Abstract
In control samples, intense acoustic “go” stimuli accelerate the central and peripheral motor processes that compose simple reaction time movements. The goal of the current study was to determine whether movements that are initiated to intense acoustic cues facilitate simple reaction times in 1) adults with chronic stroke as compared to age matched controls and 2) in older as compared to younger adults. EMG and force data were collected from three groups (stroke, older adults, and younger adults) during a ballistic wrist and finger extension task. Movements were made to the onset of 80dB and 107dB acoustic cues and simple reaction times were fractionated into premotor and motor components. The present findings offer two important contributions to the literature. First, increases in stimulus intensity led to faster motor times in the impaired limb of stroke subjects. Second, increased stimulus intensity led to faster premotor reaction times across all groups, although an age rather than a stroke-specific motor deficit was evidenced, with the younger control group displaying significantly faster premotor times. Findings are integrated with previous evidence concerning post stroke corticospinal tract integrity and are interpreted via mechanisms which address stroke and age related changes in motoneurons and activity in motor units.
Keywords: Stroke, aging, reaction time, stimulus intensity
Chronic unilateral motor dysfunctions in the upper extremity severely limit instrumental movements of daily living [1]. However, empirical evidence from multiple treatment interventions indicates that many stroke survivors are able to regain voluntary control after a considerable amount of movement-based activity [6–9]. Indeed, neural plasticity in chronic stroke is at the forefront of relearning voluntary movements. Identifying viable variables that facilitate voluntary movements in chronic stroke remains a focus of considerable interest.
Over a century ago, researchers discovered that increases in stimulus intensity lead to a reduction in reaction time (RT) [28]. Quicker RT under these conditions is thought to reflect accelerated sensory and perceptual processing manifested in the presence of more intense physical stimuli [18]. More recent evidence confirmed that during movement planning, simple premotor RT’s are significantly faster when an unexpected intense acoustic stimulus replaces or accompanies a visual ‘go’ signal [e.g., 4, 5, 24]. For instance, Carlsen et al. (2007) reported that premotor RTs progressively decreased across dB levels (83dB, 93dB, 103dB, 113dB, 123dB) in healthy young adults, reaching asymptote at 113dB. Notably, however, when intense acoustic cues elicited a startle response (as indexed via muscle activity in the sternocleidomastoid), the authors argued that the startle circuit was activated, leading to the conclusion that the startle circuit is distinct from other circuits underlying stimulus intensity effects.
Extending the implications of this work, recent evidence indicates that movements are facilitated by the presentation of startle cues among a sample of stroke patients [16, 22]. Rothwell (2006) reported data from eight stroke participants in which preplanned movements of the wrist and ankle were executed to the onset of a loud acoustic stimulus. EMG amplitudes were greater and the onset latency of movement was much shorter following the loud acoustic stimulus than when voluntary movements were made alone, or when unplanned movements were executed to the startle cue. Further, Jankelowitz and Colebatch (2004) presented auditory cues at 120dB and reported that approximately one quarter of the patients tested had exaggerated startle responses in the biceps of the clinically affected side. Together these data suggest that intense startle triggering acoustic cues facilitate activity in the motor system. However, each of these protocols used startle eliciting acoustic cues and did not investigate whether sub-startle stimulus intensity effects [4, 11] were evident in prepared voluntary movements post stroke. As such, the primary aim in the current study was to determine the extent of intensity related facilitation post stroke.
The second goal of the current study was to determine the extent of intensity related facilitation in the aging motor system. Simple RT responses begin to steadily slow once a person reaches 30 years of age, and continue to slow throughout the remaining lifespan [12]. Evidence suggests that age-related changes in the organization of the motor system are such that as age progresses, so too does the level/intensity of stimulation that the motor system requires to achieve the same outcome. For instance, Pitcher and colleagues used TMS to demonstrate that increased stimulus intensities were necessary to induce equivalent amplitude MEPs in older as compared to younger adults [21]. To date, although independent studies suggest that stimulus intensity is negatively associated with RT across age ranges [4, 11, 22], the impact of stimulus intensity on the RT’s of varying age groups has not been tested inone study.
