Abstract
In order to regulate blood pressure, the brain exercises control over circulating hormones, and circulating hormones influence the brain by binding to brain neurons that lie outside of the blood-brain barrier. Recent work has demonstrated that “cardiovascular” hormones are synthesized and released in the brain as neurotransmitters/neuromodulators and can, in some cases, signal through the blood-brain barrier. The renin-angiotensin system is a prototype for these newly appreciated mechanisms. The brain’s intrinsic renin-angiotensin system plays an important role in blood pressure control. Angiotensin II in brain neurons affects other neurons both through activation of angiotensin receptors and via the generation of nitric oxide and reactive oxygen molecules. In a similar way, angiotensin in blood vessels activates endothelial nitric oxide, which can diffuse across the blood-brain barrier and thereby alter neuronal activity in cardiovascular control nuclei. The relative importance of these mechanisms to blood pressure control remains to be fully elucidated.
Introduction
For over a century, researchers have known that circulating hormones regulate arterial pressure. Recently, research has demonstrated that some of these hormones act via influences on the central nervous system. The prototype for most of these interactions has been angiotensin II (AII), a circulating peptide that regulates cardiovascular homeostasis, including alterations of vascular function. AII has long been known to act via the central nervous system (CNS), but these interactions were typically as mediated primarily via the circumventricular organs, areas of the brain that lack the blood-brain barrier and can, therefore, monitor peptides in the circulation. However, emerging evidence strongly indicates that AII and its active metabolites are capable of modifying neuronal activity in cardiovascular nuclei by other pathways. This paper reviews recent findings that show that AII can bypass the blood-brain barrier through a vascular-brain signaling mechanism that involves AII-induced nitric oxide generation. Further data document an intrinsic renin-angiotensin system (RAS) in the brain that modulates neuronal activity. Both of these pathways appear to act in part through the generation of reactive oxygen species.
Angiotensin and Hypertension
Hormonal imbalances have been long recognized as contributors to hypertension, and probably the most thoroughly studied of these involve the RAS. Studies over the past 60 years demonstrate that peripheral AII is intimately involved in volume homeostasis and blood pressure regulation, and AII exerts a potent dipsogenic response, stimulates vasopressin release by the brain and increases renal salt and water reabsorption. Several of the primary rodent models of hypertension display a strong linkage to AII, e.g., the spontaneously hypertensive rat (SHR), the TGR mRen2 rat, the Dahl salt-sensitive rat, the DOCA-salt rat and renal hypertensive rats [1]. In these models, AII appears to raise arterial pressure, at least in part, through inappropriate volume retention or elevated peripheral resistance. These experimental models also have elevated sympathetic nervous system activity, leading many to hypothesize a link between the RAS and sympathetic nervous system activity in hypertension. Thus, an overactive RAS may elevate arterial pressure directly through peripheral actions, through influences on CNS control of sympathetic nervous system activity and vasopressin release, and/or by blunting baroreceptor feedback to the brainstem.
Many investigators have dismissed a contribution of baroreceptors to hypertension, because baroreceptor denervation does not appreciably alter arterial pressure; it only increases lability of arterial pressure and heart rate. However, recent evidence implicates baroreceptors in the development and maintenance of hypertension. For instance, baroreceptors chronically reset to a higher “setpoint” when arterial pressure is chronically elevated. Once reset, the baroreceptor system defends the higher pressure, until the setpoint is again adjusted [2]. Second, baroreceptor sensitivity is altered in many experimental models of hypertension, and baroreceptor impairment appears to precede the onset of hypertension [1]. There is a substantial amount of data indicating that AII inhibits baroreceptor function. For example, normally in response to an increase in arterial pressure due to phenylephrine infusion, activation of baroreceptors leads to a decrease in heart rate and inhibition of sympathetic nervous system activity. In contrast, following an AII infusion, heart rate and sympathetic responses to the rise in arterial pressure are significantly blunted [3]. When rats are treated with an angiotensin II AT1 receptor blocker, baroreflex sensitivity is restored [4]. Such an effect has been documented in several models of hypertension, e.g., in SHR [4] and TGR(mREN2)27 rats [5]. Similarly, in the high renin, 2-kidney 1-clip hypertensive model [6;7] and Lyon hypertensive rat [8] baroreflex control of heart rate [6;8] and lumbar sympathetic nerve activity [7] are suppressed. In this model, treatment with an angiotensin converting enzyme (ACE) inhibitor restores sensitivity to that of normotensive controls. In contrast, angiotensinogen transgenic rats [TGR(ASrAOGEN)], which are characterized by low levels of AII, have an enhanced baroreflex response compared to non-transgenic controls. As expected in this model, infusion with AII decreases sensitivity [9].
The observation that circulating AII inhibits baroreflex activity [4] suggests that AII binds to receptors in a circumventricular organ to exert this effect. Circumventricular organs lack a blood-brain barrier, and therefore, neurons in these regions can detect and respond to circulating endocrine factors. Several circumventricular nuclei display AII binding sites, including organun vasculosum of the lamina terminalis, area postrema, subfornical organ and median preoptic nucleus [1]. The area postrema is the closest of these nuclei to the “baroreceptor nucleus” (the nucleus tractus solitarius; NTS), which is the site of afferent baroreceptor termination in the medulla. Thus, the area postrema is well situated to modulate baroreceptor input in response to circulating AII. Ablation of area postrema abolishes AII-induced desensitization of baroreflex in rabbits [3] and eliminates AII-induced hypertension in rats [10]. Further, in SHR, removal of the area postrema prevents the beneficial effects of AII receptor blockade on baroreceptor function [11].
