Abstract
The nuclear receptor retinoid X receptor-α (RXR-α)–peroxisome proliferator-activated receptor-γ (PPAR-γ) heterodimer was recently reported to have a crucial function in mediating the deleterious effects of organotin compounds, which are ubiquitous environmental contaminants. However, because organotins are unrelated to known RXR-α and PPAR-γ ligands, the mechanism by which these compounds bind to and activate the RXR-α–PPAR-γ heterodimer at nanomolar concentrations has remained elusive. Here, we show that tributyltin (TBT) activates all three RXR–PPAR-α, -γ, -δ heterodimers, primarily through its interaction with RXR. In addition, the 1.9 Å resolution structure of the RXR-α ligand-binding domain in complex with TBT shows a covalent bond between the tin atom and residue Cys 432 of helix H11. This interaction largely accounts for the high binding affinity of TBT, which only partly occupies the RXR-α ligand-binding pocket. Our data allow an understanding of the binding and activation properties of the various organotins and suggest a mechanism by which these tin compounds could affect other nuclear receptor signalling pathways.
Keywords: nuclear receptor, 3D structure, organotins, environment
Introduction
Organotin compounds are ubiquitously present throughout the environment owing to their widespread use since the 1960s in many industrial and agricultural processes (Fent, 1996; Appel, 2004). In the 1980s, these compounds were found to be responsible for a wide variety of deleterious effects in the marine ecosystem, and tributyltin (TBT) has been designated as ‘the most toxic chemical ever released into the seas' by the World Wildlife Fund. Despite some restrictions on their use, organotins persist in the environment and are absorbed by higher organisms, in which they accumulate (Antizar-Ladislao, 2008). TBT and its derivatives are so-called endocrine disruptors, inducing deregulation of vertebrate and invertebrate endocrine systems (Golub & Doherty, 2004). In many marine species, exposure to TBT results in the abnormal induction of male sexual characteristics in females as a consequence of decreased aromatase—the enzyme that converts androgens to estrogens—activity (Matthiessen & Gibbs, 1998; McAllister & Kime, 2003). In mammals, exposure to organotins induces immunosuppressive, metabolic, reproductive or developmental effects (Boyer, 1989; Ogata et al, 2001; Nakanishi, 2008). Interestingly, recent studies showed that members of the nuclear receptor family are crucial targets of organotins (Kanayama et al, 2005; Nakanishi et al, 2005; Gumy et al, 2008). In particular, it was shown that nanomolar concentrations of TBT can activate the retinoid X receptor-α (RXR-α)–peroxisome proliferator-activated receptor-γ (PPAR-γ) heterodimer to promote adipocyte differentiation (Kanayama et al, 2005; Grun & Blumberg, 2006; Grun et al, 2006) and deregulation of the aromatase gene (Nakanishi et al, 2005). Part of the endocrine disruptive action of organotins could therefore be mediated through the RXR-α–PPAR-γ signalling pathway. Intriguingly, organotins (Fig 1) neither structurally nor chemically resemble known RXR (rexinoids) or PPARγ (thiazolidinediones) ligands (Michalik et al, 2006; de Lera et al, 2007), and the mechanism of activation of RXR-α–PPAR-γ by organotins has remained enigmatic. In the following, we report on a study in which we combined functional and biophysical approaches to provide insights as to how TBT activates RXR–PPAR heterodimers.
Figure 1.
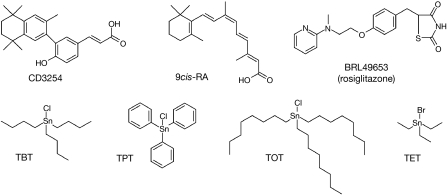
Chemical structures of some ligands used in this study. CD3254 and 9cis-RA are RXR-α agonists, whereas BRL49653 is a PPAR-γ agonist. Tributyltin (TBT), triphenyltin (TPT), trioctyltin (TOT) and triethyltin (TET). PPAR-γ, peroxisome proliferator-activated receptor-γ; RA, retinoic acid; RXR-α, retinoid X receptor-α.