The present experiment quantified the effect of acoustic stimulus intensity (80dB and 107dB) on initiating upper extremity movements in chronic stroke, while comparing these effects with younger and older healthy adult control groups. Consistent with contemporary and classic fractionated RT studies [3, 4, 10, 11, 26], we calculated two traditional components: (a) a central index; represented by premotor RT, and (b) a peripheral index; represented by motor time. Three hypotheses were tested: 1) Premotor RT and motor time displayed by the impaired limb of the stroke group will be slowest compared to all other Group × Limb × Stimulus Intensity conditions. 2) Premotor RT and motor time will be faster to 107dB cues as compared to 80dB cues for each limb across group. 3) The younger control group will display faster premotor RT and motor time when compared to the stroke and older control group.
Table 1 shows the demographic characteristics of the stroke group. Admission criteria for the stroke group was: (1) diagnosis of no more than two strokes; (2) a lower limit of 10° of voluntary wrist/fingers extension from an 80° wrist flexed position; (3) the absence of other neurological deficits; (4) normal or corrected to normal vision and hearing; 5) Mini-Mental State Examination (MMSE) score ≥ 24. All participants in the older control group (N = 6, 5 males, Mean Age = 64.60, SD = 10.45) and the younger control group (N = 9, 8 males, Mean Age = 19.63, SD = .74) self-reported right-hand dominance, no central nervous system disorders that would affect movement, and normal or corrected to normal vision and hearing.
Table 1.
Stroke participant characteristics
| Sex | Age (years) | Hand Dominance | Side of Lesion | Impaired Side | Months Since Stroke | # Strokes | Box & Blocks * Recovery Ratio (%) |
|---|---|---|---|---|---|---|---|
| M | 75 | RH | R | LH | 82 | 1 | 17/51 = 33 |
| M | 72 | RH | L | RH | 136 | 1 | 26/70 = 37 |
| M | 63 | RH | L | RH | 168 | 1 | 21/77 = 27 |
| M | 75 | RH | R | LH | 84 | 1 | 2/54 = 4 |
| M | 67 | RH | R | LH | 36 | 1 | 52/58 = 90 |
| M | 59 | RH | R | LH | 95 | 1 | 14/42 = 33 |
| M | 76 | RH | R | LH | 90 | 1 | 21/48 = 44 |
| F | 68 | RH | R | LH | 43 | 1 | 66/68 = 97 |
| M | 50 | RH | L | RH | 113 | 2** | 2/56 = 4 |
# blocks moved by each limb. Recovery ratio = (# impaired/# unimpaired) × 100.
Both strokes were in the left hemisphere.
The study was conducted in accordance with the Declaration of Helsinki and all procedures were carried out with an adequate understanding by participants. All subjects read and signed an approved Institutional Review Board informed consent.
During each trial, participants were required to respond as quickly as possible to an acoustic stimulus (50 ms cue delivered binaurally through headphones) by initiating an isometric bimanual contraction of the wrist and finger extensor muscles against two independent load cells (left/right limb) (see Figure 1). No participants were instructed to control the duration or amplitude of their brief contractions.
Figure 1.
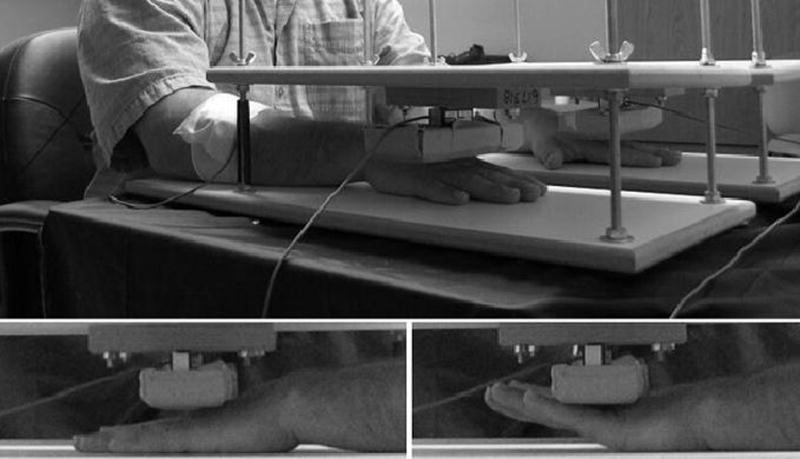
Experimental setup. Top: postures of arms, forearms, and shoulders before and during the task. Bottom left: posture of hands relative to load cells during movement preparation and ITI. Bottom right: posture of hands relative to load cells during movement execution.