Research by Dampney and colleagues indicates that microinjection of AII into the area postrema blunts baroreceptor sensitivity, and microinjection of an ACE inhibitor into the area postrema inhibits this AII effect [12••]. Further, ablation of the area postrema greatly reduces AII-induced desensitization of baroreflex. Also, microinjection of an ACE inhibitor directly into the adjacent NTS enhances baroreflex sensitivity, and microinjection of AII into the NTS blunts baroreflex sensitivity. This later effect occurs whether the area postrema is intact or lesioned, suggesting that neurons in the NTS can respond to AII both directly and indirectly via the circumventricular organ. Since the NTS has a blood brain barrier that AII cannot normally penetrate, circulating AII does not gain access to the NTS neurons under normal conditions. But AT1 receptors in the NTS appear to mediate some AII-induced alterations in baroreflex control [13–15]. The discovery of an endogenous AII system in the brain has shed new light on the pathways that affect these AII receptors in the NTS.
The Endogenous Brain Renin-Angiotensin System
AII increases sympathetic nervous system activity and arterial pressure via binding to AII receptors in several circumventricular organs, but the ability of AII to modify arterial pressure also involves an endogenous, brain renin-angiotensin system. In the past two decades, research has demonstrated that the brain contains all of the necessary components for renin-angiotensin signaling, including angiotensinogen, renin and angiotensin converting enzyme [1;16]. The pattern of localization of these components in the brain suggests that angiotensinogen is released from glial cells and modified by neuronal renin and ACE to form AII (see [17]), which in turn can act as a paracrine neuromodulator or be released as a neurotransmitter. The existence of an endogenous brain renin angiotensin signaling system is further supported by the widespread distribution of neuronal AII receptors that are localized in almost all of the nuclei involved in cardiovascular regulation [16]. These include paraventricular nucleus (PVN), parabrachial nucleus, rostral ventrolateral medulla (RVLM) and the NTS, along with widespread distribution in all of the circumventricular regions [1]. While these studies have provided detailed localization of an intrinsic renin angiotensin system in the central nervous system, research is only beginning to elucidate its role in cardiovascular control.
The endogenous renin-angiotensin system has been extensively studied in relation to the subfornical organ (SFO). Circulating AII binds to receptors in the SFO and elicits a potent dipsogenic response, which is transmitted to other neurons in the brain, at least in part, by the SFO neurons using AII as a neurotransmitter. During the past five years, Sigmund, Davisson and colleagues have developed novel lines of transgenic mice that express human renin and/or angiotensin genes, and these mice have deleted the endogenous matching genes in selected areas of the brain. Their findings in these models demonstrate that arterial pressure is increased in mice expressing either human renin (hREN) or human angiotensinogen (hAGT). In both models, interventricular administration of losartan (an AT1 antagonist) ameliorates this hypertension [18]. However, since both circulating and tissue RAS components are elevated in the models, the resulting hypertension could be due to intrinsic brain AII or circulating AII. To differentiate these alternatives, they used a single transgenic mouse that expressed hAGT, but they flanked the angiotensinogen gene with loxP sites [19••]. In this model, the angiotensionogen gene is fully functional unless an Ad/Cre adenovirus is present, in which case the gene is non-functional. Injection of renin into the cerebral ventricle of these mice increases arterial pressure and decreases heart rate, without changing circulating angiotensin levels. Intracerebral losartan injection blocks this response. In contrast, Ad/Cre adenovirus microinjection into the SFO renders the hAGT gene nonfunctional and abolish the blood pressure and heart rate responses to intercerebral injection renin. In this model, intraventricular injection of AII increases arterial pressure, demonstrating that the transgene does not alter the normal response of the neurons to AII. These results support a role for the intrinsic renin-angiotensin system in the SFO in mediating cardiovascular responses to central angiotensin, and also provide a useful tool to test the role of endogenous AII in the brain.
Given that one of the primary roles of SFO in angiotensin signaling is dipsogenesis, they sought to determine whether the intrinsic RAS contributes to water homeostasis. To investigate this question, they modified their hREN and hAGT double-transgenic strain (SRA) so that hREN had a neural specific promoter, ensuring expression only in neural tissue [17••]. Compared to non-transgenic mice, these mice displayed higher arterial pressure and had a three-fold increase in water intake. They also showed increased urine and electrolyte excretion, which resulted in a preference for saline (compared to water) consumption. Chronic intercerebral losartan administration decreased water intake and urine volume to a much greater degree in the transgenic than non-transgenic mice. In contrast, subcutaneous losartan had no effect on drinking behavior or urine output. Immunocytochemistry demonstrated that AII immunoreactivity was increased only in the SFO. These data suggest that the renin-angiotensin system in the SFO plays a significant role in drinking behavior. Also, while these transgenic mice displayed elevated arterial pressure, suggesting a role for SFO RAS in arterial pressure control, no blood pressure effects of losartan injections were reported, leaving the role of the intrinsic AII in the SFO on blood pressure control unclear.
