The Sixth Biennial Meeting on Axon Guidance, Synaptogenesis & Neural Plasticity took place between 10 and 14 September 2008, at Cold Spring Harbor, New York, USA, and was organized by A. Ghosh, C. Holt & A. Kolodkin.
Glossary
Introduction
In September 2008, more than 400 neuroscientists gathered in Cold Spring Harbor (NY, USA) to discuss how axons in the nervous system navigate to their synaptic targets to create highly organized connections with intriguing properties of plasticity and adaptability. Traditionally, this meeting not only features research on axon guidance, but also includes a consideration of additional processes in neural development that are relevant to circuit formation, including cell positioning and migration, synapse formation and plasticity, dendrite development and axon regeneration. This year was no exception. Coincidentally, many of the molecules that were initially identified as axon-guidance molecules are now also known to have a role in many of these other events. A rich diversity of work was presented from the identification of guidance cues, receptors and signal-transduction pathways to the function of these cues in pathfinding and target selection. In addition, the cell-biological mechanisms by which these cues act were discussed, as well as the transcriptional and translational mechanisms that control their action. Functional imaging of developing and adult circuits was also presented. Of course, the meeting did not solve all problems in axon guidance; however, it clearly reflected new directions in axon-guidance studies. The field is becoming a more interdisciplinary and multilevel forum that addresses a central problem of developmental neuroscience: the formation of functional neural circuits. This meeting report highlights some of the exciting advances that were presented at the meeting.
Axon-guidance molecules: the classics
Many classes of axon-guidance molecules have been discovered during the past two decades (Dickson, 2002), and only a few new ones were presented at the meeting. However, new functions of known guidance molecules are still being uncovered, and the focus is now on the interaction between various families of molecules and multiple intracellular signalling pathways. Guidance at the CNS midline is still a robust model for studies of axonal navigation; this is not surprising given that almost 90% of vertebrate CNS axons cross the midline of the nervous system either in the brain or in the spinal cord. Among the first axon-guidance molecules to be described in commissural axon pathfinding were axonin-1/TAG-1 and NrCAM (Stoeckli & Landmesser, 1995). New studies from the V. Castellani laboratory were presented by H. Nawabi (Lyon, France), which suggest that NrCAM might regulate the surface expression of plexinA1—but not other plexin family members—on the surface of the growth cone of spinal cord commissural axons. PlexinAs are best known as receptor components that mediate the repellent activities of class 3 semaphorins (Negishi et al, 2005). The upregulation of plexinA1 on the surface of commissural growth cones, once they are in contact with the floor plate, would explain previously published findings showing the expulsion of commissural axons from the floor plate (Zou et al, 2000).
The necessity for changes in the responsiveness of axons to midline repellents has been widely recognized, yet little is known mechanistically at a molecular level, especially in vertebrates. M.-L. Baudet from the C. Holt laboratory (Cambridge, UK) reported that Xenopus retinal ganglion-cell axons change their responsiveness to guidance cues by means of a microRNA that acts as an intrinsic clock by regulating the expression of axon-guidance receptors. It will be interesting to see whether the switch in growth-cone response at the rodent spinal cord midline might also be mediated by microRNAs.
The members of the Robo family of proteins are well known for their role in midline guidance and longitudinal pathway selection in post-crossing commissural axons in Drosophila. Biochemical and genetic studies presented by T. Evans from the G. Bashaw laboratory (Philadelphia, PA, USA) and B. Spitzweck from the B. Dickson laboratory (Vienna, Austria) characterize the contributions of the individual family members, and found that robo1 and robo2 have distinct functions, indicating the complexity of midline guidance.
A different mechanism of regulating Robo/slit signalling was presented by C. Conway from the J. Mason laboratory (Edinburgh, UK) at the optic chiasm, where a role for the sulphation of HSPGs was implicated in setting the level of axonal repulsion in response to slits at the optic chiasm. In the absence of Hs6st1, axons experienced reduced repulsion from the chiasm, resulting in projections of retinal ganglion-cell axons to the contralateral eye. Similarly, in the absence of Hs2st, slit1 seemed to be less repulsive, which resulted in axons navigating along the midline, rather than crossing the chiasm.