Participants completed a total of 64 experimental trials: 24 trials in which an 80dB cue was presented, 24 trials in which a 107dB cue was presented, and 16 trials in which no cue was presented (catch trials). Catch trials were included to prevent habituation and anticipation. The order of the trials was randomized and manipulated for each participant to ensure that the same condition (i.e., 80 dB, 107 dB, or catch) was not presented more than twice in succession. The beginning of each trial and the cue to get ready to respond was demarcated by the onset of a visual stimulus which remained on the screen for 6 s1. Acoustic stimuli were presented randomly between intervals of 2 – 4 s after trial onset. Intertrial intervals were 10 – 14 s.
EMG surface electrodes (silver–silver chloride electrodes, 1 cm in diameter and 2 cm apart with an epoxy-mounted preamplifier) were placed over the belly of the extensor digitorum communis and extensor carpi ulnaris muscles of the left and right arms. To index force generation during each wrist/finger extension, two 75lb load cells embedded in cushioned platforms were altered in height to accommodate individual hand sizes. Upper limb EMG (bandpass filter 1 – 500 Hz) and force data were amplified by 5 K and collected at 1000 Hz via Biopac software (3.8.1, Biopac Systems Inc, Goleta, CA, USA). Trial onset/offset and auditory stimuli were controlled via a custom Labview program. Data were streamed to disk for offline analyses.
After answering questions and obtaining informed consent, participants sat in a comfortable chair positioned 1.0 m from a 19″ LCD presentation screen. Force platform heights were adjusted, load cells were calibrated, and EMG sensors were attached to the forearm muscles. Following calibration, participants were familiarized to the protocol by completing 4 practice trials (1 × 107dB, 2 × 80dB, and 1 catch). At the end of testing, hands were removed from the force platforms, EMG sensors were removed, and participants were debriefed.
Two dependent variables were calculated for each trial: premotor RT and motor time. Calculations were derived from onset of muscle contractions and force production. Baseline EMG and force scores were calculated for each trial (mean score during the 150 ms preceding acoustic stimulus onset). Onset of muscle contraction was identified by locating the first time point (after presentation of the acoustic cue) where EMG signal amplitude was greater than double the baseline value [27]. Thus, premotor RT equaled the elapsed time from the onset of the acoustic cue until the initiation of muscle contraction [27]. The onset of force production was identified as the first time point where force data exceeded double the force baseline value. Motor time was then calculated as the duration of time from muscle contraction to force onset [19]. Summary statistics for each acoustic cue were created by averaging premotor RT and motor time for each group (stroke, older control and younger control) and for each limb (impaired, unimpaired). For the control groups, the impaired limb corresponded to their non-dominant limb and the unimpaired limb was matched to their dominant limb.
Premotor RT and motor time were each analyzed in a separate mixed design Group (3: stroke, older control, younger control) × Limb (2: impaired, unimpaired) × Stimulus Intensity (2: 80dB, 107dB,) analysis of variance (ANOVA) with repeated measures on the last two factors. Tukey-Kramer’s follow-up procedure was used when appropriate.
Table 2 shows premotor RT data for each group and both limbs as a function of stimulus intensity levels. The three-way ANOVA indicated two significant main effects: (1) Group, F (1, 20) = 4.19, p = .030, η2 = .30) and (2) Stimulus Intensity, F (1, 20) = 18.32, p < .001, η2 = .48). Post hoc analyses on the group main effect revealed that the younger control group displayed faster premotor RTs relative the older control group and the stroke group (younger control: M = 195.81 ms, SE = 17.55; older control: M = 256.76 ms, SE = 20.26; stroke: M = 259.97 ms, SE = 16.54). In addition, the stimulus intensity findings indicated faster premotor RTs for the 107dB acoustic cues (M = 218.14 ms, SE = 12.07) as compared to 80dB acoustic cues (M = 256.89 ms, SE = 10.00). The main effect of limb and all interactions failed to reach significance (p > .05).