Similar to the SFO, the rostral ventrolateral medulla (RVLM) also displays an intrinsic renin-angiotensin system, including a significant expression of AII receptors. Although protected by the blood-brain barrier, recent studies have also documented that the RAS in this nucleus contributes to regulation of sympathetic nervous system activity and arterial pressure. The RVLM, which is a major site that controls sympathetic nervous system activity, receives baroreceptor-related input from the NTS and descending hypothalamic input, which together modulate the amount of sympathetic tone generated by the RVLM. Microinjection of AII into the RVLM increases sympathetic activity and arterial pressure, suggesting a role for intrinsic AII in modulating RVLM output [20]. Microinjection of an adenovirus expressing a constitutively active AT1 receptor into the RVLM results in a significant increase in blood pressure, likely attributable to an increase in sympathetic outflow [21•]. Interestingly, the constitutively active AT1 receptors in this study were localized to the surrounding glial (vs. neuronal) cells, suggesting that the glial cells can modify RVLM activity. Further support for a role of RVLM AT1 receptors in blood pressure and sympathetic nervous system control is provided by studies in SHR [22], Dahl-sensitive rats [23] and TGR(mRen2)27 rats [24], in which microinjection of AT1 receptor blocker into RVLM lowers arterial pressure. However, other studies, particularly in normotensive and/or anesthetized animals, have failed to demonstrate that AT1 receptor blockade alters arterial pressure or a depressor response (see [25••]). These discrepancies suggest that endogenous AII does not tonically alter RVLM control of sympathetic nervous system activity, except under conditions in which the renin angiotensin system is disturbed [25••]. Some studies suggest that endogenous AII exerts both sympatho-excitatory and -inhibitory effects on the RVLM, thus modifying the setpoint of sympathetic outflow and arterial pressure in an additive manner [25••]. In this model, any imbalance of endogenous AII could alter this push-pull relationship, and correspondingly change arterial pressure in either a hypertensive or hypotensive manner.
The paraventricular nucleus (PVN) in the hypothalamus is a major source of input to the RVLM. Dampney and colleagues have demonstrated a role for AII in PVN-modulation of RVLM activity. In anesthetized rats, activation of PVN neurons elicits an increase in arterial pressure and sympathetic activity [26;27]. Microinjection of an AT1 receptor antagonist into the RVLM significantly reduces these responses, but blockade of glutamate [26;27] and GABA [27] receptors in the RVLM does not alter the responses. These results suggest that AT1 receptors in the RVLM mediate excitatory synaptic inputs from the PVN to the RVLM. Interestingly, PVN neurons that project to the RVLM express AT1 receptors and appear to be themselves activated by AII. To elucidate the mechanism by which AII modulates PVN activity, Li, et al., used brain slices and whole-cell patch clamp techniques to measure cell responses to AII [28•]. After identifying PVN neurons that projected to the RVLM and utilized AII, they measured whole cell current response to AII. AII increased activity of the PVN neurons, and losartan treatment eliminated the effect. In assessing the effect of AII on specific currents, the authors found that AII decreased the amplitude of evoked GABAergic inhibitory postsynaptic currents in a dose-dependent manner, and the addition of bicuculline blocked AII activation of PVN neurons. In contrast, AII did not alter excitatory currents. In a similar study, Chen, et al., examined the effect of AII on the activity of RVLM-projecting neurons in the PVN [29]. Using whole brain slices, they demonstrated that application of AII decreased GABAergic postsynaptic inhibitory currents via a G-protein dependent pathway. These results indicate that AII decreases GABAergic inhibition of PVN neurons, thereby increasing their firing rate and leading to excitation of RVLM neurons.
Angiotensin and Reactive Oxygen Species
AII receptors are widely distributed throughout cardiovascular centers in the brain, and these receptors respond to AII (described above). Therefore, researchers are examining the intracellular signaling pathway by which AII exerts its effects. Recent studies have focused on angiotensin-induced generation of reactive oxygen species (ROS). ROS, including oxygen ions, free radicals and peroxides, are a natural byproduct of the normal metabolism from enzymes such as NADPH-oxidase. As ROS are generated, they are converted by intracellular superoxide dismutase (SOD) into hydrogen peroxide. There are two forms of SOD, i.e., mitochondrial SOD, which contains manganese (MnSod) and cytoplasmic SOD, which contains copper and zinc (CuZnSod). Since hydrogen peroxide is a potent free radical species, it must be quickly degraded by enzymes such as catalase, glutathione peroxidase and peroxiredoxins.
While the role of ROS in cell damage and apoptosis is well documented, increasing evidence suggests that ROS also are involved in intracellular signaling pathways, including those utilized by AII. Campese, et al., found that when AII was infused centrally, mean arterial pressure and renal sympathetic nerve activity were significantly elevated [30••]. When tempol (a SOD mimetic) was co-administered with AII to reduce ROS levels, the AII effects on arterial pressure and sympathetic nerve activity were abolished. Similarly, Zimmerman, et al., have observed that AII induces pressor and drinking responses were accompanied by increased superoxide production in the SFO, and SOD overexpression via central administration of an SOD transgene into the SFO (causing overexpression localized to the SFO) eliminates these responses [31;32••]. These results suggest that elevated AII can increase arterial pressure by increasing ROS generation within SFO neurons, thereby increasing activation of hypothalamic centers that control sympathetic nervous system activity. This is supported by studies in cultured neuroblastoma cells in which AII induces a rapid increase in cytosolic calcium, while reduction of ROS generation via adenoviral overexpression of SOD greatly reduced this calcium influx [33].
