Abstract
Cocaine-primed reinstatement is an animal model of drug relapse. The neurocircuitry underlying cocaine-primed reinstatement includes a decrease in GABA in the ventral pallidum (VP) that is inhibited by a μ opioid receptor antagonist, suggesting that opioid peptides colocalized with GABA in the projection from the nucleus accumbens to the VP may mediate this effect. Neurotensin is also colocalized with GABA and has been shown to increase GABA release in several brain regions. Therefore, the present study determined whether neurotensin increases GABA release in the VP, antagonizes cocaine-induced decreases in GABA, and prevents reinstatement of cocaine seeking. In vivo microdialysis revealed that the neurotensin agonist neurotensin peptide fragment 8–13 [NT(8–13)] increased GABA in the VP in a neurotensin receptor and tetrodotoxin-dependent manner and blocked the cocaine-induced decrease in GABA. NT(8–13) (3 nmol) microinjected into the VP prevented cue-induced reinstatement without affecting cocaine self-administration. In contrast, 3 nmol NT(8–13) potentiated cocaine-primed reinstatement. The neurotensin antagonist SR142948 (2-[[[5-(2,6-dimethoxyphenyl)-1-[4-[[[3-(dimethylamino)propyl]methylamino]carbonyl]-2-(1-methylethyl)phenyl]-1H -pyrazol-3-yl]carbonyl]amino]-tricyclo-[3.3.1.13,7]decane-2-carboxylic acid) had no effect on any behavioral measure when infused in the VP at the dose tested but attenuated cocaine-primed reinstatement when administered systemically. In contrast to reinstatement, NT(8–13) did not alter the motor response to acute cocaine or the development of motor sensitization by chronic cocaine. Three conclusions can be drawn from these data: 1) neurotensin promotes GABA release in the VP and correspondingly inhibits cue-induced reinstatement, 2) neurotensin and cocaine interact in a manner that countermands the neurotensin-induced increase in GABA and promotes reinstatement, and 3) endogenous release of neurotensin in the VP is not necessary for reinstatement.
Drug addiction is a serious public health issue, and one of the most problematic aspects of addiction is the tendency to relapse even after extended periods of abstinence. Relapse can be studied in rodents using a combination of neurochemical and behavioral methods, including reinstatement of drug seeking. In this model, animals are trained to self-administer a drug of abuse, this behavior is then extinguished by removal of the reinforcer, and then drug seeking is reinstated by exposure to a stressor, drug-associated cue, or the drug itself (Shalev et al., 2002; Shaham et al., 2003).
Much of the neurocircuitry underlying cocaine-primed reinstatement has been established. By reversible inactivation of specific brain regions with a mixture of GABAA and GABAB agonists or blockade of voltage-dependent sodium channels, it has been shown that cocaine- or stress-induced reinstatement of cocaine-seeking requires activity of the ventral tegmental area (VTA), dorsal medial prefrontal cortex, nucleus accumbens (NAc) core, and the ventral pallidum (VP; McFarland and Kalivas, 2001; McFarland et al., 2004). Additional studies have further established that reinstatement requires glutamatergic activity at α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in the NAc core, and a decrease in GABA release in the VP (Di Ciano and Everitt, 2001; McFarland et al., 2003; Tang et al., 2005). The decrease in GABA in the VP produced by a cocaine-priming injection seems to be mediated by the μ opioid receptor (MOR) because microinjection of the MOR antagonist CTAP into the VP prevents both the cocaine-induced decrease in GABA and the reinstatement of cocaine seeking (Tang et al., 2005). Enkephalins are endogenous ligands for the MOR that are colocalized with GABA in the projection from the NAc core to the VP (Zahm et al., 1985), and MORs are localized both pre- and postsynaptically, allowing for enkephalin-mediated negative feedback regulation of GABA release (Olive et al., 1997). Therefore, one strategy for preventing drug relapse is to increase GABAergic tone in the VP, countermanding the MOR-mediated disinhibition of VP output neurons produced by cocaine and thereby inhibiting reinstatement behavior. This was done previously by microinjecting GABA agonists into the VP (McFarland and Kalivas, 2001) but might also be accomplished by exploiting colocalized neuropeptides that positively regulate GABA release in the NAc projection to the VP (Torregrossa and Kalivas, 2007).
Several neuropeptides in addition to the enkephalins are colocalized with GABA in the projection from the NAc to the VP and may feedback to regulate GABA release. Neurotensin is one colocalized neuropeptide that has been shown to increase the extracellular concentration of GABA locally in the dorsal striatum, NAc, prefrontal cortex, and hippocampus and to increase GABA in the globus pallidus when administered into the striatum (Tanganelli et al., 1994; Ferraro et al., 1997, 1998; Rakovska et al., 1998; O’Connor, 2001; Petrie et al., 2005). Therefore, neurotensin is a candidate endogenous peptide to increase GABA release within the VP and inhibit reinstatement to drug seeking. The present study aimed to determine the neurochemical effects of neurotensin on GABA release in the VP, the ability of neurotensin to prevent cocaine-induced decreases in GABA, and to determine the effect of neurotensin and a neurotensin receptor antagonist administered into the VP on cocaine-induced behaviors including locomotor sensitization, self-administration, extinction, and reinstatement.
Materials and Methods
Animals
Male Sprague-Dawley rats (Charles River, Indianapolis, IN) delivered weighing 250 to 275 g were used for all experiments. Rats were housed individually in humidity- and temperature-controlled rooms. Rats used in microdialysis and locomotor activity studies were maintained on a regular light/dark cycle, whereas rats used in self-administration and reinstatement experiments were maintained on a reverse light/dark cycle. Rats were allowed ad libitum access to food and water, except when rats were in self-administration experiments, in which case they were allowed ad libitum water and were given 20 g of rat chow per day. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were approved by the Medical University of South Carolina Animal Care and Use Committee.
Drugs
Cocaine hydrochloride was provided by the National Institute on Drug Abuse (Bethesda, MD) and was dissolved in 0.9% sterile saline for i.v. and i.p. injections. Neurotensin peptide fragment 8 –13 [NT(8 –13)], which is known to possess all of the pharmacological activity of the full-length neurotensin peptide, was purchased from Sigma/RBI (Natick, MA) and was dissolved in artificial cerebral spinal fluid (aCSF) for microinjection and microdialysis studies. The neurotensin antagonist SR48692 was a generous gift from Dr. Charles Nemeroff, and the neurotensin antagonist SR142948 was purchased from Tocris Cookson Inc. (Ellisville, MO). Initial experiments were conducted using SR48692, but this compound became unavailable, and the neurotensin (NT) antagonist SR142948 was employed in subsequent experiments. Both were dissolved in dimethyl sulfoxide and diluted in aCSF to a final concentration of 1% dimethyl sulfoxide.
