Abstract
The present study tests the sex-dependent effect of perinatal taurine exposure on arterial pressure control in adults. Female Sprague-Dawley rats were fed normal rat chow with 3% beta-alanine (taurine depletion, TD), 3% taurine (taurine supplementation, TS) or water alone (C) from conception to weaning. Their male and female offspring were then fed normal rat chow and tap water with 5% glucose (C with glucose, CG; TD with glucose, TDG; TS with glucose, TSG) or water alone (CW, TDW or TSW). At 7–8 weeks of age, they were studied in a conscious condition. Body weights were lower in male and female TDG and male TDW rats. Kidney to body weights increased in female TSW but not TSG. Plasma sodium and potassium were not significantly different among males. In the females, plasma sodium levels were lower in all glucose treated groups while plasma potassium levels were lower only in TDG. Hematocrit, fasting blood glucose, and glucose tolerance were not significantly different among sexes. Mean arterial pressures increased in male TDG, TSW, and TSG while in the females, mean arterial pressures increased in TDW, TDG, and TSG. Heart rates were not significantly different among sexes. The present data indicate that perinatal exposure alters arterial pressure control of adult rats and this effect is gender specific.
Introduction
In both human and animal models, fetal environment in utero has significant impact on adult health and disease (Harding 2001; Langley-Evans et al. 2003). Undernutrition or imbalanced food consumption (for instance low protein-high carbohydrate diets) in the prenatal period results in low birth weight and subsequently induces several cardiovascular disorders in adults, including coronary vascular diseases, hypertension, insulin resistance, diabetes mellitus, and ultimately renal damage (Barker et al. 2002; Forrester 2004). Hypertension and diabetes mellitus also appear to be related to obesity developed in the later life (Mendez et al. 2004). Epidemiologic studies indicate that African-American women have a higher prevalence of low birth weight and adult obesity (Ventura et al. 2002). Although the mechanism(s) of these effects is still unclear, abnormalities of the renin-angiotensin and sympathetic nervous systems have been characterized in humans and animal models (Eriksson et al. 2007; Lackland et al. 2002). The perinatal programming of adult function and diseases has been recognized for a decade (Barker 2007). Low birth weight has been associated with many changes, including taurine deficiency in the perinatal period and later life (Aerts and Van Assche 2002). In adult animals, taurine supplementation decreases hypertension, likely by increased renal Na excretion, inhibition of the renin-angiotensin system, and decreased sympathetic nerve activity (Militante and Lombardini 2002).
Taurine, a 2-aminoethane sulfonic acid, is a phylogenetically ancient compound that is present in high concentration in many organs including brain, heart, kidneys, and reproductive organs. Its content is highest in these organs during fetal life and gradually decreases after birth (Aerts and Van Assche 2002). During lactation it appears to be an essential amino acid, since taurine synthesis is minimal in the organism with maternal milk as the main source. Several lines of evidence indicate that perinatal taurine status programs cells for adult function, especially organs related to the cardiovascular system. Perinatal taurine supplementation prevents hypertension in spontaneously hypertensive rats (SHR), partly by its antioxidant activity (Racasan et al. 2004). Our previous experiments indicated that either taurine depletion or supplementation in early life alters renal function (Roysommuti et al. 2004) and autonomic nervous control of arterial pressure (Roysommuti et al. 2007; Suvanich et al. 2006) in adult, male rats. Perinatal taurine depletion increased arterial pressure but not heart rate in adult, female offspring. Their renal hemodynamics and excretory function were also modified by the perinatal taurine exposure (Lerdweerapol et al. 2007). In addition, the autonomic nervous system and renal function responses to high sugar intake in young adult animals appear to be altered by perinatal taurine exposure. The protection of arterial pressure in females may be due to the putative antihypertensive effects of estrogen in female animal models, including spontaneously hypertensive and salt-induced hypertension in ovariectomized rats (Clark et al. 2004; Peng et al. 2003a). This study compares the long-term effect of perinatal taurine exposure on arterial pressure control in male and female adult offspring fed with a high sugar diet.