The importance of SOD in AII signaling raises the question of how superoxide is generated in this pathway. While there are a number of potential enzymes by which reactive oxygen species could be generated in response to AII, most data suggests a NADPH-oxidase. AII-induced NADPH-oxidase activity occurs in similar areas to those areas that display endogenous AII. In NTS, AII infusion increases ROS production, and this is blocked by NADPH oxidase inhibitors [34]. Nozoe, et al., have demonstrated that NADPH-oxidase is elevated in the NTS of stroke prone SHR (SHR-SP), and selective inhibition of NADPH-oxidase significantly reduces arterial pressure and heart rate in SHR-SP but not in normotensive WKY [35••]. In addition, CuZnSod was significantly lower in SHR-SP, indicating a reduced ability to remove generated ROS. A similar role for NADPH-oxidase generation of ROS has been demonstrated in the RVLM. Chronic intraventricular infusion of AII in rabbits increases renal sympathetic nerve activity and elevates AT1 receptor density, NADPH oxidase levels, and superoxide production in the RVLM [36]. Baroreflex control of heart rate is also impaired by the infusion. This suggests that NADPH-derived superoxide production in the RVLM contributes to elevated sympathetic activity in response to intracerebroventricular administration of AII. Similarly, Chan et al. observed that injection of AII into the RVLM increased glutamanergic excitatory postsynaptic potentials and activated the MAPK signaling pathway in RVLM neurons, and these effects were attenuated by the addition of either an antisense oligonucleotide against NADPH oxidase or the SOD mimetic tempol [37•]. These results suggest that AII enhances pre-synaptic glutamate release via NADPH oxidase-derived superoxide that activates the MAPK pathway. Other studies indicate that SOD generation plays a role in AII signaling in the PVN. In PVN, SOD treatment abolishes AII-induced decreases in GABA currents in cells projecting to the RVLM [29]. While the above research supports ROS generation as a contributor to AII-induced sympathoexcitation, other hypertensive agents and models also show a correlation with ROS generation. In vitro research suggests that endothelin stimulates superoxide production in sympathetic ganglia via ETB receptors [38]. A subsequent in vivo study by Lau et al. found that the ETB-specific agonist safrotoxin increases superoxide formation in the inferior mesenteric ganglion, although this response is due in part to pressor effects [39]. Similar results were demonstrated in DOCA rats, which display elevated NAPDH oxidase activity and increased superoxide levels in sympathetic ganglia versus normotensive rats [40]. In Dahl salt-sensitive rats, salt-loading increases mean arterial pressure and NADPH-dependent superoxide production, along with upregulating NADPH mRNA levels in the brain [41]. In Dahl salt-sensitive, compared to salt-resistant, rats, tempol administration in the brain induces a significantly greater drop in arterial pressure, renal sympathetic nerve activity and heart rate. Thus, ROS levels may contribute to elevated sympathetic nervous system activity induced by dietary sodium in this model.
Nitric Oxide
Nitric oxide (NO) is another agent that appears to modulate sympathetic nervous system activity in blood pressure control. NO is a metabolic product of arginine metabolism that is produced by nitric oxide synthase (NOS), of which there are three forms, i.e., endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS). Of these eNOS and nNOS are distributed in the central nervous system including, regions responsible for cardiovascular regulation. Although the mechanisms by which NO modulates neuronal activity are unclear, research suggests that NO alters neuronal responses to excitatory amino acids. One region of interest in relation to the actions of NO is the NTS, where neurons demonstrate nNOS immunoreactivity (see [42]) and express glutamanergic AMPA receptors. Since the NTS is the site of initial baroreceptor input to the medulla, and the NTS relays this information to the RVLM, it is possible that NO generation in these neurons contributes to baroreflex control of autonomic output. Mifflin and colleagues have investigated this role. They demonstrated that activity of AMPA-containing NTS neurons that receive vagal input are facilitated by NO [42]. To further identify whether such neurons participate in baroreceptor and cardiopulmonary reflex control of autonomic tone, they studied the effect of NO blockade on the renal sympathetic nerve activity response to baroreceptor and cardiopulmonary reflex input [43••]. Microinjection of glutamate agonists AMPA or NMDA decreased mean arterial pressure, heart rate and renal sympathetic nerve activity. Microinjection of the NOS antagonist L-NAME prior to AMPA or NMDA treatment greatly reduced the response of arterial pressure, heart rate and renal nerve activity to the glutamate agonists. To determine the functional significance of NO in baroreflex control of sympathetic activity, they further examined the effect of NOS inhibition on both baroreflex and cardiopulmonary activation. Both the baroreflex and cardiopulmonary reflex response were attenuated when NO production was blocked. These results support the hypothesis that NO production facilitates glutamanergic signaling in baroreceptor and cardiopulmonary reflexes.
While the work from Mifflin’s laboratory demonstrates a role for NO in facilitating baro- and cardiopulmonary reflexes, there are a number of studies that suggest the opposite. Research from Paton’s laboratory indicate that eNOS is overexpressed in SHR compared to normotensive Wistar Kyoto rats (WKY) [44••]. Blockade of eNOS activity by adenoviral-mediated gene transfer increased cardiac baroreceptor reflex sensitivity in both SHR and WKY (defined by decreased cardiac sympathetic tone and increased vagal output). This suggests that basal levels of NO contribute constitutively to reflex control, which was not observed in Mifflin’s studies [43]. Additionally, eNOS blockade decreased arterial pressure in SHR but not in WKY. These studies help explain how baroreflexes may be desensitized in SHR; overexpression of eNOS resulting in elevated NO levels in the NTS neurons, desensitizing the baroreflexes and cardiopulmonary reflexes. However, it is unclear what accounts for the conflicting results reported in numerous other studies (see [45•]). To further investigate these discrepancies, Wang, et al., measured current responses to NO in rat brainstem slices and found that both NO and an NO donor could induce both excitatory and inhibitory responses, with a lower dose required to elicit an excitatory response [45•]. Both soluble guanylate cyclase and a non-NO-dependent guanylate cyclase activator mimicked these effects. These studies indicate that these effects of NO are presynaptic, suggesting that NO modulates neurotransmitter release in NTS neurons. These observations indicate that the amount of NO generated determines the response of the NTS neurons. Low concentrations of NO facilitate glutaminergic transmission in NTS neurons, while higher concentrations inhibit the transmission. The careful consideration of the concentration of NO to which neurons are exposed will be critical for the interpretation of all future studies of NO’s role in brain control of arterial pressure.
