Abstract
Several microRNAs (miRNAs) have recently been described as crucial regulators of epithelial-to-mesenchymal transition (EMT) and metastasis. By comparing the expression profiles of miRNAs, we found upregulation of miR-29a in mesenchymal, metastatic RasXT cells relative to epithelial EpRas cells. Overexpression of miR-29a suppressed the expression of tristetraprolin (TTP), a protein involved in the degradation of messenger RNAs with AU-rich 3′-untranslated regions, and led to EMT and metastasis in cooperation with oncogenic Ras signalling. We also observed enhanced miR-29a and reduced TTP levels in breast cancer patient samples, indicating relevance for human disease. Previously, miR-29 family members were shown to have tumour-suppressive effects in haematopoietic, cholangiocytic and lung tumours. Therefore, miRNAs can act as either oncogenes or tumour suppressors, depending on the context.
Keywords: breast cancer, metastasis, miR-29a, miRNA, tristetraprolin
Introduction
Cancer is the second most frequent cause of death in the Western world, and tumours of epithelial origin—carcinomas—constitute more than 80% of all cases. Mortality is caused mainly by metastatic spread to secondary sites concomitant with organ failure. A characteristic of metastasizing cells is their transition from an epithelial state to a mesenchymal phenotype, a process known as epithelial-to-mesenchymal transition (EMT; Grunert et al, 2003; Yang & Weinberg, 2008). EMT, which is characterized by downregulation of the epithelial gatekeeper protein E-cadherin and upregulation of vimentin, can be used to model metastasis in vitro. A suitable system to identify microRNAs (miRNAs) that are regulated during EMT and/or metastasis is the well-characterized EpH4/EpRas/RasXT cell culture system (Grunert et al, 2003). Parental EpH4 cells are non-tumorigenic murine mammary epithelial cells and show typical physiological responses to relevant growth factors and cytokines. EpRas cells, which are derived from EpH4 cells, are still epithelial but tumorigenic because of constitutive overexpression of oncogenic H-Ras-V12. EpRas cells can undergo EMT and metastasize in response to transforming growth factor-β (TGF-β). The resulting cells, called RasXT, stably maintain their mesenchymal phenotype through autocrine TGF-β loops (Grunert et al, 2003). Genome-wide messenger RNA (mRNA) profiling of these cells showed regulation of a plethora of known genes involved in tumour progression and identified new genes relevant for EMT and metastasis in human patients (Jechlinger et al, 2003; Waerner et al, 2006).
MicroRNAs have been identified as important developmental regulators and their mis-expression was shown to contribute to tumorigenesis (Calin & Croce, 2006). Recently, several studies have implicated dysregulated expression of miRNAs in the metastatic progression of carcinomas (Ma et al, 2007; Gregory et al, 2008; Huang et al, 2008). Although the metastasis-relevant miRNAs identified so far are not sufficient to transform non-tumorigenic cells alone, but rather exert their effects in cooperation with other oncogenes or tumour suppressors, they hold great therapeutic promise (Calin & Croce, 2006; Ma et al, 2007).
In this study, we used the EpH4/EpRas/RasXT cell system and identified miR-29a as one of the first miRNAs with the capacity to both interfere with and promote tumour progression, depending on the oncogenic context. Previously, reduction and/or loss of miR-29 family members and upregulation of their oncogenic targets, Tcl1, Mcl1 and DNMT3, have been implicated in chronic lymphocytic leukaemia, cholangio-carcinoma and lung cancer (Pekarsky et al, 2006; Fabbri et al, 2007; Mott et al, 2007).
Our findings show tumour- and metastasis-promoting roles for miR-29a and tristetraprolin (TTP) in breast carcinomas, suggesting a context-dependent pattern for miRNAs in tumorigenicity.
Results And Discussion
Identification of microRNAs regulated during EMT
To identify miRNAs that are up- or downregulated during EMT and metastasis, we performed an miRNA array profiling (Kauppinen et al, 2006) of epithelial EpRas and mesenchymal, metastatic RasXT cells. The miRNA array showed approximately ten miRNA families with level changes >twofold. In agreement with results from others, the strongest downregulation was observed for miR-200 family members, and we found upregulation of miR-10b (Ma et al, 2007; Gregory et al, 2008; data not shown). The most upregulated miRNA in RasXT cells was miR-29a; levels were analysed by northern blot analysis and quantitative PCR (qPCR; Fig 1A; see also supplementary Fig S1a online). In addition, miR-29a levels have been shown to be also enhanced in two mesenchymal, highly invasive breast cancer cell lines (MDA-MB-231 and MDA-MB-451) of the NCI-60 panel of cancer cell lines, but not in the epithelial ones (MCF7 and T47D; Park et al, 2008).
