When cells become infected with a virus, they mount a coordinated antiviral response, a key component of which is the production of interferons (IFNs). This family of cytokines is present only in vertebrates, and mediates its antiviral effects both directly on infected cells and indirectly by activating other important effectors of the innate immune response, including natural killer cells, dendritic cells and macrophages (Sadler & Williams, 2008). There are three groups of IFNs: type I, type II and the poorly characterized type III. The type I IFNs are produced by virus-infected cells, whereas the only type II member—IFN-γ—is expressed in T cells and natural killer cells. The type I IFNs bind to a receptor complex at the cell surface—which is composed of two subunits, IFN-α receptor 1 (IFNAR1) and IFNAR2—and activate intracellular signalling pathways, the best characterized of which is the Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway. JAKs are tyrosine kinases that associate with the receptor complex and phosphorylate STAT proteins, which subsequently translocate to the nucleus and regulate the expression of IFN-stimulated genes (ISGs; Sadler & Williams, 2008).
In addition to JAK–STAT signalling, IFNs activate other intracellular pathways, including mitogen-activated protein (MAP) kinase pathways. It has been shown recently that the c-Jun amino-terminal kinase (JNK) group of MAP kinases can mediate the IFN-α-induced apoptosis of cells (Yanase et al, 2005; Jeon et al, 2008). JNK signalling is required for normal development; however, it is also a major regulator of the response of cells to stress (Davis, 2000). The JNK pathway—similar to other MAP kinase pathways—features a core triple-kinase module consisting of a MAP kinase kinase kinase (MKKK), a MAP kinase kinase (MKK) and JNK. Members of the Rho family of small GTPases—including Rac1 and cell-division cycle 42 (Cdc42)—feed signals into this module. The specificity of signalling through the JNK pathway is partly mediated by scaffold proteins that bind to the components of the module, thereby directing their subcellular localization and restricting the signals that can activate the module (Davis, 2000).
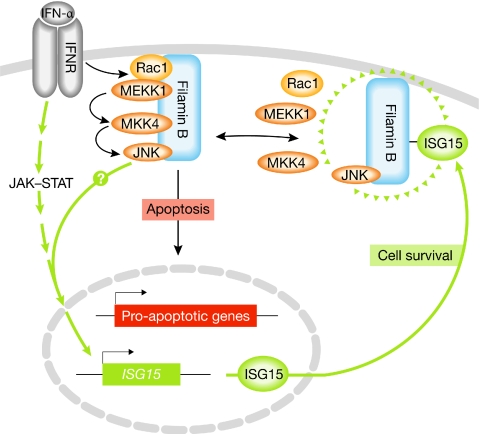
Two recent papers by Chung and colleagues—one of them in this issue of EMBO reports—have shed new light on how JNK signalling is regulated by type I IFNs (Jeon et al, 2008, 2009). They found that IFN-α activates a specific JNK signalling module consisting of the Rac1 GTPase, the mitogen-activated extracellular-signal-regulated kinase kinase 1 (MEKK1), MKK4 and JNK (Fig 1). Significantly, JNK activation by IFN-α depends on the binding of this module to filamin B (Jeon et al, 2008). Filamin proteins bind to actin through an amino-terminal domain, and facilitate the cross-linking of actin filaments and their connection to cellular membranes. They also feature a large rod-like domain of tandem repeats and—in addition to actin—interact with diverse proteins, which imply that they have important roles in regulating many cellular processes. Chung and colleagues have shown that filamin B facilitates JNK activation and apoptosis independently of its actin-binding ability, but dependent on its binding to the JNK module through a subset of tandem repeats (Jeon et al, 2008). They also found that IFN-α significantly enhances complex formation between filamin B and the JNK module, and leads to the colocalization of filamin B with Rac1 in membrane ruffles (Jeon et al, 2008). How this occurs is unclear; however, a likely explanation put forward by the authors is that filamin B could be modified in response to IFN-α, perhaps by phosphorylation, to facilitate its translocation and binding to the JNK module. Indeed, several uncharacterized phosphorylation sites have been identified on filamin B in vivo (Gnad et al, 2007). Further studies will be needed to determine how filamin B promotes Rac1 activation and the formation of membrane ruffles. For example, it could recruit a specific guanine nucleotide-exchange factor (GEF) to activate Rac1 and initiate signalling through the JNK module. In addition, Rac1 might activate the p21-activated kinase (PAK1), which has a crucial role in membrane-ruffle formation and that the authors report can also bind to filamin B (Jeon et al, 2008).
Figure 1.