Results And Discussion
Tributyltin activates RXR-α–PPAR-γ through RXR-α
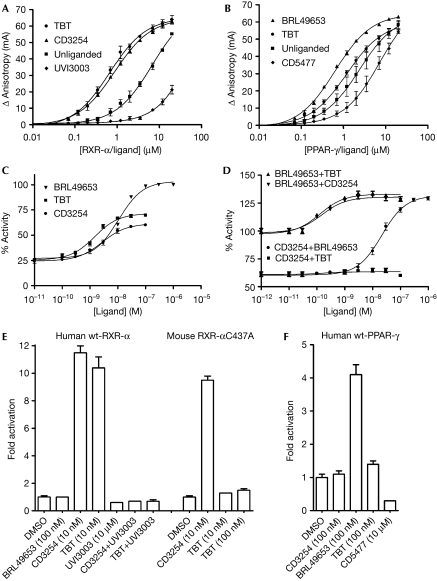
To unravel the mechanism of activation of RXR-α–PPAR-γ by organotins, we compared the ability of reference ligands with TBT to promote the recruitment of a PPAR-γ coactivator 1α (PGC-1α) LxxLL motif by RXR-α and PPAR-γ using fluorescence anisotropy. As expected, the synthetic agonists, CD3254 and BRL49653, strongly enhance the recruitment of the fluorescein-labelled PGC-1α peptide by RXR-α and PPAR-γ, respectively (Fig 2A,B). By contrast, binding of the antagonists UVI3003 and CD5477 impairs the recruitment of the LxxLL motif by their specific receptors RXR-α and PPAR-γ. Interestingly, RXR-α liganded with TBT recruits the PGC-1α peptide as efficiently as with CD3254, suggesting that both ligands are equally strong agonists. By contrast, TBT poorly increases the basal interaction of PPAR-γ with the LxxLL motif and seems as a weak PPAR-γ agonist as compared with BRL49653.
Figure 2.
The effect of tributyltin on coactivator recruitment and activation of RXR-α and PPAR-γ. Titration of fluorescein-labelled PGC-1α peptide by (A) RXR-α and (B) PPAR-γ, in the absence of ligand or in the presence of CD3254 (RXR agonist), UVI3003 (RXR antagonist), BRL49653 (PPAR-γ agonist), CD5477 (PPAR-γ antagonist) or TBT. (C) Stably transfected HGELN PPAR-γ cells were incubated with BRL49653, CD3254 or TBT to measure their effect on the transcriptional activity of the heterodimer formed by Gal4-PPAR-γ and endogenous RXR. (D) To measure the specific effect of the organotin on PPAR-γ and RXR, BRL49653, CD3254 or TBT were also tested in the presence of saturating concentrations of RXR (CD3254)- or PPAR-γ (BRL49653)-specific ligands. Here, 100% activity corresponds to the activity obtained with 1 μM BRL49653. (E) HeLa cells transiently transfected with the reporter recombinant (RXRE)6-TK-Luc and pSG5-RXR-α (human wild-type or mouse RXR-αC437A) were incubated with BRL49653, CD3254 or TBT to assess their agonist potential on an RXR-α homodimer. The antagonist UVI3003 (10 μM) was also used to check the reversibility of TBT (10 nM) binding. (F) The same experiment as in (E) but using (PPRE)3-TK-Luc and pSG5-hPPAR-γ. The antagonist CD5477 was used as a control. HGELN, HeLa ERE (estrogen responsive element) luciferase neomycin; PGC-1α, PPAR-γ coactivator 1α; PPAR-γ, peroxisome proliferator-activated receptor-γ; RXR-α, retinoid X receptor-α; TBT, tributyltin.
