Abstract
Background
Prolonged myocardial ischemia results in cardiomyocyte loss despite successful revascularization. We have reported that retrograde application of embryonic endothelial progenitor cells (eEPCs) provides rapid paracrine protection against ischemia-reperfusion injury. Here, we investigated the role of thymosin β4 (Tβ4) as a mediator of eEPC-mediated cardioprotection.
Methods and Results
In vitro, neonatal rat cardiomyocytes were subjected to hypoxia-reoxygenation in the absence or presence of eEPCs with or without Tβ4 short hairpin RNA (shRNA) transfection. In vivo, pigs (n=9 per group) underwent percutaneous left anterior descending artery occlusion for 60 minutes on day 1. After 55 minutes of ischemia, control eEPCs (5×106 cells) or cells transfected with Tβ4 shRNA when indicated or 15 mg Tβ4 alone were retroinfused into the anterior interventricular vein. Segmental endocardial shortening in the infarct zone at 150-bpm atrial pacing, infarct size (triphenyl tetrazolium chloride viability and methylene blue exclusion), and inflammatory cell influx (myeloperoxidase activity) were determined 24 hours later. Survival of neonatal rat cardiomyocytes increased from 32±4% to 90±2% after eEPC application, an effect sensitive to shRNA transfection compared with Tβ4 (45±7%). In vivo, infarct size decreased with eEPC application (38±4% versus 54±4% of area at risk; P<0.01), an effect abolished by Tβ4 shRNA (62±3%). Segmental subendocardial shortening improved after eEPC treatment (22±3% versus −3±4% of control area) unless Tβ4 shRNA was transfected (−6±4%). Retroinfusion of Tβ4 mimicked eEPC application (infarct size, 37±3%; segmental endocardial shortening, 34±7%). Myeloperoxidase activity (3323±388 U/mg in controls) was decreased by eEPCs (1996±546 U/mg) or Tβ4 alone (1455±197 U/mg) but not Tβ4 shRNA–treated eEPCs (5449±829 U/mg).
Conclusion
Our findings show that short-term cardioprotection derived by regional application of eEPCs can be attributed, at least in part, to Tβ4.
Keywords: progenitor cells, ischemia, molecular biology, myocardial infarction, reperfusion
Since the landmark studies of Asahara and coworkers1 and Rafii et al,2 the bone marrow has been viewed as a source of circulating endothelial progenitor cells (EPCs), which contribute to endothelialization after vascular injury, angiogenesis in response to ischemia, and reversal of endothelial dysfunction. In addition to the identification of circulating EPCs as prognostic markers in coronary artery disease,3,4 therapeutic aspects have been investigated in the field of myocardial protection after ischemic events. In contrast to mostly inefficient attempts to enhance mobilization of EPCs from the bone marrow by, for example, granulocyte colony-stimulating factor,5–8 Ficoll gradient–enriched progenitor cells from the bone marrow resident pool were successfully applied in a randomized placebo-controlled study,9 even though not reproduced in a smaller trial10 that applied a different cell conservation protocol.11
With regard to the mechanism of action of circulatory progenitor cells, paracrine supply of growth and survival factors for vascular12,13 and parenchymal cells14,15 has gained recent support. Consistent with this paradigm, embryonic endothelial cells exert postischemic cardioprotection by paracrine factors activating the phosphoinositide 3-kinase (PI3K)/AKT signaling pathway in cardiomyocytes in vitro and in vivo.16 Screening the transcriptome of the embryonic EPC (eEPC) population by the Affymetrix array technique revealed that among the known PI3K/AKT activating factors, thymosin β4 (Tβ4), a short peptide of 43 amino acids, is expressed most abundantly.17 Because Tβ4 has recently been reported to exert a profound cardioprotective effect after exogenous application in an acute myocardial infarction model in mice,18 we hypothesized that this peptide is of relevance for eEPC-derived cardioprotection after ischemia and reperfusion in a preclinical pig model. Therefore, in the present study, we modulated the Tβ4 production of eEPCs by specific short hairpin RNA (shRNA) transfection or exogenously applied Tβ4 via retroinfusion and investigated the corresponding postischemic myocardial injury.
Methods
Animals
German pigs were purchased from a local farm (Oberschleissheim, Germany). Animal care and all experimental procedures were performed in strict accordance to the German and National Institutes of Health animal legislation guidelines and were approved by the local animal care and use committees.
Reagents
All chemicals were purchased from Sigma (Deisenhofen, Germany). Contrast agent Solutrast 370 was provided by Byk Gulden (Konstanz, Germany). 125I and 123I were from Amersham/GE (Braunschweig, Germany).
eEPC Studies
Mouse eEPCs were generated by isolating genetically marked cells from a transgene mouse strain carrying a β-galactosidase reporter gene instead of 1 thrombomodulin allele.19 Cells were selected by reporter gene expression, cultivated on a feeder layer as isolated colonies, and then propagated on gelatin-coated plates.20 All experiments were performed with cells of an individual colony.
shRNAs were obtained with the pSuperior.retro kit from Oligoengine (Seattle, Wash) according to the manufacturer’s instructions. Sequences for scrambled shRNA were 5′-TTCTCCGAAC-GTGTCACGT-3′ and 5′-ACGTGACACGTTCGGAGAA-3′ and for Tβ4 were 5′-AGAAGCAAGCTGGCGAATCGTAA-3′ and 5′-TTACGATTCGCCAGCTTGCTTCT-3′. Transfection of eEPCs with shRNA was performed with Lipofectamine (Invitrogen, Karlsruhe, Germany). Verification of shRNA efficacy was achieved by real-time polymerase chain reaction.