Materials and Methods
Sprague-Dawley (SD) rats were bred from the animal unit of Faculty of Medicine, Khon Kaen University and maintained at constant humidity (60 ± 5%), temperature (24 ± 1 °C), and light cycle (0600–1800 h). Female SD rats were fed with normal rat chow and free access to tap water alone (control, C), tap water with 3% beta-alanine (taurine depletion, TD) or tap water with 3% taurine (taurine supplementation, TS) from conception until weaning. Then, their male and female offspring were fed with the normal rat chow with either 5% glucose in tap water (TD with glucose, TDG; TS with glucose, TSG; C with glucose, CG) or tap water alone (TDW, TSW, and CW) throughout the experiment.
At 7–8 weeks of age, under thiopental anesthesia, all male and female offspring were implanted with femoral arterial and venous catheters. Two or three days later and after an overnight fasting, arterial blood samples were obtained from conscious animals for Na, K, hematocrit, and fasting blood sugar determinations. Then, glucose tolerance tests were started by intravenous injection of glucose (2 g/kg in saline) and blood glucose levels were subsequently measured at 30, 60, and 120 minutes, respectively. Twenty-four hours later, non-fasting blood samples were collected and then arterial pressure pulses were continuously recorded (Bio-pac system, CA) in a conscious condition. At the end of experiment, all animals were sacrificed and kidney and heart weights were collected.
Experimental protocols and animal care were approved by the university animal committee. Plasma sodium and potassium concentrations were determined by a flame photometry, hematocrit by a standard technique, fresh blood sugar by a glucometer and glucostrips ((Accu-chek®, Germany), and mean arterial pressure and heart rate by Acknowledge software version 3.8.1 (Biopac system, CA)
All data were expressed as mean ± SEM. Statistical comparisons among groups (p < 0.05) were done by using one-way ANOVA and Dun-can’Multi-Range.
Results
Perinatal taurine depletion caused significantly growth retardation in male offspring which could be partially recovered by a high sugar supplementation after weaning (Table 1). In contrast, growth in female rats was not retarded by that perinatal taurine depletion alone but together with a high sugar diet after weaning, it did retard growth. Perinatal taurine supplementation had no effect on body and heart weight but slightly increased kidney weight when compared to CG and CW female rats. High sugar treatment alone had no effect on growth. While plasma Na levels were not significantly different among male groups, they significantly decreased in all glucose-treated female offspring (Table 2). Both male and female offspring displayed similar plasma potassium concentrations, hematocrit, and fasting blood sugar among groups. In males, non-fasting blood sugar levels were slightly increased in CG rats, while they were significantly increased in all glucose-treated female offspring when compared to their corresponding controls (CW, TDW, and TSW). Both and female offspring displayed similar glucose tolerance (Fig. 1).
Table 1.
Body (BW), kidney (KW), and heart (HW) weights in male (M) and female (F) offspring
| Treatment | BW (g) | KW (g) | HW (g) | KW/BW (%) | HW/BW (%) | |
|---|---|---|---|---|---|---|
| CW | M (n=6) | 233±4 | 1.12±0.02 | 0.90±0.02 | 0.48±0.01 | 0.39±0.01 |
| F (n=7) | 194±5 | 1.66±0.04 | 0.6±0.02 | 0.85±0.03 | 0.36±0.01 | |
|
| ||||||
| CG | M (n=5) | 234±7 | 1.13±0.03 | 0.91±0.02 | 0.48±0.02 | 0.39±0.02 |
| F (n=8) | 196±4 | 1.61±0.04 | 0.74±0.02 | 0.82±0.01 | 0.37±0.00 | |
|
| ||||||
| TDW | M (n=5) | 210±5* | 1.07±0.04 | 0.85±0.02 | 0.51±0.03 | 0.41±0.01 |
| F (n=6) | 196±2 | 1.72±0.08 | 0.72±0.04 | 0.88±0.04 | 0.37±0.02 | |
|
| ||||||
| TDG | M (n=5) | 214±6 | 1.02±0.06 | 0.89±0.03 | 0.48±0.04 | 0.42±0.02 |
| F(n=10) | 179±5*,α | 1.59±0.02 | 0.67±0.03 | 0.89±0.02 | 0.37±0.01 | |
|
| ||||||
| TSW | M (n=6) | 238±6 | 1.08±0.04 | 0.90±0.02 | 0.45±0.01 | 0.38±0.01 |
| F (n=6) | 197±4 | 1.88±0.0*,α | 0.75±0.01 | 0.95±0.02*,α | 0.38±0.01 | |
|
| ||||||
| TSG | M (n=6) | 228±7 | 1.11±0.05 | 0.93±0.02 | 0.49±0.02 | 0.41±0.01 |
| F (n=8) | 191±5 | 1.67±0.04 | 0.73±0.02 | 0.88±0.04 | 0.38±0.01 | |
Data were mean±SEM.
denoted significant difference when compared to CW or CG, respectively. See text for abbreviations.