The differential involvement of the two receptors in TBT-induced RXR–PPAR-γ signalling was validated by several cell-based assays. Initial transactivation experiments using the stably transfected HeLa ERE (estrogen responsive element) luciferase neomycin (HGELN) Gal4-PPAR-γ cell line were carried out in the presence of CD3254, BRL49653 or TBT and confirmed the ability of the organotin to activate the RXR–PPAR-γ heterodimer at nanomolar concentrations (Fig 2C). Interestingly, the activation curves obtained with TBT or CD3254 show similar profiles with a maximum activity corresponding to roughly 60–70% of that induced by 1 μM BRL49653. To assess the specific effect of TBT on RXR and PPAR-γ, cells were co-incubated with saturating concentrations of the agonists CD3254 or BRL49653 and increasing concentrations of BRL49653, CD3254 or TBT (Fig 2D). Similar to CD3254, TBT is able to further activate the BRL49653-saturated heterodimer. However, in contrast with BRL49653, TBT seems unable to act in conjunction with CD3254 to enhance the activity of RXR–PPAR-γ (Fig 2D). Similarly, transient transactivation experiments showed that TBT activates RXR-α as efficiently as CD3254 (Fig 2E), whereas it behaves as a very weak PPAR-γ agonist (Fig 2F).
Tributyltin binds covalently to RXR-α Cys 432
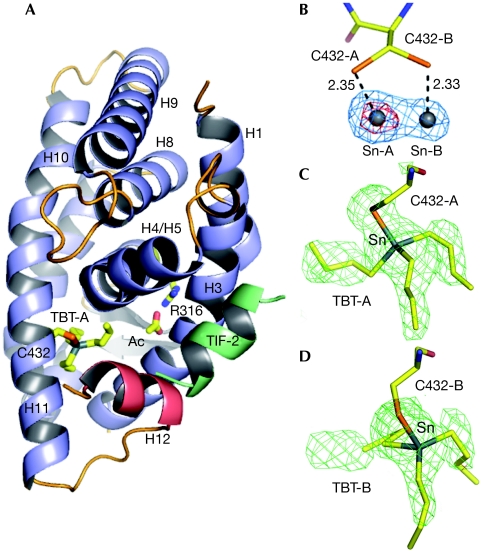
To gain structure-based insight into the binding mode of organotins to RXR, we solved the crystal structure (Protein Data Bank accession code 3E94) of the RXR-α ligand-binding domain (LBD) that is bound to TBT and a transcriptional intermediary factor 2 (TIF2) coactivator fragment (LxxLL motif) at 1.9 Å resolution (supplementary Table S1 online). The RXR-α LBD adopts a canonical agonist-bound conformation (Fig 3A). The TBT tin anomalous difference map allowed the unambiguous identification of two positions of the metal in close proximity to two alternative conformations of residue Cys432 in helix H11 (Fig 3B). Sn-A had a stronger anomalous signal than Sn-B (25σ compared with 11σ), and accordingly the best model refinement was obtained with two TBT molecules with occupations of 0.70 and 0.30 for TBT-A and TBT-B, respectively (Fig 3C,D). In addition, the distances between the sulphur atoms of Cys432-A and Cys 432-B and the tin atoms (Fig 3B) are shorter than the sum of their ionic radii (2.54 Å), suggesting the presence of a covalent bond between TBT and Cys432.
Figure 3.
Structure of the RXR-α ligand-binding domain in complex with TBT and TIF2. (A) Overall structure of the complex in cartoon representation. The TBT is shown bound to Cys432 through a covalent interaction, and an acetate molecule (Ac) involved in a salt bridge with Arg316 are depicted. This acetate, not essential for RXR activity, derives from the crystallization condition. (B) Anomalous difference electron density map contoured at 15.0σ (red) and 5.0σ (blue) showing two tin sites facing the two alternative positions of Cys432 (A,B). (C,D) The two positions of TBT (TBT-A and TBT-B) bound to Cys432-A and Cys432-B are shown in the FO–FC omit map contoured at 3.0 σ. LBD, ligand-binding domain; RXR-α, retinoid X receptor-α; TBT, tributyltin; TIF2, transcriptional intermediary factor 2.