Morphogens in axon guidance and synaptogenesis
Morphogens are best known for their effect on neural patterning in early development. However, approximately 10 years ago, it was shown that morphogens can also have an effect on axon guidance that is independent of cell-fate determination (Zou & Lyuksyutova, 2007). In fact, morphogens go beyond axon guidance: Wnts and BMPs have been implicated in regulating the formation or growth of synapses between cerebellar mossy fibres and granule cells in the mouse (Hall et al, 2000), and at the Drosophila NMJ (Packard et al, 2002; Marques et al, 2002; Aberle et al, 2002; McCabe et al, 2003; Haghighi et al, 2003; Salinas & Zou, 2008). More recent work has linked Wnts to synapse formation in Caenorhabditis elegans (Klassen & Shen, 2007). E. Dickins (London, UK) reported data implicating Wnt3 in NMJ development in the chicken limb bud through a non-canonical pathway (Henriquez et al, 2008). Work in the P. Salinas laboratory has also implicated a divergent canonical Wnt signalling pathway in synaptogenesis in the CNS.
Several studies focusing on signalling downstream of morphogens in axon guidance or synapse formation in both the CNS and the PNS were presented at the meeting. Wnt–Frz signalling controls the anterior–posterior guidance of commissural axons in rodents (Lyuksyutova et al, 2003). How Wnt proteins signal in growth cones is beginning to be deciphered, and work presented by B. Shafer from the Y. Zou laboratory (San Diego, CA, USA) implicates components of the PCP pathway in postcommissural axon guidance, in addition to aPKC, as published last year (Wolf et al, 2008). Crucial components of PCP are not only present in the growth cones of commissural axons, but are also required for anterior–posterior guidance, which is thought to involve Wnt attraction. Wnt signals seem to affect the growth and guidance of axons in many ways because Wnts also repel corticospinal axons when Ryk is expressed as the receptor or co-receptor (Liu et al, 2005). L. Li from the K. Kalil laboratory (Madison, WI, USA) reported that calcium entry through TRP channels is required for the repulsive effect of Wnt5a and for neurite outgrowth, whereas IP3-derived Ca2+ elevations seem to be required only for neurite outgrowth. Ca2+ transients were observed downstream of both Ryk and Frz receptors. Although much less is known about signalling downstream of Shh and BMPs, a requirement for transcription in Shh-mediated attraction is unlikely.
Cell-biological mechanisms of growth-cone guidance
Although many axon-guidance molecules have been identified, and their receptors and signalling mechanisms are being elucidated, the cell-biological mechanisms of growth-cone guidance remain unknown. According to the generally accepted model, extracellular cues are thought to trigger cascades of intracellular signalling pathways, which, in turn, influence and/or remodel cytoskeletal structures to mediate turning responses. However, it is not clear what these cellular processes are and which are crucial for regulating the direction of growth-cone turning.
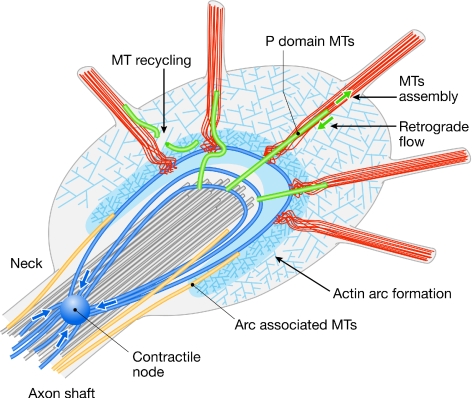
P. Forscher (New Haven, CT, USA) discussed his research using the Aplysia growth cone (Fig 1). He reported that fluorescent-speckle microscopy has revealed a remarkable polarized retrograde actin flow in the peripheral domain, at least one function of which might be to act as a kinetic barrier to microtubule advance, providing a negative force to oppose growth-cone advance.
Figure 1.
Structure of an Aplysia growth cone. Redrawn from an original kindly provided by P. Forscher (New Haven, CT, USA). MT, microtubule.
The actin arc is a remarkable structure, which might provide a severing force for filaments causing them to be trimmed as they grow towards the inside of the growth cone. The actin arc is also involved in holding the microtubule bundles together, particularly at the growth-cone neck (Burnette et al, 2008), and myosin II seems to be the motor essential for the functioning of actins. These exciting findings shed light on how axon guidance cues might regulate growth-cone turning, although a coherent picture is still lacking.