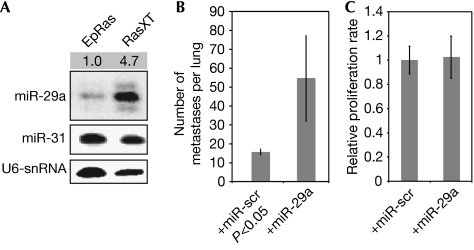
Figure 1.
The microRNA miR-29a is upregulated in mesenchymal cells and promotes epithelial-to-mesenchymal transition and metastasis. (A) Northern blot analysis using total RNA extracted from confluent epithelial EpRas or mesenchymal RasXT cells was performed to monitor the expression levels of miR-29a. miR-31 and U6-snRNA were used as controls. The numbers indicate normalization by densitometry. (B) EpRas cells were transfected with miR-29a followed by injection into the tail vein of nude mice. Mice were killed after 14 days and lung metastases were counted. (C) EpRas cells were transfected with miR-29a, and after 48 h proliferation was measured. miR, microRNA; miR-scr, microRNA-scrambled; snRNA, small nuclear RNA.
miR-29a promotes metastasis and suppresses TTP
We therefore investigated whether miR-29a was able to promote metastatic growth. For this purpose, we overexpressed miR-29a in EpRas, injected these cells into the tail veins of nude mice and monitored lung colonization after 14 days. As shown in Fig 1B, enhanced miR-29a levels in murine mammary EpRas cells led to an increase in the number of metastatic foci in the lung. The proliferation rate of EpRas cells did not increase on overexpression of miR-29a (Fig 1C). Conversely, functional inhibition of miR-29a (supplementary Fig S1b online) did not reduce the number or size of metastatic foci, possibly because of the upregulation of oncogenic targets (data not shown and supplementary Fig S1c online).
To identify targets of miR-29a, we compared a list of genes downregulated from EpRas to RasXT cells (Jechlinger et al, 2003) with miRNA target predictions obtained through the algorithm TargetScan (www.targetscan.org; Lewis et al, 2005). TTP was found to be strongly downregulated from EpRas to RasXT cells (Jechlinger et al, 2003), a finding that we confirmed by qPCR (Fig 2A). RasXT cells also showed lower amounts of TTP protein than epithelial EpRas cells (Fig 2B).
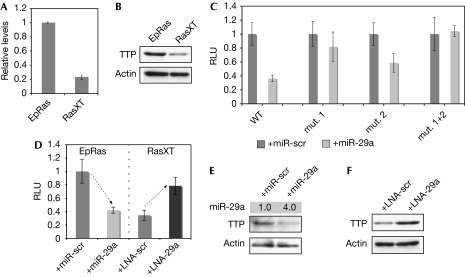
Figure 2.
The microRNA miR-29a downregulates tristetraprolin. (A) Total RNA was extracted from EpRas and RasXT cells. The levels of TTP as normalized to GAPDH were determined by qPCR. (B) Western blot analysis using whole-cell extracts of EpRas and RasXT cells was performed to monitor the expression of TTP protein. (C) NIH3T3 cells were transfected with miR-scr or miR-29a. Constructs containing the luciferase ORF coupled to the WT and mutated (mut.) versions of the TTP-3′-UTR were used to assess the regulatory impact of miR-29a on TTP. Relative light units (RLUs) are shown. (D) EpRas cells were transfected with miR-scr or miR-29a, and RasXT cells were transfected with LNA-scr or LNA complementary to miR-29a (LNA-29a). Constructs containing the luciferase ORF coupled to the TTP-3′-UTR were used to assess the regulatory impact of endogenous miR-29a on TTP. RLUs are shown. (E) Western blot analysis using whole-cell extracts of EpRas cells and EpRas cells overexpressing miR-29a was performed to monitor the expression of TTP. (F) Western blot analysis using whole-cell extracts of RasXT cells and RasXT cells transfected with LNA-29a was performed to monitor the expression of TTP. LNA, locked nucleic acid; miR-scr, microRNA-scrambled; ORF, open reading frame; qPCR, quantitative PCR; TTP, tristetraprolin; UTR, untranslated region; WT, wild type.