Table 2.
Plasma sodium, plasma potassium, hematocrit, non-fasting blood sugar (NFBS), and fasting blood sugar (FBS) in male (M) and female (F) offspring
| Treatment | Na (mEq/L) | K (mEq/L) | Hematocrit (%) | NFBS (mg/dl) | FBS (mg/dl) | |
|---|---|---|---|---|---|---|
| CW | M(n=6) | 139.6±1.2 | 3.75±0.19 | 42.2±0.83 | 83.5±2.91 | 80.3±3.73 |
| F (n=7) | 132.5±3.3 | 4.44±2.23 | 41.9±1.17 | 115.0±5.17 | 86.4±3.60 | |
|
| ||||||
| CG | M(n=5) | 138.6±1.5 | 3.82±0.04 | 42.2±1.02 | 105.6±5.28* | 80.0±1.65 |
| F (n=8) | 118.3±3.1* | 4.51±0.33 | 40.8±0.77 | 129.4±3.02* | 91.3±2.74 | |
|
| ||||||
| TDW | M(n=5) | 139.4±0.5 | 3.86±0.02 | 41.4±0.60 | 89.8±5.59 | 81.2±4.13 |
| F(n=6) | 122.7±2.4 | 4.49±0.08 | 40.5±0.82 | 125.7±2.86 | 91.2±2.81 | |
|
| ||||||
| TDG | M(n=5) | 134.6±2.1 | 3.74±0.18 | 42.2±0.80 | 107.0±4.94* | 84.6±3.37 |
| F(n=10) | 117.3±2.4* | 3.45±0.18 | 40.5±0.93*,α | 120.4±5.35 | 86.6±2.83 | |
|
| ||||||
| TSW | M(n=6) | 139.0±1.3 | 3.88±0.05 | 42.8±0.65 | 88.0±5.95 | 82.8±3.13 |
| F (n=6) | 124.0±2.4 | 3.97±0.17 | 39.2±1.09 | 126.7±3.56 | 94.0±3.44 | |
|
| ||||||
| TSG | M(n=6) | 134.8±1.8 | 3.80±0.15 | 41.2±0.79 | 102.5±4.40* | 85.7±2.20 |
| F (n=8) | 114.3±6.0* | 4.06±0.12 | 41.3±1.10 | 121.5±3.92 | 90.3±1.45 | |
Data were mean±SEM.
denoted significant difference when compared to CW or CG, respectively. See text for abbreviations.
Fig. 1.