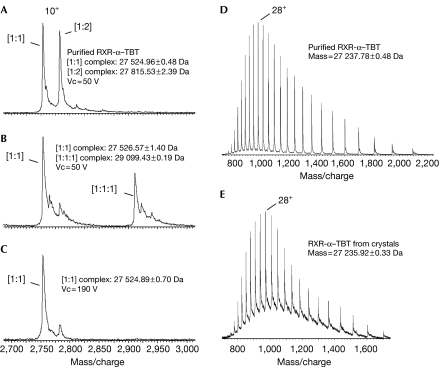
To characterize further the binding mode of TBT, the RXR-α–TBT complex was analysed by electrospray ionization mass spectrometry (ESI-MS) under native conditions. Unexpectedly, the spectrum showed two equally abundant species corresponding to complexes of RXR-α with TBT in 1:1 or 1:2 molar ratios, that is, one or two TBT bound to one RXR molecule, respectively (Fig 4A). The addition of the TIF2 peptide induced strong competition of the TBT bound to the second RXR-binding site, and simultaneously the formation of the ternary complex RXR–TBT–TIF2 was observed (Fig 4B). These results suggested that the second organotin molecule was bound nonspecifically to the hydrophobic coactivator binding groove. Interestingly, the interaction of TBT with RXR-α could not be disrupted in the mass spectrometer interface when the most stringent parameters were applied—190 V and 3 mbar, generating collisions extremely energetic and disruptive for non-covalent interactions—supporting a covalent coupling (Fig 4C). Subsequently, ESI-MS analysis was carried out under denaturing conditions after complexes, either purified or solubilized from crystals, were separated by high-performance liquid chromatography and eluted with a gradient of acetonitrile. In both cases, the mass calculated from the spectra corresponded to the mass of the unbound RXR-α (Fig 4D,E), substantiating the idea that TBT and Cys432 are connected through a fragile Sn-S covalent bond. The weakness of the covalent bond could also be observed in transactivation experiments in which the antagonist UVI3003 at high concentration was able to compete with TBT (Fig 2E).
Figure 4.
Mass spectrometry analysis. Non-denaturing ESI-MS was used to characterize RXR-α–TBT complexes in (A) the absence or in (B,C) the presence of a fourfold molar excess of TIF2 peptide. Increasing ion acceleration voltage (Vc) from 50 V (A,B) to 190 V (C) had no effect on the interaction of TBT with RXR. LC-MS analysis of RXR-α–TBT complexes either (D) purified or (E) solubilized from crystals was carried out to assess the reversibility of TBT binding. Measured mass corresponds to unbound RXR-α LBD. ESI-MS, electrospray ionization mass spectrometry; LBD, ligand-binding domain; LC-MS, liquid chromatography-mass spectrometry; RXR-α, retinoid X receptor-α; TBT, tributyltin; TIF2, transcriptional intermediary factor 2.
To confirm the importance of the Sn-S interaction for the activation of RXR-α by TBT, the residue equivalent to Cys432 in mouse RXR-α (Cys437) was mutated into alanine. Consistent with the prediction, this mutation abrogated TBT-induced RXR-α activity, whereas the activation of RXR-α by CD3254 was only modestly affected (Fig 2E).