TTP is predicted to be a target of miR-29a as it contains two regions complementary to its seed region in the 3′-untranslated region (3′-UTR; supplementary Fig S2 online). To validate this, we performed a reporter gene assay in NIH3T3 cells by using the 3′-UTR of TTP fused to the luciferase open reading frame (supplementary Fig S2 online). Indeed, co-transfection of miR-29a led to a strong reduction of the luciferase signal (Fig 2C, WT). Mutations at the first (Fig 2C, mut. 1) or the second (Fig 2C, mut. 2) putative miR-29a-binding site in the 3′-UTR of TTP reduced this inhibition, and combining both mutations (Fig 2C, mut. 1+2) abolished the effect. Thus, miR-29a regulates the expression of TTP through binding to two regions in the 3′-UTR complementary to the seed region of miR-29a.
To test whether miR-29a regulates TTP in the EpRas/RasXT cell system, we transfected the TTP-3′-UTR–luciferase construct into EpRas and RasXT cells. Co-transfection of miR-29a into EpRas cells strongly reduced the luciferase signal (Fig 2D, left side), similar to that in RasXT, which has higher levels of endogenous miR-29a (Fig 1A) and therefore lower expression of basal luciferase, as shown in a reporter gene assay. Notably, inhibition of endogenous miR-29a in RasXT cells by complementary locked nucleic acid (LNA) rescued the luciferase expression (Fig 2D, right side). This effect also applied to the endogenous TTP protein, as overexpression of miR-29a reduced TTP protein levels in EpRas cells (Fig 2E), and complementary LNA restored TTP protein levels in RasXT cells (Fig 2F). In conclusion, these results show that miR-29a negatively regulates TTP by binding to two regions in the TTP-3′-UTR, and that the endogenous miR-29a levels are crucial to control TTP during EMT from EpRas to RasXT cells.
miR-29a promotes disruption of epithelial polarity
To investigate the effect of miR-29a on cell polarity and EMT, we generated cell lines stably overexpressing miR-29a. Although EpH4 cells appeared to be unaffected (data not shown), in EpRas cells we observed disruption of epithelial polarity, depending on the levels of overexpression of miR-29a (Fig 3A). Expression of TTP, as well as of the survival protein Mcl1, a known miR-29a target (Mott et al, 2007), negatively correlated with miR-29a levels in EpRas cells (Fig 3A). In addition, E-cadherin and vimentin levels also correlated with the degree of upregulation of miR-29a and downregulation of TTP (Fig 3A). Thus, enhanced miR-29a levels influenced the expression of TTP and epithelial polarity in EpRas cells. We verified whether the short interfering RNA (siRNA) mediated knockdown of TTP could also induce EMT in cooperation with Ras. As shown in Fig 3B, strongly reduced levels of TTP correlated with loss of E-cadherin expression and strong upregulation of vimentin. Corresponding protein levels of selected clones were detected by Western blot analysis (Fig 3C). To rule out off-target effects of siRNAs directed against TTP, we reverted EMT by expressing a variant of TTP (vTTP), which was resistant to degradation by siRNA (Fig 3C). The loss of epithelial polarization on overexpression of miR-29a or knockdown of TTP was also obvious in cells grown on porous supports, and was reverted on expression of vTTP (Fig 3D).
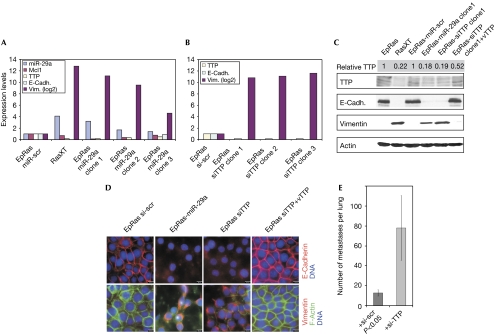
Figure 3.
