Abstract
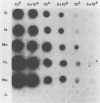
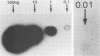
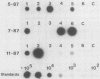
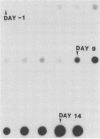
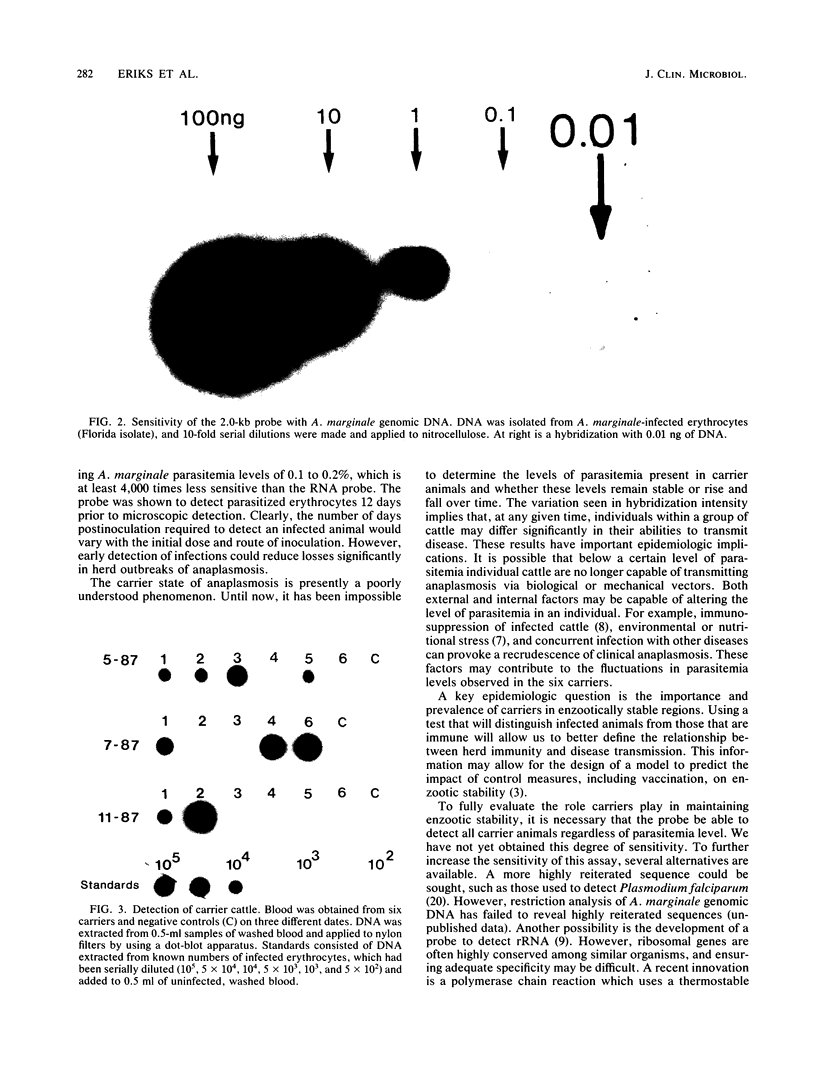
Cattle which have recovered from acute infection with Anaplasma marginale, a rickettsial hemoparasite of cattle, frequently remain persistently infected with a low-level parasitemia and serve as reservoirs for disease transmission. To fully understand the role of these carriers in disease prevalence and transmission, it is essential that low levels of parasitemia can be accurately detected and quantitated. We have developed a nucleic acid probe, derived from a portion of a gene encoding a 105,000-molecular-weight surface protein, that can detect A. marginale-infected erythrocytes. The probe is specific for A. marginale and can detect 0.01 ng of genomic DNA and 500 to 1,000 infected erythrocytes in 0.5 ml of blood, which is equivalent to a parasitemia of 0.000025%. This makes the probe at least 4,000 times more sensitive than light microscopy. Hybridization of the probe with treated blood from animals proven to be carriers of anaplasmosis showed that parasitemia levels were highly variable among carriers, ranging from greater than 0.0025 to less than 0.000025%. Parasitemia levels of individual animals on different dates were also variable. These results imply that, at any given time, individuals within a group of cattle may differ significantly in their abilities to transmit disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrosio R. E., Potgieter F. T., Nel N. A column purification procedure for the removal of leucocytes from parasite-infected bovine blood. Onderstepoort J Vet Res. 1986 Sep;53(3):179–180. [PubMed] [Google Scholar]
- Anderson R. M., May R. M. Vaccination and herd immunity to infectious diseases. 1985 Nov 28-Dec 4Nature. 318(6044):323–329. doi: 10.1038/318323a0. [DOI] [PubMed] [Google Scholar]
- Barbet A. F., Palmer G. H., Myler P. J., McGuire T. C. Characterization of an immunoprotective protein complex of Anaplasma marginale by cloning and expression of the gene coding for polypeptide Am105L. Infect Immun. 1987 Oct;55(10):2428–2435. doi: 10.1128/iai.55.10.2428-2435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker R. H., Jr, Suebsaeng L., Rooney W., Alecrim G. C., Dourado H. V., Wirth D. F. Specific DNA probe for the diagnosis of Plasmodium falciparum malaria. Science. 1986 Mar 21;231(4744):1434–1436. doi: 10.1126/science.3513309. [DOI] [PubMed] [Google Scholar]
- Brandsma J., Miller G. Nucleic acid spot hybridization: rapid quantitative screening of lymphoid cell lines for Epstein-Barr viral DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6851–6855. doi: 10.1073/pnas.77.11.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrier D. E., Wagner G. G., Adams L. G. Recrudescence of Anaplasma marginale induced by immunosuppression with cyclophosphamide. Am J Vet Res. 1981 Jan;42(1):19–21. [PubMed] [Google Scholar]
- Dame J. B., McCutchan T. F. The four ribosomal DNA units of the malaria parasite Plasmodium berghei. Identification, restriction map, and copy number analysis. J Biol Chem. 1983 Jun 10;258(11):6984–6990. [PubMed] [Google Scholar]
- Engleberg N. C., Eisenstein B. I. The impact of new cloning techniques on the diagnosis and treatment of infectious diseases. N Engl J Med. 1984 Oct 4;311(14):892–901. doi: 10.1056/NEJM198410043111406. [DOI] [PubMed] [Google Scholar]