Structural basis for RXR activation by organotins
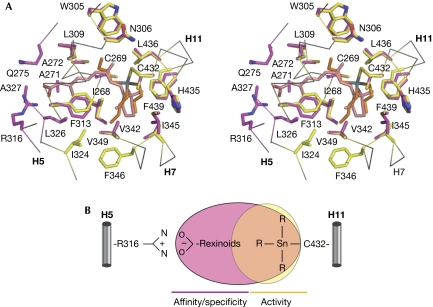
Classically, rexinoids contain an acidic head group and a long aliphatic/aromatic chain (Fig 1) bridging Arg316 (H5) on one side and helix H11 on the other (Fig 5A,B). Although TBT interacts with only a subset of binding pocket residues in the H11 region, it is engaged in enough essential contacts to stabilize RXR-α in its active conformation. Comparison of the RXR-α–TBT structure with that of RXR-α bound to its natural agonist 9cis-retinoic acid (Egea et al, 2000) shows that, for the most part, residues in contact with the organotin also belong to the 9cis-retinoic-acid-binding pocket (Fig 5A). In addition, both ligands induce identical residue positioning and side-chain orientations. It is noted that, TBT preserves the conformation of Leu436, which has previously been shown to be crucial for full agonism (Nahoum et al, 2007). This observation shows that interactions involving residues of the TBT-binding site are crucial for stabilization of the receptor-active form, whereas interactions made with residues on the Arg316 side act to increase the number of protein–ligand contacts and enhance the affinity and specificity of classical rexinoids (Fig 5B).
Figure 5.
Structural determinants of recognition of tributyltin by the RXR-α. (A) Stereo view of the superposition of the RXR-α LBD in complex with TBT (orange) or 9cis-RA (pink, Protein Data Bank code 1FBY). Residues involved in TBT and 9cis-RA binding are coloured in yellow and purple, respectively. (B) Schematic representation of the parts of RXR-α LBP occupied by TBT and classical rexinoids. LBD, ligand-binding domain; LBP, ligand-binding pocket; RA, retinoic acid; RXR-α, retinoid X receptor-α; TBT, tributyltin.
Organotins form a group of more than 250 tin compounds containing a variety of mono-, di-, tri- or tetra-substituted organic groups. Our structure shows that the high affinity of TBT for RXR (12.5 nM; Grun et al, 2006) derives from the covalent interaction linking the tin atom to residue Cys432 and the direct van der Waals contacts between all TBT atoms and RXR-α residues. Accordingly, modelling studies indicate that the most active organotins such as triphenyltin show such features (supplementary Fig S1 and Table S2 online). By contrast, organotins such as triethyltin with fewer and/or shorter alkyl groups do not establish enough contacts with the protein to position the tin atom correctly against residue Cys432 and ensure high-affinity binding. Finally, RXR is unable to accommodate compounds with longer alkyl groups such as trioctyltin, as the volume of which in some directions exceeds that of the ligand-binding pocket (LBP).
Organotins as nuclear receptor modulators?
TBT is able to activate RXR–PPAR-γ through RXR because this heterodimer interacts poorly with corepressors in vivo and belongs to the group of so-called ‘permissive' heterodimers, which can be stimulated by RXR ligands on their own (Germain et al, 2002). Accordingly, significant activation of other permissive heterodimers such as RXR–LXR or RXR–NURR1 has been reported (Grun et al, 2006). Using stably transfected HGELN Gal4-PPAR-α and HGELN Gal4-PPAR-δ cell lines, we showed that TBT is able to activate all three RXR–PPAR-α, -γ and -δ heterodimers (supplementary Fig S2 online).