Downregulation of tristetraprolin promotes epithelial-to-mesenchymal transition and metastasis. (A) The levels of miR-29a, TTP, Mcl1, E-cadherin and vimentin were determined by qPCR in EpRas cells stably overexpressing scrambled miRNA or miR-29a (three independent clones are shown), and levels in RasXT cells are shown as a control. (B) Levels of TTP, E-cadherin and vimentin were determined by qPCR in EpRas cells stably overexpressing scrambled siRNA or siTTP (three independent clones are shown). (C) Western blot analysis was performed to monitor the expression of E-cadherin and vimentin using whole-cell extracts of the indicated cell types. vTTP is a TTP variant resistant to siRNA-mediated degradation. (D) EpRas cells stably expressing an shRNA harbouring either miR-scr, miR-29a, siTTP or siTTP+vTTP were cultured on porous supports. Immunofluorescence stainings for E-cadherin, vimentin and F-actin are shown. (E) EpRas cells were transiently transfected with si-scr or siTTP followed by injection into the tail vein of nude mice. Mice were killed after 14 days and lung metastases were counted. qPCR, quantitative PCR; scr, scrambled; shRNA, short hairpin RNA; si, short interfering; TTP, tristetraprolin.
Knockdown of tristetraprolin can promote metastasis
To determine whether downregulation of TTP in EpRas cells in addition to the promotion of EMT could also stimulate metastasis in vivo, we performed tail vein injections and monitored the number of metastatic colonies in the lung. Indeed, after knocking down TTP, the number of metastases in the lung increased strongly (Fig 3E). Tumour-suppressing effects of TTP might be exerted by controlling the degradation of many different AU-rich elements (ARE)-containing targets known to promote tumorigenesis, including tumour necrosis factor-α (TNF-α), cyclooxygenase 2 (Cox2), interleukin (IL)-3, IL-8, vascular endothelial growth factor, c-Myc and cyclin D1 (Taylor et al, 1996; Shim & Karin, 2002; Stoecklin et al, 2003). It has been shown previously that forced expression of TTP interfered with Ras-driven and IL-3-dependent tumour progression in a mast cell tumour model (Stoecklin et al, 2003).
High miR-29a and low TTP in human breast cancer
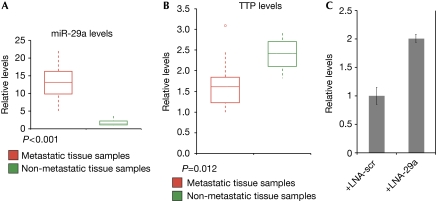
Considering the marked phenotypes observed above and the prognostic or therapeutic potential of miR-29a and TTP, we questioned whether these factors might be dysregulated in human breast cancer. We measured the levels of miR-29a in a population of randomly selected breast cancer specimens, including 20 patients with invasive tumours (ductal carcinomas) and 4 control samples of benign, non-invasive hyperplasias (for details, see supplementary Table 1 online). Strikingly, we confirmed that the levels of miR-29a were also greatly enhanced in patients with invasive breast carcinomas by using a padlock rolling circle amplification assay (Fig 4A), in contrast to miR-31 (supplementary Fig S3a online). In addition, TTP was significantly downregulated in the set of invasive breast cancers (Fig 4B), although miR-29a is clearly not the sole factor controlling TTP levels. The p38–MK2 pathway, TNF-α, protein phosphatase 2A (PP2A), epidermal growth factor receptor (EGFR) signalling, 14-3-3 proteins and miR-16 also influence TTP expression, stability and function (Jing et al, 2005; Brook et al, 2006; Hitti et al, 2006; Amit et al, 2007; Sun et al, 2007). This might explain why the correlation between miR-29a levels and TTP in a complex setting such as cancer is only slightly negative (supplementary Fig S3b online). Nevertheless, in the metastatic human breast cancer cell line MDA-MB-468, we were able to show the regulatory input of endogenous miR-29a by functional inhibition (Fig 4C).
Figure 4.