AII, NO and ROS: How the Vasculature Signals the Brain
Paton and his associates have introduced an intriguing additional mechanism by which circulating hormones can alter neuronal activity. The studies considered above, and others, demonstrate both an endogenous AII signaling system in the brain and a mechanism by which circulating AII binds to, and activates, brain neuronal receptors outside of the blood-brain barrier (in circumventricular organs). It is increasingly clear that both circulating and endogenous angiotensin contribute to regulation of sympathetic activity through both of these mechanisms. The work of Paton and colleagues suggest that AII can bind to receptors in the vascular endothelium and thereby induce the release of signaling molecules that can cross the blood-brain barrier and stimulate neurons in the brain. According to their hypothesis, AII stimulates AT1 receptors on blood vessels, and activation of these receptors causes the release of NO from endothelial cells and diffusion of NO across the blood-barrier. This results in activation of neurons in the brain in a paracrine manner [46;47••].
As detailed above, several lines of evidence indicate that an interaction between AII and NO in NTS alters baroreceptor responses [47••]. Using electron-paramagnetic resonance spectroscopy (which traps NO as a stable form that is localized to the area of production), Patton and colleagues demonstrated that AII microinjection into the NTS stimulates local NO, blunting baroreflex gain, and that microinjection of an NO donor similarly reduces baroreflex sensitivity. Direct injection of AII in this model does not alter baroreflex gain when an inhibitor of soluable guanylate cyclase is injected prior to AII injection. Together this suggests that AII acts via an NO-dependent pathway in the NTS. Using immunocytochemistry, this group also demonstrated that AT1 receptors are localized on the vascular endothelium in the NTS and on a few NTS neurons. Together, this supports the ability of AII to modulate baroreceptor function via endothelial cell release of NO in the area of the NTS (see [46]). Differential central versus vascular signaling in the NTS may help to explain the conflicting observations on the role of AII in the NTS discussed above [13;14].
Conclusions
Circulating AII acting via activation of brain neurons outside of the blood-brain barrier is a well-known modulator of drinking behavior and cardiovascular function, but current research has uncovered novel AII signaling mechanisms that also appear to regulate these systems (see Figure 1). First, neurons in cardiovascular areas inside of the blood-brain barrier have AII receptors on their surface, and these neurons use AII (produced via their endogenous, brain renin-angiotensin system) to signal other cardiovascular neurons. This endogenous system may play an important role in some forms of hypertension. While the exact pathways, and their specific roles, remain to be fully elucidated, research indicates that activation of NADPH oxidase and formation of reactive oxygen species is critical in the II pathway. Second, circulating AII may regulate cardiovascular neurons in the brain by activating endothelial NO, which readily diffuses into the brain and alters neuronal activity of neurons in cardiovascular regulatory nuclei. Thus, the circulating AII can circumvent the blood-brain barrier and activate signaling mechanisms in central nuclei. Whether this mechanism is important in cardiovascular nuclei other than those in the ventral medulla remains to be determined. ROS may also be signaling molecules in the vascular-brain signaling pathway, but this requires further exploration.
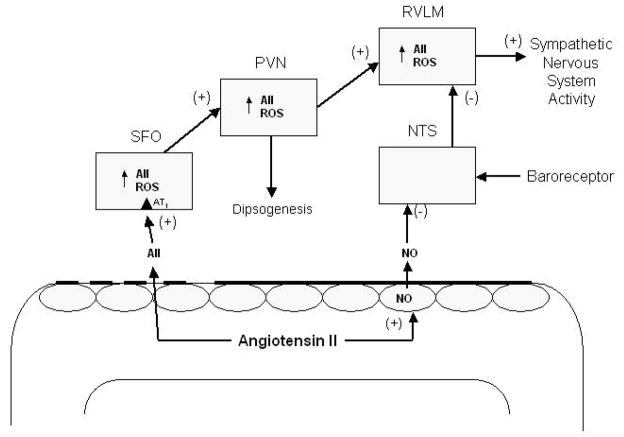
Figure 1.
Angiotensin II (AII) can directly access AT1 receptors on neurons in the SFO, which lacks the blood brain barrier (dashed line), thereby increasing the activity of neurons that project to the PVN, resulting in the release of AII and other transmitters. This stimulates drinking behavior and increases activity of neurons projecting to the RVLM, resulting in an increase in sympathetic nervous system activity and elevation in arterial pressure. Neuronal activity in the SFO, PVN and RVLM may be directly or indirectly altered by intrinsic brain AII binding to AII receptors and through AII-induced increases of reactive oxygen species (ROS) via stimulation of NADPH-oxidase activity. AII can also stimulate formation of nitric oxide (NO) from vascular endothelial cells, which can diffuse across the endothelial blood-brain barrier (solid dark line) and stimulate intracellular mechanisms, resulting in a depression of baroreceptor sensitivity. NTS, nucleus tractus solitarius; PVN, paraventricular nucleus of the hypothalamus; RVLM, rostral ventrolateral medulla; SFO, subfornical organ.