Rho activity is known to be essential to axon repulsion, although its mechanism of action is unclear. A new model of the growth-cone cytoskeletal mechanism proposed by P. Forscher suggests that Rho activity might act on the actin arc, and cause it to contract and retract, rather than causing actin depolymerization in the peripheral domain, such as in the filopodia. This argument is consistent with the observation that axon repellents often do not cause growth-cone collapse (Zhang et al, 2003). Conversely, axon attractants seem to function by blocking retrograde actin flow in the filopodia, allowing microtubules to invade them and thereby allowing growth-cone advance.
It remains to be seen whether these are universal mechanisms of growth-cone guidance. Bundled actin filaments in the filopodia of vertebrate growth cones also flow in a retrograde manner; however, it is not yet known whether they affect microtubules. S. Wanner from the L. Lanier laboratory (Minneapolis, MN, USA) showed that an alternative actin architecture, formed by the ARP2/3 complex, seems to regulate microtubule dynamics in the growth cone by limiting the number of actively polymerizing microtubules in the periphery. In addition, ARP2/3 regulates the distribution of cell adhesions. Therefore, ARP2/3 is a negative regulator of axon elongation. The role of the Arp2/3 complex was also investigated in C. elegans growth cones. E. Lundquist (Lawrence, KA, USA) reported that Arp2/3, UNC-115/abLIM and UNC-34/Ena formed three redundant pathways that regulate filopodia formation and pathfinding. In this case, Arp2/3 probably contributes to filopodia formation by providing a supporting network to allow actin bundles to be formed, anchored or stabilized. S. Gupton from the F. Gertler laboratory (Cambridge, MA, USA) showed that filopodia are required for the initiation of neurites, and that they can be induced through mechanisms that are either Ena/VASP dependent or Ena/VASP independent but laminin dependent. They found that laminin induces an integrin-dependent switch in membrane trafficking that drives Ena/VASP-independent neuritogenesis. Gowth-cone and cell migration have interesting similarities, as well as differences. D. Solecki (Memphis, TN, USA) suggested that myosin II motors, which are localized in the leading processes of migrating cerebellar granule cells, just forward of the centrosome and neuronal soma, provide the force that polarizes centrosomal motility in migrating neurons. J. Shieh from the S. McConnell laboratory (Stanford, CA, USA) suggested that endocytosis might have an important role in weakening or removing cell-adhesion sites during neuronal soma migration. Although cellular migration might require distinct mechanisms from those involved in axon guidance, as the latter does not involve cell-body translocation, it will be interesting to see whether endocytosis in neuronal growth cones has a similar role.
Target recognition and synaptogenesis
One of the least understood aspects of neural circuit formation is target recognition. We have learned the principles of how axon-guidance cues cooperate to steer axons to their target area; however, we have little idea why axons choose a particular cell as a partner for synapse formation, partly because it is much harder to detect molecular differences at this level. A possible mechanism would be the mutual recognition of presynaptic and postsynaptic cells by the expression of cell-adhesion molecules. Indeed, some candidates have been identified that are sufficient to induce synapses, even when expressed in HEK cells. These molecules include the SynCAMs, the LRRTM proteins and the neuroligins/neurexins, all of which were initially characterized for their roles in synaptogenesis (Yamagata et al, 2003; Lauren et al, 2003). Together, they organize the scaffold that is required for the formation of synapses, although the story is clearly not yet complete. In some cases, for example, for the LRRTMs, the presynaptic partner has not yet been identified. A. Ghosh (San Diego, CA, USA) showed that a member of the LRRTM family is sufficient to recruit scaffolding proteins to the membrane in order to induce excitatory synapses. In other cases, presynaptic and postsynaptic partners are known—as in the case of neuroligin and neurexin—but nothing is known about the mechanism by which scaffold components such as PSD-95 and NMDARs are recruited. New studies presented by T. Biederer (New Haven, CT, USA) suggest that, at least for the SynCAMs, there seems to be a function before target innervation. Additional molecules involved in synaptogenesis have been discovered from the other direction, having initially been identified as axon-guidance molecules. For example, class 3 semaphorins and their receptors—formed by neuropilins and plexins—were well described in regards to axon guidance before they were linked to synaptogenesis (Yazdani & Terman, 2006). By using a range of single-knockout and double-knockout mouse lines, which were generated in the laboratories of A. Kolodkin and D. Ginty, T. Tran (Baltimore, MD, USA) showed an increase in dendritic spine size and number in the dentate gyrus and layer 5 cortical neurons of Sema3F-mutant and neuropilin-2-mutant mice. The misshaped spines of these mice are unusually large in size, contain novel multivesicular structures and are tenfold more likely to contain multiple postsynaptic densities than wild-type spines. Sema3F reduces the number of coincident presynaptic and postsynaptic contacts, and also the overall number of postsynaptic specializations, as assessed in vitro by vGlut1 and PSD-95 localization.
