The Cantoblanco Workshop on the Initiation of Antigen Receptor Signaling took place between 20 and 22 October 2008, in Cantoblanco, Madrid, Spain, and was organized by B. Alarcón, M. Davis & A. Weiss.
Glossary
Introduction
In the back of our minds, we all know how antigen receptors trigger the activation of T- and B-lymphocytes; it seems relatively well worked out. The binding of an antigen to the T-cell receptor (TCR) and the B-cell receptor (BCR) results in the phosphorylation of the cytoplasmic domains of these receptors, and off the cells go. Yet how exactly does that happen?
For a group of researchers gathered in Madrid, Spain, in the autumn of 2008, that was the question. How does antigen binding to the ectodomains of the TCR and the BCR change the receptors so as to initiate intracellular signalling? Since the discovery of the TCR and the BCR, immunologists have learned a tremendous amount about the signalling cascades that are triggered by antigen binding and ultimately lead to T-cell and B-cell activation. However, the mechanisms that translate antigen binding into intracellular signalling remain among the real mysteries of adaptive immunity. How can that be if the mechanisms underlying the activation of a range of receptors—such as growth factor receptors—have been well worked out? In contrast to these conventional receptors, several unique characteristics of antigen receptors suggest that their inner workings are more complicated, including the enormous variability of their antigen-binding domains and the need to learn to discriminate self from non-self. How do the numerous unique interactions of antigens with the TCRs and BCRs expressed by each lymphocyte converge on a common change in the intracellular domains of the receptors that is sensitive to foreign antigens, but not self?
To foster a discussion of antigen-receptor activation, B. Alarcón, M. Davis and A. Weiss brought together a group of researchers who take diverse approaches to the topic, including the characterization of protein structure, genetic analyses, mathematical modelling and live-cell imaging. During the three days of the workshop, there seemed to be a growing belief that in order to initiate signalling, antigen receptors must undergo specific, antigen-induced structural changes; however, the nature of these changes largely remains unknown. Solving the conundrum of whether TCRs and BCRs undergo changes in conformation brings to mind the photographs of Eadweard Muybridge. In the late 1800s, Muybridge used an ingenious set-up consisting of lenses and cameras to capture the progressive movement of animals and humans. He produced images that showed what could be considered ‘conformational changes' that could not be perceived by the unaided eye. One of his earliest images, which was produced in 1878, was commissioned by Leland Stanford to settle a debate among the horsemen of the American ‘new West': whether, in a gallop, all four hooves of a horse were ever off the ground at the same time (Fig 1). They were indeed—end of debate (for more information, see http://americanhistory.si.edu/muybridge/htm/htm_sec1/sec1.htm). It seems that we will need to have far clearer progressive images of the silhouettes of immune–receptor complexes in their native environment—the plasma membranes of T and B cells—as they bind to antigens before we will know whether antigen-induced conformational changes do indeed occur, putting an end to our debate.
Figure 1.
The Horse in Motion by Eadweard Muybridge. All four hooves leave the ground as the horse gallops, as can be seen in frames 2 and 3 (http://americanhistory.si.edu/muybridge/htm/htm_sec1/sec1.htm).
Here, we review a small subset of the many excellent presentations at the meeting that focused most closely on the earliest steps in antigen-receptor activation. This omits many great talks such as those describing the interwoven signalling pathways emanating from the receptors. However, by limiting our scope, we hope to serve better the core issues of the workshop—the initial events in the antigen receptors that are triggered by antigen binding.