As RXR-α Cys432 has a crucial function in the mechanisms of binding and activation by TBT, we looked at whether this residue is conserved in other nuclear receptors and found that the presence of a cysteine residue at this particular position is unique to RXR-α, -β and -γ. Conversely, the PPAR-γ LBP contains a cysteine (Cys285) that couples covalently with conjugated oxo fatty acids (Itoh et al, 2008) and might act as an anchoring point for TBT. However, in contrast to Cys432, which is located in RXR-α helix H11, Cys285 of PPAR-γ resides in helix H3. Hence, TBT could bind to PPAR-γ in a region of the LBP, which does not allow efficient stabilization of the active receptor conformation. Finally, it was recently reported that dibutyltin acts as a potent antagonist of the glucocorticoid receptor (Gumy et al, 2008). Similarly to other oxo-steroid receptors, glucocorticoid receptor contains cysteine residues that could help fix dibutyltin in the hormone-binding site. The involvement of cysteine residues in the binding of organotins to receptors other than RXR remains to be established. Nevertheless, our data suggest that tin compounds could use the specific Sn-S interaction to modulate the transcriptional activity of several nuclear receptors, the functional outcome being dictated by the structure of the organotin and the position of the anchoring cysteine in the LBP.
Methods
Ligands and peptides. TBT and BRL49653 were purchased from Sigma-Aldrich (St Quentin Fallavier, France) and Interchim (Montlucon, France), respectively. CD5477 and CD3254 were kindly provided by Galderma (Sophia-Antipolis, France). UVI3003 was synthesized by AR de Lera (University of Vigo, Spain). GW327647 and GW610742 were gifts from Dr TM Wilson (GlaxoSmithKline, Research Triangle Park, NC, USA). The fluorescein-139EEPSLLKKLLLAPA152 peptide corresponding to the NR box 2-binding motif of PGC-1α and the NR box 2 peptide (686KHKILHRLLQDSS698) of TIF2 were purchased from EZbiolab (Westfield, IN, USA).
Fluorescence assays. Fluorescence anisotropy assays were performed using a Safire Microplate Reader (TECAN) with the excitation wavelength set at 470 nm and emission measured at 530 nm. Experiments were performed as described previously (Pogenberg et al, 2005). The reported data are the average of at least three independent experiments and error bars correspond to standard deviations.
Stable transactivation experiments. HGELN human PPAR-α, -δ and -γ cell lines were generated as described in the supplementary information online. To measure the activity of TBT, HGELN Gal4-PPAR cells were seeded at a density of 40,000 cells per well in 96-well white opaque tissue culture plates (Dutscher, Brumath, France). Compounds were added 8 h later and cells were incubated for 16 h. At the end of incubation, the culture medium was replaced by a medium containing 0.3 mM luciferin. Luciferase activity was measured for 2 s in intact living cells using a microBeta Wallac luminometer (Perkin Elmer, Villebon sur Yvette, France). Tests were performed in quadruplicate in at least three independent experiments and data were expressed as mean±s.d. Dose–response curves were fitted using the sigmoid dose–response function of a graphics and statistics software package (Graph-Pad Prism, version 4, 2003, Graph-Pad Software Inc., San Diego, CA, USA).
Transient transactivation experiments. PPAR-γ and RXR-α homodimer activities were monitored on (PPRE)3-TK- and (RXRE)6-TK luciferase reporter constructs. pSG5-hPPAR-γ and (PPRE)3-TK-Luc are gifts from Dr L Fajas (IRCM, Montpellier, France). pSG5-hRXR-α, mRXR-α C437A and (RXRE)6-TK-Luc were kindly provided by Dr H Gronemeyer (IGBMC, Illkirch, France). Transient transfection assays were performed in HeLa cells using Jet-PEI (Ozyme, Saint-Quentin en Yvelines, France) according to the manufacturer's instructions. Luciferase assays were performed with the Promega dual-reporter kit, according to the manufacturer's instructions. Renilla luciferase encoded by the normalization vector phRLTK (Promega, Charbonnières les Bains, France) was used as an internal control for firefly luciferase normalization. Tests were performed in duplicate in at least three independent experiments and data were expressed as mean±s.d.