Surgical Procedures
Rats were anesthetized with a combination of 87.5 mg/kg ketamine (Ketaset; Fort Dodge Laboratories, Fort Dodge, IA) and 5 mg/kg xylazine (Rompun; Bayer, Shawnee Mission, KS) administered i.m. Animals were also given the analgesic Ketoralac (3 mg/kg i.p., Hospira Inc., Lake Forest, IL) before surgery. Animals used for microdialysis studies were implanted bilaterally with a 20-gauge guide cannula (Plastics One, Roanoke, VA) aimed 2 mm dorsal to the VP (from bregma: 0 mm AP, ±3.2 mm ML, −5.8 mm DV at an angle of 6°). Cannula were secured with three miniature screws and dental acrylic resin (Lang Dental Mfg. Co., Inc., Wheeling, IL), and dummy cannula (C313DC, Plastics One, Roanoke, VA) were inserted the length of the guide cannula to maintain patency.
Animals used for reinstatement studies were implanted with i.v. jugular catheters and with bilateral 26-gauge microinjection guide cannula (Small Parts Inc., Miami Lakes, FL). Catheters were constructed with silastic tubing (11 cm; Dow Corning, Midland, MI) attached to a bent steel guide cannula (C313-5UP 22GA; Plastics One), surrounded by dental acrylic and attached to a 2-inch square piece of mesh (Prolite Mesh; Atrium Medical Corp., Hudson, NH). Catheters were implanted in the right jugular vein 3 cm and fed s.c. to the back, where they exited through a 3-mm biopsy punch between the shoulder blades. Microinjection cannula were implanted aimed 1 mm dorsal to the VP (from bregma: 0 mm AP, ± 2.3 mm ML, −7.5 mm DV) based on the atlas of Paxinos and Watson. Cannula were secured with three miniature screws and dental acrylic as above. Obturators were inserted the length of the cannula to maintain patency. After surgery, catheters were flushed daily with 0.2 ml of Timentin antibiotic (GlaxoSmithKline, Research Triangle Park, NC) dissolved in 13 ml of heparin saline (100 IU/ml). Animals were allowed 7 days to recover from surgery before experimentation began.
Microdialysis Procedure
The dialysis probes were constructed of 1.5 to 2.0 mm of active membrane (regenerated cellulose, 13,000-Da molecular mass cut-off; Spectrum Laboratories, Inc., Rancho Dominguez, CA) and secured inside a 24-gauge internal cannula (Plastics One) with epoxy. Inlet and outlet fused silica tubing (152-μm o.d., 75 μm i.d.; Polymicro Technologies, LLC, Phoenix, AZ) were inserted in the guide cannula and secured with epoxy and 3 cm of PE50 tubing (BD Biosciences, San Jose, CA) surrounding the inlet and outlet. The other end of the inlet was inserted into a 3-cm length of PTFE tubing (Cole-Parmer Instrument Co., Vernon Hills, IL) that was then inserted into an 8-cm length of PE50 tubing and secured with epoxy. This end was used to attach the inlet to a swivel that was connected through a liquid switch to the infusion syringe and to a coiled tether that protected the dialysis probe tubing and allowed the animal to move freely about the dialysis chamber.
The night before microdialysis experiments, animals were implanted with dialysis probes extending into the VP. The next morning, the probes were perfused for 2 h with aCSF (dialysis buffer; 5mM D-glucose, 2.5 mM KCl, 140 mM NaCl, 1.4 mM CaCl2, 1.2 mM MgCl2, and 0.15% phosphate-buffered saline, pH 7.4) at a rate of 2 μl/min. Then, six baseline samples were collected over a period of 2 h (20 min/sample), followed by infusion of one dose of a test compound dissolved in aCSF by means of the liquid switch. Every dose of any test compound was perfused for 1 h (three samples collected) before switching to another dose of compound or other experimental manipulation. Samples were stored at −80°C until analysis.
The concentration of GABA was determined from samples by electrochemical detection with Coularray hardware and software (ESA Inc., Chelmsford, MA). The method was based on the ESA application note for glutamate and GABA analysis. The mobile phase for high-performance liquid chromatography consisted of 100 mM sodium phosphate dibasic in 22% methanol and 3.5% acetonitrile to pH 6.75 with o-phosphoric acid. The flow rate was 0.65 ml/min. Twenty microliters of each sample was derivatized with 30 μl of a solution of o-phthalaldehyde and β-mercaptoethanol dissolved in 0.1 M tetraborate buffer, pH 9.3. Derivatized samples were then separated on a C18 analytical column (Capcell Pak MG, 3 μm, 3.0-mm i.d. × 50 mm; ESA Inc.) and then passed through two oxidizing electrodes that applied potentials of 150 and 550 mV. The concentration of GABA in the sample was linearly related to the amount of current change. GABA peaks were determined based on the retention time of GABA in standard solutions, and the concentration was determined based on the peak area compared with a standard calibration curve. Peak area and concentration were determined using ESA CoulArray Win software.
Microinjection Procedure
Animals receiving microinjections were given sham injections 2 to 3 days before the experiment, where obturators were removed, and injection cannula extending 1 mm below the guide cannula were inserted in each brain hemisphere for several seconds. Injectors were then removed, and the obturators were replaced. During experimental sessions, obturators were removed, and injection cannula were inserted that were connected by Tygon tubing (Norton Performance Plastics, Akron, OH) to a syringe pump. The test drug or its vehicle was then infused in a volume of 0.3 μl/side over 1 min, and injectors were left in for an additional minute to allow for diffusion. Injectors were then removed and obturators replaced.
Self-Administration and Reinstatement Procedures
Animals used for self-administration studies were first trained to lever press for food on a fixed ratio 1 schedule of reinforcement. Animals were food deprived 24 h prior to a single, overnight 14-h food training session where pressing the active (right) lever resulted in the presentation of one 45-mg food pellet, whereas pressing the inactive (left) lever had no programmed consequences. The next day, cocaine self-administration began on a fixed ratio 1 schedule where active lever presses resulted in presentation of a stimulus light above the lever, a 5-s tone, and infusion of 0.05 ml over 2.7 s of 0.2 mg of cocaine (0.6 mg/kg/infusion), followed by a 20-s unsignaled time out. Inactive lever presses were recorded but had no programmed consequences. The house light was on throughout the session, which lasted 2 h or until 200 infusions were earned, whichever occurred first. The program was controlled by and the data were collected using Schedule Manager for Windows software version 2.09 (MED Associates, St. Albans, VT). Rats completing 12 self-administration sessions and obtaining at least 15 cocaine infusions on the last 3 days of self-administration were used for extinction and reinstatement testing. Extinction consisted of a 2-h daily session where rats were placed in the operant chamber, and pressing of the previously active or inactive lever had no programmed consequences. Rats went through a minimum of 10 days of extinction training or until they achieved 2 consecutive days of less than 25 active lever presses. Using these criteria, it is theoretically possible that animals receiving a minimum of 15 cocaine infusions in self-administration might have fewer lever presses in self-administration than the 25 lever-press criteria set for extinction; however, animals respond at much higher rates than the number of infusions received due to responding during the infusion and during time out. Therefore, in the present study, no animal responded less in self-administration than in extinction.