The microRNA miR-29a is overexpressed and tristetraprolin is downregulated in metastatic human breast cancers. (A) Total RNA was extracted from patient samples with invasive breast carcinomas (red) or non-invasive hyperplasias (green). Levels of miR-29a were determined in a padlock assay followed by detection with a 32P-labelled probe. Results are shown in a box–whisker plot. (B) The level of TTP mRNA was determined by qPCR, and expression of GAPDH was used as normalization control. (C) RT–qPCR of total RNA extracts from MDA-MB-468 cells transfected with LNA-scr or LNA-29a for 72 h was performed to monitor the expression of TTP. LNA, locked nucleic acid; mRNA, messenger RNA; RT–qPCR, reverse transcription–quantitative PCR; scr, scrambled; TTP, tristetraprolin.
TTP is known to contribute to cytokine homeostasis, including the degradation of TNF-α mRNA (Taylor et al, 1996), and is crucial for the reduction of inflammation. As TTP controls the degradation of a plethora of ARE-containing target mRNAs (Shim & Karin, 2002), including its own (Brooks et al, 2004), the downregulation of TTP could contribute to the genetic complexity of breast cancers. As EMT and carcinogenesis have many commonalities with inflammation (Coussens & Werb, 2002), the role of TTP in carcinoma progression and metastasis requires closer investigation.
The microRNA miR-29a in tumorigenesis
Our findings, together with those of others (Pekarsky et al, 2006; Fabbri et al, 2007; Mott et al, 2007) show that miR-29a can control opposing cellular functions. A possible explanation for this phenomenon might rely on the large number and diverse nature of miR-29a target genes—the algorithm Targetscan predicted 682 conserved targets for the miR-29 family. In general, the net effect of changes in the level of an miRNA will be the sum of all impacts on its targets in a cell-type-specific manner. In addition, owing to the high number of mRNA targets, overexpression and functional inhibition of a miRNA might have diverse effects. Similar observations were made for other miRNAs, including the miR-17–92 cluster (Calin & Croce, 2006). Thus, anticancer therapies administered by application of miRNA inhibitors might only be possible for a limited number of miRNAs.
Methods
Isolation of RNA. Isolation of RNA from cultured cells or human samples was performed as described previously (Obernosterer et al, 2006).
Cell culture. The culture conditions for EpH4, EpRas and RasXT cells have been described previously (Janda et al, 2002). EpRas cells overexpressing miR-29a or short hairpin RNA against TTP in a pMSCV-puro vector were generated by retroviral gene transfer as described previously (Janda et al, 2002). Clones were isolated from retrovirally infected and drug-selected cell populations by single-cell sorting into a 96-well dish.
Short interfering RNA. To knock down TTP, the sequences 5′-AAAAGGAACAAGAGGACAGGG-3′ or 5′-AUUGAAGAUGGGGAGACGCCU-3′ were used either as canonical 2-nucleotide (nt) 3′-overhang duplexes with their respective complementary sequences or cloned into pShag and shuttled into pMSCV through the Invitrogen Gateway system.
Site-directed mutagenesis. The QuickChange Mutagenesis Kit from Stratagene (www.stratagene.com) was used. The respective primers were 5′-TTTCCCTGGGTCTGGGCTGGGGC-3′ and 5′-CCAGCCCAGACCCAGGGAAATGG-3′ for the first putative miR-29a seed-binding site (TGGTGCT) starting at nt 143, and 5′-GAATCCTGGGTCTCAAATTTCCC-3′ and 5′-AAATTTGAGACCCAGGATTCTCAG-3′ for the second putative miR-29a seed-binding site starting at nt 498 of the TTP-3′-UTR. Mutations of the binding site of siTTP were carried out with 5′-GCCCTTCCCTATCATCATGCTCCTTTTCGCC-3′ and 5′-GCGAAAAGGAGCATGATGATAGGGAAGGGCC-3′ for siTTP 5′-AAAAGGAACAAGAGGACAGGG-3′.
MicroRNA array profiling. Total RNA of EpRas and RasXT cells was profiled by Exiqon (www.exiqon.com) using miRCURY LNA arrays (Kauppinen et al, 2006).
Northern blotting. Northern blots were performed as described before (Obernosterer et al, 2006). The sequences of the probes were 5′-TA*ACC*GAT*TTC*AGA*TGG*TGC*TA-3′ (miR-29a), 5′-CA*GCT*ATG*CCA*GCA*TCT*TGC*CT-3′ (miR-31) and 5′-GCAGGGGCCATGCTAATCTTCTCTGTATCG-3′ (U6-snRNA). LNA nucleotides are indicated by ‘*N'.