Table 2.
Mean (and SD) premotor RT’s and motor times to 80dB and 107dB acoustic cues for the impaired and unimpaired limb for each group. Ratio columns [*] for both premotor and motor times represent an index of stimulus intensity: Ratio = (80dB/107dB) × 100
| Premotor RT (msec) | Motor Time (msec) | |||||
|---|---|---|---|---|---|---|
| 80 dB | 107 dB | 80 dB | 107 dB | |||
| Group-Limb | Mean (SD) | Mean (SD) | Ratio * | Mean (SD) | Mean (SD) | Ratio * |
| Young | ||||||
| Unimpaired | 227.73 (45.28) | 169.82 (47.21) | 134.10 | 35.16 (10.14) | 31.32 (7.61) | 112.22 |
| Impaired | 221.81 (41.76) | 163.88 (44.69) | 135.34 | 42.67 (15.67) | 35.00 (10.58) | 121.92 |
| Old | ||||||
| Unimpaired | 271.73 (70.45) | 246.88 (71.45) | 110.06 | 40.63 (21.39) | 41.90 (19.63) | 96.97 |
| Impaired | 256.12 (49.76) | 252.33 (73.88) | 101.50 | 37.97 (13.01) | 36.03 (8.44) | 105.40 |
| Stroke | ||||||
| Unimpaired | 267.73 (47.81) | 224.47 (65.14) | 119.28 | 42.74 (10.26) | 37.80 (7.90) | 113.04 |
| Impaired | 296.22 (57.93) | 251.47 (64.98) | 117.80 | 69.43 (14.14) | 53.53 (14.80) | 129.71 |
Note: for the younger and older control groups the unimpaired limb corresponds to the dominant limb and the impaired limb corresponds to the non-dominant limb.
Analysis of the motor times indicated that reliable main effects for each factor were superseded by three significant two-way interactions: (1) Group × Limb, F(2, 20) = 9.29, p = .001, η2 = .48, (2) Group × Stimulus Intensity, F(2, 20) = 12.63, p < .001, η2 = .56, (3) Limb × Stimulus Intensity, F(1, 20) = 20.13, p < .001, η2 = .50. Moreover, our findings were further qualified by a reliable Group × Limb × Stimulus Intensity interaction, F(2, 20) = 3.75, p = .041, η2 = .27. The significant three-way interaction confirmed our prediction that motor times would be slowest for the impaired limb of the stroke group at 80dB relative to all other conditions. In addition, motor times were slower for the impaired limb of the stroke group to 107dB cues relative to all other conditions, aside from the unimpaired limb of the stroke group at 80dB and the impaired limb of the younger group at 80dB. Finally, motor times were faster for the unimpaired limb of the younger control group following 107dB cues as compared to the impaired limb of the younger control group at 80dB and the unimpaired limb of the stroke group at 80dB.
The primary goal of the current study was to determine whether movements that are initiated to intense acoustic cues would facilitate central and peripheral motor processes in adults with chronic stroke, particularly in their impaired limb. A secondary aim was to assess the impact of acoustic stimulus intensity on voluntary movements in the aging motor system. The present findings offer two important contributions to the literature.
First, an age effect rather than a stroke-specific effect was responsible for slower premotor RTs, with the younger control group displaying faster premotor RT’s relative to both older groups. Indeed, the similarity between the older control group and the stroke group suggests that the corticospinal tracts (CST) of participants in the chronic stroke group were largely intact. Second, increases in stimulus intensity resulted in faster motor times in the impaired limb of chronic stroke participants. Although a similar pattern was evidenced in the premotor RT data, the trend was not significant. We therefore suggest that whereas mild/moderate upper limb motor deficits following stroke are reflected in both central and peripheral processes, the deficit appears to be more clearly distinguishable in peripheral motor processes (i.e., motor time). Each of these findings is elaborated further.