Cocaine-primed reinstatement tests consisted of a microinjection immediately before injection of 3, 10, or 30 mg/kg cocaine i.p. Rats were then placed in the operant chamber where pressing of the active or inactive lever had no programmed consequences. Cue-induced reinstatement consisted of microinjection followed by placement in the operant chamber where pressing of the active lever resulted in presentation of the light, tone, and syringe pump activation cues, but no infusion was given.
Experimental Protocol
The effect of a neurotensin receptor agonist and antagonist on GABA release in the VP was determined in drug-naive animals by reverse dialysis of increasing doses of each compound in separate groups of animals. After baseline collection, the perfusion fluid was switched to dialysis buffer containing the lowest dose of the test compound by means of a liquid switch. Samples were collected for 1 h (three samples) before switching to the next highest dose. Three doses were tested for each compound in one dialysis session. The doses tested covered a range that encompassed effective doses in previous microdialysis experiments and often included higher doses to verify a lack of effect (Ferraro et al., 1998; Petrie et al., 2005).
To determine whether the increase in GABA produced by NT(8–13) was receptor dependent and sodium channel dependent, two experiments were conducted. In the first, the neurotensin receptor antagonist SR48692 (10 μM) was reverse dialyzed for 1 h, then a combination of 100 μM NT(8–13) and 10 μM SR48692 was reverse dialyzed for an additional hour. SR48692 is a selective neurotensin receptor (NTR) 1 antagonist that has been shown to be effective in blocking the effect of neurotensin or NT(8–13) in microdialysis and electrophysiological experiments at doses lower than 10 μM (Petrie et al., 2005; Martorana et al., 2006). Ten micromolar was used because it was shown to have no effect on GABA release and based upon the aforementioned studies should be more than sufficient to inhibit the effect of 100 μM NT(8–13). In the second experiment, 1 μM sodium channel blocker tetrodotoxin (TTX) was reverse dialyzed for 1 h, after which the combination of 1 μM TTX and 100 μM NT(8–13) was reverse dialyzed for another hour.
Next, the ability of NT(8–13) to increase GABA release in animals chronically treated with cocaine was determined because of the possibility that chronic cocaine would produce neuroadaptations that might alter the capacity of NT(8–13) to affect GABA release. All rats were given a saline injection on day 0, and then they were divided into two groups to receive 7 days of cocaine or saline. The cocaine regimen was 15 mg/kg i.p. on days 1 and 7 and 30 mg/kg on days 2 to 6. Locomotor activity was determined on days 0, 1, and 7 by measuring activity for a 1-h habituation period and then for 2 h after injection of cocaine or saline. Rats were then abstinent from cocaine for 3 weeks, during which time the animals had surgery to implant dialysis cannula. The dialysis experiment was conducted as before, where increasing doses of NT(8–13) were reverse dialyzed for 1 h at each dose.
In addition, the ability of neurotensin to prevent a cocaine-induced decrease in GABA release was determined in rats chronically treated with cocaine. Rats were treated with cocaine as described above. The dialysis experiment was conducted as described above, except that after baseline sample collection, 3 μM NT(8–13) was reversed dialyzed for 1 h, and then a 15 mg/kg i.p. cocaine injection was given, and samples were collected for an additional 2 h. The dose of NT(8–13) employed was a subthreshold dose for increasing GABA based upon the first dialysis experiment and was used to minimize a concern that NT-induced increases in extracellular GABA could mask the effect of cocaine.
The abilities of the neurotensin receptor agonist NT(8–13) and NT receptor antagonist SR142948 to affect behavior during cocaine self-administration, extinction, and reinstatement were tested. The ability of NT(8–13) to increase GABA in the VP suggested that infusion of this compound in the VP should inhibit both cue- and cocaine-primed reinstatement (McFarland and Kalivas, 2001; Tang et al., 2005). The 3 nmol/side dose of NT(8–13) was chosen for reinstatement experiments based on preliminary studies that examined the effect of a wide range (0.01–10 nmol) of cocaine-induced locomotor activity. These studies indicated that 3 nmol NT(8–13) could alter activity, whereas lower and higher doses did not (however, in the final analysis, the 3 nmol dose did not have a significant effect on locomotor activity). In addition, we wanted to determine whether endogenous neurotensin release could modulate self-administration or reinstatement. Thus, we also infused the NT antagonist in the VP and analyzed the effect on cocaine self-administration and reinstatement behavior. Although the NTR antagonist SR142948 is a less selective NTR1 receptor antagonist than SR48692, SR48692 became unavailable, and we changed to a commercially available antagonist. Because SR48692 blocked the NT(8–13)-induced increase in GABA, this effect is probably mediated by NTR1 receptors, and these two antagonists have been shown to have equivalent capacities to inhibit the effect of NT in several studies (Binder et al., 2002; Martorana et al., 2006). The microinjection dose was chosen based on electrophysiological studies suggesting that 1 nmol is more than effective in blocking the effects of neurotensin (Martorana et al., 2006).
Immediately before a self-administration or extinction session, one dose of the test compound or vehicle was infused according to the above protocol. Then, the rat was placed in the operant chamber for a 2-h session. For reinstatement tests, the test compound or vehicle was infused immediately before either a 3, 10, or 30 mg/kg cocaine priming injection and placement in the operant chambers where lever presses were recorded but had no programmed consequences (cocaine-primed reinstatement) or were not given a priming injection but were placed in the operant chambers, where right lever presses resulted in the presentation of the cues previously associated with cocaine self-administration (cue-primed reinstatement). In one experiment, 10 μg/kg NT antagonist SR142948 or its vehicle was injected i.p., 1 h before a 10 mg/kg cocaine-priming injection, followed by immediate placement in the operant chamber for a 2-h reinstatement test. This experiment was conducted to determine whether general antagonism of neurotensin receptors throughout the brain could affect cocaine-primed reinstatement because intra-VP infusion had no effect. The dose was chosen based on effective behavioral doses in the literature (Binder et al., 2002).
Finally, the ability of neurotensin to alter locomotor activity induced by an acute injection of saline or cocaine was also determined to verify that the effect of NT(8–13) on cue- and cocaine-primed reinstatement was not due to nonspecific locomotor effects. Animals were habituated to photobeam activity boxes for 1 h before receiving a microinjection of vehicle or 0.3 or 3 nmol/side NT(8–13) into the VP followed by an injection of saline or 15 mg/kg cocaine i.p. Then, animals were returned to the activity boxes for 2 h, where the number of beam breaks was tracked by computer software that calculated the total distance traveled and the amount of stereotypy (continuous breaking of the same photobeam) among other activity measures (Omnitech Technologies Inc., Columbus, OH).
