Abstract
Reactivation of stalled replication forks requires specialized mechanisms that can recognize the fork structure and promote downstream processing events. Fork regression has been implicated in several models of fork reactivation as a crucial processing step that supports repair. However, it has also been suggested that regressed forks represent pathological structures rather than physiological intermediates of repair. To investigate the biological role of fork regression in bacteriophage T4, we tested several mechanistic models of regression: strand exchange-mediated extrusion, topology-driven fork reversal and helicase-mediated extrusion. Here, we report that UvsW, a T4 branch-specific helicase, is necessary for the accumulation of regressed forks in vivo, and that UvsW-catalysed regression is the dominant mechanism of origin-fork processing that contributes to double-strand end formation. We also show that UvsW resolves purified fork intermediates in vitro by fork regression. Regression is therefore part of an active, UvsW-driven pathway of fork processing in bacteriophage T4.
Keywords: DNA damage, fork regression, helicase, replication
Introduction
It has become evident that replication forks routinely stall and become inactivated (Mirkin & Mirkin, 2007). Inactivation probably involves the dissociation of replisome components, which presumably facilitates DNA repair pathways. However, exposed fork structures are inherently fragile, contributing to the formation of DNA breaks, genome instability and cell death. Reactivation of stalled forks requires specialized processing mechanisms that can both recognize the fork structure and facilitate reloading of the replisome.
Several reports argue that replication fork regression can be involved in fork processing pathways in vivo (Michel et al, 2001; Courcelle et al, 2003; Long & Kreuzer, 2008). Fork regression involves extrusion and annealing of leading and lagging strands from the template DNA, resulting in a double-stranded DNA end (DSE) and a Holliday junction. Some models of fork reactivation invoke fork regression as a crucial processing step, including the strand switching-mediated lesion bypass (Higgins et al, 1976), replisome/branch clearance for lesion repair (McGlynn & Lloyd, 2002) and recombination-dependent replication (Michel et al, 2001).
Other studies have suggested that regressed forks represent pathological structures, rather than physiological intermediates of repair (Lopes et al, 2001; Sogo et al, 2002; Meister et al, 2005). The accumulation of aberrant DNA structures—including regressed forks—is correlated with cell death in rad53 mutant (checkpoint-defective) budding yeast cells, which led to the suggestion that stalled forks are normally stabilized during the checkpoint response to prevent spontaneous regression (Lopes et al, 2001; Sogo et al, 2002; Yoon et al, 2004). However, an alternative interpretation is that stalled forks are actively regressed to facilitate reactivation, but that regressed forks are extremely transient intermediates in wild-type cells. In this view, the accumulation of regressed intermediates in the rad53 mutant is due to a defect in regressed fork processing by some Rad53-dependent pathway.
Understanding the mechanism of fork regression should shed light on its biological role—that is, pathological structures versus physiological intermediates. Although the requirements for in vivo fork regression are still unknown, several models of regression have emerged, mostly from in vitro experiments.
(i) Strand exchange-mediated extrusion. Strand exchange proteins might localize to regions of single-stranded DNA within stalled fork structures and promote strand exchange between the two arms of a fork intermediate, leading to extrusion and annealing of the two daughter strands. This reaction has been shown in vitro on model fork structures with Escherichia coli RecA (Robu et al, 2001), human Rad51 (Yoon et al, 2004) and bacteriophage T4 UvsX (Kadyrov & Drake, 2004). Strand exchange between fork arms has also been proposed to function in fork stability by preventing regression. In this case, a limited region of strand exchange is proposed to link the fork arms together and prevent spontaneous fork regression or inappropriate recombination reactions (Donaldson et al, 2006).
(ii) Topology-driven fork reversal. During replication fork progression, positive supercoils can accumulate ahead of the fork if topoisomerase activity is insufficient (for review, see Schvartzman & Stasiak, 2004). This positive supercoiling can be relieved by spontaneous fork regression, as shown in vitro with partly replicated plasmid intermediates (Postow et al, 2001).