RNA Modulation and Detection
Total RNA was extracted from eEPCs, treated with DNaseI (Invitrogen), and converted to cDNA. Real-time polymerase chain reaction was performed with SYBR green dye (iQ SYBR Green Supermix, Bio-Rad, Munich, Germany) and run on the iQ-Cycler (Bio-Rad). Primer pairs for Tβ4 were 5′-CACGAGCATTGCCTTCTTAT-3′ and 5′-TCTCTGCTAGCCAGACCATC-3′. These were normalized to GAPDH (5′-AATTCAACGGCACAGTCAAG-3′ and 5′-ATGGTGGTGAAGACACCAGT-3′). Polymerase chain reaction–amplified products also were separated electrophoretically on 2% agarose gels to confirm that single bands were amplified (data not shown). shRNAs were obtained using the pSuperior.retro kit from Oligoengine according to the manufacturer’s instructions. Sequences for scrambled shRNA were 5′-TTCTCCGAACGTGTCACGT-3′ and 5′-ACGTGACACGTTCGGAGAA-3′ and for Tβ4 were 5′-AGAAGCAAGCTGGCGAATCGTAA-3′ and 5′-TTACGATTCGCCAGCTTGCTTCT-3′.
Cardiomyocyte and Endothelial Cell Culture
Rat neonatal ventricular cardiomyocytes were prepared as previously described.16 After plating on 6-well plates, hypoxia was induced for 4 hours, followed by reoxygenation for 1 hour. When indicated, eEPCs were placed in a permeable insert (Biocoat, Becton Dickinson, Heidelberg, Germany) in the well before hypoxia. The Tβ4 antibody sc-32405 was from Santa Cruz (Heidelberg, Germany). At the end of the experiment, Trypan blue was added for 2 minutes, and 5 microscopic fields were photographed and quantified (Zeiss AxioVision, Göttingen, Germany).
Rat neonatal coronary endothelial cells were obtained by collagenase and trypsin digestion of neonatal rat hearts, seeding for 1 hour on collagen-coated dishes, removal of nonadherent cells, and cultivation in DMEM containing 10% FBS. Neonatal coronary endothelial cells were subjected to hypoxia (18 hours) and reoxygenation (4 hours). Thereafter, cells were fixated with paraformaldehyde (4% in PBS) and analyzed with a commercial terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling staining kit (Roche Diagnostics GmbH, Mannheim, Germany). Application of eEPCs with or without Tβ4 shRNA was performed at the onset of hypoxia.
Leukocyte Adhesion
To test the influence of eEPCs or Tβ4 on leukocyte adhesion under flow conditions, we seeded primary endothelial cells on microslides (ibidi, Martinsried, Germany). After incubation with eEPCs (50 000 per slide for 4 hours) or Tβ4 transfection (24 hours before the experiment with Effectene, Qiagen, Hilden, Germany), microslides were subjected to endothelial activation by interleukin 1β (10 ng/mL). Then, we superfused DiI-labeled monocytic cells (THP-1, a gift from P. Nelson, Munich, Germany) at 4 mL/h for 10 minutes. Slides were then rinsed with PBS for 3 minutes. Finally, adherent cells were quantified in 10 high-power fields per slide under fluorescence microscopy.
Pig Ischemia-Reperfusion Model
All pig experiments were conducted at the Institute for Surgical Research at the University of Munich. Pigs (n=9 per experimental group) were anesthetized and instrumented as described previously.16 Briefly, a balloon was placed in the left anterior descending artery (LAD) distal to the bifurcation of the first diagonal branch and inflated with 4 atm (0.41 MPa). Correct localization of the coronary occlusion and patency of the first diagonal branch were ensured by injection of contrast agent. Animals with an obviously abnormal perfusion pattern (area at risk [AAR] <25% of the left ventricle and/or infarct size <20% of AAR) were excluded from further analysis (n=2).
eEPC Application
As described in detail before, 5×106 eEPCs or 15 mg Tβ4 (supplied by I. Bock-Marquette, University of Texas, Dallas) was retroinfused into the anterior interventricular vein, draining the LAD-perfused myocardium, after 55 minutes of ischemia for 10 minutes.16 In all groups, the percutaneous transluminal coronary angioplasty balloon was deflated after 60 minutes of ischemia, and the onset of reperfusion was documented angiographically.
Hemodynamic and Tissue Measurements
After 24 hours of reperfusion, sternotomy was performed and ultrasonic crystals were placed in the noninfarcted and infarcted AAR, as well as in the circumflex perfusion area in a standardized manner. Subendocardial segment shortening was performed under resting heart rate and at 120- and 150-bpm atrial pacing (for 1 minute each). After assessment of infarct size (triphenyl tetrazolium chloride viability and methylene blue exclusion), myeloperoxidase assays were performed as previously described.16
Radioactive Labeling of eEPCs
To assess recruitment of native and Tβ4 shRNA–treated eEPCs to the ischemic region, tracking via a well-described sodium iodide symporter (NIS) was used.21,22 eEPCs were stably transfected with an NIS-containing plasmid (pCDNA3-CMV-NIS, provided by Dr S.M. Jhiang, Ohio State University, Columbus) using Lipofectamine. After selection by G418 for ≈2 weeks, surviving cell clones were isolated and subjected to screening for 125I uptake activity as described earlier.23
In vivo, 123I (40 MBq) was systemically applied 24 hours after ischemia. Twenty-five minutes later, hearts were excised. The tissue in the LAD and right circumflex region was separated according to an established segmentation pattern24 and then analyzed for 123I accumulation by gamma counter analysis.