Model for the regulation of type 1 interferon signalling by filamin B. IFN-α binds to the IFNR and this induces the formation of a complex of filamin B bound to a specific JNK signalling module, Rac1–MEKK1–MKK4–JNK, at the plasma membrane. Filamin B and JNK can mediate IFN-α-induced apoptosis, potentially by upregulating the expression of pro-apoptotic genes such as TRAIL-R1. IFN-α stimulation of cells also leads to the expression of ISG15, in part mediated by the JAK–STAT pathway and possibly by JNK. ISG15 conjugates to filamin B and interferes with its binding to components of the JNK module, thereby downregulating JNK signalling and protecting cells from apoptosis. Cell fate might therefore be determined by the balance between non-modified and ISG15-modified forms of filamin B. IFN-α, interferon-α; IFNR, interferon receptor; ISG15, IFN-stimulated gene 15; JAK–STAT, Janus kinase–signal transducer and activator of transcription; JNK, c-Jun amino-terminal kinase; MEKK1, mitogen-activated extracellular-signal-regulated kinase kinase 1; MKK4, mitogen-activated protein kinase kinase 4; TRAIL-R1, tumour necrosis factor-related apoptosis-inducing ligand receptor 1.
Chung and colleagues have now extended their analysis of the molecular events that underpin the regulation of JNK signalling by IFN (Jeon et al, 2009). A recent screen for putative conjugation targets of the IFN-inducible ubiquitin-like molecule ISG15 identified filamin B (Zhao et al, 2005), and Chung and colleagues have shown that ISG15 modification of filamin B regulates IFN-induced JNK activation. Specifically, the conjugation of ISG15 to a specific lysine residue in the carboxyl terminus of filamin B was found to abrogate its binding to Rac1, MEKK1 and MKK4. Although JNK could still bind to filamin B, the loss of binding of the upstream components of the module correlated with a loss of JNK activation (Jeon et al, 2009). These results point to the existence of a negative-feedback loop whereby the exposure of cells to IFN-α causes filamin B-mediated JNK activation and upregulation of ISG15 expression, which leads to its conjugation to filamin B and subsequent downregulation of JNK signalling (Fig 1). Whether the ISG15 expression is induced by JNK signalling is not known. This model is supported by experiments using a non-conjugatable mutant of filamin B, as well as RNA interference (RNAi)-mediated knockdown of the ISG15-conjugating enzyme ubiquitin-activating enzyme-E1-like protein (UBE1L), which both lead to persistent IFN-α-induced JNK activity and apoptosis (Jeon et al, 2009).
Type I IFNs can induce the apoptosis of virus-infected cells, but can also protect uninfected cells. The results of Chung and colleagues indicate that modulating JNK signalling might be an important mechanism for determining cell fate. One possible scenario is that JNK signalling contributes to apoptosis in virus-infected cells, whereas in neighbouring uninfected cells the modification of filamin B by ISG15 downregulates JNK signalling and thereby promotes their protection. If this is the case, the ISG15 modification of filamin B might need to be suppressed in cells destined for apoptosis. Further work is clearly required to determine precisely how ISG15 modification of filamin B is regulated and how this integrates with other signalling pathways initiated by type 1 IFN. How JNK contributes to type 1 IFN-induced apoptosis is also unclear, although one mechanism might be through the transcriptional upregulation of pro-apoptotic genes. Indeed, it has been reported that filamin B and JNK are both required for IFN-α-induced expression of the death-receptor TRAIL-R1 (tumour necrosis factor-related apoptosis-inducing ligand receptor 1; Yanase et al, 2005; Jeon et al, 2008); however, how this occurs is unclear, as filamin B is proposed to colocalize with the JNK module in membrane ruffles (Jeon et al, 2008). A pool of activated JNK could perhaps be released from the scaffold complex and relocalize to the nucleus, where it targets its substrates—such as members of the activator protein 1 (AP-1) family of transcription factors. A more intriguing possibility is that filamin B itself might transport active JNK to the nucleus. There is a precedent for a nuclear role of filamins: truncated C-terminal versions of the related protein, filamin A, occur naturally following proteolytic cleavage and can localize to the nucleus with transcription factors (Loy et al, 2003). These cleavage sites are conserved among filamins, raising the possibility that C-terminal fragments of filamin B—which would contain the JNK module binding sites—could participate in IFN-α-stimulated nuclear signalling.