Neurotensin’s effect on cocaine-induced locomotor sensitization was also determined to verify that after chronic cocaine exposure, NT(8–13) does not significantly alter cocaine-induced locomotor activity. Animals received a regimen of 1 day of saline and 7 days of cocaine as described above followed by 3 weeks of abstinence. Locomotor activity measurements were taken on days 1 and 7 of cocaine exposure and on day 28 (3 weeks later), where the amount of increase in activity on day 28 compared with day 1 was taken as an index of sensitization magnitude. On day 28, animals received a microinjection of vehicle or 0.3 or 3 nmol NT(8–13) bilaterally into the VP before a 15 mg/kg cocaine injection. Activity was monitored for 2 h after cocaine, and the total distance traveled and amount of stereotypy were evaluated. Thus, the ability of neurotensin to modulate the expression of cocaine-induced sensitization was determined.
Histology and Statistics
At the end of each experiment, animals were anesthetized with pentobarbital and were transcardially perfused with saline. Brains were dissected and placed in 4% para-formaldehyde. Brains were sectioned on a Vibratome at a thickness of 80 μm and placed on gelatin-subbed slides. Sections were then stained with cresyl violet, coverslipped, and correct cannula placements were determined by visualization under a light microscope. Animals with incorrect cannula placements were not included in the study.
Statistical analyses were performed using Prism software (Graph-Pad Software Inc., San Diego, CA). Repeated measures one-way ANOVA with Dunnett’s post-hoc test was used to compare the effects of different doses of compounds on GABA release compared with its baseline. Differences between two different groups over time were analyzed with repeated measures two-way ANOVA and Bonferroni’s post-hoc test. Reinstatement tests were analyzed with two-way ANOVA, where the two factors were treatment and test phase with Bonferroni’s post-hoc test. For all experiments, p < 0.05 was considered significant.
Results
Neurotensin Increases Extracellular GABA in the VP
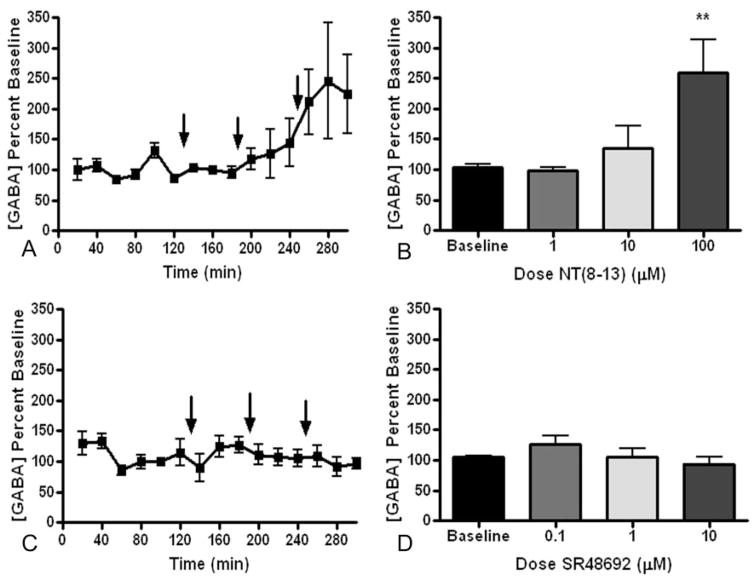
Neurotensin dose-dependently increased the extracellular concentration of GABA after reverse dialysis into the VP. The concentration of GABA in each sample is expressed as a percentage of the average baseline concentration of GABA calculated for the last hour of baseline sample collection (Fig. 1A), and the average concentration of the last two samples taken at each dose of NT(8–13) was compared with the baseline average (Fig. 1B). The 100 μM dose of NT(8–13) increased the extracellular concentration of GABA [F(3,27) = 6.207, p = 0.004]. The increase in GABA by NT(8–13) was mediated by NT receptors because the neurotensin antagonist SR48692 abolished the capacity of 100 μM NT(8–13) to elevate extracellular GABA [F(2,20) = 1.347, p = 0.297] (Fig. 2, A and B). However, SR48692 alone had no effect on the concentration of GABA at any of the doses tested [F(3,19) = 2.533, p = 0.106] (Fig. 1, C and D). The increase in extracellular GABA also depended on neuronal activity because the increase in extracellular GABA by NT(8–13) was abolished by the voltage-dependent sodium channel inhibitor TTX. However, TTX pretreatment alone did not alter basal extra-cellular levels of GABA [F(2,14) = 1.543, p = 0.271].
Fig. 1.
Effect of NT(8–13) and SR48692 on extracellular concentrations of GABA in the VP. A and C, effect of increasing doses of NT(8–13) (n = 7) (A) or SR48692 (n = 5) (C) over time. The arrows represent where the drug was added, and dose was increased. The amount of GABA is expressed as a percentage of the average of the baseline samples. The average basal concentration of GABA in the VP was 0.59 ± 0.04 pmol/40 μl in A and B and 0.56 ± 0.05 pmol/40 μl in C and D. B and D, dose-effect representation of the effect of NT(8–13) (B) and SR48692 (D) on the concentration of GABA in the VP where the baseline bar represents the average of the last hour of baseline samples collected, and each dose represents the average of the last two samples collected at that dose.**, p < 0.01, compared with baseline using a Dunnett’s post hoc analysis.
Fig. 2.
Blockade of the increase in VP GABA produced by 100 μM NT(8–13) by SR48692 and TTX. A and C, time course of the amount of GABA collected as a percentage of baseline, where the first arrow represents the addition of 10 μM SR48692 (n = 7) (A) or 1 μM TTX (n = 5) (C) to the dialysis buffer, and the second arrow represents the addition of 100 μM NT(8–13) to the dialysis buffer containing SR48692 or TTX (which were kept in the dialysis buffer for the duration of the experiment). The basal concentration of GABA in the VP was 1.2 ± 0.23 pmol/40 μl sample in A and B and 0.53 ± 0.06 pmol/40 μl sample in C and D. B and D, bar graph representation of the average of the last hour of baseline compared with the average of the last two samples collected after each change to SR48692 (B) or TTX (D) alone or in combination with 100 μM NT(8–13).
NT(8–13) also increased extracellular GABA in the VP of rats withdrawn from chronic cocaine administration. Figure 3A shows that NT(8–13) dose-dependently increased extracellular GABA. Figure 3B shows that the average increase in GABA at both 10 and 100 μM NT(8–13) was increased over baseline [F(3,23) = 7.543, p = 0.003]. To evaluate whether neurotensin and cocaine have an interactive effect on GABA, rats had either 3 μM NT(8–13) (a subthreshold dose for increasing extracellular GABA; see Fig. 1) or vehicle reverse dialyzed into the VP and after 1 h received an i.p. injection of 15 mg/kg cocaine. A subthreshold dose was used so that any effect of cocaine would not be masked by neurotensin-induced high concentrations of GABA. In animals receiving vehicle into the VP, the cocaine injection caused a decrease in extracellular GABA, estimated using a repeated measures one-way ANOVA to evaluate the effect of cocaine in control animals [F(6,76) = 2.231, p = 0.052] (Fig. 4). In addition, a repeated measures two-way ANOVA comparing treatment [vehicle or 3 μM NT(8–13)] over time revealed an effect of treatment [F(1,280) = 10.26, p = 0.0045], an effect of time [F(14,280) = 4.66, p < 0.0001], and an interaction [F(14,280) = 5.61, p < 0.0001]. Bonferroni’s post-hoc test revealed that the NT(8–13)-treated group had a greater concentration of extracellular GABA over the last 5 time points after the cocaine injection than the vehicle-treated group, but there was no effect of 3 μM NT(8–13) on GABA release before the cocaine injection (Fig. 4). The average basal concentration of GABA in both treatment groups was significantly higher in this experiment than the other dialysis experiments. It is unclear why this occurred, but it is unlikely that the chronic cocaine treatment had an effect on basal levels because basal levels were not increased in other dialysis experiments conducted in animals treated with chronic cocaine. It should be noted that basal levels were consistent among the animals in this experiment, and differences in basal levels compared with other experiments are possibly due to differences in probe recovery.