Plasticity
Synaptic connections are often initially imprecise and are later refined into specific patterns, a process that is thought to be mediated by neural activity. In the visual system, neural activity is important for the eye-specific segregation of retinal ganglion-cell axons in the LGN, as well as for topographic mapping. However, how neural activity shapes axon connections and what forms of activity are involved have remained crucial unanswered questions.
H. Xu from the M. Crair laboratory (New Haven, CT, USA) showed that retinotopic mapping and eye-specific segregation depend on different patterns of neural activity. Local waves of synchronized retinal activity are sufficient to drive retinotopic refinement in the dorsal LGN and superior colliculus. However, eye-specific segregation requires global waves of retinal activity. J. Trachtenberg (Los Angeles, CA, USA) suggested that the establishment and refinement of cortical maps of the ipsilateral eye are sensitive to experience-dependent binocular interactions in the cortex, whereas contralateral eye maps depend solely on the quality of vision through that eye. Therefore, the role and mechanism of neural activity in regulating the specificity of connections is far more complex than previously thought, and might be divergent in different visual centres.
The plasticity of connections is also being addressed outside of the visual system. C. Cowan (Dallas, TX, USA) shed light on the mechanism of cocaine-induced structural changes in dendritic spines in the nucleus accumbens, and identified MEF2 as a crucial mediator of this process. Synaptic plasticity is an important regulator of circuit function. N. Nadif Kasri from the L. Van Aelst laboratory (Cold Spring Harbor, NY, USA) linked OPHN1—a Rho-GTPase activating protein—to the activity-dependent maturation and plasticity of excitatory synapses through its control of structural and functional stability. A defective OPHN1 function during early circuit development might contribute to X-linked mental retardation. Collaborative work from the laboratories of R. Klein (Munich, Germany) and E. Pasquale (La Jolla, CA, USA) presented by S. Paixao showed that glial glutamate transporters might regulate synaptic glutamate concentration—and thereby modulate long-term synaptic plasticity—in the hippocampus. This process seems to be regulated by the interaction between dendritic EphA4 and astrocytic ephrinA3 proteins.
Regeneration
It has long been appreciated that the axons of the adult CNS are not able to regenerate after injury, owing to both the hostile extrinsic environment—inhibitors associated with CNS myelin, proteoglycans in the glial scar and reinduced repulsive axon-guidance molecules—and the lack of growth potential of the neurons themselves. New progress in understanding both components was presented at the meeting. Three myelin-inhibitory proteins, MAG, Nogo and OMgp, are all thought to signal through a receptor complex that includes the NgR, P75 or TROY, and LINGO-1. However, NgR-knockout mice had only a modest improvement of regeneration. J. Pinkston-Gosse from the M. Tessier-Lavigne laboratory (San Francisco, CA, USA), in collaboration with the C. Shatz laboratory, identified PirB—an immune system receptor previously reported to be a neuronal receptor of MHC I (Syken et al, 2006)—as a high-affinity receptor for MAG and Nogo. PirB might therefore be another receptor that mediates myelin inhibition (Atwal et al, 2008). Interestingly, the Shatz laboratory has shown that PirB limits the amount of ocular dominance plasticity, not only during the critical period but also in adulthood (Syken et al, 2006). Together with work presented by S. Strittmatter (New Haven, CT, USA), which implicates NgR as a regulator of the critical period and plasticity during the development of ocular dominance maps, these studies suggest an intriguing mechanistic connection between neural plasticity and regeneration (McGee et al, 2005).