(iii) Helicase-mediated extrusion. Several DNA helicases have been shown to promote regression of model forks in vitro (McGlynn et al, 2001; Machwe et al, 2006; Ralf et al, 2006; Blastyák et al, 2007; Li et al, 2008). In addition, Michel et al (2001) have implicated the E. coli helicase RecG in a RuvAB-mediated fork regression pathway in vivo based on genetic results and an indirect assay involving RuvC-dependent chromosomal breakage. Similar approaches argue that RuvAB participates in fork regression, either through regressed fork stabilization (Seigneur et al, 1998) or by directly catalysing fork regression (Masson et al, 2008).
To analyse pathways of fork processing in bacteriophage T4, we have used the ori(34) origin-fork as a model fork intermediate (Long & Kreuzer, 2008). The origin-fork is formed naturally during T4 infection when one fork exits the origin region but the retrograde fork has not yet started. The amount of origin-fork detected as a function of time presumably depends on various factors such as the efficiency of origin R-loop formation and removal, the efficiency of leading-strand priming from the R-loop and the efficiency of retrograde fork initiation. Retrograde fork initiation is similar to stalled fork reactivation, and thus the origin-fork allows simplified analysis of fork processing mechanisms without the complications of replication inhibitors or DNA damage. Previously, we have shown that the origin-fork undergoes regression in vivo, and that regression supports two mechanisms of fork processing (Long & Kreuzer, 2008).
Here, we show that the T4-encoded UvsW protein is necessary for the accumulation of regressed origin-fork intermediates in vivo and catalyses fork regression in vitro. UvsW is a T4 branch-specific helicase that shares structural homology to the eukaryotic SF2 helicase, Rad54 (Kerr et al, 2007). We also show that UvsW-driven fork regression is the dominant mechanism of DSE formation at the origin-fork. These results argue that regression is part of a biologically relevant pathway of fork processing in bacteriophage T4.
Results
uvsW is required for accumulation of regressed forks
Previously, we have detected regressed origin-forks at early times of T4 infection, and observed that the amount of regressed forks increased in a gene 46 mutant infection (Long & Kreuzer, 2008). Gene 46 encodes an essential component of the gp46/47 exonuclease–ATPase complex, which is a member of the Rad50/Mre11 family. gp46/47 is known to degrade DSEs in vivo (see Kreuzer, 2000), explaining the increase in regressed origin-fork accumulation. Therefore, we used a 46− background to test particular gene mutations for their effect on regressed origin-fork accumulation.
To test each of the three models of fork regression (see above), we introduced uvsX, gene 39, dda and uvsW mutations into the T4 46− background. uvsX encodes the T4 strand-exchange protein, a member of the RecA/Rad51/RadA family of recombinases. Gene 39 encodes a required subunit of the T4 type II topoisomerase. According to the topology model, mutation of gene 39 might be expected to increase fork regression owing to the accumulation of unresolved positive DNA supercoils. T4 encodes three DNA helicases: gp41, Dda and UvsW. gp41 is the replicative helicase and is required for extensive, coordinated replication of both the leading and lagging strands. Therefore, we could not investigate whether regressed origin-forks accumulate in the absence of gp41. Dda is a well-characterized DNA helicase, the in vivo function of which is unclear. UvsW, a T4 helicase that promotes branch migration of Holliday junctions (Webb et al, 2007), is perhaps the best candidate for an enzyme that catalyses fork regression. This protein was shown to resolve Y-shaped DNA intermediates in vitro, but the intermediates and products were not identified and thus the mechanism of resolution was ambiguous.
DNA intermediates from each mutant infection were assayed for regressed fork accumulation using two-dimensional agarose gel electrophoresis (Fig 1). In two-dimensional gels, regressed origin-fork intermediates form an arc that is similar in appearance to the X-arc but that originates from the origin-fork position on the Y-arc (Fierro-Fernandez et al, 2007; Long & Kreuzer, 2008).