Models of the TCR architecture
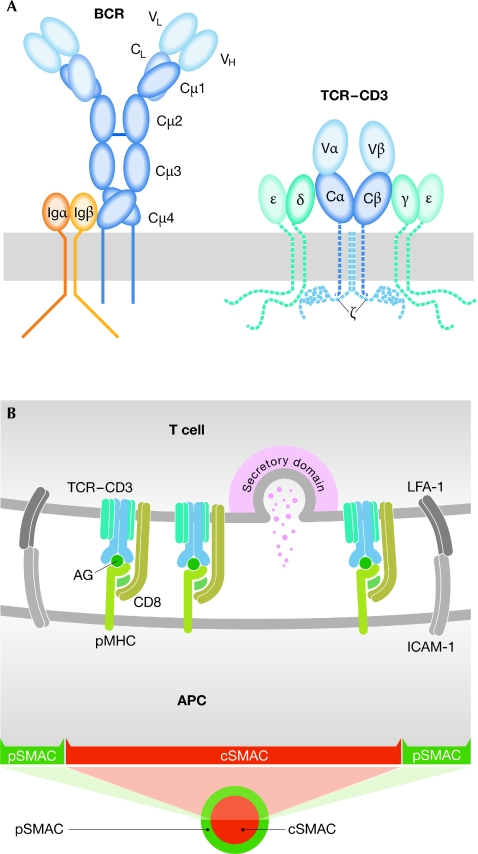
The αβ-TCR is the most complex of the antigen receptors. It consists of the TCR-αβ heterodimer that binds to the pMHC, two CD3 heterodimers—CD3-γδ and CD3-γε—and one CD3-ζ homodimer (Fig 2A). Initially, the only component of the TCR for which there was a high-resolution structure was the TCR-αβ heterodimer, both alone and bound to pMHC complexes. Although these studies were seminal to our understanding of the structural basis of TCR–pMHC recognition, they revealed little about the mechanism of TCR activation. However, recent efforts have resulted in a growing list of structures of the remaining TCR components: the extracellular domains of both the CD3-γε and the CD3-δε heterodimers (Sun et al, 2004), the homodimer of CD3-ζ transmembrane domains (Call et al, 2006) and recently the cytoplasmic domain of CD3-ε, which we discuss separately below. With the structure of the extracellular domains of the TCR components available, it seems that the time is ripe to probe the overall architecture of the complex.
Figure 2.
Antigen receptors and immune synapse. (A) Schematic structures of the B-cell and T-cell antigen receptors. (B) Scheme of the immune synapse, indicating its bull's eye pattern. AG, antigenic peptide; APC, antigen-presenting cell; BCR, B-cell receptor; CD, cluster of differentiation; cSMAC, central supramolecular activation cluster; ICAM-1, intercellular adhesion molecule 1; LFA-1, leukocyte functional antigen 1; pMHC, peptide–major histocompatibility complex; pSMAC, peripheral supramolecular activation cluster; TCR, T-cell receptor.
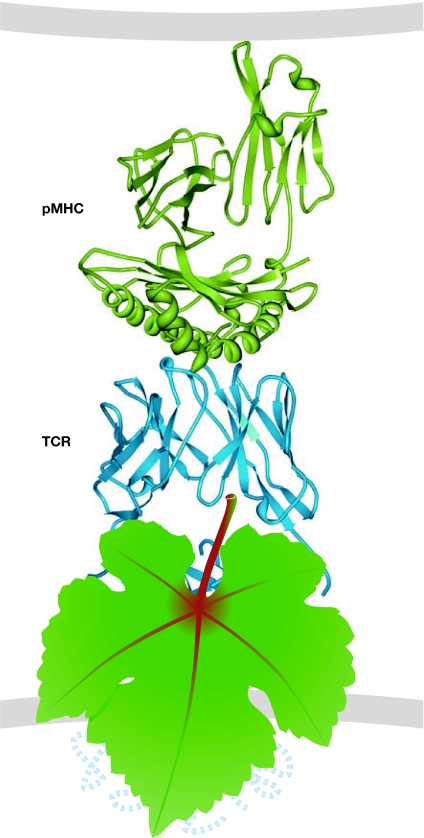
E. Reinherz (Boston, MA, USA) proposed a model of the complete TCR complex based on in silico docking of the available TCR-αβ and CD3 structures (Sun et al, 2004). This model suggests that the CD3 subunits bind to opposite sides of the TCR-αβ, with CD3-γε interacting with the Cβ domain and CD3-γδ interacting with the Cα domain. An alternative model, based on the results of mutational studies of the TCR-αβ constant domains, predicts that the binding sites of TCR-α for CD3-γδ and of TCR-β for CD3-δε might be close to each other, positioning both CD3 heterodimers on one side of the TCR-αβ (Kuhns & Davis, 2007). It seems that the discrepancies between these two models—as well as the numerous previous models of TCR–CD3 association—will be adequately resolved only by a direct visualization of the whole TCR complex, which is currently hidden from our view (Fig 3).
Figure 3.