Quantitative PCR. qPCR for mRNA was carried out by using the 2 × SYBR Green Master mix from Applied Biosystems (www.appliedbiosystems.com). qPCR primers for GAPDH, TTP, Mcl1, E-cadherin and vimentin were from Qiagen (www.qiagen.com). qPCR for miRNAs was carried out by using the probes for miR-29a, the small, nucleolar RNA snoRNA-135 (normalization for murine samples), U18-snRNA (normalization for human samples), the TaqMan miRNA Reverse Transcription Kit and the 2 × TaqMan Gene Expression Kit from Applied Biosystems.
Padlock assay. Padlock assays based on rolling circle amplification were performed as described by others (Jonstrup et al, 2006) using 100 ng of human patient samples and 500 ng of cellular extracts. Padlock probes were 5′-AGATGGTGCTATTTATTTCCTCAATGCTGCTGCTGTACTACTAGTGATTTACTTGGATGTCTGTAACCGATTTC-3′ (miR-29a); 5′-GCATCTTGCCTTTTATTTCCTCAATGCTGCTGCTGTACTACTAGTGATTTACTTGGATGTCTGAGCTATGCCA-3′ (miR-31) and 5′-ATCACTACTGTTTATTTCCTCAATGCTGCTGCTGTACTACTAGTGATTTACTTGGATGTCTGGTGGAATTTC-3′ (U18-snRNA).
Western blotting and immunofluorescence stainings. Western blots and immunofluorescence stainings were carried out as described before (Janda et al, 2002). The antibodies used were rb-α-TTP (sc-14030; SCBT (www.scbt.com)), rb-α-actin (A2066; Sigma (www.sigmaaldrich.com)), ms-α-E-cadherin (610182; BD Biosciences (www.bdbiosciences.com)) and ms-α-vimentin (V5255; Sigma). Phalloidin (A12379) was from Invitrogen (www.invitrogen.com).
Reporter gene assays. In total, 40,000 NIH3T3 or EpRas cells were seeded per well of a 24-well plate. Here, 200 ng of pGL3 vector containing the indicated 3′-UTR coupled to firefly luciferase plus 100 nM miR-29a or scrambled siRNA was transfected per well and 20 ng Renilla luciferase was used for normalization.
Proliferation assays. EpRas cells were transfected with miR-29a and 2,000 cells were seeded in each well of a 96-well-plate. After 48 h, proliferation was measured by the incorporation of [H3+]thymidine.
Patient samples. Human patient samples were obtained from the Biobank of the Department of Pathology, Medical University Graz, Austria. All tissues were snap-frozen in methyl butane pre-cooled with liquid nitrogen within 20 min of the operation. For details, see supplementary Table 1 online.
Induction of tumours and metastases in mice. To test the impact of overexpression of miR-29a, inhibition of miR-29a or knockdown of TTP, eight athymic nude mice (MF1nu/nu; 8–10 weeks old) were used for each condition. miR-29a or siTTP (100 nM; as duplexes) was transfected into EpRas cells using Dharmafect 1. For inhibition of miR-29a, 200 nM complementary DNA/LNA (5′-TA*ACC*GAT*TTC*AGA*TGG*TGC*TA-3′) was transfected into RasXT cells. In total, 150,000 cells suspended in 200 μl PBS were injected into the tail vein. Mice were killed after 14 days. Lungs were fixed in 4% paraformaldehyde in PBS and 70% ethanol, and embedded in paraffin. Sections were sliced at 8 μm steps and stained with haematoxylin and eosin according to standard protocols, and metastases were counted.
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
Supplementary Information
Acknowledgments
We are indebted to Exiqon for miRNA profiling. We thank Andrew Pospisilik and Pavel Kovarik for comments on the paper, and Gregor Obernosterer, Philipp Leuschner, Andrea Salvá Giró, Sabine Maschler and Daniela Pabst for help with experiments. This study was financially supported by GEN-AU (Genomic Research in Austria) and FWF (Fonds zur Förderung der wissenschaftlichen Forschung) grant P20115-B11.
Footnotes
The authors declare that they have no conflict of interest.