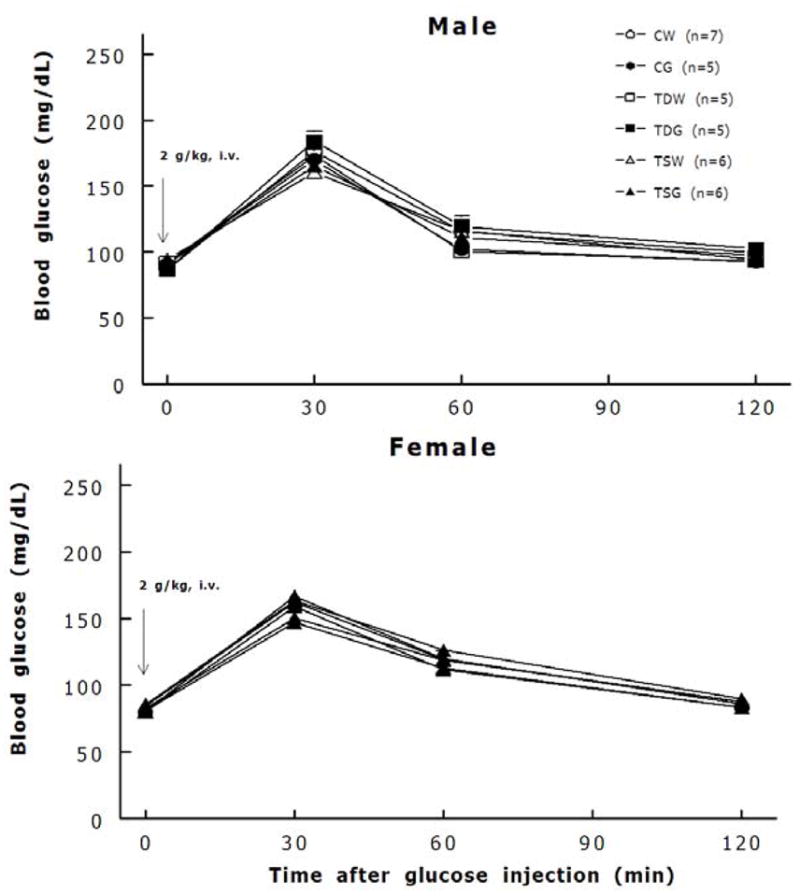
Both male and female offspring displayed well glucose tolerance.
Perinatal taurine depletion alone increased mean arterial pressures in female but not male TDW while taurine supplementation alone increased them in male but not female TSW (Fig. 2). These perinatal taurine effects were not altered by the high sugar diet. The high sugar diet significantly increased mean arterial pressure in perinatal taurine depleted males (CW, 101.2 ± 2.5 mm Hg; CG, 96.0 ± 3.0 mm Hg; TDW, 97.6 ± 2.7 mm Hg; TDG, 109.6 ± 2.1 mm Hg; P < 0.05) and perinatal taurine-supplemented female (CW, 112.8 ± 1.8 mm Hg; CG, 117.16 ± 3.39 mm Hg; TSW, 118.0 ± 2.5 mm Hg; TSG, 121.2 ± 3.0 mm Hg; P < 0.05) when compared to CW or CG rats (Figure 2). Heart rates were not significantly different among male or female rats (Figure 3). Although the females displayed lower body weight than the males (Table 1), their mean arterial pressures were significantly higher in all corresponding groups (Fig. 2).
Fig. 2.
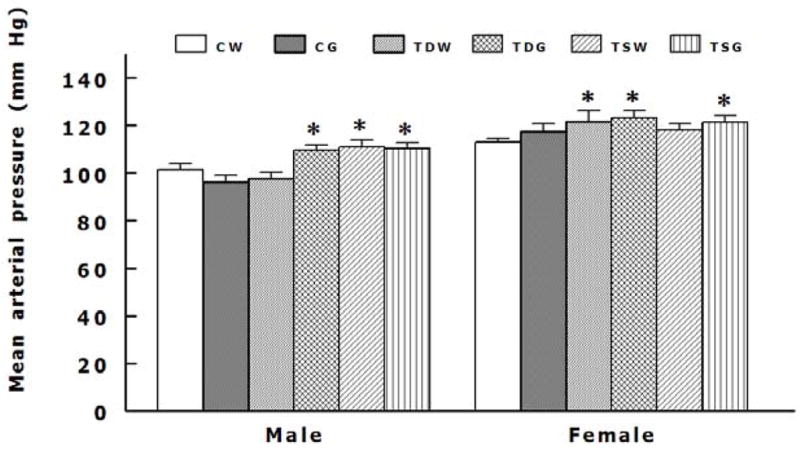
Mean arterial pressures were significantly higher in male TDG, male TSW, male TSG, female TDW, female TDG, and female TSG (P < 0.05 compared to CW). See text for abbreviations.
Fig. 3.
Heart rates were not significantly different among male or female offspring. See text for abbreviations.