The unknown T-cell receptor. The structure of a TCR (blue) bound to a pMHC complex (green) on an APC surface. This structure was originally published by Garboczi et al (1996) and its Protein Data Bank accession code is . A grape leaf covers the private region of the TCR. APC, antigen-presenting cell; pMHC, peptide–major histocompatibility complex; TCR, T-cell receptor.
As reported at the meeting, we might be close to seeing the TCR complex by electron microscopy. J. Valpuesta (Madrid, Spain) isolated the full TCR complex by detergent lysis from cells, and was able to locate the TCR-αβ and CD3-εγ subunits in electron-microscopy images of the protein particles by labelling them with specific antibodies. The details of the interactions between the TCR-αβ chains and the CD3-εγ were still obscured by the limited resolution; however, although it is early days, the approach seems promising.
The role of extracellular domains in signalling
One of the main intellectual underpinnings for the models of antigen-receptor signalling was the observation that antigen binding to the hypervariable loops of the immunoglobulins and of TCR-αβ in solution does not propagate allosteric changes to the constant domains of the receptors. As a result, most of these models considered the extracellular domains of the receptors to be inert in terms of signal transduction. The predominant thinking in the field was that antigen binding serves only to bring into close proximity or to cluster either two or more antigen receptors, or an antigen receptor and a co-receptor. Although there is a large body of evidence showing that physical crosslinking of antigen receptors can initiate signalling, how such crosslinking can be achieved by the binding of apparently monovalent ligands, under the physiological conditions of interactions of B and T cells with antigens on APC surfaces, is not clear.
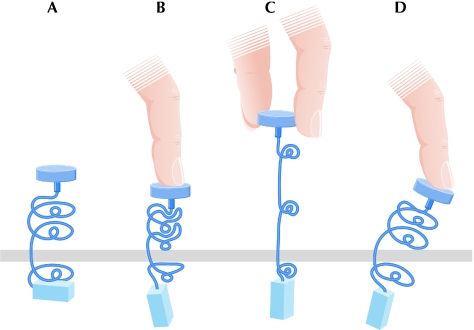
Is there support for a model of signal transduction elicited by conformational changes in antigen receptors? Specific proof of a conformational change in the TCR was provided by the demonstration that the binding of the pMHC to the TCR exposes a binding site for the cytoplasmic signalling adaptor Nck in the intracellular domains of CD3-ε (Gil et al, 2002). However, the mechanism by which binding of the pMHC initiates structural changes in the cytoplasmic domains remains unknown. Several presentations focused on this enigma and two general possibilities emerged: the binding of the pMHC might induce yet unseen changes in the TCR complex directly through changes in the hypervariable loops (Fig 4A,B) or might apply a force that would induce changes in the TCR complex distal to the pMHC binding site (Fig 4A,C). The idea of a pulling force on the TCR during the movement of a T cell over the APC—possibly enhanced by active cytoskeletal forces (van der Merwe, 2001)—has recently received considerable attention (Ma et al, 2008). This mechanism could translate the highly variable interactions of TCRs with their respective pMHC complexes to a common change in the constant domains of the TCR, and seemed appealing to the attendees of the meeting. However, there is still no direct evidence for a force-induced change in the TCRs.
Figure 4.
Ways to think about antigen-induced conformational changes in immune receptors; three types of conformational change induced by antigen binding that are propagated across the membrane. (A) The receptor at rest. (B) Conformational changes induced in the antigen-interaction site. (C) Conformational changes induced by forces created by APC-associated antigens ‘pulling' on the receptor. (D) Changes in a receptor composed of rigid subunits, on which the antigen pushes as a lever. APC, antigen-presenting cell.
In order for a force to induce a conformational change, there must be conformational flexibility in the receptor. There could be movement of the TCR-αβ and the CD3 subunits with respect to each other (Fig 4D). As the CD3 subunits form rigid dimers through the interaction of their G strands and interdomain residues, they could be used as mechanical lateral supports to transduce pMHC ligation signals from the TCR-αβ. Reinherz proposed that the FG loop of the TCR Cβ ectodomain, which protrudes out of the domain and possibly rests on the top of the CD3-γε heterodimer, could act as a lever that could push on the CD3-εγ piston. Reinherz and colleagues used nuclear magnetic resonance to localize the binding of several monoclonal antibodies to CD3, and found that Fabs that bound to the top of the CD3-ε stimulated T cells, whereas Fabs that bound to the cleft between CD3-ε and CD3-γ did not. These observations suggest that there are specific physical forces or torques applied to the CD3-εγ heterodimer that are necessary for signal transduction.