A further point of interest is whether there is a wider role for the filamin family in coordinating JNK signalling. Filamin A also binds to Rac1 and MKK4, and indirectly to MEKK1, and has been shown to be required for JNK activation in response to tumour necrosis factor-α (TNF-α) and Wnt5a, which might contribute to inflammatory responses and cell migration, respectively (Marti et al, 1997; Nomachi et al, 2008). Chung and colleagues found no evidence for a role of filamin A in IFN-α signalling and did not detect ISG15 modification of filamin A (Jeon et al, 2008, 2009), although the same screen that identified filamin B as a target of ISG15 conjugation also identified filamin A (Zhao et al, 2005). These studies indicate that individual members of the filamin family might regulate JNK signalling in response to distinct stimuli in order to mount a coordinated immune response to microbial insult and injury.
The two studies by Chung and colleagues have uncovered a novel mechanism by which ISG15 can modulate immune signalling. In recent years, it has become evident that the expression of ISG15 is crucial for host responses to virus infection (Pitha-Rowe & Pitha, 2007). For example, ISG15-deficient mice are more susceptible to several viruses, including the influenza virus, herpes simplex virus and Sindbis virus (Lenschow et al, 2007). However, the molecular mechanisms that account for the antiviral actions of ISG15 have remained largely unknown and there have been some conflicting reports in the literature. This might be due to the complexity of ISG15 function as, apart from conjugating to proteins—more than 200 potential targets have now been identified—there is evidence for a regulatory role of free ISG15 in cells, and it can also be secreted from cells and act as a cytokine to modulate other immune responses (Pitha-Rowe & Pitha, 2007). For example, free ISG15 has recently been found to inhibit the activity of the E3 ubiquitin ligase Nedd4, which is utilized by viruses such as the Ebola virus and rabies virus to facilitate viral release from cells (Okumura et al, 2008; Malakhova & Zhang, 2008). The antiviral actions of ISG15 are therefore likely to be mediated by distinct mechanisms and by a diverse group of proteins. Future studies will undoubtedly reveal further interesting mechanisms, some of which could be exploited for antiviral therapies.

References
- Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252 [DOI] [PubMed] [Google Scholar]
- Gnad F, Ren S, Cox J, Olsen JV, Macek B, Oroshi M, Mann M (2007) PHOSIDA (phosphorylation site database): management, structural and evolutionary investigation and prediction of phosphosites. Genome Biol 8: R250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YJ et al. (2008) Filamin B serves as a molecular scaffold for type I interferon-induced c-Jun NH2-terminal kinase signaling pathway. Mol Biol Cell 19: 5116–5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YJ et al. (2009) ISG15 modification of filamin B negatively regulates type I interferon-induced JNK signaling pathway. EMBO Rep 10: 374–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow DJ et al. (2007) IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza and Sindbis viruses. Proc Natl Acad Sci USA 104: 1371–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy CJ, Sim KS, Yong EL (2003) Filamin-A fragment localizes to the nucleus to regulate androgen receptor and coactivator functions. Proc Natl Acad Sci USA 100: 4562–4567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakhova DA, Zhang DE (2008) ISG15 inhibits Nedd4 ubiquitin ligase E3 activity and enhances the innate antiviral response. J Biol Chem 283: 8783–8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti A, Luo Z, Cunningham C, Ohta Y, Hartwig J, Stossel TP, Kyriakis JM, Avruch J (1997) Actin-binding protein-280 binds to stress-activated protein kinase (SAPK) activator SEK1 and is required for tumor necrosis factor-alpha activation of SAPK in melanoma cells. J Biol Chem 272: 2620–2626 [DOI] [PubMed] [Google Scholar]
- Nomachi A, Nishita M, Inaba D, Enomoto M, Hamasaki M, Minami Y (2008) Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarised cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. J Biol Chem 283: 27973–27981 [DOI] [PubMed] [Google Scholar]
- Okumura A, Pitha PM, Harty RN (2008) ISG15 inhibits Ebola VP40 VLP budding in an L-domain-dependent manner by blocking Nedd4 ligase activity. Proc Natl Acad Sci USA 105: 3974–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitha-Rowe IF, Pitha PM (2007) Viral defence, carcinogenesis and ISG15: new roles for an old ISG. Cytokine Growth Factor Rev 18: 409–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler AJ, Williams BRG (2008) Interferon-inducible antiviral effectors. Nat Rev Immunol 8: 559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanase N, Hata H, Shimo K, Hayashida M, Evers BM, Mizuguchi J (2005) Requirement of c-Jun NH2-terminal kinase activation in interferon-α-induced apoptosis through upregulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in Daudi B lymphoma cells. Exp Cell Res 310: 10–21 [DOI] [PubMed] [Google Scholar]
- Zhao C, Denison C, Huibregtse JM, Gygi S, Krug RM (2005) Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc Natl Acad Sci USA 102: 10200–10205 [DOI] [PMC free article] [PubMed] [Google Scholar]