Discussion
Perinatal treatment can significantly alter adult organ function and health, including cardiovascular function. Female rats treated with testosterone during the first 4 days of life develop a male pattern of gonadotropin secretion with abnormal female sexual behavior in the mature life (BARRACLOUGH 1961; Clark et al. 2004; Peng et al. 2003a). Handling or various stressors in the neonates results in permanent changes in hypothalamic structure and abnormal stress responses in the adult period (Cella et al. 1990). Perinatal administration of angiotensin converting enzyme inhibitors attenuates hypertension in the adult SHR but does not prevent salt-induced hypertension (Wyss et al. 1994). Perinatal taurine treatment also attenuates hypertension in the adult SHR, likely via its antioxidant properties (Racasan et al. 2004). Our previous experiments indicate that pre- or postnatal (lactation) taurine supplementation increased mean arterial pressure in adult male offspring (Roysommuti et al. 2004). The present data further demonstrates that perinatal taurine supplementation can increase the mean arterial pressure in adult male rats but not female rats. Further, perinatal taurine depletion can increase arterial pressure in adult female but not male rats. This study thus demonstrates gender specific responses to perinatal taurine exposure.
It is well-known that sugar consumption is a significant risk factor for the development of hypertension. Sugar-induced hypertension is associated with hyperinsulinemia, insulin resistance, renin-angiotensin system overactivity, sympathetic nervous system overactivity, and renal dysfunction (Johnson et al. 2007). However, previous experiments indicate that glucose supplementation induces renal dysfunction before insulin resistance and hypertension, and that these effects can be abolished by treatment with an angiotensin converting enzyme inhibitor (captopril) (Roysommuti et al. 2002). Taurine inhibits the renin-angiotensin system (Azuma et al. 2000; Schaffer et al. 2000) and prevents fructose-induced hypertension in rats (Harada et al. 2004). Moreover, in many forms of hypertension taurine supplementation in young or adult life reduces arterial pressure (Militante and Lombardini 2002), improves renal function and inhibits the sympathetic nervous system. The present study reports the interaction between perinatal taurine exposure and the subsequent effect of high sugar intake on arterial pressure in both sexes. Perinatal taurine depletion induces a pressor effect from high sugar consumption in male but not female rats and vice versa for perinatal taurine supplementation. Theses opposite effects may be due to gender differences in the body taurine content, sex hormones, autonomic nervous system, renin-angiotensin system or renal function at the adult life. The permanent changes and programming at the early life is hypothesized to be the primary factor.
Obesity, insulin resistance, hyperinsulinemia, and electrolyte disturbances are associated with the development of hypertension, and perinatal imbalances of nutrition have been reported to be a predisposing factor to these dysfunctions (Barker et al. 2002; Harding 2001; Langley-Evans 2006). Exposure to taurine in fetuses and neonates is primarily from diets through the placenta or maternal milk (Aerts and Van Assche 2002). Thus, taurine deficiency is observed in rat neonates of the pregnant mother that are fed low protein diets (Cherif et al. 1998). Though body taurine content can return to normal levels within 5–6 weeks after end of its supplementation or depletion (Pacioretty et al. 2001), permanent changes appear to continue into adult life, as shown by the present data. In the present study, the pressor effect of perinatal taurine with or without sugar supplementation did not relate to adult body weight, insulin resistance or Na-K imbalance. Periodic fluctuation of blood glucose level and hyperinsulinemia in all sugar-treated rats might play a role in sugar-induced hypertension in more aged rats. Also, sugar treatment with this dose has been reported to alter renal function without insulin resistance, glucose intolerance, and hypertension in male Sprague-Daley rats (Roysommuti et al. 2002).
Although taurine supplementation may improve or prevent hypertension in humans and animal models (Militante and Lombardini 2002), its mechanisms of action are complicated. It prevents fructose-induced hypertension but exacerbates hyperinsulinemia and hypertriglyceridemia in rats (Anuradha and Balakrishnan 1999; Harada et al. 2004). This anti-hypertensive action is likely mediated by kinins and renal fluid excretion (Gentile et al. 1994; Nandhini et al. 2004; Nandhini and Anuradha 2004). In contrast, hypertension, insulin resistance, and renal damage in adult offspring that are induced by perinatal imbalance of nutrition may be prevented or improved by taurine treatment in the early or later life (Hoet et al. 2000; Militante and Lombardini 2002). In addition, taurine may inhibit the sympathetic activity and the renin-angiotensin system in many forms of hypertension. Thus, the pressor effect of perinatal taurine exposure and its interaction with high sugar consumption at later life need further clarified.