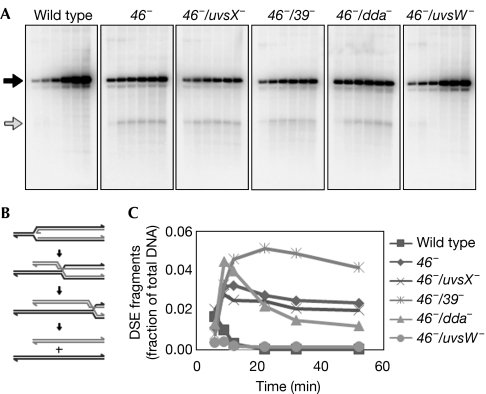
Figure 1.
Requirements for origin-fork regression in vivo. (A) Total DNA from the indicated T4 infections was digested with PacI and separated by two-dimensional gel electrophoresis. The time point shown for each infection was the time of maximal origin-fork accumulation (see Long & Kreuzer, 2008; complete time courses in supplementary Fig 1 online). Gels were visualized by Southern blot with a probe specific to the ori(34) region. (B) The two-dimensional gel region containing the regressed origin-fork arc is shown at increased magnification and contrast, with the regressed origin-fork arc indicated by an arrow.
Mutation of genes uvsX, 39 or dda had no apparent effect on regressed origin-fork accumulation (Fig 1). Although these results indicate that UvsX and Dda are not required for fork regression in vivo, they do not rule out the possibility that topology has a function in the regression of authentic stalled replication forks (as opposed to the origin-forks studied here). The most notable result is that the 46−/uvsW− infections showed no discernible accumulation of regressed origin-fork intermediates, showing directly that UvsW is required for regression in vivo (Fig 1).
Previously, we found that in a 46−/49− background, regressed origin-forks accumulate to a level about twice that found in 46− infections (Long & Kreuzer, 2008). This result implied that the product of gene 49, EndoVII, cleaves Holliday junctions formed at some of the regressed origin-forks. The triple mutant 46−/49−/uvsW− showed no discernible accumulation of regressed origin-forks (supplementary Fig 1 online), supporting the conclusion that UvsW is required for regression in vivo.
uvsW is required for accumulation of ori(34) fragments
Fork stalling is often associated with the appearance of DSEs, presumably owing to fork processing by any of several possible mechanisms: fork breakage, regression and head-to-tail fork collisions. Previously, we have shown that origin-fork accumulation is associated with the formation of DSEs, with a 1.48-kb DSE fragment resulting from restriction enzyme digestion with AseI (Long & Kreuzer, 2008). This DSE fragment could be generated by origin-fork regression if the Holliday junction created by regression undergoes branch migration past the AseI restriction site (Fig 2B).
Figure 2.
Accumulation of double-stranded DNA end fragments in the ori(34) region. (A) Total DNA from the indicated T4 infections at 6, 9, 12, 22, 32 and 52 min post-infection was digested with AseI and separated by agarose gel electrophoresis. DNA was visualized by Southern blot with a probe specific to the region downstream from ori(34). Full-length restriction fragments and the 1.48-kb DSE fragment are indicated by black and gray arrows, respectively. (B) Schematic diagram depicting the formation of DSE fragments following origin-fork regression and extensive HJ branch migration. (C) Relative DSE fragment accumulation over time for each infection as a fraction of total DNA (average of three experiments). DSE, double-stranded DNA end; HJ, Holliday junction.
To test the requirements for DSE formation, we analysed the accumulation of ori(34) DSE fragments in 46− infections—as noted above, inactivation of gene 46 prevents degradation of DSEs (Fig 2). DSE fragments accumulated in the 46−/uvsX−, 46−/39− and 46−/dda− infections to levels similar to that of a 46− single mutant infection. However, 46−/uvsW− infections were almost completely deficient for DSE fragment accumulation, implying that UvsW-catalysed regression is the dominant mechanism of origin-fork processing that leads to DSE formation.