Fig. 3.
Effect of increasing doses of NT(8–13) on extracellular GABA in the VP of rats chronically treated with cocaine for 7 days and withdrawn for 21 to 28 days. A, effect of NT(8–13) (n = 6) over time. The arrows represent where the drug was added, and dose was increased. The amount of GABA is expressed as a percentage of the average of the baseline samples. The average basal concentration of GABA in this experiment was 0.43 ± 0.03 pmol/40 μl sample. B, dose-effect representation of the increase in GABA produced by NT(8–13) in the VP.*, p < 0.05;**, p < 0.01, comparing NT(8–13) with baseline.
Fig. 4.
NT(8–13) reverses the cocaine-induced decrease in GABA in the VP of rats chronically treated with cocaine. A, extracellular concentration of GABA in the VP is expressed as a percentage of baseline, where the first arrow represents a switch to vehicle (n = 11) or 3 μM NT(8–13) (n = 11), and the second arrow represents the point where all animals received an injection of cocaine (15 mg/kg i.p.). The basal concentration of GABA in the VP for these experiments was 63.4 ± 8.7 pmol/40 μl sample for the vehicle-treated group and 47.3 ± 5.3 pmol/40 μl sample for the NT(8–13)-treated group. B, bar graph representation of the data shown in A, showing that 1 and 2 h after cocaine, the 3 μM NT(8–13)-treated group had a significantly higher concentration of GABA than the aCSF group.**, p < 0.01;***, p < 0.001, comparing between the two groups using a Dunnett’s post-hoc test.
Effect of Neurotensin in the VP on Cocaine Self-Administration and Reinstatement
In the next set of experiments, the effect of NT(8–13) microinjected into the VP on cocaine self-administration and reinstatement behavior was determined. At the end of self-administration training, the average active lever presses for all groups of rats in this set of experiments was 54.2 ± 6.0 and average inactive lever presses was 5.7 ± 1.8. NT(8–13), 3 nmol/side, microinjected into the VP had no effect on the number of self-administered cocaine infusions (or active lever presses, data not shown) compared with the average number of infusions self-administered in the 2 days before the test (pretest) and the average number of infusions self-administered in the 2 days after the test (post-test) (Fig. 5A). However, 3 nmol NT(8–13) inhibited cue-primed reinstatement of cocaine seeking. Animals received a microinjection of aCSF or 3 nmol/side NT(8–13) immediately before being placed in the operant chamber, where right (active) lever presses resulted in the presentation of the light-tone cue, and a two-way ANOVA revealed an effect of test phase (extinction versus reinstatement) [F(1,12) = 9.07, p = 0.01] but no effect of treatment or interaction. Post hoc analysis comparing reinstatement to extinction within the aCSF or the NT(8–13) groups revealed that animals receiving aCSF injections reinstated active lever pressing, whereas the NT(8–13)-treated group did not show reinstatement (Fig. 5B).
Fig. 5.
Effect of NT(8–13) micro-injected into the VP on cocaine self-administration and reinstatement related behaviors. A, during the maintenance phase of self-administration, rats received a microinjection of vehicle (n = 4) or 3 nmol/side NT(8–13) (n = 4) into the VP, and the number of cocaine infusions self-administered was recorded (test) and compared with the average number of infusions self-administered on the 2 days before the test (pretest) and the 2 days after the test (post-test). B, after microinjection of vehicle (n = 8) or 3 nmol/side NT(8–13) (n = 6) in the VP, rats were tested for cue-primed reinstatement.*, p < 0.05. C, 3 nmol/side NT(8–13) (n = 5, n = 7, n = 6, respectively) or vehicle (n = 6, n = 9, n = 6, respectively) was microinjected into the VP before a 3, 10, or 30 mg/kg i.p. injection of cocaine. D, several doses of NT(8–13) (n = 4–7) or vehicle (n = 5) were administered into the VP, and the amount of active lever presses during extinction was recorded.*, p < 0.05;***, p < 0.001, compared with extinction pressing; ##, p < 0.01, comparing Reinst-3 nmol NT with Reinst-aCSF.
In contrast, when 3 nmol NT(8–13) was administered before a 10 or 30 mg/kg cocaine-priming injection, the reinstatement response was greater in the NT(8–13) versus the aCSF group. A two-way ANOVA revealed an effect of cocaine dose [F(2,66) = 10.28, p = 0.0001], an effect of test phase (extinction or reinstatement) [F(3,66) = 9.48, p < 0.0001], and an interaction [F(6,66) = 3.306, p = 0.0066]. Bonferroni’s post-hoc test revealed that there was no reinstatement to a 3 mg/kg cocaine prime regardless of treatment but that the NT(8–13)-treated group had a significant reinstatement effect over extinction behavior at the 10 and 30 mg/kg cocaine-priming doses. In contrast, the aCSF group did not show a statistically significant reinstatement at any dose of cocaine when all doses were analyzed together. In addition, the NT(8–13)-treated group had a statistically greater amount of reinstatement at the 30 mg/kg cocaine-priming dose than the aCSF-treated group (Fig. 5C).
Because of the ability of NT(8–13) to potentiate cocaine-primed reinstatement, we next examined whether NT(8–13) could reinstate drug seeking when given alone, at a range of doses. There was no effect of vehicle or any dose of NT(8–13) on extinction responding (Fig. 5D). Thus, microinjection of NT(8–13) alone in the VP was not sufficient to induce reinstatement. There was also no effect of NT(8–13) on inactive lever pressing in any of the experiments (data not shown).