Gender differences in pathogeneses of cardiovascular diseases have been shown in many experimental models and humans (D’Amore and Mora 2006; Meyer et al. 2006). Estrogen rather than testosterone plays a protective action for these diseases. Estrogen protects against increases in arterial pressure by acting on blood vessels and on cardiovascular centers in the brain (Ashraf and Vongpatanasin 2006; Maturana et al. 2007; Peng et al. 2003b; Wyss and Carlson 2003). Recently, it was reported that estrogen treatment improves or prevents hypertension in the female growth-restricted offspring (Ojeda et al. 2007). Taurine depletion is also observed in these animal models (Aerts and Van Assche 2002). In addition, prenatal testosterone treatment could induce cardiovascular diseases in adult offspring, similar to prenatal malnutrition (Dumesic et al. 2007; King et al. 2007). Thus, perinatal taurine exposure likely alters the sex hormone status in the early life and programs the subsequent organ function and adult diseases.
In summary, the present study indicates a gender disparity in the long-term effects of perinatal taurine depletion and supplementation on arterial pressure control. An imbalance of taurine exposure in early life will thus predispose or program the pressor effect of high sugar consumption in the later life.
Acknowledgments
This work was supported by grants from the Faculty of Medicine, Khon Kaen University and National Institutes of Health grants, AT 00477 (JMW) from the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Complementary and Alternative Medicine, the Office of Dietary Supplements, or the National Institutes of Health.
Contributor Information
Sanya Roysommuti, Department of Physiology, Khon Kaen University, Faculty of Medicine, Khon Kaen 40002, Thailand.
Atchariya Suwanich, Department of Physiology, Khon Kaen University, Faculty of Medicine, Khon Kaen 40002, Thailand.
Wichaporn Lerdweeraphon, Department of Physiology, Khon Kaen University, Faculty of Medicine, Khon Kaen 40002, Thailand.
Atcharaporn Thaeomor, Department of Physiology, Khon Kaen University, Faculty of Medicine, Khon Kaen 40002, Thailand.
Dusit Jirakulsomchok, Department of Physiology, Khon Kaen University, Faculty of Medicine, Khon Kaen 40002, Thailand.
J. Michael Wyss, Department of Cell Biology, School of Medicine, University of Alabama at Birmingham, AL 35294, USA.
References
- Aerts L, Van Assche FA. Taurine and taurine-deficiency in the perinatal period. J Perinat Med. 2002;30:281–6. doi: 10.1515/JPM.2002.040. [DOI] [PubMed] [Google Scholar]
- Anuradha CV, Balakrishnan SD. Taurine attenuates hypertension and improves insulin sensitivity in the fructose-fed rat, an animal model of insulin resistance. Can J Physiol Pharmacol. 1999;77:749–54. [PubMed] [Google Scholar]
- Ashraf MS, Vongpatanasin W. Estrogen and hypertension. Curr Hypertens Rep. 2006;8:368–76. doi: 10.1007/s11906-006-0080-1. [DOI] [PubMed] [Google Scholar]
- Azuma M, Takahashi K, Fukuda T, Ohyabu Y, Yamamoto I, Kim S, Iwao H, Schaffer SW, Azuma J. Taurine attenuates hypertrophy induced by angiotensin II in cultured neonatal rat cardiac myocytes. Eur J Pharmacol. 2000;403:181–8. doi: 10.1016/s0014-2999(00)00483-0. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–7. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–9. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- BARRACLOUGH CA. Production of anovulatory, sterile rats by single injections of testosterone propionate. Endocrinology. 1961;68:62–7. doi: 10.1210/endo-68-1-62. [DOI] [PubMed] [Google Scholar]