Analyses corroborated an age related slowing of premotor RT [12, 21] with no differences between the chronic stroke and the older control group emerging. In the current sample of stroke participants, therefore, signal conduction speed from the brain to the periphery was not significantly compromised as compared to the older control group. Although we accept that unequal group sizes and the relatively small sample size may have been partly responsible, our robust effect sizes suggest that a more plausible explanation may reside in the characteristics of our Stroke group. All stroke participants were required to demonstrate partial movement prior to inclusion (i.e., 10 degrees of initial movement), which was further qualified by our Box and Block tests derived recovery index which showed some level of recovery in all participants. Each of these indices, coupled with the premotor RT data, suggest that the corticospinal tract (CST) in the stroke group was largely intact. Hence, although recovery rates (as indexed by the Box and Blocks test) indicated mild/moderate severity, our findings suggest that premotor RT may function independently from the efficacy of performing a functional motor task (i.e., picking up, moving, and releasing a block).
Using a goal directed force pulse task, Ward and colleagues [25] demonstrated that the integrity of the CST post stroke negatively correlates with the recruitment of secondary motor areas. However, given that ~30 % of the corticospinal tract fibers originate in primary motor cortex, 30 % from the premotor cortex and ~40% from the somatosensory cortex [13, 15, 17], we suggest that premotor RT may be more affected by CST integrity as compared to the extent of post stroke cortical reorganization from primary to secondary motor areas. In the current study, the task was a simple RT response to an auditory stimulus with no limitations placed on task duration, strength, or complexity. As such, our data show that cortical motor areas and the CST were able to transfer a simple go signal from the brain to the periphery without any significant temporal deficit (relative to age-matched controls). However, our findings do not permit us to speculate on whether the group differences/similarities were driven by a reorganization of the motor system post stroke, or whether the initial stroke location spared these systems. Nevertheless, how CST integrity and motor cortex reorganization alter the speed of muscle activity onset as compared to the quality and complexity of the functional movement that may follow is important to establish and a topic for future investigation.
The impact of stroke and stimulus intensity on motor time was consistent with our first hypothesis. Specifically, the more intense 107dB acoustic cue decreased motor times of the impaired limb to a similar level as the unimpaired limb at 80dB. With regard to the impact of a cerebral infarction on peripheral motor processes, previous evidence has shown that: a) the number of electrically excitable motor axons is reduced [2], b) the range of motoneuron recruitment forces is compressed [14], c) the ability to increase motor unit discharge rate during voluntary force increases is diminished [14], and d) fewer high threshold motor units are recruited on the impaired as compared to the non-impaired side [20]. Collectively, the consequences of these changes in the peripheral system may lead to a reduction in the efficiency of a muscle contraction, an increase in effort and fatigue, and a sense of weakness for force generation [14]. However, given that motor units can be driven to fire at higher frequencies after a period of audiovisual feedback training [23], we postulate that in the current study an increase in auditory stimulus intensity may have partially offset the deficits that manifest as a result of one or a potential combination of these factors.
Aside from the robust effect of stimulus intensity on the impaired limb of the stroke group, the facilitatory effect of stimulus intensity was generally less pronounced in older as compared to younger participants. This finding complements previous evidence which has shown that increased stimulus intensities are necessary to induce equivalent amplitude MEPs in older as compared to younger adults [21]. Pitcher and colleagues offer two potential mechanisms that may underlie their age related findings, each of which can be extrapolated to the current data. Fewer spinal motoneurons may have been activated synchronously in the older as compared to the younger participants. Conversely, older participants may have engaged a similar quantity of motoneurons, but in a less synchronous manner. In the current study, either of these mechanisms may have been responsible for the aging effect as well as the lack of a stimulus intensity effect in older participants. More specifically, whereas the 27dB increase in stimulus intensity led to reliable reductions in premotor RT and motor time in the younger group, this increase in intensity was not large enough to elicit reliable changes in the older control group or the unimpaired limb of the stroke group.