Next, we determined whether the NT receptor antagonist could alter cocaine self-administration or reinstatement behavior. On the last day of self-administration training for these experiments, the average number of active lever presses was 54.4 ± 6.1, and the average number of inactive lever presses was 7.4 ± 2.5. Similar to neurotensin receptor stimulation, 1 nmol of the NT receptor antagonist SR142948 microinjected into the VP had no effect on the number of cocaine infusions self-administered compared with the amount administered on the 2 days previous to the test and the 2 days after the test (Fig. 6A). In addition, 1 nmol SR142948 did not alter responding on day 1 of extinction (Fig. 6B), nor did it alter the reinstatement of cocaine seeking elicited by a 10 mg/kg cocaine-priming injection. A two-way ANOVA revealed that there was an effect of test phase (extinction versus reinstatement) [F(1,16) = 21.34, p = 0.0003], but no effect of treatment and no interaction. Therefore, 10 mg/kg cocaine produced reinstatement in both SR142948-treated animals and controls (Fig. 6C). Likewise, when SR142948 was administered systemically (10 μg/kg i.p.) 1 h before a cocaine-priming injection, there was an effect of test phase [F(1,10) = 9.357, p = 0.012], but no effect of treatment or interaction between treatment and test phase (Fig. 6D). Post-hoc analysis revealed that the vehicle-treated group showed reinstatement compared with extinction responding, whereas the SR142948-treated group did not show reinstatement.
Fig. 6.
Effect of SR142948 i.p. and microinjected into the VP on cocaine self-administration and reinstatement related behaviors. A, 1 nmol/side SR142948 (n = 6) or vehicle (n = 8) was microinjected into the VP, and the number of cocaine infusions self-administered was recorded (test) and compared with the average number of infusions self-administered on the 2 days before the test (pretest) and the 2 days after the test (post-test). B, 1 nmol/side SR142948 (n = 9) or vehicle (n = 9) was microinjected into the VP before placing the animal in the operant chamber for day 1 of extinction to determine whether SR142948 could alter extinction pressing. C, 1 nmol/side SR142948 (n = 9) or vehicle (n = 9) was microinjected into the VP before a 10 mg/kg i.p. cocaine injection to determine whether SR142948 could prevent cocaine-primed reinstatement. D, 10 μg/kg SR142948 (n = 6) or vehicle (n = 6) was injected i.p. 1 h. before a 10 mg/kg cocaine-priming injection.*, p < 0.05;**, p < 0.01, compared with extinction pressing.
Effect of Neurotensin in the VP on Cocaine-Induced Locomotor Activity
Finally, we determined whether NT(8 –13) microinjected into the VP had any effect on locomotor activity or cocaine-induced locomotor sensitization. Figure 7A shows that there was no effect of NT(8 –13) microinjection into the VP on spontaneous motor activity [F(2,187) = 0.14, p = 0.8699], although there was an effect of time [F(11,187) = 14,54, p < 0.0001]. Likewise, there was no effect of NT(8 –13) on acute cocaine-induced (15 mg/kg i.p.) motor activity. Although it seems that the 0.3 nmol dose of NT(8 –13) may reduce the amount of locomotor activity induced by cocaine and that 3 nmol NT(8 –13) may increase the amount of activity produced by cocaine, repeated measures two-way ANOVA revealed only an effect of time [F(11,65) = 7.51, p < 0.0001], with no effect of treatment [F(2,165) = 1.47, p = 0.262] and no interaction [F(22,165) = 1.37, p = 0.138].
Fig. 7.
Effect of NT(8–13) on locomotor activity induced by a saline injection, 15 mg/kg cocaine, and on the expression of cocaine-induced locomotor sensitization. A, animals were microinjected with vehicle (n = 8) or 0.3 (n = 4) or 3 (n = 8) nmol NT(8–13) into the VP, followed by an i.p. injection of saline. Locomotor activity is shown as the total distance traveled in 10-min bins. B, experiment was carried out as described in A, except that animals received a 15 mg/kg injection of cocaine instead of saline (aCSF; n = 7, 0.3 nmol, n = 4; 3 nmol, n = 7). C, animals were given a sensitizing regimen of cocaine for 7 days, where locomotor activity was measured on day 1 of cocaine administration. After 21 to 28 days of withdrawal, animals were tested for the expression of locomotor sensitization after microinjection of vehicle (n = 6) or 0.3 (n = 7) or 3 (n = 6) nmol/side NT(8–13) into the VP, followed by an i.p. injection of 15 mg/kg cocaine. The graph shows the amount of locomotor activity in the 2 h after the cocaine injection expressed as the sensitization magnitude where the amount of locomotor activity produced on day 1 of cocaine was subtracted from the amount of locomotor activity measured on the sensitization test day in each of the 10-min bins. All values above zero are an increase or sensitization in the amount of locomotor activity produced by cocaine. NT(8–13) had no significant effect on sensitization magnitude.
The ability of NT(8–13) to alter the expression of cocaine-induced locomotor sensitization was also determined. Three weeks after discontinuing repeated cocaine administration, vehicle or 0.3 or 3 nmol NT(8–13) was microinjected in the VP immediately before a 15 mg/kg priming injection of cocaine and placement in a locomotor activity recording chamber. For each animal, the amount of motor activity recorded during the 2 h after the very first injection of cocaine was subtracted from the amount of locomotor activity recorded on the sensitization test day (occurring 4 weeks later) at each of the 10-min time intervals to obtain a measure of sensitization magnitude for each of the three treatment groups. When these data were analyzed by repeated measures two-way ANOVA, an effect of time was identified [F(11,176) = 5.226, p < 0.0001], but no effect of treatment [F(2,176) = 0.384, p = 0.687] or time/treatment interaction [F(22,176) = 0.516, p = 0.965], indicating that neither dose of NT(8–13) had an effect on the amount of locomotor sensitization produced by cocaine (Fig. 7C). The effect of NT(8–13) on cocaine-induced stereotypy was also evaluated in the acute cocaine and locomotor sensitization experiments to determine whether NT(8–13) altered the expression of cocaine-induced stereotypy preferentially over total locomotor activity, which would be an indication of an increased psychostimulant effect of cocaine. However, there was also no effect of any treatment on the estimation of stereotypy (data not shown).
Histology
Figure 8 shows illustrated representations of the microdialysis probe and microinjection cannula placements for all experiments. Animals with cannula placements outside the VP were excluded from data analysis.
Fig. 8.
Location of all microdialysis probes and microinjections sites used for data analysis. A, illustrations based upon the atlas of Paxinos and Watson (1986) showing the placement of the active zone of the dialysis probes for all experiments. One brain hemisphere was used for each dialysis experiment, but in some animals, the other hemisphere was used for a second experiment. B, illustrative representation of the micro-injection sites for all experiments. Animals with placements outside of the VP were not included in the data analysis and are not shown.