High-Performance Liquid Chromatography Analysis
The concentration of Tβ4 was determined as described.25,26 Briefly, cells were disrupted by adding perchloric acid to the cell suspension to a final concentration of 0.4 mol/L. After incubating for 30 minutes on ice, the mixture was centrifuged for 5 minutes at 20 000g. The supernatant solution was adjusted to pH 4 with NaOH, and an aliquot of the sample was analyzed by reverse-phase high-performance liquid chromatography.27
Statistical Analysis
The results are given as mean±SEM. Statistical analysis was done with 1-way ANOVA (SPSS 13.0, SPSS Inc, Chicago, Ill) and, where appropriate, 2-way ANOVA (Figures 2C and 4C) or 2-way ANOVA with repeated measures on 1 factor (Figures 3D, 3E, 6C, and 6D) (SigmaStat, Systat Software Inc, Chicago, Ill). Whenever a significant effect was obtained with ANOVA, we used multiple-comparison tests between groups with the Student-Newman-Keul procedure. Comparisons of 2 groups were performed by 2-sided independent t tests.
Figure 2.
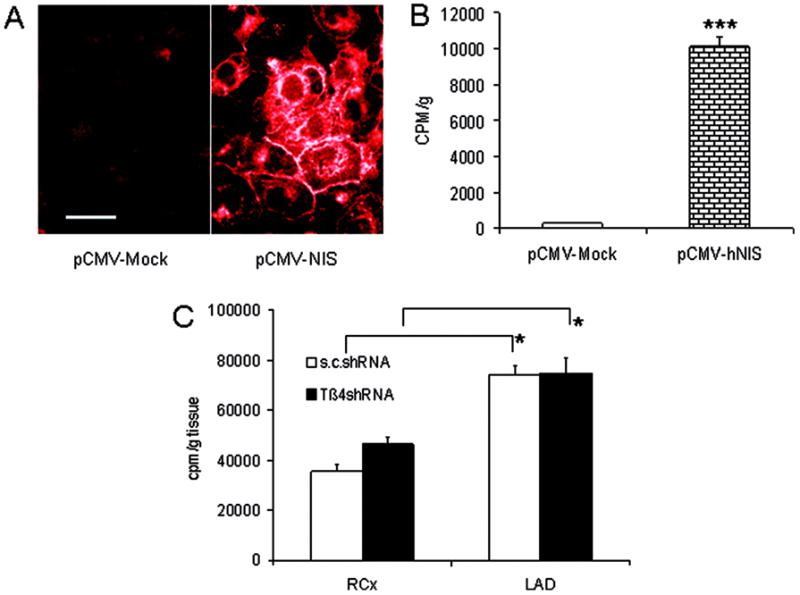
Effect of Tβ4 shRNA on eEPC adhesion. A, Fluorescence microscopy of NIS expression (mock-transfected cells [left] vs NIS-transfected cells [right]; white bar=10 μm). B, 125I uptake of untransfected and NIS-transfected eEPCs in vitro. C, Uptake of 123I 24 hours after ischemia-reperfusion and retroinfusion of 5×106 eEPCs with or without Tβ4 shRNA transfection. *P<0.05 vs right circumflex (RCx) perfused tissue; ***P<0.0001 vs mock transfection.
Figure 4.
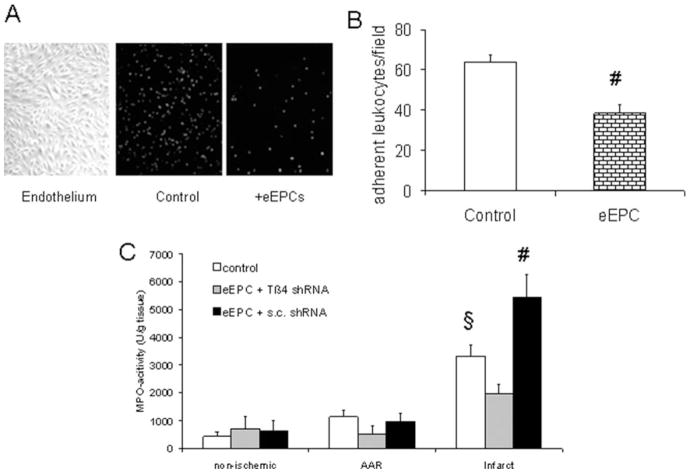
Tβ4 moderates postischemic inflammation. A, Example of neonatal endothelial cells grown on microslides with or without eEPC preincubation (1 hour) and superfused with fluorescing monocytic THP-1 cells (see Methods). B, Quantitative adhesion of THP1 cells on activated endothelium without or with eEPC coincubation (n=3; #P<0.05 vs control). C, Myeloperoxidase (MPO) activity in nonischemic, AAR (noninfarcted), and infarcted regions after retroinfusion of eEPCs transfected with Tβ4 or scrambled (s.c.) shRNA (n=6; §P<0.05 vs nonischemic control; #P<0.05 vs nonischemic eEPCs plus control shRNA).
Figure 3.