Importantly, the stimulus intensity effect was only evidenced in motor times of the impaired limb of the stroke group, suggesting that the stimulus intensity levels used in the current study may offset stroke related peripheral slowing but not an age related slowing of central motor processes. Whether increasing stimulus intensity would impact an age-related slowing of central motor processes, and whether the intensity of an auditory startle eliciting stimulus would be greater in older as compared to younger adults has not been empirically tested. Moreover, the suggestion that the startle circuit is distinct from the circuit that mediates stimulus intensity effects [4] makes further investigation of this issue all the more compelling.
We offer four suggestions to extend the current line of research: a) manipulation of a broader range of stimulus intensities; b) focus on upper and lower extremity motor function; c) record EMG data from the sternocleidomastoid and orbicularis occuli muscles to control for trials in which a startle response is elicited [4]; and d) index and correlate CST integrity and primary/secondary motor cortex reorganization with RT measures in addition to functional measures.
In conclusion, deficits in premotor RT should not be assumed post stroke even when functional tasks show mild/moderate impairment. Indeed, results from the current stroke group suggest a level of independence between the central and peripheral motor systems and their susceptibility to stimulus intensity effects of movement initiating cues. We emphasize, however, that the current results and subsequent conclusions may well be qualified by stroke lesion location and should not be generalized to all chronic stroke patients. Finally, we did not find evidence to support the notion that increasing stimulus intensity can offset an age-related slowing of RT, although empirical tests that further manipulate increases in stimulus intensity are necessary to qualify this position.
Acknowledgments
Grants
Funding: This research was supported by American Heart Association grant 0515120B and in part by American Heart Association grant 00061194, National Institute of Mental Health grants T32-MH-067631 and R03-MH-70678, and National Institute of Child Health and Human Development grant 5R03-HD-044534.
Footnotes
Visual stimuli were a range of emotional and neutral pictures. No significant between group findings for picture content were evidenced and so data were collapsed across the picture content condition for brevity. The interested reader is directed to reference # 14 which addressed picture content, stimulus intensity, and fractionated RT in healthy young adults.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Heart Association. Heart Disease and Stroke Statistics - 2008 Update. American Heart Association; Dallas, Texas: [Google Scholar]
- 2.Arasaki K, Igarashi O, Ichikawa Y, Machida T, Shirozu I, Hyodo A, Ushijima R. Reduction in the motor unit number estimate (MUNE) after cerebral infarction. J Neurol Sci. 2006;250:27–32. doi: 10.1016/j.jns.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Botwinick J, Thompson LW. Premotor and motor components of reaction time. J Exp Psychol. 1966;71:9–15. doi: 10.1037/h0022634. [DOI] [PubMed] [Google Scholar]
- 4.Carlsen AN, Dakin CJ, Chua R, Franks IM. Startle produces early response latencies that are distinct from stimulus intensity effects. Exp Brain Res. 2007;176:199–205. doi: 10.1007/s00221-006-0610-8. [DOI] [PubMed] [Google Scholar]
- 5.Carlsen AN, Hunt MA, Inglis JT, Sanderson DJ, Chua R. Altered triggering of a prepared movement by a startling stimulus. J Neurophysiol. 2003;89:1857–1863. doi: 10.1152/jn.00852.2002. [DOI] [PubMed] [Google Scholar]
- 6.Cauraugh J, Light K, Kim S, Thigpen M, Behrman A. Chronic motor dysfunction after stroke: recovering wrist and finger extension by electromyography-triggered neuromuscular stimulation. Stroke. 2000;31:1360–1364. doi: 10.1161/01.str.31.6.1360. [DOI] [PubMed] [Google Scholar]
- 7.Cauraugh JH, Kim S. Two coupled motor recovery protocols are better than one: electromyogram-triggered neuromuscular stimulation and bilateral movements. Stroke. 2002;33:1589–1594. doi: 10.1161/01.str.0000016926.77114.a6. [DOI] [PubMed] [Google Scholar]