References
- Amit I et al. (2007) A module of negative feedback regulators defines growth factor signaling. Nat Genet 39: 503–512 [DOI] [PubMed] [Google Scholar]
- Brook M, Tchen CR, Santalucia T, McIlrath J, Arthur JS, Saklatvala J, Clark AR (2006) Posttranslational regulation of tristetraprolin subcellular localization and protein stability by p38 mitogen-activated protein kinase and extracellular signal-regulated kinase pathways. Mol Cell Biol 26: 2408–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SA, Connolly JE, Rigby WF (2004) The role of mRNA turnover in the regulation of tristetraprolin expression: evidence for an extracellular signal-regulated kinase-specific, AU-rich element-dependent, autoregulatory pathway. J Immunol 172: 7263–7271 [DOI] [PubMed] [Google Scholar]
- Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6: 857–866 [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420: 860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M et al. (2007) MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA 104: 15805–15810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ (2008) The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10: 593–601 [DOI] [PubMed] [Google Scholar]
- Grunert S, Jechlinger M, Beug H (2003) Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol 4: 657–665 [DOI] [PubMed] [Google Scholar]
- Hitti E, Iakovleva T, Brook M, Deppenmeier S, Gruber AD, Radzioch D, Clark AR, Blackshear PJ, Kotlyarov A, Gaestel M (2006) Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol Cell Biol 26: 2399–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q et al. (2008) The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol 10: 202–210 [DOI] [PubMed] [Google Scholar]
- Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, Beug H, Grunert S (2002) Ras and TGF[β] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol 156: 299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jechlinger M, Grunert S, Tamir IH, Janda E, Ludemann S, Waerner T, Seither P, Weith A, Beug H, Kraut N (2003) Expression profiling of epithelial plasticity in tumor progression. Oncogene 22: 7155–7169 [DOI] [PubMed] [Google Scholar]
- Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, Di Padova F, Lin SC, Gram H, Han J (2005) Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell 120: 623–634 [DOI] [PubMed] [Google Scholar]
- Jonstrup SP, Koch J, Kjems J (2006) A microRNA detection system based on padlock probes and rolling circle amplification. RNA 12: 1747–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppinen S, Vester B, Wengel J (2006) Locked nucleic acid: high-affinity targeting of complementary RNA for RNomics. Handb Exp Pharmacol 173: 405–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20 [DOI] [PubMed] [Google Scholar]
- Ma L, Teruya-Feldstein J, Weinberg RA (2007) Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 449: 682–688 [DOI] [PubMed] [Google Scholar]
- Mott JL, Kobayashi S, Bronk SF, Gores GJ (2007) mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene 26: 6133–6140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernosterer G, Leuschner PJ, Alenius M, Martinez J (2006) Post-transcriptional regulation of microRNA expression. RNA 12: 1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Gaur AB, Lengyel E, Peter ME (2008) The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 22: 894–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarsky Y et al. (2006) Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res 66: 11590–11593 [DOI] [PubMed] [Google Scholar]
- Shim J, Karin M (2002) The control of mRNA stability in response to extracellular stimuli. Mol Cell 14: 323–331 [PubMed] [Google Scholar]
- Stoecklin G, Gross B, Ming XF, Moroni C (2003) A novel mechanism of tumor suppression by destabilizing AU-rich growth factor mRNA. Oncogene 22: 3554–3561 [DOI] [PubMed] [Google Scholar]
- Sun L, Stoecklin G, Van Way S, Hinkovska-Galcheva V, Guo RF, Anderson P, Shanley TP (2007) Tristetraprolin (TTP)–14-3-3 complex formation protects TTP from dephosphorylation by protein phosphatase 2a and stabilizes tumor necrosis factor-α mRNA. J Biol Chem 282: 3766–3777 [DOI] [PubMed] [Google Scholar]
- Taylor GA et al. (1996) A pathogenetic role for TNF α in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity 4: 445–454 [DOI] [PubMed] [Google Scholar]
- Waerner T, Alacakaptan M, Tamir I, Oberauer R, Gal A, Brabletz T, Schreiber M, Jechlinger M, Beug H (2006) ILEI: a cytokine essential for EMT, tumor formation, and late events in metastasis in epithelial cells. Cancer Cell 10: 227–239 [DOI] [PubMed] [Google Scholar]
- Yang J, Weinberg RA (2008) Epithelial–mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 14: 818–829 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information