- Cella SG, Locatelli V, Mennini T, Zanini A, Bendotti C, Forloni GL, Fumagalli G, Arce VM, de GCV, Wehrenberg WB, et al. Deprivation of growth hormone-releasing hormone early in the rat’s neonatal life permanently affects somatotropic function. Endocrinology. 1990;127:1625–34. doi: 10.1210/endo-127-4-1625. [DOI] [PubMed] [Google Scholar]
- Cherif H, Reusens B, Ahn MT, Hoet JJ, Remacle C. Effects of taurine on the insulin secretion of rat fetal islets from dams fed a low-protein diet. J Endocrinol. 1998;159:341–8. doi: 10.1677/joe.0.1590341. [DOI] [PubMed] [Google Scholar]
- Clark JT, Chakraborty-Chatterjee M, Hamblin M, Wyss JM, Fentie IH. Estrogen depletion differentially affects blood pressure depending on age in Long-Evans rats. Endocrine. 2004;25:173–86. doi: 10.1385/ENDO:25:2:173. [DOI] [PubMed] [Google Scholar]
- D’Amore S, Mora S. Gender-specific prediction of cardiac disease: importance of risk factors and exercise variables. Cardiol Rev. 2006;14:281–5. doi: 10.1097/01.crd.0000244460.25429.c8. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007;8:127–41. doi: 10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JG, Forsen TJ, Kajantie E, Osmond C, Barker DJ. Childhood growth and hypertension in later life. Hypertension. 2007;49:1415–21. doi: 10.1161/HYPERTENSIONAHA.106.085597. [DOI] [PubMed] [Google Scholar]
- Forrester T. Historic and early life origins of hypertension in Africans. J Nutr. 2004;134:211–6. doi: 10.1093/jn/134.1.211. [DOI] [PubMed] [Google Scholar]
- Gentile S, Bologna E, Terracina D, Angelico M. Taurine-induced diuresis and natriuresis in cirrhotic patients with ascites. Life Sci. 1994;54:1585–93. doi: 10.1016/0024-3205(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Harada H, Tsujino T, Watari Y, Nonaka H, Emoto N, Yokoyama M. Oral taurine supplementation prevents fructose-induced hypertension in rats. Heart Vessels. 2004;19:132–6. doi: 10.1007/s00380-003-0757-1. [DOI] [PubMed] [Google Scholar]
- Harding JE. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol. 2001;30:15–23. doi: 10.1093/ije/30.1.15. [DOI] [PubMed] [Google Scholar]
- Hoet JJ, Ozanne S, Reusens B. Influences of pre- and postnatal nutritional exposures on vascular/endocrine systems in animals. Environ Health Perspect. 2000;108(Suppl 3):563–8. doi: 10.1289/ehp.00108s3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sanchez-Lozada LG. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- King AJ, Olivier NB, Mohankumar PS, Lee JS, Padmanabhan V, Fink GD. Hypertension caused by prenatal testosterone excess in female sheep. Am J Physiol Endocrinol Metab. 2007;292:E1837–41. doi: 10.1152/ajpendo.00668.2006. [DOI] [PubMed] [Google Scholar]
- Lackland DT, Egan BM, Syddall HE, Barker DJ. Associations between birth weight and antihypertensive medication in black and white medicaid recipients. Hypertension. 2002;39:179–83. doi: 10.1161/hy0102.100545. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC. Developmental programming of health and disease. Proc Nutr Soc. 2006;65:97–105. doi: 10.1079/pns2005478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley-Evans SC, Langley-Evans AJ, Marchand MC. Nutritional programming of blood pressure and renal morphology. Arch Physiol Biochem. 2003;111:8–16. doi: 10.1076/apab.111.1.8.15136. [DOI] [PubMed] [Google Scholar]
- Lerdweerapol W, Jirakulsomchok D, Wyss JM, Roysommuti S. Perinatal taurine supplementation influences renal hemodynamics and excretory function in adult, female rats. FASEB J. 2007;21(5 Part I):A501–2. [Google Scholar]
- Maturana MA, Irigoyen MC, Spritzer PM. Menopause, estrogens, and endothelial dysfunction: current concepts. Clinics. 2007;62:77–86. doi: 10.1590/s1807-59322007000100012. [DOI] [PubMed] [Google Scholar]