UvsW protein resolves origin-forks by regression in vitro
Previously, we reported that UvsW is able to resolve Holliday junction-containing substrates in vitro by promoting extensive branch migration (Webb et al, 2007). In that study, we also found that UvsW resolves Y-shaped intermediates in vitro. However, as the substrate was total DNA from a T4 infection—containing various T4-modified DNA intermediates—we could not readily identify the products or intermediates of resolution.
To analyse the mechanism of fork resolution by UvsW, we introduced inter-strand DNA crosslinks into total DNA before treatment with UvsW protein and visualization by a two-dimensional gel (supplementary Fig 2 online). Increasing amounts of crosslinks prevented the complete resolution of both X- and Y-shaped intermediates. In addition, a collection of partly resolved intermediates accumulated within a cone-shaped region between the X- and Y-arcs. This accumulation is consistent with the expected migration pattern for partly regressed fork structures of various sizes (see schematic diagram, supplementary Fig 2 online; Fierro-Fernandez et al, 2007).
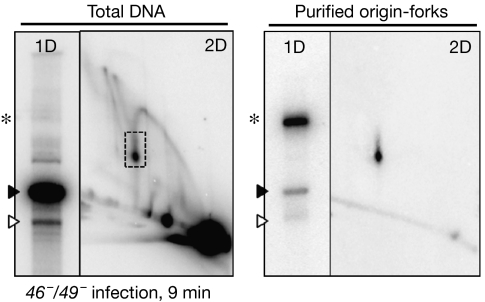
To perform a simpler analysis on the mechanism of fork resolution by UvsW, we purified the origin-fork intermediate so that it became the main DNA form detected by Southern blotting with an ori(34) probe. This was accomplished by separating total DNA samples from a T4 infection by using a variation of the two-dimensional gel electrophoresis procedure and extracting DNA from gel slices. Total DNA and the purified origin-fork intermediates are compared in Fig 3, and visualized by both one- and two-dimensional gels (with Southern blotting). In addition to the intact origin-fork intermediate, the preparation also contained small amounts of 6.2- and 5.2-kb linear fragments, which correspond to the full-length linear molecule and one arm of the origin-fork, respectively. These fragments presumably arise from origin-fork breakage during preparation and/or co-purification from total DNA.
Figure 3.
Origin-fork purification. Total DNA from a 46−/49− phage infection (9 min post-infection; left) and purified origin-fork intermediates (right) were analysed by one- and two-dimensional gel electrophoresis followed by Southern blotting with an ori(34) probe. The one-dimensional gel position of the origin-fork is indicated by an asterisk, and the 6.2- and 5.2-kb linear fragments are indicated by closed and open arrowheads, respectively. 1D, one dimensional; 2D, two dimensional.
By using the purified origin-fork intermediates, we analysed the mechanism of fork resolution by UvsW in vitro. Increasing amounts of UvsW resolved the origin-fork into 6.2- and 5.2-kb linear fragments as visualized by a one-dimensional gel (Fig 4A). When identical reactions were analysed by using two-dimensional gels, increasing amounts of UvsW were found to generate a regressed fork arc that was identical to that seen in Fig 1 (Fig 4B; arc from total DNA is also shown for comparison). At the highest concentration of UvsW (250 nM), most origin-fork intermediates were resolved to linear fragments not visible in the two-dimensional gel image. As determined using both one- and two-dimensional gels, resolution of origin-forks by UvsW required the presence of ATP and was blocked by inter-strand DNA crosslinks in the substrate (Fig 4C,D). In addition, purified UvsW protein with a mutation in the Walker A motif (K141R; deficient in ATP hydrolysis and branch migration) was unable to carry out origin-fork resolution (Fig 4C,D). These results show that UvsW promotes resolution of fork intermediates through fork regression, followed by extensive branch migration until the resulting Holliday junction falls off the end of the restriction fragment.
Figure 4.