B. Alarcón (Madrid, Spain) analysed the conformational flexibility of the extracellular domain of CD3-εδ by modelling the vibrations in the CD3-εδ dimer. These simulations showed that the binding of an agonist antibody to CD3-ε stiffened not only the antibody-binding site at the upper loops of the CD3-ε, but also the membrane-proximal region of the heterodimer, including the stalk residues that connect the extracellular domains to the transmembrane helix. Mutational analysis of the membrane-proximal region of CD3-ε revealed that the mutation of the first cysteine in a CXXC motif in the stalk or of a nearby lysine had a strong negative effect on the conformational change of the CD3 cytoplasmic domains after TCR stimulation, as measured by Nck binding in a pull-down assay. These mutations also severely impaired the phosphorylation of TCR-ζ and IL-2 production. Alarcón also showed that mice expressing the mutated form of CD3-ε on a CD3-ε-deficient background had a severe block of T-cell development in the thymus—at the pre-T-cell stage—and compromised TCR signalling in peripheral T cells. He pointed out that a strong phenotype could be observed even in a CD3-ε-sufficient background, in which the mutated CD3-ε was expressed at only about 10% of the level of the wild-type CD3-ε. This alludes to the possibility that the conformational changes occur cooperatively in several TCRs, for example by conformational spreading in TCR clusters. Along the same lines, J. Kappler (Denver, CO, USA) mutated the cysteine residues of the CXXC motif in CD3-ε stalks to serines, and showed that mice expressing this mutated form of CD3-ε had a partial block in T-cell development at the pre-TCR signalling checkpoint, and a reduced efficiency of the activation of Erk after the stimulation of peripheral T cells by crosslinking the TCRs. Eventually, any changes induced in the extracellular domains of the TCR by pMHC binding have to be transduced across the plasma membrane to the cytoplasmic domains. The results of Alarcón and Kappler indicate that the stalks of the CD3 dimers have an important role in this process.
Shifting the focus to the BCR, S. Pierce (Rockville, MD, USA) provided evidence for a model of BCR activation in which antigen-induced changes in the extracellular domains of the membrane immunoglobulin of the BCR lead to BCR clustering (Tolar et al, 2009). By using single-molecule tracking of the BCR after the engagement of membrane antigens, Pierce showed that the formation of signalling-active BCR clusters and B-cell activation depended on the presence of the Cμ4 membrane proximal domain of the constant region of the immunoglobulin heavy chain, as well as on a motif in the transmembrane helices. Conversely, an amino-terminally truncated membrane immunoglobulin containing only the Cμ4 domain spontaneously clustered and activated B cells. These findings suggest that BCR clustering and signalling are regulated by antigen-mediated changes in the membrane immunoglobulin of the BCR.
Structure of the intracellular domains
What type of changes in the cytoplasmic domains of the receptors might be elicited by antigen-induced changes in the ectodomains? In answering this question, we are hampered by the limited knowledge about the structure of the cytoplasmic domains. So far, the cytoplasmic domains of all antigen receptors have been refractory to structural analyses, as they typically behave as unstructured peptides when analysed in solution. However, it is possible that the cytoplasmic domains present in the full antigen–receptor complexes at the plasma membrane are much more structured than previously thought. In the case of the BCR, fluorescence resonance-energy transfer analyses suggested that antigen binding induces changes in the conformation of the cytoplasmic domains related to phosphorylation of ITAMs (Tolar et al, 2005). Studies of the TCR-ζ chain showed that the cytoplasmic domain of TCR-ζ adopts a partly helical structure when analysed bound to negatively charged lipids, such as those found in the cytoplasmic leaflet of the plasma membrane (Aivazian & Stern, 2000). K. Wucherpfennig (Boston, MA, USA) presented the nuclear magnetic resonance structure of a large part of the cytoplasmic domain of CD3-ε bound to lipid bicelles, which confirmed that numerous basic residues of the CD3-ε bind to the charged lipid head groups (Xu et al, 2008). Remarkably, the structure also revealed that the ITAM tyrosines point into the bilayer, with their hydroxyl groups completely embedded in its hydrophobic core. The cytoplasmic domain therefore has to dissociate from the membrane to allow Src kinases to phosphorylate the ITAMs. This structure also showed that the N-terminal region of the cytoplasmic domain—which connects it to the transmembrane helix—is flexible, making it unlikely that a mechanical force could readily dislodge the ITAM from the membrane. A possibility raised by Wucherpfennig was that the interactions of the CD3-ε cytoplasmic domains with the plasma membrane might be regulated by changes in the local lipid composition. These findings suggest that there are unique structural switches in the cytoplasmic domains of antigen receptors that regulate the transition from an inactive state to an active state.