- 8.Cauraugh JH, Kim SB. Stroke motor recovery: active neuromuscular stimulation and repetitive practice schedules. J Neurol Neurosurg Psychiatry. 2003;74:1562–1566. doi: 10.1136/jnnp.74.11.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cauraugh JH, Summers JJ. Neural plasticity and bilateral movements: A rehabilitation approach for chronic stroke. Prog Neurobiol. 2005;75:309–320. doi: 10.1016/j.pneurobio.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Coombes SA, Cauraugh JH, Janelle CM. Dissociating motivational direction and affective valence: specific emotions alter central motor processes. Psychol Sci. 2007;18:938–942. doi: 10.1111/j.1467-9280.2007.02005.x. [DOI] [PubMed] [Google Scholar]
- 11.Coombes SA, Cauraugh JH, Janelle CM. Emotional state and initiating cue alter central and peripheral motor processes. Emotion. 2007;7:275–284. doi: 10.1037/1528-3542.7.2.275. [DOI] [PubMed] [Google Scholar]
- 12.Der G, Deary IJ. Age and sex differences in reaction time in adulthood: results from the United Kingdom Health and Lifestyle Survey. Psychol Aging. 2006;21:62–73. doi: 10.1037/0882-7974.21.1.62. [DOI] [PubMed] [Google Scholar]
- 13.Galea MP, Darian-Smith I. Multiple corticospinal neuron populations in the macaque monkey are specified by their unique cortical origins, spinal terminations, and connections. Cereb Cortex. 1994;4:166–194. doi: 10.1093/cercor/4.2.166. [DOI] [PubMed] [Google Scholar]
- 14.Gemperline JJ, Allen S, Walk D, Rymer WZ. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve. 1995;18:1101–1114. doi: 10.1002/mus.880181006. [DOI] [PubMed] [Google Scholar]
- 15.Jane JA, Yashon D, DeMyer W, Bucy PC. The contribution of the precentral gyrus to the pyramidal tract of man. J Neurosurg. 1967;26:244–248. doi: 10.3171/jns.1967.26.2.0244. [DOI] [PubMed] [Google Scholar]
- 16.Jankelowitz SK, Colebatch JG. The acoustic startle reflex in ischemic stroke. Neurology. 2004;62:114–116. doi: 10.1212/01.wnl.0000101711.48946.35. [DOI] [PubMed] [Google Scholar]
- 17.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. Appleton and Lange; Norwalk: 1991. [Google Scholar]
- 18.Levick WR. Variation in the response latency of cat retinal ganglion cells. Vision Research. 1973;13:837–853. doi: 10.1016/0042-6989(73)90047-3. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Stevens JA, Kamper DG, Rymer WZ. The movement-specific effect of motor imagery on the premotor time. Motor Control. 2005;9:119–128. doi: 10.1123/mcj.9.2.119. [DOI] [PubMed] [Google Scholar]
- 20.Lukacs M, Vecsei L, Beniczky S. Large motor units are selectively affected following a stroke. Clin Neurophysiol. 2008;119:2555–2558. doi: 10.1016/j.clinph.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Pitcher JB, Ogston KM, Miles TS. Age and sex differences in human motor cortex input-output characteristics. J Physiol. 2003;546:605–613. doi: 10.1113/jphysiol.2002.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothwell JC. The startle reflex, voluntary movement, and the reticulospinal tract. Suppl Clin Neurophysiol. 2006;58:223–231. doi: 10.1016/s1567-424x(09)70071-6. [DOI] [PubMed] [Google Scholar]
- 23.Shahani BT, Connors L, Mohr JP. Electromyographic audiovisual training effect in the motor performance in patients with lesions of the central nervous system. Arch Phys Med Rehabil. 1977;58:519. [Google Scholar]
- 24.Valls-Solé J, Rothwell JC, Goulart F, Cossu G, Munoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol. 1999;516(Pt 3):931–938. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RS. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129:809–819. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss AD. The Locus of Reaction Time Change with Set, Motivation, and Age. J Gerontol. 1965;20:60–64. doi: 10.1093/geronj/20.1.60. [DOI] [PubMed] [Google Scholar]
- 27.Wong YM, Ng GY. The double peak-to-peak analysis for determining EMG onset of muscle contraction. Electromyogr Clin Neurophysiol. 2005;45:267–271. [PubMed] [Google Scholar]
- 28.Woodworth RS. Experimental Psychology. Henry Holt; New York: 1938. [Google Scholar]