UvsW resolves purified origin-forks by fork regression. (A,B) Purified origin-fork intermediates were treated with increasing concentrations of UvsW protein for 30 min at 37 °C in 1 × reaction buffer. Identical reactions were analysed by (A) one-dimensional and (B) two-dimensional gels. (C,D) Purified origin-fork intermediates were treated with UvsW (Input, −ATP, Full Rxn and +Triox) or UvsW-K141R (+K141R) proteins (250 nM) for 30 min at 37 °C in 1 × reaction buffer in the presence of ATP and in the absence of interstrand DNA crosslinks unless otherwise indicated (−ATP and +Triox, respectively). Identical reactions were analysed by (C) one-dimensional and (D) two-dimensional gels. All gels were visualized by Southern blot with an ori(34) probe. The one-dimensional gel position of the origin-fork is indicated by an asterisk, and the 6.2- and 5.2-kb linear fragments are indicated by closed and open arrowheads, respectively. Rxn, reaction; Triox, trioxsalen.
Discussion
Here, we have shown that the branch-specific helicase UvsW is necessary for the accumulation of regressed origin-forks in vivo, and that the UvsW protein is sufficient for origin-fork regression in vitro. In addition, 46−/uvsW− infections are almost completely deficient for DSE fragment accumulation. Thus, fork regression is the dominant mechanism of fork processing that leads to DSE formation, rather than fork breakage or head-to-tail fork collisions. Previously, we reported that regression supports two mechanisms of processing that can lead to fork reactivation—regressed DSE degradation by gp46/47 and Holliday-junction cleavage by T4 EndoVII (Long & Kreuzer, 2008). Taken together, these results indicate that UvsW-catalysed regression is part of an active helicase-driven pathway of fork processing that can stimulate fork reactivation in T4. This conclusion is supported by the fact that UvsW-deficient mutants—including deletion and K141R substitution—are hypersensitive to hydroxyurea, which causes fork stalling owing to nucleotide starvation (Carles-Kinch et al, 1997, and references therein). The hypersensitivity indicates that UvsW has a crucial function in reactivating stalled forks after hydroxyurea treatment, although further experiments are needed to test directly whether this crucial function involves UvsW-promoted fork regression.
Origin-fork resolution is severely delayed in 46−/49− infections that are deficient in regressed DSE degradation by gp46/47 and Holliday-junction cleavage by EndoVII (Long & Kreuzer, 2008). Interestingly, the uvsW mutation seems to rescue this delay (supplementary Fig 1 online), suggesting that alternative processing mechanisms are sufficient for wild-type levels of origin-fork resolution if fork regression by UvsW is prevented. This alternative processing mechanism could involve direct loading of the replicative helicase gp41 by the helicase loading protein gp59. These observations might relate to the association of genome instability and cell death with replication fork regression (Lopes et al, 2001). Once a fork structure becomes regressed, it might be ‘committed' to resolution by specific processing mechanisms and the absence of such mechanisms might be particularly detrimental.
In addition to UvsW, several other helicases have recently been shown to promote fork regression in vitro (McGlynn et al, 2001; Machwe et al, 2006; Ralf et al, 2006; Blastyák et al, 2007; Li et al, 2008). Of particular interest are WRN and BLM, which are implicated in human diseases characterized by premature ageing and cancer predisposition, respectively. Although the biological role of these proteins has not been established in vivo, the implication is that fork regression might have a crucial function in genome stability by facilitating stalled fork processing and reactivation. Indeed, a crucial function of fork regression in vivo might be to avoid the formation of overt DNA breaks that contribute to genome instability.
Methods
Bacterial phage and strains. The bacterial host for phage infections is a derivative of E. coli CAG12135 with the following additional mutations: acrA∷Tn10-kan, recA∷Tn10-cam and recD. The phage T4 strains used in this study are derivatives of strain K10 (amB262 (gene 38), amS29 (gene 51), nd28 (denA), rIIPT8 (rII-denB deletion); Selick et al, 1988). The additional phage mutations used here include: amB14 (gene 46), amE727 (gene 49), amHL628 (gene 59), ddaΔ (T4 coordinates 10 449–10 629), am11 (uvsX), amN116 (gene 39) and uvsWam (CT → AG mutation at T4 coordinates 113 075–113 076, constructed using the T4 I/S system (Selick et al, 1988)].