- Goff W., Barbet A., Stiller D., Palmer G., Knowles D., Kocan K., Gorham J., McGuire T. Detection of Anaplasma-marginale-infected tick vectors by using a cloned DNA probe. Proc Natl Acad Sci U S A. 1988 Feb;85(3):919–923. doi: 10.1073/pnas.85.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A., Prediger E., Huecas M. E., Nogueira N., Lizardi P. M. Minichromosomal repetitive DNA in Trypanosoma cruzi: its use in a high-sensitivity parasite detection assay. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3356–3360. doi: 10.1073/pnas.81.11.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel U. B., Stanbridge E. J. Cloned mycoplasma ribosomal RNA genes for the detection of mycoplasma contamination in tissue cultures. Science. 1984 Dec 7;226(4679):1211–1213. doi: 10.1126/science.6505688. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T. Estimate of the genome size of various microorganisms. J Bacteriol. 1969 Jun;98(3):1400–1401. doi: 10.1128/jb.98.3.1400-1401.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan S. C., Doherty M., Gitschier J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N Engl J Med. 1987 Oct 15;317(16):985–990. doi: 10.1056/NEJM198710153171603. [DOI] [PubMed] [Google Scholar]
- Luther D. G., Cox H. U., Nelson W. O. Comparisons of serotests with calf inoculations for detection of carriers in anaplasmosis- vaccinated cattle. Am J Vet Res. 1980 Dec;41(12):2085–2086. [PubMed] [Google Scholar]
- McGuire T. C., Palmer G. H., Goff W. L., Johnson M. I., Davis W. C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984 Sep;45(3):697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin G. L., Collins W. E., Campbell G. H. Comparison of genomic, plasmid, synthetic, and combined DNA probes for detecting Plasmodium falciparum DNA. J Clin Microbiol. 1987 May;25(5):791–795. doi: 10.1128/jcm.25.5.791-795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao C. C., Lin S. S., Wu S. Y., Juang W. M., Chang C. H., Lin J. Y. The detection of mycobacterial DNA sequences in uncultured clinical specimens with cloned Mycobacterium tuberculosis DNA as probes. Tubercle. 1988 Mar;69(1):27–36. doi: 10.1016/0041-3879(88)90037-2. [DOI] [PubMed] [Google Scholar]
- RISTIC M., WATRACH A. M. Anaplasmosis. VI. Studies and a hypothesis concerning the cycle of development of the causative agent. Am J Vet Res. 1963 Mar;24:267–277. [PubMed] [Google Scholar]
- Razin S., Gross M., Wormser M., Pollack Y., Glaser G. Detection of mycoplasmas infecting cell cultures by DNA hybridization. In Vitro. 1984 May;20(5):404–408. doi: 10.1007/BF02619586. [DOI] [PubMed] [Google Scholar]
- Taylor M. A., Wise K. S., McIntosh M. A. Selective detection of Mycoplasma hyorhinis using cloned genomic DNA fragments. Infect Immun. 1985 Mar;47(3):827–830. doi: 10.1128/iai.47.3.827-830.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth D. F., Rogers W. O., Barker R., Jr, Dourado H., Suesebang L., Albuquerque B. Leishmaniasis and malaria: new tools for epidemiologic analysis. Science. 1986 Nov 21;234(4779):975–979. doi: 10.1126/science.3535070. [DOI] [PubMed] [Google Scholar]
- Zaugg J. L., Stiller D., Coan M. E., Lincoln S. D. Transmission of Anaplasma marginale Theiler by males of Dermacentor andersoni Stiles fed on an Idaho field-infected, chronic carrier cow. Am J Vet Res. 1986 Oct;47(10):2269–2271. [PubMed] [Google Scholar]