Membrane organization of antigen receptors
Since the discovery of the immunological synapse—a structure induced by the engagement of the antigen receptors in contact with an APC, which leads to the reorganization of the lymphocyte surface proteins in an intriguing bull's eye-like pattern—there has been intense interest in the composition of synapses, the mechanisms by which they form, and the relationship between synapse formation and cell activation (Fig 2B). Recent studies have shown that receptor microclusters—which ultimately concentrate in the cSMAC—form during the spreading of lymphocytes on APC surfaces. These antigen-induced receptor microclusters move by the treadmilling of actin fibres that extend from the leading edges of the lamellipodia towards the centre of the contact area. Eventually, the microcluster movement produces the segregation of the immune synapse into the pSMAC and cSMAC areas. M. Dustin (New York, NY, USA) pointed out that the architecture of retrograde actin flow in T-cell synapses resembles a migrating cell that extends lamellipodia at the leading edge and retracts its uropod at the trailing edge. The symmetry of the synapse, however, can be disturbed, resulting in a mobile structure that Dustin referred to as a kinapse (Sims et al, 2007). In vivo, the sequential formation of synapses has been observed in the initial phases of the T-cell immune response and might be important for T cells to integrate signals from many APCs.
Dustin also showed that synapses do not always develop clear pSMAC and cSMAC segregation. In response to a strong agonist, the TCRs in the cSMAC recruit the ESCRT complex, which binds to ubiquitinated membrane proteins to target them for degradation. Blocking ESCRT-complex recruitment by knocking down the ubiquitin-binding component TSG101 blocked TCR incorporation into the cSMAC and led to a tenfold increase in TCR signalling in a peri-cSMAC area. By contrast, weak ligands induced TCR microclusters that spontaneously dissolved in the cSMAC, without recruitment of the ESCRT complex. These results help to explain the mechanism by which the organization of the TCR in the synapse modulates T-cell activation.
Co-stimulation
Additional complexity in the initiation of antigen-receptor signalling comes from its regulation by co-receptors, which assures the lymphocyte that the antigen is a valid target and is presented in an immunologically meaningful context. In T cells, one of the crucial co-receptors is CD28, which recognizes CD80 on professional APCs. By using spectacular total internal reflection-fluorescence images, T. Saito (Osaka, Japan) showed that when engaged with CD80 in synapses, CD28 is recruited to the TCR microclusters and moves with them to a distinct ring outside of the cSMAC (Yokosuka et al, 2008). Interestingly, the same ring is the site of recruitment of the inhibitory counterpart of CD28, CTLA4, which is delivered to these sites from intracellular lysosomal compartments. The co-localization of CD28 and CTLA4 in the peripheral ring of the cSMAC indicates that this is where these receptors compete for dominance of the positive or negative signalling.
F. Batista (London, UK) showed that CD19 has an essential role in the activation of B cells, by enhancing calcium flux in response to membrane antigens (Depoil et al, 2008). CD19 serves as a LAT-like adaptor to recruit Vav and PI(3)K to BCR microclusters. Indeed, single-particle tracking with dual-colour acquisition showed a dynamic association of CD19 with BCR microclusters, leading to the amplification of signalling through the BCR. Further investigation of the spatial and temporal dynamics of CD19 on the cell surface will probably lead to new insights into both the initiation of BCR signalling and the role of CD19 in B-cell activation.
Conclusions
The Cantoblanco workshop left many of us feeling that, despite the latest endeavours in the exploration of antigen-mediated activation of lymphocytes, the mechanism of antigen-receptor activation remains a puzzle. However, thanks to the progress made in understanding the structure of antigen-receptor components, we are getting closer to a complete picture of the full antigen-receptor complex, which will probably be a cornerstone for the mechanistic understanding of the activation process triggered by antigen binding. The structures of the antigen receptors along with live-cell microscopy should provide images in our time that are every bit as exciting as those produced by Muybridge in the late 1800s.