Crystallization of the RXR-α LBD–TBT complex. Expression and purification of the human RXR-α LBD have been described previously (Nahoum et al, 2007). Fractions containing the purified receptor were pooled, mixed with a threefold molar excess of TBT and a fivefold molar excess of the TIF2 NR2 coactivator peptide and concentrated to 10.5 mg/ml. Crystals were obtained by vapour diffusion at 293 K. The well buffer contained 28% PEG 4000, 0.1 M Tris–HCl pH7.5 and 1.0 M ammonium acetate. Crystals were of space group P43212. A single crystal was mounted from the mother liquor onto a cryoloop (Hampton Research, Aliso Viejo, CA, USA), soaked in the reservoir solution containing an additional 20% glycerol and frozen in liquid nitrogen.
Crystallographic data collection, processing and structure refinement. Diffraction data were collected using an ADSC Q315r CCD detector at the BM30A beamline of the European Synchrotron Radiation facility (ESRF, Grenoble, France) at 1.9 Å resolution. Diffraction data were processed using MOSFLM (Leslie, 2006) and scaled with SCALA from the CCP4 program suite (Collaborative Computational Project, 1994). The structure was solved by using the previously reported structure 2P1T (Nahoum et al, 2007) from which the ligand was omitted. Initial FO–FC difference maps showed a strong signal for the ligand, which could be fitted accurately into the electron density. The structure was modelled with COOT (Emsley & Cowtan, 2004) and refined with REFMAC (Collaborative Computational Project, 1994) using rigid body refinement, restrained refinement and individual B-factor refinements.
Mass spectrometry. For liquid chromatography-mass spectrometry (LC-MS) analysis, 80 μg of purified RXR-α LBD–TBT complexes and 15 μl of solubilized crystals (eight crystals in 15 μl of 5 M ammonium acetate, pH 7.0) were injected into a C4 reversed-phase high-performance liquid chromatography column (Vydac, Grace, Deerfield, IL, USA). Separation was obtained with a linear gradient (10–60%) of acetonitrile, and detection of eluting protein was carried out at 205 nm. Here, 300 μl fractions were collected, dried under vacuum, solubilized in 20 μl of 50% acetonitrile solution acidified with 1% HCOOH, and infused for mass spectrometry analysis using automated Triversa Nanomate (Advion, Ithaca, NY, USA) coupled with LC-TOF mass spectrometer (Waters, Versailles, France). Data acquisition was carried out with the following interface parameters: Vc of 30 V, Pi of 2.6 mbar. Non-denaturing mass spectrometry analysis requires the final purification buffer of RXR-α LBD–TBT complexes to be exchanged for ammonium acetate buffer (50 mM, pH 7.5) using the Zeba spin column system (Pierce, Rockford, IL, USA). Mass spectrometer interface parameters were set up as follows: Vc varied from 190 V to 50 V and Pi varied from 5 mbar to 3 mbar.
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
Supplementary Information
Acknowledgments
We acknowledge the technical assistance of the BM30A beamline managers at the ESRF (Grenoble, France). This study was financially supported by the French National Research Agency (ANR-07-PCVI-0001-01 to W.B.), the Agence Française de Sécurité Sanitaire de l'Environnement et du Travail (AFSSET, RD-2005-007 to P.B.) and the European Union Commission (CASCADE FOOD-CT-2004-506319 to P.B.).
Footnotes
The authors declare that they have no conflict of interest.