- Mendez MA, Wynter S, Wilks R, Forrester T. Under- and overreporting of energy is related to obesity, lifestyle factors and food group intakes in Jamaican adults. Public Health Nutr. 2004;7:9–19. doi: 10.1079/phn2003508. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension. 2006;47:1019–26. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- Militante JD, Lombardini JB. Treatment of hypertension with oral taurine: experimental and clinical studies. Amino Acids. 2002;23:381–93. doi: 10.1007/s00726-002-0212-0. [DOI] [PubMed] [Google Scholar]
- Nandhini AT, Anuradha CV. Hoe 140 abolishes the blood pressure lowering effect of taurine in high fructose-fed rats. Amino Acids. 2004;26:299–303. doi: 10.1007/s00726-003-0003-2. [DOI] [PubMed] [Google Scholar]
- Nandhini AT, Thirunavukkarasu V, Anuradha CV. Potential role of kinins in the effects of taurine in high-fructose-fed rats. Can J Physiol Pharmacol. 2004;82:1–8. doi: 10.1139/y03-118. [DOI] [PubMed] [Google Scholar]
- Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension. 2007;50:679–85. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacioretty L, Hickman MA, Morris JG, Rogers QR. Kinetics of taurine depletion and repletion in plasma, serum, whole blood and skeletal muscle in cats. Amino Acids. 2001;21:417–27. doi: 10.1007/s007260170006. [DOI] [PubMed] [Google Scholar]
- Peng N, Clark JT, Wei CC, Wyss JM. Estrogen depletion increases blood pressure and hypothalamic norepinephrine in middle-aged spontaneously hypertensive rats. Hypertension. 2003b;41:1164–7. doi: 10.1161/01.HYP.0000065387.09043.2E. [DOI] [PubMed] [Google Scholar]
- Peng N, Clark JT, Wei CC, Wyss JM. Estrogen depletion increases blood pressure and hypothalamic norepinephrine in middle-aged spontaneously hypertensive rats. Hypertension. 2003a;41:1164–7. doi: 10.1161/01.HYP.0000065387.09043.2E. [DOI] [PubMed] [Google Scholar]
- Racasan S, Braam B, van der Giezen DM, Goldschmeding R, Boer P, Koomans HA, Joles JA. Perinatal L-arginine and antioxidant supplements reduce adult blood pressure in spontaneously hypertensive rats. Hypertension. 2004;44:83–8. doi: 10.1161/01.HYP.0000133251.40322.20. [DOI] [PubMed] [Google Scholar]
- Roysommuti S, Khongnakha T, Jirakulsomchok D, Wyss JM. Excess dietary glucose alters renal function before increasing arterial pressure and inducing insulin resistance. Am J Hypertens. 2002;15:773–9. doi: 10.1016/s0895-7061(02)02974-6. [DOI] [PubMed] [Google Scholar]
- Roysommuti S, Malila P, Jirakulsomchok D, Jirakulsomchok S, Wyss JM. Perinatal taurine status influences renal hemodynamics in adult conscious rats. FASEB J. 2004;18(4 Part I):A292–3. [Google Scholar]
- Roysommuti S, Suvanich A, Jirakulsomchok D, Wyss JM. Perinatal taurine depletion causes autonomic dysregulation in rats on a high glucose diet. FASEB J. 2007;21(6 Part II):A887. [Google Scholar]
- Schaffer SW, Lombardini JB, Azuma J. Interaction between the actions of taurine and angiotensin II. Amino Acids. 2000;18:305–18. doi: 10.1007/pl00010320. [DOI] [PubMed] [Google Scholar]
- Suvanich A, Jirakulsomchok D, Muchimapura S, Wyss JM, Roysommuti S. Perinatal taurine depletion impairs autonomic control in conscious rats. FASEB J. 2006;20(5 Part II):A1406–7. [Google Scholar]
- Ventura SJ, Martin JA, Curtin SC, Mathews TJ, Park MM. Department of Health and Human Services. In: Hyattsville MD, editor. National Vital Statistics Report; 2002. pp. 1–100. [Google Scholar]
- Wyss JM, Carlson SH. Effects of hormone replacement therapy on the sympathetic nervous system and blood pressure. Curr Hypertens Rep. 2003;5:241–6. doi: 10.1007/s11906-003-0027-8. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Roysommuti S, King K, Kadisha I, Regan CP, Berecek KH. Salt-induced hypertension in normotensive spontaneously hypertensive rats. Hypertension. 1994;23:791–6. doi: 10.1161/01.hyp.23.6.791. [DOI] [PubMed] [Google Scholar]