References and Recommended Reading
- 1.Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol. 2003;139:191–202. doi: 10.1038/sj.bjp.0705262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andresen MC, Yang M. Arterial baroreceptor resetting: contributions of chronic and acute processes. Clin Exp Pharmacol Physiol Suppl. 1989;15:19–30. doi: 10.1111/j.1440-1681.1989.tb02993.x. [DOI] [PubMed] [Google Scholar]
- 3.Sanderford MG, Bishop VS. Central mechanisms of acute ANG II modulation of arterial baroreflex control of renal sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2002;282:H1592–H1602. doi: 10.1152/ajpheart.00222.2001. [DOI] [PubMed] [Google Scholar]
- 4.Kawano Y, Yoshida K, Matsuoka H, Omae T. Chronic effects of central and systemic administration of losartan on blood pressure and baroreceptor reflex in spontaneously hypertensive rats. Am J Hypertens. 1994;7:536–542. doi: 10.1093/ajh/7.6.536. [DOI] [PubMed] [Google Scholar]
- 5.Schiffer S, Pummer S, Witte K, Lemmer B. Cardiovascular regulation in TGR(mREN2)27 rats: 24h variation in plasma catecholamines, angiotensin peptides, and telemetric heart rate variability. Chronobiol Int. 2001;18:461–474. doi: 10.1081/cbi-100103969. [DOI] [PubMed] [Google Scholar]
- 6.Berenguer LM, Garcia-Estan J, Ubeda M, et al. Role of renin-angiotensin system in the impairment of baroreflex control of heart rate in renal hypertensionJ Hypertens 199191127–1133. [PubMed] [Google Scholar]
- 7.Heesch CM, Crandall ME, Turbek JA. Converting enzyme inhibitors cause pressure-independent resetting of baroreflex control of sympathetic outflow. Am J Physiol. 1996;270:R728–R737. doi: 10.1152/ajpregu.1996.270.4.R728. [DOI] [PubMed] [Google Scholar]
- 8.Lantelme P, Cerutti C, Lo M, et al. Mechanisms of spontaneous baroreflex impairment in lyon hypertensive ratsAm J Physiol 1998275R920–R925. [DOI] [PubMed] [Google Scholar]
- 9.Baltatu O, Janssen BJ, Bricca G, et al. Alterations in blood pressure and heart rate variability in transgenic rats with low brain angiotensinogen. Hypertension. 2001;37:408–413. doi: 10.1161/01.hyp.37.2.408. [DOI] [PubMed] [Google Scholar]
- 10.Fink GD, Bruner CA, Mangiapane ML. Area postrema is critical for angiotensin- induced hypertension in rats. Hypertension. 1987;9:355–361. doi: 10.1161/01.hyp.9.4.355. [DOI] [PubMed] [Google Scholar]
- 11.Matsumura K, Averill DB, Ferrario CM. Role of AT1 receptors in area postrema on baroreceptor reflex in spontaneously hypertensive rats. Brain Res. 1999;850:166–172. doi: 10.1016/s0006-8993(99)02128-9. [DOI] [PubMed] [Google Scholar]
- 12••.Tan PS, Killinger S, Horiuchi J, Dampney RA. Baroreceptor reflex modulation by circulating angiotensin II is mediated by AT1 receptors in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2267–R2278. doi: 10.1152/ajpregu.00267.2007. Demonstrates that AII binding to receptors in the NTS elicits a greater desensitization versus the AII’s effect via the area postrema. This suggests that circulating AII can access receptors in the NTS to modulate baroreflex function. [DOI] [PubMed] [Google Scholar]
- 13.Boscan P, Allen AM, Paton JF. Baroreflex inhibition of cardiac sympathetic outflow is attenuated by angiotensin II in the nucleus of the solitary tract. Neuroscience. 2001;103:153–160. doi: 10.1016/s0306-4522(00)00559-5. [DOI] [PubMed] [Google Scholar]
- 14.Wong LF, Polson JW, Murphy D, et al. Genetic and pharmacological dissection of pathways involved in the angiotensin II-mediated depression of baroreflex function. FASEB J. 2002;16:1595–1601. doi: 10.1096/fj.02-0099com. [DOI] [PubMed] [Google Scholar]
- 15.Kasparov S, Butcher JW, Paton JF. Angiotensin II receptors within the nucleus of the solitary tract mediate the developmental attenuation of the baroreceptor vagal reflex in pre-weaned rats. J Auton Nerv Syst. 1998;74:160–168. doi: 10.1016/s0165-1838(98)00149-0. [DOI] [PubMed] [Google Scholar]
- 16.Parsons KK, Coffman TM. The renin-angiotensin system: it’s all in your head. J Clin Invest. 2007;117:873–876. doi: 10.1172/JCI31856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Sakai K, Agassandian K, Morimoto S et al. Local production of angiotensin II in the subfornical organ causes elevated drinking. J Clin Invest. 2007;117:1088–1095. doi: 10.1172/JCI31242. Developed a double-transgenic strain (SRA) in which the human renin gene was expressed only in neural tissue and the human angiotensinogen gene could be functionally disabled. Using this technique they demonstrated a role for the endogenous renin-angiotensin system in the SFO in controlling drinking behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davisson RL, Yang G, Beltz TG, et al. The brain renin-angiotensin system contributes to the hypertension in mice containing both the human renin and human angiotensinogen transgenes. Circ Res. 1998;83:1047–1058. doi: 10.1161/01.res.83.10.1047. [DOI] [PubMed] [Google Scholar]