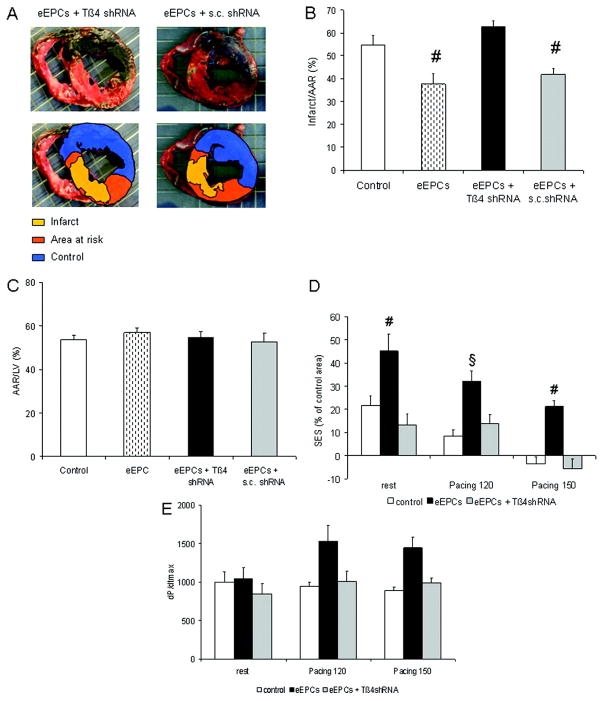
Effect of Tβ4 shRNA on eEPC-mediated cardioprotection in vivo. A, Example of infarct size (percentage of AAR) 24 hours after ischemia and retroinfusion of 5×106 eEPCs with Tβ4 shRNA. B, Quantification revealed a significant decrease in infarct size after eEPC retroinfusion with or without scrambled (s.c.) shRNA (n=9 per group; #P<0.01 vs control). C, AAR/left ventricle (LV) did not differ significantly between groups. D, Subendocardial segment shortening (SES) in the apical LAD perfused region (percentage of the nonischemic right circumflex region) at rest and at 120- and 150-bpm atrial pacing (n=9 per group; §P<0.05 vs control; #P<0.05 vs control and eEPC plus Tβ4 shRNA). E, Contraction velocity (dP/dtmax) at the given heart rates (P=0.052, 2-way ANOVA).
Figure 6.
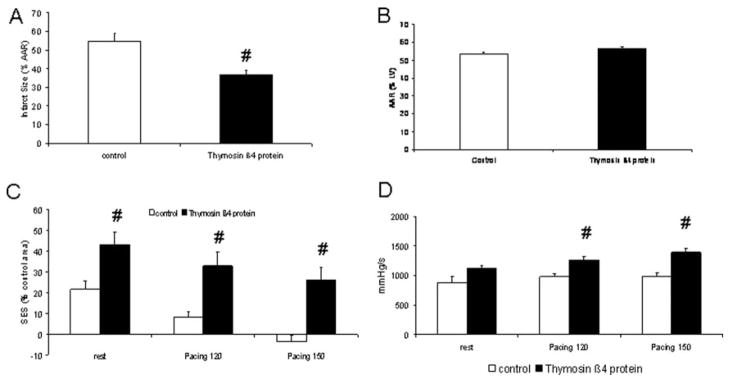
Tβ4 protein exerts cardioprotection in vivo. A, Retroinfusion of 15 mg recombinant Tβ4 reduces infarct size in a series of AAR sizes (B) similar to controls. C, Moreover, Tβ4 increases subendocardial segment shortening at rest and at atrial pacing (120 and 150 bpm) and (D) enhances dP/dtmax at rapid atrial pacing (#P<0.05 vs control; n=9). LV indicates left ventricle; SES, subendocardial segment shortening.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
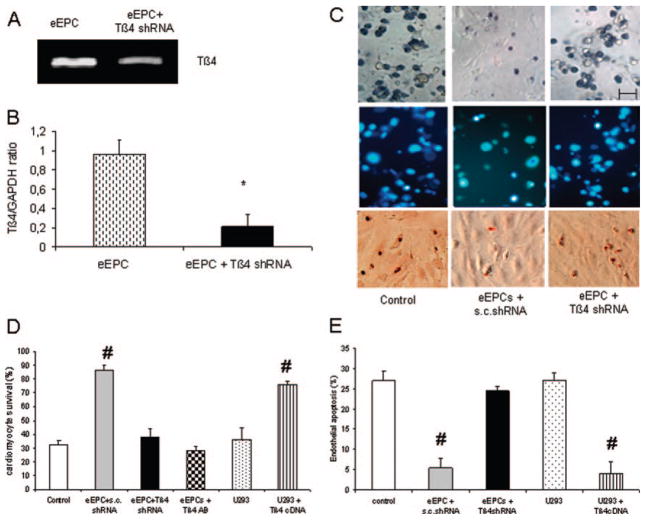
To evaluate the role of Tβ4 in vivo, transient downregulation of its RNA was attempted by shRNA transfection. As depicted in Figure 1A and 1B, this approach yielded a downregulation by 77% of the Tβ4 mRNA. In accordance, the content of Tβ4 per cell decreased from 400 to 150 fg after Tβ4 shRNA transfection (data not shown). In vitro, Tβ4-specific shRNA transfection or an antibody directed against Tβ4 decreased the survival of eEPC-treated cardiomyocytes to the extent of untreated control cells (Figure 1C and 1D). Vice versa, U293 cells, which do not contain Tβ4, had no beneficial effect on cardiomyocyte survival unless Tβ4 was transfected (Figure 1D). Endothelial apoptosis after hypoxia and reoxygenation was inhibited by eEPCs unless Tβ4 was knocked down (Figure 1E). Again, U293 cell cocultivation did not alter endothelial apoptosis unless Tβ4 was transfected.