T4 infection and DNA analyses. T4 infections, DNA purifications and gel analysis of DNA were carried out essentially as described by Long & Kreuzer (2008). Details are presented in the supplementary information online.
Purification of origin-fork intermediates. Total DNA from a 20 ml infection (46−/49− phage, 9 min post-infection) was prepared as described in the supplementary information online and treated with PacI. For the one-dimensional gel, digested DNA was loaded into a wide, trough-shaped well and subjected to electrophoresis at 0.75 V/cm for 72 h at 21°C. This extended one-dimensional gel produces greater separation of DNA intermediates and allows selection of two-dimensional gel slices that closely border the origin-fork position (based on the migration of size markers). The two-dimensional gel was then cast and run normally, and the gel was sliced to produce a narrow region that contains the origin-forks. DNA was then extracted from the gel slices by electro-elution into dialysis tubing, followed by ethanol precipitation and resuspension in TE buffer.
UvsW reactions. UvsW protein was purified as described previously (Webb et al, 2007). Enzyme reactions were carried out in 1 × reaction buffer (50 mM NaCl, 20 mM Tris–HCl (pH 7.8), 1 mM DTT, 0.1 mg/ml BSA, 3.5 mM MgCl2 and 10 mM ATP) at 37 °C for 30 min with the indicated concentration of UvsW. DNA was then extracted sequentially with phenol and chloroform–isoamyl alcohol (24:1) to remove any DNA-associated proteins. Crosslinked samples were prepared by incubating DNA with trioxsalen (200 nM; Sigma-Aldrich, St Louis, MO, USA) for 1 h, followed by exposure to long-wave ultra-violet light for 20 min. Crosslinked samples were then dialysed against TE buffer for 1 h at 4 °C.
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
Supplementary Information
Acknowledgments
We thank Michael Webb for UvsW protein. This research was supported by National Institutes of Health grants GM34622 and GM066934.
Footnotes
The authors declare that they have no conflict of interest.
References
- Blastyák A, Pintér L, Unk I, Prakash L, Prakash S, Haracska L (2007) Yeast Rad5 protein required for postreplication repair has a DNA helicase activity specific for replication fork regression. Mol Cell 28: 167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carles-Kinch K, George JW, Kreuzer KN (1997) Bacteriophage T4 UvsW protein is a helicase involved in recombination, repair, and the regulation of DNA replication origins. EMBO J 16: 4142–4151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courcelle J, Donaldson JR, Chow K-H, Courcelle CT (2003) DNA damage-induced replication fork regression and processing in Escherichia coli. Science 299: 1064–1067 [DOI] [PubMed] [Google Scholar]
- Donaldson JR, Courcelle CT, Courcelle J (2006) RuvABC is required to resolve Holliday junctions that accumulate following replication on damaged templates in Escherichia coli. J Biol Chem 281: 28811–28821 [DOI] [PubMed] [Google Scholar]
- Fierro-Fernandez M, Hernandez P, Krimer DB, Schvartzman JB (2007) Replication fork reversal occurs spontaneously after digestion but is constrained in supercoiled domains. J Biol Chem 282: 18190–18196 [DOI] [PubMed] [Google Scholar]
- Higgins NP, Kato K, Strauss B (1976) A model for replication repair in mammalian cells. J Mol Biol 101: 417–425 [DOI] [PubMed] [Google Scholar]
- Kadyrov FA, Drake JW (2004) UvsX recombinase and Dda helicase rescue stalled bacteriophage T4 DNA replication forks in vitro. J Biol Chem 279: 35735–35740 [DOI] [PubMed] [Google Scholar]
- Kerr ID, Sivakolundu S, Li Z, Buchsbaum JC, Knox LA, Kriwacki R, White SW (2007) Crystallographic and NMR analyses of UvsW and UvsW.1 from bacteriophage T4. J Biol Chem 282: 34392–34400 [DOI] [PubMed] [Google Scholar]
- Kreuzer KN (2000) Recombination-dependent DNA replication in phage T4. Trends Biochem Sci 25: 165–173 [DOI] [PubMed] [Google Scholar]