- 19••.Sinnayah P, Lazartigues E, Sakai K et al. Genetic ablation of angiotensinogen in the subfornical organ of the brain prevents the central angiotensinergic pressor response. Circ Res. 2006;99:1125–1131. doi: 10.1161/01.RES.0000250259.66683.f5. Used a novel transgenic mouse, in which the angiotensinogen gene could be functionally disabled, to study the role of the endogenous renin-angiotensin system in the subfornical organ. [DOI] [PubMed] [Google Scholar]
- 20.Dampney RA, Fontes MA, Hirooka Y, et al. Role of angiotensin II receptors in the regulation of vasomotor neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol. 2002;29:467–472. doi: 10.1046/j.1440-1681.2002.03658.x. [DOI] [PubMed] [Google Scholar]
- 21•.Allen AM, Dosanjh JK, Erac M et al. Expression of constitutively active angiotensin receptors in the rostral ventrolateral medulla increases blood pressure. Hypertension. 2006;47:1054–1061. doi: 10.1161/01.HYP.0000218576.36574.54. Selective expression of a constitutively active AT1 receptor in the RVLM significantly increased arterial pressure. [DOI] [PubMed] [Google Scholar]
- 22.Ito S, Komatsu K, Tsukamoto K, et al. Ventrolateral medulla AT1 receptors support blood pressure in hypertensive rats. Hypertension. 2002;40:552–559. doi: 10.1161/01.hyp.0000033812.99089.92. [DOI] [PubMed] [Google Scholar]
- 23.Ito S, Hiratsuka M, Komatsu K, et al. Ventrolateral medulla AT1 receptors support arterial pressure in Dahl salt-sensitive rats. Hypertension. 2003;41:744–750. doi: 10.1161/01.HYP.0000052944.54349.7B. [DOI] [PubMed] [Google Scholar]
- 24.Fontes MA, Baltatu O, Caligiorne SM, et al. Angiotensin peptides acting at rostral ventrolateral medulla contribute to hypertension of TGR(mREN2)27 rats. Physiol Genomics. 2000;2:137–142. doi: 10.1152/physiolgenomics.2000.2.3.137. [DOI] [PubMed] [Google Scholar]
- 25••.Dampney RA, Tan PS, Sheriff MJ et al. Cardiovascular effects of angiotensin II in the rostral ventrolateral medulla: the push-pull hypothesis. Curr Hypertens Rep. 2007;9:222–227. doi: 10.1007/s11906-007-0040-4. Demonstrate that endogenous AII in the RVLM exerts both sympathoexcitatory and inhibitory effects, suggesting that net sympathetic outflow is a composite result of a push-pull relationship. [DOI] [PubMed] [Google Scholar]
- 26.Tagawa T, Dampney RA. AT(1) receptors mediate excitatory inputs to rostral ventrolateral medulla pressor neurons from hypothalamus. Hypertension. 1999;34:1301–1307. doi: 10.1161/01.hyp.34.6.1301. [DOI] [PubMed] [Google Scholar]
- 27.Tagawa T, Horiuchi J, Potts PD, Dampney RA. Sympathoinhibition after angiotensin receptor blockade in the rostral ventrolateral medulla is independent of glutamate and gamma-aminobutyric acid receptors. J Auton Nerv Syst. 1999;77:21–30. doi: 10.1016/s0165-1838(99)00026-0. [DOI] [PubMed] [Google Scholar]
- 28•.Li DP, Pan HL. Angiotensin II Attenuates Synaptic GABA Release and Excites Paraventricular-Rostral Ventrolateral Medulla Output Neurons. J Pharmacol Exp Ther. 2005;313:1035–1045. doi: 10.1124/jpet.104.082495. Using whole-cell patch clamp techniques, they demonstrate that AII decreases GABAergic inhibitory postsynaptic currents in PVN neurons that project to the RVLM, subsequently increasing PVN activation of RVLM neurons. [DOI] [PubMed] [Google Scholar]
- 29.Chen Q, Pan HL. Signaling Mechanisms of Angiotensin II-Induced Attenuation of GABAergic Input to Hypothalamic Presympathetic Neurons. J Neurophysiol. 2007;97:3279–3287. doi: 10.1152/jn.01329.2006. [DOI] [PubMed] [Google Scholar]
- 30••.Campese VM, Shaohua Y, Huiquin Z. Oxidative stress mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension. 2005;46:533–539. doi: 10.1161/01.HYP.0000179088.57586.26. Central administration of tempol abolished AII-induced increases in arterial pressure and renal sympathetic nerve activity, indicating that central AII stimulates formation of reactive oxygen species in its signaling pathway. [DOI] [PubMed] [Google Scholar]
- 31.Zimmerman MC, Lazartigues E, Lang JA, et al. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- 32••.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension Caused by Angiotensin II Infusion Involves Increased Superoxide Production in the Central Nervous System. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. AII-induces pressor and drinking responses are accompanied by increased superoxide production in the SFO, and SOD overexpression in the SFO eliminates these responses. [DOI] [PubMed] [Google Scholar]
- 33.Zimmerman MC, Sharma RV, Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension. 2005;45:717–723. doi: 10.1161/01.HYP.0000153463.22621.5e. [DOI] [PubMed] [Google Scholar]
- 34•.Wang G, Anrather J, Glass MJ et al. Nox2, Ca2+, and Protein Kinase C Play a Role in Angiotensin II-Induced Free Radical Production in Nucleus Tractus Solitarius. Hypertension. 2006;48:482–489. doi: 10.1161/01.HYP.0000236647.55200.07. AII infusion increases ROS production in the NTS, and this is blocked by NADPH-oxidase inhibitors. Indicates that ROS is produced via NADPH-oxidase. [DOI] [PubMed] [Google Scholar]