Figure 1.
Effect of Tβ4 shRNA on eEPC-mediated cardiomyocyte and endothelial cell survival after hypoxia-reoxygenation. Example (A) and quantification (B) of the reduction of Tβ4 by shRNA. C, Examples of neonatal cardiomyocyte survival (Trypan blue exclusion, top) after 4 hours of hypoxia and 1 hour of reoxygenation with or without coapplication of eEPCs transfected by scrambled (s.c.) or Tβ4 shRNA (bar=20 μm). D, eEPCs but not U293 cells displayed cardiomyocyte protection. The effect of eEPCs was blunted by Tβ4 shRNA or Tβ4 antibody application, whereas U293 cells provided cardioprotection after Tβ4 transfection. E, Neonatal endothelial cells underwent apoptosis after 18 hours of hypoxia and 4 hours of reoxygenation unless eEPCs or Tβ4-transfected U293 cells were applied. *P<0.05 vs eEPC; #P<0.05 vs control, eEPCs plus Tβ4 shRNA, and U293 cells (n=4 independent experiments).
Encouraged by the notion of a Tβ4-mediated protection of cardiomyocytes and endothelial cells in vitro, we conducted in vivo experiments of ischemia and reperfusion (see Methods). To exclude an impact of Tβ4 shRNA on the adhesion capability of eEPCs in the postischemic myocardium, we used an NIS-expressing eEPC population (Figure 2A and 2B) that was transfected with Tβ4 or control shRNA (see Methods). By virtue of the selectivity of the NIS, 123I is taken up exclusively by the thyroid gland and NIS-transfected cells.22 Scintigraphic analysis of the LAD- and right circumflex–related perfusion area at 24 hours revealed a selective recruitment to the LAD perfused myocardium without a difference caused by Tβ4 shRNA transfection (Figure 2C).
Applying eEPCs pretreated with Tβ4 shRNA, however, was found to reduce the benefit of smaller infarct sizes accomplished by untreated, or control, shRNA-transfected eEPCs (Figure 3A through 3C). The impact of Tβ4 shRNA treatment on eEPC retroinfusion consistently translated into a decrease in regional myocardial function as assessed by subendocardial segment shortening (Figure 3D). Moreover, a strong trend toward a gain in dP/dtmax, a marker of global systolic left ventricular function,28 which was accomplished by eEPC treatment (P=0.052), was absent in the case of Tβ4 shRNA–transfected eEPCs (Figure 3E).
One important prognostic parameter of myocardial ischemia-reperfusion injury is postischemic inflammation. In vitro, eEPCs were capable of reducing the amount of adhesive inflammatory cells on an activated endothelial layer under flow conditions (Figure 4A and 4B). In vivo, leukocyte influx into the infarcted region, assessed by myeloperoxidase activity, was limited after application of eEPCs as opposed to Tβ4 shRNA eEPCs (Figure 4C).
To further assess the cardioprotective capability of Tβ4, we considered 2 options: application of Tβ4 cDNA in a liposomal formulation, which has been successfully applied before,29,30 or application of a recombinant protein. Because from a clinical perspective the gene transfer approach implies the disadvantage of treatment 2 days before the ischemic event, we decided to use recombinant Tβ4. In vitro, Tβ4 protein was able to protect posthypoxic, reoxygenated myocytes and endothelial cells (Figure 5A and 5B). Moreover, leukocyte adhesion was reduced by Tβ4 in vitro (Figure 5C) and in vivo (Figure 5D). Finally, Tβ4 retroinfusion at the end of the ischemic interval displayed cardioprotection similar to eEPC retroinfusion; infarct size was reduced to 37±3% (Figure 6A and 6B), whereas segmental subendocardial shortening increased (from −4±3% to 26±5% of the nonischemic region at 150 bpm; Figure 6C). Consistently, global systolic function (dP/dtmax) was found to be increased at rapid pacing (Figure 6D).
Figure 5.
Effect of Tβ4 protein on cardiomyocyte and endothelial survival and inflammation. A, Tβ4 enhances posthypoxic cardiomyocyte survival and reduces endothelial cell apoptosis (B). Leukocyte adhesion was reduced after transient Tβ4 overexpression in vitro (C) and after Tβ4 protein retroinfusion in vivo (D). n=3 per group (n=5 per group in D). TUNEL indicates terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling staining. #P<0.05 vs control.
Taken together, these results show that Tβ4, which has cardioprotective properties during hypoxia and reoxygenation in vitro, limits the extent of ischemia-reperfusion injury in vivo if present in large amounts in eEPCs or if applied solely as protein. Consistently, a reduction in Tβ4 expression by shRNA diminishes the cardioprotection achieved by an eEPC population, indicating that Tβ4 is an essential factor in the acute, eEPC-mediated cardioprotection in vitro and in vivo.