K. Park from the Z. He laboratory (Boston, MA, USA) reported a new discovery regarding the intrinsic growth potential of neurons. The He laboratory dug deeper into the gene programme that controls cell growth using a virus-assisted in vivo conditional knockout approach. They found that removing PTEN—a negative regulator of the mTOR pathway—in adult RGCs promoted robust axon regeneration. They also found that the mTOR pathway, which stimulates new protein synthesis related to growth, is indeed markedly suppressed after injury. Reactivating this pathway through the conditional knockout of TSC1, another inhibitor of the mTOR pathway, also led to significant axon regeneration. These findings open new avenues of research to promote regeneration (Park et al, 2008).
The problem of regeneration has spurred interest in using model systems that are more amenable to genetic manipulations. M. Hammarlund (New Haven, CT, USA) used a genetic screen to identify genes required for regeneration in C. elegans. The screen used a sensitized background of β-spectrin mutants, in which axons spontaneously break and regenerate. In this screen, a MAP kinase pathway was found to be essential for regeneration. Interestingly, the genes in this pathway—DLK-1, MKK-4 and PMK-3—are not required for axon outgrowth during development. By contrast, when axons break or are cut with a laser, these genes are required for regeneration.
Closing remarks
As in previous meetings, enormous amounts of information were exchanged during the four days with an intense programme of talks and poster sessions, as well as informal discussions. The focus of the axon-guidance field now seems to be on the mechanisms of wiring at all levels: the signalling mechanisms (particularly how growth cones integrate various guidance signalling pathways), the cell-biological mechanisms of growth-cone guidance, the mechanisms of how growth cones change responsiveness and how axon–axon interactions contribute to guidance. The search for molecular cues for setting up specific synaptic connections is still active and the molecular components involved in mediating neural plasticity are being identified. Extrinsic and intrinsic factors that block regeneration are being unveiled. Therefore, the next meeting in the series—to be held at Cold Spring Harbor in 2010—will surely feature new exciting breakthroughs in understanding how the nervous system wires itself.
abLIM actin-binding LIM domain protein
aPKC atypical protein kinase C
ARP2/3 seven-subunit protein complex that regulates the actin cytoskeleton, two of its subunits are actin-related proteins 2 and 3
BMP bone morphogenetic protein
CNS central nervous system
DLK-1 dual-leucine–zipper MAP KKK 1
Ena Enabled
EphA4 ephrin A4
Frz Frizzled
HEK human embryonic kidney
Hs2st heparin sulphate 2-O-sulphotransferase
Hs6st1 heparin sulphate 6-O-sulphotransferase
HSPG heparin sulphate proteoglycan
IP3 inositol triphosphate
LGN lateral geniculate nucleus
LINGO leucine-rich repeat and Ig domain-containing Nogo-receptor- interacting protein
LRRTM leucine-rich repeat transmembrane
MAG myelin-associated glycoprotein
MAP mitogen-activated protein
MEF2 myocyte-enhancer factor 2
MHC I major histocompatibility complex I
MKK-4 MAP kinase kinase 4
mTOR mammalian target of rapamycin
NgR Nogo receptor
NMDAR N-methyl-D-aspartate receptor
NMJ neuromuscular junction
Nogo also known as reticulon-4
NrCAM neuron-glia cell-adhesion molecule-related cell-adhesion molecule
OMgp oligodendrocyte-myelin glycoprotein
OPHN1 oligophrenin-1
P75 low-affinity neurotrophin receptor
PCP planar cell polarity
PirB paired immunoglobulin-like receptor B
PMK-3 p38 MAP kinase 3
PNS peripheral nervous system
PSD-95 postsynaptic density protein 95
PTEN phosphatase and tensin homologue
RGC retinal ganglion cell
Robo Roundabout
Ryk related to receptor tyrosine kinase
Sema3F semaphorin 3F
Shh Sonic hedgehog
SynCAM synaptic cell adhesion molecule
TAG-1 transiently expressed axonal glycoprotein-1
TRP transient receptor potential
TSC tuberous sclerosis complex
UNC uncoordinated
VASP vasodilator-stimulated phosphoprotein
vGlut1 vesicular glutamate transporter 1
Wnt Wingless-int-1

Esther Stoeckli

Yimin Zou
Acknowledgments
We thank A. Ghosh, C. Holt and A. Kolodkin for organizing such a stimulating and enjoyable meeting. We apologize to those speakers whose work we could not mention owing to space constraints, as well as to the authors of the excellent posters and the lively discussion around them that we were also unable to include in this report.