- 35••.Nozoe M, Hirooka Y, Koga Y et al. Inhibition of Rac1-Derived Reactive Oxygen Species in Nucleus Tractus Solitarius Decreases Blood Pressure and Heart Rate in Stroke-Prone Spontaneously Hypertensive Rats. Hypertension. 2007;50:62–68. doi: 10.1161/HYPERTENSIONAHA.107.087981. NADPH-oxidase is elevated in the NTS of stroke prone SHR, indicating that ROS levels are elevated. Selective inhibition of NADPH-oxidase significantly reduces arterial pressure and heart rate. [DOI] [PubMed] [Google Scholar]
- 36.Gao L, Wang W, Li YL, et al. Sympathoexcitation by central ANG II: Roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol. 2005;288:H2271–H2279. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- 37•.Chan SHH, Hsu KS, Huang CC et al. NADPH Oxidase-Derived Superoxide Anion Mediates Angiotensin II-Induced Pressor Effect via Activation of p38 Mitogen-Activated Protein Kinase in the Rostral Ventrolateral Medulla. Circ Res. 2005;97:772–780. doi: 10.1161/01.RES.0000185804.79157.C0. Injection of AII into the RVLM increased glutamanergic excitatory postsynaptic potentials in RVLM neurons, and the addition of either an oligonucleotide against NADPH oxidase antisense or tempol abolishes this effect. [DOI] [PubMed] [Google Scholar]
- 38.Dai X, Galligan JJ, Watts SW, et al. Increased O2{middle dot}-Production and Upregulation of ETB Receptors by Sympathetic Neurons in DOCA-Salt Hypertensive Rats. Hypertension. 2004;43:1048–1054. doi: 10.1161/01.HYP.0000126068.27125.42. [DOI] [PubMed] [Google Scholar]
- 39.Lau YE, Galligan JJ, Kreulen DL, Fink GD. Activation of ETB receptors increases superoxide levels in sympathetic ganglia in vivo. Am J Physiol Regul Integr Comp Physiol. 2006;290:R90–R95. doi: 10.1152/ajpregu.00505.2005. [DOI] [PubMed] [Google Scholar]
- 40.Dai X, Cao X, Kreulen DL. Superoxide anion is elevated in sympathetic neurons in DOCA-salt hypertension via activation of NADPH oxidase. Am J Physiol Heart Circ Physiol. 2006;290:H1019–H1026. doi: 10.1152/ajpheart.00052.2005. [DOI] [PubMed] [Google Scholar]
- 41.Fujita M, Ando K, Nagae A, Fujita T. Sympathoexcitation by Oxidative Stress in the Brain Mediates Arterial Pressure Elevation in Salt-Sensitive Hypertension. Hypertension. 2007;50:360–367. doi: 10.1161/HYPERTENSIONAHA.107.091009. [DOI] [PubMed] [Google Scholar]
- 42.Dias AC, Colombari E, Mifflin SW. Effect of nitric oxide on excitatory amino acid-evoked discharge of neurons in NTS. Am J Physiol Heart Circ Physiol. 2003;284:H234–H240. doi: 10.1152/ajpheart.00037.2002. [DOI] [PubMed] [Google Scholar]
- 43••.Dias AC, Vitela M, Colombari E, Mifflin SW. Nitric oxide modulation of glutamatergic, baroreflex, and cardiopulmonary transmission in the nucleus of the solitary tract. Am J Physiol Heart Circ Physiol. 2005;288:H256–H262. doi: 10.1152/ajpheart.01149.2003. Microinjection of glutamate agonists into the NTS decreases baroreflex sensitivity, and pretreatment with a NOS antagonist greatly reduces this effect. These results suggest that NO production in the NTS facilitates glutamanergic signaling in baroreceptor and cardiopulmonary reflexes. [DOI] [PubMed] [Google Scholar]
- 44••.Waki H, Murphy D, Yao ST et al. Endothelial NO synthase activity in nucleus tractus solitarii contributes to hypertension in spontaneously hypertensive rats. Hypertension. 2006;48:644–650. doi: 10.1161/01.HYP.0000238200.46085.c6. Bockade of eNOS activity using adenoviral-mediated gene transfer increased cardiac baroreceptor reflex sensitivity in both normotensive and hypertensive rats, while decreasing arterial pressure in the hypertensive group. This suggests that basal levels of NO contribute constitutively to reflex control, and may explain how increased NO generation may desensitize baroreflexes and contribute to hypertension. [DOI] [PubMed] [Google Scholar]
- 45•.Wang S, Paton JF, Kasparov S. Differential sensitivity of excitatory and inhibitory synaptic transmission to modulation by nitric oxide in rat nucleus tractus solitarii. Exp Physiol. 2007;92:371–382. doi: 10.1113/expphysiol.2006.036103. Found that NO presynaptically modulates neurotransmitter release in NTS neurons. Low concentrations of NO facilitate glutaminergic transmission in NTS neurons, while higher concentrations inhibit the transmission. [DOI] [PubMed] [Google Scholar]
- 46.Paton JF, Waki H, Abdala AP, et al. Vascular-brain signaling in hypertension: role of angiotensin II and nitric oxide. Curr Hypertens Rep. 2007;9:242–247. doi: 10.1007/s11906-007-0043-1. [DOI] [PubMed] [Google Scholar]
- 47••.Paton JF, Lonergan T, Deuchars J et al. Detection of angiotensin II mediated nitric oxide release within the nucleus of the solitary tract using electron-paramagnetic resonance (EPR) spectroscopy. Auton Neurosci. 2006;126–127:193–201. doi: 10.1016/j.autneu.2006.02.016. Using electron-paramagnetic resonance spectroscopy, this study demonstrated that AII microinjection into the NTS stimulates local NO, blunting baroreflex gain. This suggests that AII acts via an NO-dependent pathway in the NTS, and supports that hypothesis that AII modulate baroreceptor function via endothelial cell release of NO in the area of the NTS. This hypothesis is further explained in reference [46]. [DOI] [PubMed] [Google Scholar]