritis by combination cyclosporin A and methotrexate therapy. Arthritis Rheum 34 1282-1288. [DOI] [PubMed] [Google Scholar]
- Brahn E, Tang C, and Banquerigo ML (1994) Regression of collagen-induced arthritis with taxol, a microtubule stabilizer. Arthritis Rheum 37 839-845. [DOI] [PubMed] [Google Scholar]
- Brahn E and Trentham DE (1984a) Antigen-specific suppression of collagen arthritis by adoptive transfer of spleen cells. Clin Immunol Immunopathol 31 124-131. [DOI] [PubMed] [Google Scholar]
- Brahn E and Trentham DE (1984b) Effect of antithymocyte serum on collagen arthritis in rats: evidence that T cells are involved in its pathogenesis. Cell Immunol 86 421-428. [DOI] [PubMed] [Google Scholar]
- Campagnuolo G, Bolon B, and Feige U (2002) Kinetics of bone protection by recombinant osteoprotegerin therapy in Lewis rats with adjuvant arthritis. Arthritis Rheum 46 1926-1936. [DOI] [PubMed] [Google Scholar]
- Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438 932-936. [DOI] [PubMed] [Google Scholar]
- Caulfield JP, Hein A, Dynesius-Trentham R, and Trentham DE (1982) Morphologic demonstration of two stages in the development of type II collagen-induced arthritis. Lab Invest 46 321-343. [PubMed] [Google Scholar]
- de Bandt M, Grossin M, Weber AJ, Chopin M, Elbim C, Pla M, Gougerot-Pocidalo MA, and Gaudry M (2000) Suppression of arthritis and protection from bone destruction by treatment with TNP-470/AGM-1470 in a transgenic mouse model of rheumatoid arthritis. Arthritis Rheum 43 2056-2063. [DOI] [PubMed] [Google Scholar]
- Griffith EC, Su Z, Turk BE, Chen S, Chang YH, Wu Z, Biemann K, and Liu JO (1997) Methionine aminopeptidase (type 2) is the common target for angiogenesis inhibitors AGM-1470 and ovalicin. Chem Biol 4 461-471. [DOI] [PubMed] [Google Scholar]
- Hannig G, Bernier SG, Hoyt JG, Doyle B, Clark E, Karp RM, Lorusso J, and Westlin WF (2007) Suppression of inflammation and structural damage in experimental arthritis through molecular targeted therapy with PPI-2458. Arthritis Rheum 56 850-860. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Madden TL, Tran HT, Blumenschein GR Jr, Meyers CA, Seabrooke LF, Khuri FR, Puduvalli VK, Allgood V, Fritsche HA Jr, et al. (2002) Safety and pharmacokinetic effects of TNP-470, an angiogenesis inhibitor, combined with paclitaxel in patients with solid tumors: evidence for activity in non-small-cell lung cancer. J Clin Oncol 20 4440-4447. [DOI] [PubMed] [Google Scholar]
- Hisadome M, Fukuda T, Adachi K, and Komatsu H (2004) Combination benefit of a pyrimidylpiperazine derivative (Y-40138) and methotrexate in arthritic rats. Eur J Pharmacol 497 351-359. [DOI] [PubMed] [Google Scholar]
- Ingber D, Fujita T, Kishimoto S, Sudo K, Kanamaru T, Brem H, and Folkman J (1990) Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 348 555-557. [DOI] [PubMed] [Google Scholar]
- Kudelka AP, Levy T, Verschraegen CF, Edwards CL, Piamsomboon S, Termrungruanglert W, Freedman RS, Kaplan AL, Kieback DG, Meyers CA, et al. (1997) A phase I study of TNP-470 administered to patients with advanced squamous cell cancer of the cervix. Clin Cancer Res 3 1501-1505. [PubMed] [Google Scholar]
- Lainer DT and Brahn E (2005) New antiangiogenic strategies for the treatment of proliferative synovitis. Expert Opin Investig Drugs 14 1-17. [DOI] [PubMed] [Google Scholar]
- Lainer-Carr D and Brahn E (2007) Angiogenesis inhibition as a therapeutic approach for inflammatory synovitis. Nat Clin Pract Rheumatol 3 434-442. [DOI] [PubMed] [Google Scholar]
- Lazarus DD, Doyle EG, Bernier SG, Rogers AB, Labenski MT, Wakefield JD, Karp RM, Clark EJ, Lorusso J, Hoyt JG, et al. (2008) An inhibitor of methionine aminopeptidase type-2, PPI-2458, ameliorates the pathophysiological disease processes of rheumatoid arthritis. Inflamm Res 57 18-27. [DOI] [PubMed] [Google Scholar]
- Logothetis CJ, Wu KK, Finn LD, Daliani D, Figg W, Ghaddar H, and Gutterman JU (2001) Phase I trial of the angiogenesis inhibitor TNP-470 for progressive androgen-independent prostate cancer. Clin Cancer Res 7 1198-1203. [PubMed] [Google Scholar]
- Mankin HJ, Dorfman H, Lippiello L, and Zarins A (1971) Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am 53 523-537. [PubMed] [Google Scholar]
- Peacock DJ, Banquerigo ML, and Brahn E (1992) Angiogenesis inhibition suppresses collagen arthritis. J Exp Med 175 1135-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock DJ, Banquerigo ML, and Brahn E (1995) A novel angiogenesis inhibitor suppresses rat adjuvant arthritis. Cell Immunol 160 178-184. [DOI] [PubMed] [Google Scholar]
- Silva MA, Ishii-Iwamoto EL, Bracht A, Caparroz-Assef SM, Kimura E, Cuman RK, and Bersani-Amado CA (2005) Efficiency of combined methotrexate/chloroquine therapy in adjuvant-induced arthritis. Fundam Clin Pharmacol 19 479-489. [DOI] [PubMed] [Google Scholar]
- Sin N, Meng L, Wang MQ, Wen JJ, Bornmann WG, and Crews CM (1997) The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc Natl Acad Sci U S A 94 6099-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL (2000) Bone resorption by osteoclasts. Science 289 1504-1508. [DOI] [PubMed] [Google Scholar]
- Trentham DE, Townes AS, and Kang AH (1977) Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med 146 857-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DA (1999) Angiogenesis and arthritis. Rheumatology (Oxford) 38 103-112. [DOI] [PubMed] [Google Scholar]
- Wu Y, Liu J, Feng X, Yang P, Xu X, Hsu HC, and Mountz JD (2005) Synovial fibroblasts promote osteoclast formation by RANKL in a novel model of spontaneous erosive arthritis. Arthritis Rheum 52 3257-3268. [DOI] [PubMed] [Google Scholar]
- Yanase T, Tamura M, Fujita K, Kodama S, and Tanaka K (1993) Inhibitory effect of angiogenesis inhibitor TNP-470 on tumor growth and metastasis of human cell lines in vitro and in vivo. Cancer Res 53 2566-2570. [PubMed] [Google Scholar]
- Yeh JR, Ju R, Brdlik CM, Zhang W, Zhang Y, Matyskiela ME, Shotwell JD, and Crews CM (2006) Targeted gene disruption of methionine aminopeptidase 2 results in an embryonic gastrulation defect and endothelial cell growth arrest. Proc Natl Acad Sci U S A 103 10379-10384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yeh JR, Mara A, Ju R, Hines JF, Cirone P, Griesbach HL, Schneider I, Slusarski DC, Holley SA, et al. (2006) A chemical and genetic approach to the mode of action of fumagillin. Chem Biol 13 1001-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]