Discussion
In the present study, we investigated the mechanism of cardioprotection observed after transplantation of eEPCs.17 Given the recent finding that the PI3K/AKT signaling pathway is critically involved in eEPC-mediated limitation of ischemia-reperfusion injury, we screened the eEPC transcriptome for genes encoding secreted proteins capable of activating this pathway.17 We found that Tβ4, one of the most highly expressed AKT-activating factors in our eEPC population, is indeed essential for cardiomyocyte protection in vitro. In particular, Tβ4 downregulation by shRNA or an anti-Tβ4 antibody blocked the eEPC-mediated increase in cardiomyocyte survival (Figure 1). Moreover, endothelial apoptosis and adhesion of inflammatory cells were blunted by eEPCs unless Tβ4 was knocked down (Figures 1E, 4A, and 4B). In vivo, the decrease in infarct size and postischemic inflammation and the increase in left ventricular function achieved by eEPC retroinfusion were blocked by Tβ4 shRNA pretreatment of the eEPCs (Figures 3 and 4C). Confirming the relevance of eEPC-borne Tβ4, exogenous application of the polypeptide in vivo mimicked the cardioprotection achieved by eEPCs (Figures 5 and 6).
Because Tβ4 interacts with G-actin and enhances the migratory capacity of differentiated endothelial cells31 and epicardial vascular progenitor cells in the developing heart,32 we addressed the possibility of an impaired eEPC recruitment of Tβ4 knockdown cells in a subset of experiments. A population of eEPCs expressing NIS, which is otherwise confined to the thyroid gland,22 was analyzed for recruitment to the heart by measurement of 123I accumulation. Using this scintigraphic approach, we could rule out an impairment of eEPC recruitment as a potential mechanism for the loss of eEPC-mediated cardioprotection after Tβ4 knockdown (Figure 2).
The beneficial effect of eEPCs may be achieved by 2 distinct principles: by providing building blocks for novel tissue, ie, vasculature or cardiomyocytes, or by providing paracrine factors as software for cell survival and healing.33 The eEPC population did not display hallmarks of transdifferentiation, ie, Oct-4 expression or GATA-4 activation, in vitro (data not shown). Moreover, the number of recovered eEPCs declined and was undetectable at day 14 after application in a model of chronic hind-limb ischemia, although a proangiogenic effect persisted at later time points. Therefore, the supply of software by eEPCs aimed at resolving acute and chronic ischemic syndromes appears more likely. This possibility has previously been related to the PI3K/AKT pathway,16 which is potentially activated by a variety of eEPC-borne candidate factors.17 Among PI3K/AKT activators, the highly expressed Tβ4 emerged as a likely candidate because of its functional profile. Other proangiogenic factors such vascular endothelial growth factor-A, platelet-derived growth factor-BB, and insulin-like growth factor-1 are expressed at much lower levels. Moreover, the highly expressed wnt agonists wnt7b and wnt11, activating PI3K/AKT signaling, are not known for cardioprotection (as opposed to the wnt antagonist FrzA34). Other PI3K/AKT activators such as macrophage migration inhibitory factor35 would counteract the antiinflammatory effect observed in our study (Figure 4).
On the other hand, Tβ4 reduced infarct size in a chronic murine LAD occlusion model, requiring PI3K/AKT signaling.18 Moreover, Tβ4 is capable of mediating angiogenesis in a long-term time course.32,36
The short-term protection of reperfused myocardium, which was targeted in the present study, may involve several distinct capabilities of Tβ4. In vitro Tβ4 incubation blocks cardiomyocyte death after hypoxia and reoxygenation, comparable to the paracrine effect of eEPCs, unless Tβ4 is largely diminished by shRNA transfection (Figures 1D and 5A). Moreover, Tβ4 is capable of reducing endothelial apoptosis after the hypoxia-reoxygenation protocol, again similar to the Tβ4-containing eEPCs (Figure 1E, Figure 5B). In previous studies, we found that protection of endothelial cells, eg, by endothelial nitric oxide synthase or vascular endothelial growth factor cDNA transfection,29 sufficed to reduce infarct size in our model.
Notably, in all of these studies in the pig ischemia-reperfusion model, a reduction in postischemic inflammation was observed. A reduced polymorphonuclear neutrophil influx also has been found after preconditioning and postconditioning37 and erythropoietin.38 On the other hand, a direct antiinflammatory effect of Tβ4 has been described earlier.39 In the present study, we confirm that Tβ4 decreased the adhesion of inflammatory cells in vitro and in vivo (Figure 5C and 5D).
Is Tβ4 a major player in adult EPC-mediated cardioprotection? Although adult porcine EPCs also contain about half of the Tβ4 content of embryonic eEPCs (data not shown), we refrained from applying adult EPCs at the end of ischemia because of the potentially harmful effects of contaminating inflammatory cells on microvasculatory function and inflammation. Consistent with this notion, a clinical trial using bone marrow–derived EPCs after >24 hours of reperfusion failed to document a cardioprotective effect,40 as opposed to the successful Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) study, which applied circulating EPCs after 4 days of reperfusion.9 However, we caution against extrapolation of our data to other sources of EPCs, which may differ with respect to adhesion, migration, and paracrine effector profiles.
Taken together, these results show that Tβ4 reveals a pleiotropic pattern of cardioprotection, including activation of cardiomyocyte survival pathways, inhibition of endothelial apoptosis, and limitation of inflammatory cell recruitment. These mechanisms have been activated by eEPCs containing Tβ4 and by application of synthetic Tβ4. The therapeutic potential of early Tβ4 protein application might be of relevance in the setting of ischemia-reperfusion in patients because no side effects limiting its applicability in this scenario are known yet.
Acknowledgments
We would like to thank Susanne Helbig, Elisabeth Ronft, and Natalie Schwab for excellent technical assistance.
Sources of Funding
This work was supported by Deutsche Forschungsgemeinschaft grants KU-1019/9-3/4 to Drs Kupatt and Boekstegers and Sp 581/4-1 to Dr Spitzweg, as well as National Institutes of Health grant HL-083958 to Dr Hatzopoulos.