Discussion
The present study determined whether a neurotensin receptor agonist could increase GABA release in the VP, prevent the cocaine-induced decrease in GABA in the VP, and thereby prevent cue- and cocaine-primed reinstatement. The active peptide fragment of neurotensin, NT(8–13), increased GABA release in the VP in both drug-naive and cocaine-treated animals. The NT(8–13)-induced increase in GABA was both neurotensin receptor and TTX dependent, and NT(8–13) prevented the cocaine-induced decrease in GABA release in the VP. Moreover, NT(8–13) attenuated cue-induced reinstatement. However, contrary to our hypothesis, NT(8–13) potentiated rather than inhibited cocaine-primed reinstatement, although it should be noted that only one dose of NT(8–13) was tested in the reinstatement experiments, making it possible that other doses of NT(8–13) might have different effects. These findings do suggest that neurotensin alone in the VP may inhibit the neurocircuitry necessary for cue-induced reinstatement but that an interaction between NT(8–13) and cocaine in the VP may bypass the regulation of cocaine seeking by the GABAergic projection from the accumbens to the VP (see further discussion below). Finally, the neurotensin receptor antagonist SR142948 had no significant effect on cocaine self-administration, extinction, or cocaine-induced reinstatement when given in the VP (although only one dose was tested) but inhibited reinstatement when given systemically, suggesting that endogenous stimulation of neurotensin receptors in the VP is not necessary for these cocaine-mediated behaviors but that neurotensin acting in other regions may regulate reinstatement.
Neurotensin Induced GABA Release
Intra-VP NT(8–13) increased extracellular GABA, much as was previously demonstrated for neurotensin agonists in the prefrontal cortex (Petrie et al., 2005), NAc (Tanganelli et al., 1994; O’Connor, 2001), dorsal striatum (Ferraro et al., 1997, 1998), and hippocampus (Rakovska et al., 1998). In the present study, the blockade of the neurotensin-induced increase in extracellular GABA by TTX indicates that the increase was mediated by action potential-induced depolarization. It is possible that neurotensin increases the release of GABA from NAc projection neurons because this is the main source of neuronal GABA to the VP (Zahm and Heimer, 1988). However, GABA may also be released from VTA projection neurons (Del-Fava et al., 2007) or from recurrent collaterals within the VP itself. It is unfortunate that there are no studies to date evaluating the relative localization of NT receptors postsynaptically on VP neurons versus presynaptically on one of the afferent projections or recurrent collaterals, making the relative contribution from each potential source of GABA impossible to estimate.
Relationship among GABA Release, NT, and Drug Seeking
The systemic administration of many drugs of abuse, including cocaine, heroin, morphine, and amphetamine, decrease GABA in the VP (Bourdelais and Kalivas, 1990; Tang et al., 2005; Caillé and Parsons 2004, 2006). Moreover, cocaine-induced reinstatement requires activity of MORs in the VP, and Tang et al. (2005) hypothesized that this resulted via inhibition of GABA release from enkephalin/GABA colocalized accumbens afferents that possess presynaptic MOR (Olive et al., 1997). Based upon this study, increasing the extracellular concentration of VP GABA or blocking the drug-induced decrease in GABA was predicted to prevent reinstatement. Accordingly, NT(8–13) induced GABA release, and NT(8–13) microinjection into the VP inhibited cue-primed reinstatement. However, NT(8–13) paradoxically promoted cocaine-induced reinstatement. This distinction was unexpected given that both cue- and cocaine-primed reinstatement are known to depend on intact neurotransmission in the nucleus accumbens and VP (McFarland and Kalivas, 2001; See, 2002). Although a potential concern with these experiments is that a single microinjection is given before a 2-h behavioral test, there were significant behavioral effects, suggesting that a potentially short half-life of NT(8–13) was not a major influence. Additional explanations for the differential effect on reinstatement include the following: 1) cocaine and NT interact within the VP to countermand the effect of promoting GABA release; 2) NT may interact differentially within subregions of the VP that preferentially regulate cue- and cocaine-induced reinstatement; and 3) the dose of NT was not sufficient to increase GABA release in the VP and, thus, produced effects in a GABA-independent manner.
An interaction between cocaine and NT separate from NT regulation of GABA release is indicated by the fact that not only did cocaine and NT(8–13) synergize on cocaine-induced reinstatement (Fig. 5C), but cocaine also seemed to promote NT(8–13)-induced GABA release (Fig. 4), and 3 nmol NT(8–13) showed a tendency toward increasing cocaine-induced locomotion (Fig. 7B). Cocaine is well established to increase the extracellular concentration of dopamine by inhibiting dopamine transporters (Seiden et al., 1993), and an interaction between NT and dopamine is anatomically supported by the fact that NT is colocalized with dopamine in projection neurons in the VTA and that the VTA projects to the VP (Bayer et al., 1991; Klitenick et al., 1992). However, the literature on this colocalized projection shows a mixed interaction between NT and dopamine (Cáceda et al., 2006). Thus, although neurotensin administered in the VTA enhances dopamine release in the NAc (Kalivas and Duffy, 1990; Laitinen et al., 1990), the NT antagonist SR48692 enhances methamphetamine-induced dopamine release in the NAc (Wagstaff et al., 1994), and low doses of neurotensin in the NAc decrease dopamine release (Tanganelli et al., 1994). Likewise, there is an extensive behavioral literature indicating varied interactions between neurotensin and amphetamine-like psychostimulants. For example, neurotensin administered directly into the NAc reduces cocaine-induced locomotor activity and has no effect on cocaine self-administration (Kalivas et al., 1984; Robledo et al., 1993), and a systemically active analog of neurotensin, NT69L, blocks the hyperactivity produced by cocaine and d-amphetamine (Boules et al., 2001). However, chronic administration of a neurotensin antagonist during cocaine withdrawal decreases sensitization to cocaine (Felszeghy et al., 2007). Given the current literature, it will be necessary to directly measure effects of NT on dopamine release in the VP to evaluate possible synergism between cocaine-induced dopamine release and NT.
Regarding the possibility that NT and enkephalin may interact differentially with cue- and cocaine-induced reinstatement circuitry, it is important to note that enkephalin is preferentially colocalized with GABA in the NAc core projection to the lateral VP, whereas the NAc shell projection to the ventromedial part of the VP colocalizes neurotensin with GABA (Zahm and Heimer, 1988, 1990). Supporting this anatomical distinction, administration of a neurotensin receptor antagonist inhibits firing of neurons in the ventromedial VP, but not lateral VP (Michaud et al., 2000). In addition, although cocaine-primed reinstatement requires neuronal activity of the NAc core, but not the NAc shell (McFarland and Kalivas, 2001), dopamine- and cue-induced drug seeking may involve the NAc shell (see Di Ciano and Everitt, 2001; See, 2002; Phillips et al., 2003). Thus, the primary physiological action of neurotensin may be in a compartment of the VP that is not required for cocaine-induced reinstatement but that can influence cue-induced reinstatement. Unfortunately, it is not technically possible to dissociate the effects of NT in the two compartments with microdialysis probes or microinjections due to the anatomical structure of the VP. Finally, it is possible that the dose of NT(8–13) used in the behavioral studies was not sufficient to increase GABA release in the VP. It is unfortunate that this is a limitation of these experiments because it is difficult to determine whether a faster bolus infusion given by microinjection has the same effect on neurotransmitter release as a steady-state lower concentration of NT supplied by reverse dialysis over a longer period of time. However, the concentrations of NT perfused into the brain with microdialysis that increased GABA could yield a dose as high as 1.2 to 12 nmol (i.e., 10 and 100 μM over 60 min at 2 μl/min, assuming 100% access across the membrane), which is in the range of the 3 nmol working dose of NT employed in the microinjection studies.