APC antigen-presenting cell
CD cluster of differentiation
cSMAC central supramolecular activation cluster
CTLA4 cytotoxic T lymphocyte antigen 4
Erk extracellular signal-regulated kinase
ESCRT endosomal sorting complex required for transport
Fab fragment antigen binding; a fragment of an antibody that includes the region that binds to antigen
IL-2 interleukin 2
ITAM immunoreceptor tyrosine-based activation motif
LAT linker of activated T cells
Nck non-catalytic region of tyrosine kinase
PI(3)K phosphatidylinositol-3-kinase
pMHC peptide–major histocompatibility complex
pSMAC peripheral supramolecular activation cluster
TSG101 tumour-suppressor gene 101
Pavel Tolar & Susan K. Pierce
Acknowledgments
We thank the organizers, B. Alarcón, M. Davis and A. Weiss, for a stimulating and delightful conference, the speakers for permission to refer to unpublished data and C. Shields for reminding us of the Muybridge images. We apologize to the speakers whose talks we could not mention owing to space constraints.
References
- Aivazian D, Stern LJ (2000) Phosphorylation of T cell receptor ζ is regulated by a lipid dependent folding transition. Nat Struct Biol 7: 1023–1026 [DOI] [PubMed] [Google Scholar]
- Call ME, Schnell JR, Xu C, Lutz RA, Chou JJ, Wucherpfennig KW (2006) The structure of the zetazeta transmembrane dimer reveals features essential for its assembly with the T cell receptor. Cell 127: 355–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoil D, Fleire S, Treanor BL, Weber M, Harwood NE, Marchbank KL, Tybulewicz VL, Batista FD (2008) CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat Immunol 9: 63–72 [DOI] [PubMed] [Google Scholar]
- Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, Wiley DC (1996) Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature 384: 134–141 [DOI] [PubMed] [Google Scholar]
- Gil D, Schamel WW, Montoya M, Sanchez-Madrid F, Alarcon B (2002) Recruitment of Nck by CD3ε reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell 109: 901–912 [DOI] [PubMed] [Google Scholar]
- Kuhns MS, Davis MM (2007) Disruption of extracellular interactions impairs T cell receptor-CD3 complex stability and signaling. Immunity 26: 357–369 [DOI] [PubMed] [Google Scholar]
- Ma Z, Sharp KA, Janmey PA, Finkel TH (2008) Surface-anchored monomeric agonist pMHCs alone trigger TCR with high sensitivity. PLoS Biol 6: e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims TN et al. (2007) Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell 129: 773–785 [DOI] [PubMed] [Google Scholar]
- Sun ZY, Kim ST, Kim IC, Fahmy A, Reinherz EL, Wagner G (2004) Solution structure of the CD3εδ ectodomain and comparison with CD3εγ as a basis for modeling T cell receptor topology and signaling. Proc Natl Acad Sci USA 101: 16867–16872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolar P, Hanna J, Krueger PD, Pierce SK (2009) The constant region of the membrane immunoglobulin mediates B cell-receptor clustering and signaling in response to membrane antigens. Immunity 30: 44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolar P, Sohn HW, Pierce SK (2005) The initiation of antigen-induced B cell antigen receptor signaling viewed in living cells by fluorescence resonance energy transfer. Nat Immunol 6: 1168–1176 [DOI] [PubMed] [Google Scholar]
- van der Merwe PA (2001) The TCR triggering puzzle. Immunity 14: 665–668 [DOI] [PubMed] [Google Scholar]
- Xu C, Gagnon E, Call M, Schnell J, Schwieters C, Carman CV, Chou J, Wucherpfennig K (2008) Regulation of T cell receptor activation by dynamic membrane binding of the CD3ε cytoplasmic tyrosine-based motif. Cell 135: 702–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka T, Kobayashi W, Sakatasogawa K, Takamatsu M, Hashimototane A, Dustin M, Tokunaga M, Saito T (2008) Spatiotemporal regulation of t cell costimulation by tcr-cd28 microclusters and protein kinase C Θ translocation. Immunity 29: 589–601 [DOI] [PMC free article] [PubMed] [Google Scholar]