Footnotes
The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circ.ahajournals.org/cgi/content/full/117/17/2232
Disclosures
None.
CLINICAL PERSPECTIVE
Myocardial organ detriment and dysfunction are common during a prolonged period of ischemia, although subsequent reperfusion as standard therapy is established. Among the pleiotropic causes of ischemia-reperfusion injury, loss of cardiomyocytes, microcirculatory disturbances preventing perfusion, and an overwhelming inflammatory reaction have been observed frequently. Novel therapeutic approaches, including application of progenitor cells, have provided new avenues to this decade-old problem. In the present study, we provide preclinical evidence that a particular source of embryonic endothelial progenitor cells is capable of cardioprotection against acute ischemia-reperfusion injury (24 hours). Moreover, we were able to confine the paracrine effect of the progenitor cell population to a single, highly expressed protein, thymosin β4. Depriving the progenitor cell population of thymosin β4 abolished cardioprotection, whereas regional application of thymosin β4 peptide provided cardioprotection similar to the embryonic endothelial progenitor cells. Mechanistically, thymosin β4 supports cardiomyocyte cell survival, prevents endothelial apoptosis, and limits postischemic inflammation. Because no side effects were observed, thymosin β4 might represent a novel therapeutic agonist in the scenario of clinical ischemia-reperfusion injury.
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 3.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 4.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 5.Ince H, Petzsch M, Kleine HD, Schmidt H, Rehders T, Korber T, Schumichen C, Freund M, Nienaber CA. Preservation from left ventricular remodeling by front-integrated revascularization and stem cell liberation in evolving acute myocardial infarction by use of granulocyte-colony-stimulating factor (FIRSTLINE-AMI) Circulation. 2005;112:3097–3106. doi: 10.1161/CIRCULATIONAHA.105.541433. [DOI] [PubMed] [Google Scholar]
- 6.Gyongyosi M, Khorsand A, Zamini S, Sperker W, Strehblow C, Kastrup J, Jorgensen E, Hesse B, Tagil K, Botker HE, Ruzyllo W, Teresinska A, Dudek D, Hubalewska A, Ruck A, Nielsen SS, Graf S, Mundigler G, Novak J, Sochor H, Maurer G, Glogar D, Sylven C. NOGA-guided analysis of regional myocardial perfusion abnormalities treated with intramyocardial injections of plasmid encoding vascular endothelial growth factor A-165 in patients with chronic myocardial ischemia: subanalysis of the EUROINJECT-ONE multicenter double-blind randomized study. Circulation. 2005;112(suppl):I-157–I-165. doi: 10.1161/01.CIRCULATIONAHA.105.525782. [DOI] [PubMed] [Google Scholar]
- 7.Zohlnhofer D, Ott I, Mehilli J, Schomig K, Michalk F, Ibrahim T, Meisetschlager G, von Wedel J, Bollwein H, Seyfarth M, Dirschinger J, Schmitt C, Schwaiger M, Kastrati A, Schomig A. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. JAMA. 2006;295:1003–1010. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]
- 8.Engelmann MG, Theiss HD, Hennig-Theiss C, Huber A, Wintersperger BJ, Werle-Ruedinger AE, Schoenberg SO, Steinbeck G, Franz WM. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization: final results from the G-CSF-STEMI (Granulocyte Colony-Stimulating Factor ST-Segment Elevation Myocardial Infarction) Trial. J Am Coll Cardiol. 2006;48:1712–1721. doi: 10.1016/j.jacc.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 9.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 10.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grogaard HK, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 11.Seeger FH, Tonn T, Krzossok N, Zeiher AM, Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- 12.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 13.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 14.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, Zhang J. The role of the sca-1+/C31− cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–1788. doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]
- 16.Kupatt C, Hinkel R, Lamparter M, von Bruhl ML, Pohl T, Horstkotte J, Beck H, Muller S, Delker S, Gildehaus FJ, Buning H, Hatzopoulos AK, Boekstegers P. Retroinfusion of embryonic endothelial progenitor cells attenuates ischemia-reperfusion injury in pigs: role of phosphatidyl-inositol 3-kinase/AKT kinase. Circulation. 2005;112(suppl):I-117–I-122. doi: 10.1161/CIRCULATIONAHA.104.524801. [DOI] [PubMed] [Google Scholar]
- 17.Kupatt C, Horstkotte J, Vlastos GA, Pfosser A, Lebherz C, Semisch M, Thalgott M, Buttner K, Browarzyk C, Mages J, Hoffmann R, Deten A, Lamparter M, Muller F, Beck H, Buning H, Boekstegers P, Hatzopoulos AK. Embryonic endothelial progenitor cells expressing a broad range of proangiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005;19:1576–1578. doi: 10.1096/fj.04-3282fje. [DOI] [PubMed] [Google Scholar]