References
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS (2002) wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33: 545–558 [DOI] [PubMed] [Google Scholar]
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M (2008) PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science 322: 967–970 [DOI] [PubMed] [Google Scholar]
- Burnette DT, Ji L, Schaefer AW, Medeiros NA, Danuser G, Forscher P (2008) Myosin II activity facilitates microtubule bundling in the neuronal growth cone neck. Dev Cell 15: 163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ (2002) Molecular mechanisms of axon guidance. Science 298: 1959–1964 [DOI] [PubMed] [Google Scholar]
- Haghighi AP, McCabe BD, Fetter RD, Palmer JE, Hom S, Goodman CS (2003) Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron 39: 255–267 [DOI] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC (2000) Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 100: 525–535 [DOI] [PubMed] [Google Scholar]
- Henriquez JP, Webb A, Bence M, Bildsoe H, Sahores M, Hughes SM, Salinas PC (2008) Wnt signaling promotes AChR aggregation at the neuromuscular synapse in collaboration with agrin. Proc Natl Acad Sci USA 105: 18812–18817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen MP, Shen K (2007) Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell 130: 704–716 [DOI] [PubMed] [Google Scholar]
- Lauren J, Airaksinen MS, Saarma M, Timmusk T (2003) A novel gene family encoding leucine-rich repeat transmembrane proteins differentially expressed in the nervous system. Genomics 81: 411–421 [DOI] [PubMed] [Google Scholar]
- Liu Y, Shi J, Lu CC, Wang ZB, Lyuksyutova AI, Song X, Zou Y (2005) Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci 8: 1151–1159 [DOI] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y (2003) Anterior–posterior guidance of commissural axons by Wnt-frizzled signaling. Science 302: 1984–1988 [DOI] [PubMed] [Google Scholar]
- Marques G, Bao H, Haerry TE, Shimell MJ, Duchek P, Zhang B, O'Connor MB (2002) The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron 33: 529–543 [DOI] [PubMed] [Google Scholar]
- McCabe BD, Marques G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, Goodman CS, O'Connor MB (2003) The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron 39: 241–254 [DOI] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM (2005) Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science 309: 2222–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi M, Oinuma I, Katoh H (2005) Plexins: axon guidance and signal transduction. Cell Mol Life Sci 62: 1363–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V (2002) The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell 111: 319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z (2008) Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322: 963–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas PC, Zou Y (2008) Wnt signaling in neural circuit assembly. Annu Rev Neurosci 31: 339–358 [DOI] [PubMed] [Google Scholar]
- Stoeckli ET, Landmesser LT (1995) Axonin-1, Nr-CAM, and Ng-CAM play different roles in the in vivo guidance of chick commissural neurons. Neuron 14: 1165–1179 [DOI] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO, Shatz CJ (2006) PirB restricts ocular-dominance plasticity in visual cortex. Science 313: 1795–1800 [DOI] [PubMed] [Google Scholar]
- Wolf AM, Lyuksyutova AI, Fenstermaker AG, Shafer B, Lo CG, Zou Y (2008) Phosphatidylinositol-3-kinase-atypical protein kinase C signaling is required for Wnt attraction and anterior–posterior axon guidance. J Neurosci 28: 3456–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR, Weiner JA (2003) Synaptic adhesion molecules. Curr Opin Cell Biol 15: 621–632 [DOI] [PubMed] [Google Scholar]
- Yazdani U, Terman JR (2006) The semaphorins. Genome Biol 7: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X-F, Schaefer AW, Burnette DT, Schoonderwoert VT, Forscher P (2003) Rho-dependent contractile responses in the neuronal growth cone are independent of classical perpheral retrograde actin flow. Neuron 40: 931–944 [DOI] [PubMed] [Google Scholar]
- Zou Y, Lyuksyutova AI (2007) Morphogens as conserved axon guidance cues. Curr Opin Neurobiol 17: 22–28 [DOI] [PubMed] [Google Scholar]
- Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M (2000) Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell 102: 363–375 [DOI] [PubMed] [Google Scholar]