- Li Z, Lu S, Hou G, Ma X, Sheng D, Ni J, Shen Y (2008) Hjm/Hel308A DNA helicase from Sulfolobus tokodaii promotes replication fork regression and interacts with Hjc endonuclease in vitro. J Bacteriol 190: 3006–3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long DT, Kreuzer KN (2008) Regression supports two mechanisms of fork processing in phage T4. Proc Natl Acad Sci USA 105: 6852–6857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes M, Cotta-Ramusino C, Pellicioli A, Liberi G, Plevani P, Muzi-Falconi M, Newlon CS, Foiani M (2001) The DNA replication checkpoint response stabilizes stalled replication forks. Nature 412: 557–561 [DOI] [PubMed] [Google Scholar]
- Machwe A, Xiao L, Groden J, Orren DK (2006) The Werner and Bloom syndrome proteins catalyze regression of a model replication fork. Biochemistry 45: 13939–13946 [DOI] [PubMed] [Google Scholar]
- Masson ML, Baharoglu Z, Michel B (2008) ruvA and ruvB mutants specifically impaired for replication fork reversal. Mol Microbiol 70: 537–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn P, Lloyd RG (2002) Genome stability and the processing of damaged replication forks by RecG. Trends Genet 18: 413–419 [DOI] [PubMed] [Google Scholar]
- McGlynn P, Lloyd RG, Marians KJ (2001) Formation of Holliday junctions by regression of nascent DNA in intermediates containing stalled replication forks: RecG stimulates regression even when the DNA is negatively supercoiled. Proc Natl Acad Sci USA 98: 8235–8240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister P, Taddei A, Vernis L, Poidevin M, Gasser SM, Baldacci G (2005) Temporal separation of replication and recombination requires the intra-S checkpoint. J Cell Biol 168: 537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel B, Flores M-J, Viguera E, Grompone G, Seigneur M, Bidnenko V (2001) Rescue of arrested replication forks by homologous recombination. Proc Natl Acad Sci USA 98: 8181–8188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkin EV, Mirkin SM (2007) Replication fork stalling at natural impediments. Microbiol Mol Biol Rev 71: 13–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow L, Ullsperger C, Keller RW, Bustamante C, Vologodskii AV, Cozzarelli NR (2001) Positive torsional strain causes the formation of a four-way junction at replication forks. J Biol Chem 276: 2790–2796 [DOI] [PubMed] [Google Scholar]
- Ralf C, Hickson ID, Wu L (2006) The Bloom's syndrome helicase can promote the regression of a model replication fork. J Biol Chem 281: 22839–22846 [DOI] [PubMed] [Google Scholar]
- Robu ME, Inman RB, Cox MM (2001) RecA protein promotes the regression of stalled replication forks in vitro. Proc Natl Acad Sci USA 98: 8211–8218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvartzman JB, Stasiak A (2004) A topological view of the replicon. EMBO J 5: 256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneur M, Bidnenko V, Ehrlich S, Michel B (1998) RuvAB acts at arrested replication forks. Cell 95: 419–430 [DOI] [PubMed] [Google Scholar]
- Selick H, Kreuzer K, Alberts B (1988) The bacteriophage T4 insertion/substitution vector system. A method for introducing site-specific mutations into the virus chromosome. J Biol Chem 263: 11336–11347 [PubMed] [Google Scholar]
- Sogo JM, Lopes M, Foiani M (2002) Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science 297: 599–602 [DOI] [PubMed] [Google Scholar]
- Webb MR, Plank JL, Long DT, Hsieh T-s, Kreuzer KN (2007) The phage T4 protein UvsW drives Holliday junction branch migration. J Biol Chem 282: 34401–34411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon D, Wang Y, Stapleford K, Wiesmüller L, Chen J (2004) p53 inhibits strand exchange and replication fork regression promoted by human Rad51. J Mol Biol 336: 639–654 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information