References
- Antizar-Ladislao B (2008) Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. Environ Int 34: 292–308 [DOI] [PubMed] [Google Scholar]
- Appel KE (2004) Organotin compounds: toxicokinetic aspects. Drug Metab Rev 36: 763–786 [DOI] [PubMed] [Google Scholar]
- Boyer IJ (1989) Toxicity of dibutyltin, tributyltin and other organotin compounds to humans and to experimental animals. Toxicology 55: 253–298 [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr 50: 760–763 [DOI] [PubMed] [Google Scholar]
- de Lera AR, Bourguet W, Altucci L, Gronemeyer H (2007) Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nat Rev Drug Discov 6: 811–820 [DOI] [PubMed] [Google Scholar]
- Egea PF, Mitschler A, Rochel N, Ruff M, Chambon P, Moras D (2000) Crystal structure of the human RXRα ligand-binding domain bound to its natural ligand: 9-cis retinoic acid. EMBO J 19: 2592–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60: 2126–2132 [DOI] [PubMed] [Google Scholar]
- Fent K (1996) Ecotoxicology of organotin compounds. Crit Rev Toxicol 26: 1–117 [PubMed] [Google Scholar]
- Germain P, Iyer J, Zechel C, Gronemeyer H (2002) Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature 415: 187–192 [DOI] [PubMed] [Google Scholar]
- Golub M, Doherty J (2004) Triphenyltin as a potential human endocrine disruptor. J Toxicol Environ Health B Crit Rev 7: 281–295 [DOI] [PubMed] [Google Scholar]
- Grun F, Blumberg B (2006) Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 147: S50–S55 [DOI] [PubMed] [Google Scholar]
- Grun F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, Gardiner DM, Kanno J, Iguchi T, Blumberg B (2006) Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol 20: 2141–2155 [DOI] [PubMed] [Google Scholar]
- Gumy C, Chandsawangbhuwana C, Dzyakanchuk AA, Kratschmar DV, Baker ME, Odermatt A (2008) Dibutyltin disrupts glucocorticoid receptor function and impairs glucocorticoid-induced suppression of cytokine production. PLoS ONE 3: e3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Fairall L, Amin K, Inaba Y, Szanto A, Balint BL, Nagy L, Yamamoto K, Schwabe JW (2008) Structural basis for the activation of PPARγ by oxidized fatty acids. Nat Struct Mol Biol 15: 924–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J (2005) Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor γ/retinoid X receptor pathway. Mol Pharmacol 67: 766–774 [DOI] [PubMed] [Google Scholar]
- Leslie AG (2006) The integration of macromolecular diffraction data. Acta Crystallogr D Biol Crystallogr 62: 48–57 [DOI] [PubMed] [Google Scholar]
- Matthiessen P, Gibbs PE (1998) Critical appraisal of the evidence for tributyltin-mediated endocrine disruption in mollusks. Environ Toxicol Chem 17: 37–43 [Google Scholar]
- McAllister BG, Kime DE (2003) Early life exposure to environmental levels of the aromatase inhibitor tributyltin causes masculinisation and irreversible sperm damage in zebrafish (Danio rerio). Aquat Toxicol 65: 309–316 [DOI] [PubMed] [Google Scholar]
- Michalik L et al. (2006) International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev 58: 726–741 [DOI] [PubMed] [Google Scholar]
- Nahoum V et al. (2007) Modulators of the structural dynamics of the retinoid X receptor to reveal receptor function. Proc Natl Acad Sci USA 104: 17323–17328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T et al. (2005) Trialkyltin compounds bind retinoid X receptor to alter human placental endocrine functions. Mol Endocrinol 19: 2502–2516 [DOI] [PubMed] [Google Scholar]
- Nakanishi T (2008) Endocrine disruption induced by organotin compounds; organotins function as a powerful agonist for nuclear receptors rather than an aromatase inhibitor. J Toxicol Sci 33: 269–276 [DOI] [PubMed] [Google Scholar]
- Ogata R, Omura M, Shimasaki Y, Kubo K, Oshima Y, Aou S, Inoue N (2001) Two-generation reproductive toxicity study of tributyltin chloride in female rats. J Toxicol Environ Health A 63: 127–144 [DOI] [PubMed] [Google Scholar]
- Pogenberg V, Guichou JF, Vivat-Hannah V, Kammerer S, Perez E, Germain P, de Lera AR, Gronemeyer H, Royer CA, Bourguet W (2005) Characterization of the interaction between retinoic acid receptor/retinoid X receptor (RAR/RXR) heterodimers and transcriptional coactivators through structural and fluorescence anisotropy studies. J Biol Chem 280: 1625–1633 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information