- 18.Bock-Marquette I, Saxena A, White MD, DiMaio JM, Srivastava D. Thymosin [beta]4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 19.Weiler-Guettler H, Aird WC, Rayburn H, Husain M, Rosenberg RD. Developmentally regulated gene expression of thrombomodulin in postimplantation mouse embryos. Development. 1996;122:2271–2281. doi: 10.1242/dev.122.7.2271. [DOI] [PubMed] [Google Scholar]
- 20.Hatzopoulos AK, Folkman J, Vasile E, Eiselen GK, Rosenberg RD. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development. 1998;125:1457–1468. doi: 10.1242/dev.125.8.1457. [DOI] [PubMed] [Google Scholar]
- 21.Spitzweg C, Morris JC. The sodium iodide symporter: its pathophysiological and therapeutic implications. Clin Endocrinol. 2002;57:559–574. doi: 10.1046/j.1365-2265.2002.01640.x. [DOI] [PubMed] [Google Scholar]
- 22.Miyagawa M, Beyer M, Wagner B, Anton M, Spitzweg C, Gansbacher B, Schwaiger M, Bengel FM. Cardiac reporter gene imaging using the human sodium/iodide symporter gene. Cardiovasc Res. 2005;65:195–202. doi: 10.1016/j.cardiores.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Weiss SJ, Philp NJ, Grollman EF. Iodide transport in a continuous line of cultured cells from rat thyroid. Endocrinology. 1984;114:1090–1098. doi: 10.1210/endo-114-4-1090. [DOI] [PubMed] [Google Scholar]
- 24.Raake P, von Degenfeld G, Hinkel R, Vachenauer R, Sandner T, Beller S, Andrees M, Kupatt C, Schuler G, Boekstegers P. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins: comparison with surgical and percutaneous intramyocardial gene delivery. J Am Coll Cardiol. 2004;44:1124–1129. doi: 10.1016/j.jacc.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 25.Hannappel E. One-step procedure for the determination of thymosin beta 4 in small tissue samples and its separation from other thymosin beta 4-like peptides by high-pressure liquid chromatography. Anal Biochem. 1986;156:390–396. doi: 10.1016/0003-2697(86)90270-8. [DOI] [PubMed] [Google Scholar]
- 26.Hannappel E. Beta-thymosins. Ann N Y Acad Sci. 2007;1112:21–37. doi: 10.1196/annals.1415.018. [DOI] [PubMed] [Google Scholar]
- 27.Huff T, Muller CSG, Hannappel E. Thymosin beta4 is not always the main beta-thymosin in mammalian platelets. Ann N Y Acad Sci. 2007;1112:451–457. doi: 10.1196/annals.1415.029. [DOI] [PubMed] [Google Scholar]
- 28.Dell’Italia LJ, Evanochko WT, Blackwell GG, Pearce DJ, Pohost GM. Relationship between shortening load, contractility, and myocardial energetics in intact dog. Am J Physiol. 1993;264:H2180–H2187. doi: 10.1152/ajpheart.1993.264.6.H2180. [DOI] [PubMed] [Google Scholar]
- 29.Kupatt C, Hinkel R, Vachenauer R, Horstkotte J, Raake P, Sandner T, Kreuzpointner R, Muller F, Dimmeler S, Feron O, Boekstegers P. VEGF165 transfection decreases postischemic NF-kappa B-dependent myocardial reperfusion injury in vivo: role of eNOS phosphorylation. FASEB J. 2003;17:705–707. doi: 10.1096/fj.02-0673fje. [DOI] [PubMed] [Google Scholar]
- 30.Kupatt C, Dessy C, Hinkel R, Raake P, Daneau G, Bouzin C, Boekstegers P, Feron O. Heat shock protein 90 transfection reduces ischemia-reperfusion-induced myocardial dysfunction via reciprocal endothelial NO synthase serine 1177 phosphorylation and threonine 495 dephosphorylation. Arterioscler Thromb Vasc Biol. 2004;24:1435–1441. doi: 10.1161/01.ATV.0000134300.87476.d1. [DOI] [PubMed] [Google Scholar]
- 31.Malinda KM, Goldstein AL, Kleinman HK. Thymosin beta 4 stimulates directional migration of human umbilical vein endothelial cells. FASEB J. 1997;11:474–481. doi: 10.1096/fasebj.11.6.9194528. [DOI] [PubMed] [Google Scholar]
- 32.Smart N, Risebro CA, Melville AAD, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 33.Heil M, Ziegelhoeffer T, Mees B, Schaper W. A different outlook on the role of bone marrow stem cells in vascular growth: bone marrow delivers software not hardware. Circ Res. 2004;94:573–574. doi: 10.1161/01.RES.0000124603.46777.EB. [DOI] [PubMed] [Google Scholar]
- 34.Barandon L, Couffinhal T, Ezan J, Dufourcq P, Costet P, Alzieu P, Leroux L, Moreau C, Dare D, Duplaa C. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation. 2003;108:2282–2289. doi: 10.1161/01.CIR.0000093186.22847.4C. [DOI] [PubMed] [Google Scholar]
- 35.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 36.Philp D, Huff T, Gho YS, Hannappel E, Kleinman HK. The actin binding site on thymosin beta4 promotes angiogenesis. FASEB J. 2003;17:2103–2105. doi: 10.1096/fj.03-0121fje. [DOI] [PubMed] [Google Scholar]
- 37.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 38.Rui T, Feng Q, Lei M, Peng T, Zhang J, Xu M, Dale Abel E, Xenocostas A, Kvietys PR. Erythropoietin prevents the acute myocardial inflammatory response induced by ischemia/reperfusion via induction of AP-1. Cardiovasc Res. 2005;65:719–727. doi: 10.1016/j.cardiores.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 39.Badamchian M, Fagarasan MO, Danner RL, Suffredini AF, Damavandy H, Goldstein AL. Thymosin [beta]4 reduces lethality and down-regulates inflammatory mediators in endotoxin-induced septic shock. Int Immunopharmacol. 2003;3:1225–1233. doi: 10.1016/S1567-5769(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 40.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]