Conclusions
NT(8–13) increases GABA release in the VP and inhibits cue-induced reinstatement of cocaine seeking. This is consistent with a postulate that reducing GABA transmission in the VP promotes drug-seeking (McFarland and Kalivas, 2001; Caillé and Parsons 2004, 2006; Tang et al., 2005). However, NT-(8–13) also potentiated cocaine-primed reinstatement, suggesting more complicated mechanisms regulating drug seeking related to subcompartments of the VP and/or that NT and cocaine may interact in the VP in a manner to countermand the NT-induced release of GABA.
Acknowledgments
This study was supported by United States Public Health Service Grants DA03906, DA12513, and T32 DA07288-15.
We thank Connie Towles and Ashley Hawkins for technical assistance in the completion of the experiments.
ABBREVIATIONS
- VTA
ventral tegmental area
- NAc
nucleus accumbens
- VP
ventral pallidum
- MOR
μ opioid receptor
- NT(8–13)
neurotensin peptide fragment 8–13
- aCSF
artificial cerebral spinal fluid
- SR48692
2-(1-(7-chlor-oquinolin-4-yl)-5-(2,6-dimethoxyphenyl)-1H-pyrazol-3-carbonyl]-amino]-adamantane-2-carboxylic acid
- SR142948
2-[[[5-(2,6-dimethoxyphenyl)-1-[4-[[[3-(dimethylamino)propyl]methylamino]carbonyl]-2-(1-methylethyl)phenyl]-1H-pyrazol-3-yl]carbonyl]amino]-tricyclo[3.3.1.13,7]decane-2-carboxylic acid
- NT
neurotensin
- NTR
neurotensin receptor
- TTX
tetrodotoxin
- ANOVA
analysis of variance
- CTAP
D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2
- NT69L
N-methyl-L-Arg, L-Lys, L-Pro, L-neo-Trp, L-tert-Leu, L-Leu
Footnotes
Parts of this work were previously presented at the following meeting: Torregrossa MM and Kalivas PW (2006) Regulation of GABA and glutamate release in the ventral palladium by co-localized neuropeptides: implications for cocaine reinstatement behavior. Society for Neuroscience Annual Meeting; 2006 Oct 14–18; Atlanta, GA. Society for Neuroscience, Washington, DC.
References
- Bayer VE, Towle AC, Pickel VM. Ultrastructural localization of neurotensin-like immunoreactivity within dense core vesicles in perikarya, but not terminals, colocalizing tyrosine hydroxylase in the rat ventral tegmental area. J Comp Neurol. 1991;311:179–196. doi: 10.1002/cne.903110202. [DOI] [PubMed] [Google Scholar]
- Binder EB, Gross RE, Nemeroff CB, Kilts CD. Effects of neurotensin receptor antagonism on latent inhibition in Sprague-Dawley rats. Psychopharmacology. 2002;161:288–295. doi: 10.1007/s00213-002-1031-4. [DOI] [PubMed] [Google Scholar]
- Boules M, Warrington L, Fauq A, McCormick D, Richelson E. A novel neurotensin analog blocks cocaine- and D-amphetamine-induced hyperactivity. Eur J Pharmacol. 2001;426:73–76. doi: 10.1016/s0014-2999(01)01197-9. [DOI] [PubMed] [Google Scholar]
- Bourdelais A, Kalivas PW. Amphetamine lowers extracellular GABA concentration in the ventral pallidum. Brain Res. 1990;516:132–136. doi: 10.1016/0006-8993(90)90907-s. [DOI] [PubMed] [Google Scholar]
- Cáceda R, Kinkead B, Nemeroff CB. Neurotensin: role in psychiatric and neurological diseases. Peptides. 2006;27:2385–2404. doi: 10.1016/j.peptides.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Caillé S, Parsons LH. Intravenous heroin self-administration decreases GABA efflux in the ventral pallidum: an in vivo microdialysis study in rats. Eur J Neurosci. 2004;20:593–596. doi: 10.1111/j.1460-9568.2004.03497.x. [DOI] [PubMed] [Google Scholar]
- Caillé S, Parsons LH. Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway. Neuropsychopharmacology. 2006;31:804–813. doi: 10.1038/sj.npp.1300848. [DOI] [PubMed] [Google Scholar]
- Del-Fava F, Hasue RH, Ferreira JG, Shammah-Lognado SJ. Efferent connections of the rostral linear nucleus of the ventral tegmental area in the rat. Neuroscience. 2007;145:1059–1076. doi: 10.1016/j.neuroscience.2006.12.039. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Felszeghy K, Espinosa JM, Scarna H, Berod A, Rostene W, Pelaprat D. Neurotensin receptor antagonist administered during cocaine withdrawal decreases locomotor sensitization and conditioned place preference. Neuropsychopharmacology. 2007;32:2601–2610. doi: 10.1038/sj.npp.1301382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, O’Connor WT, Fuxe K, Soubrie P, Tanganelli S. The striatal neurotensin receptor modulates striatal and pallidal glutamate and GABA release: functional evidence for a pallidal glutamate-GABA interaction via the pallidal-subthalamic nucleus loop. J Neurosci. 1998;18:6977–6989. doi: 10.1523/JNEUROSCI.18-17-06977.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, O’Connor WT, Antonelli T, Fuxe K, Tanganelli S. Differential effects of intrastriatal neurotensin(1–13) and neurotensin(8–13) on striatal dopamine and pallidal GABA release: a dual-probe microdialysis study in the awake rat. Eur J Neurosci. 1997;9:1838–1846. doi: 10.1111/j.1460-9568.1997.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals. 7. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council; Washington DC: 1996. [Google Scholar]
- Kalivas PW, Nemeroff CB, Prange AJ. Neurotensin microinjection into the nucleus accumbens antagonizes dopamine-induced increase in locomotion and rearing. Neuroscience. 1984;11:919–930. doi: 10.1016/0306-4522(84)90203-3. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effect of acute and daily neurotensin and enkephalin treatments on extracellular dopamine in the nucleus accumbens. J Neurosci. 1990;10:2940–2949. doi: 10.1523/JNEUROSCI.10-09-02940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitenick MA, Deutch AY, Churchill L, Kalivas PW. Topography and functional role of dopaminergic projections from the ventral mesencephalic tegmentum to the ventral pallidum. Neuroscience. 1992;50:371–386. doi: 10.1016/0306-4522(92)90430-a. [DOI] [PubMed] [Google Scholar]
- Laitinen K, Crawley JN, Mefford IN, De Witte P. Neurotensin and cholecystokinin microinjected into the ventral tegmental area modulate microdialysate concentrations of dopamine and metabolites in the posterior nucleus accumbens. Brain Res. 1990;523:342–346. doi: 10.1016/0006-8993(90)91511-e. [DOI] [PubMed] [Google Scholar]
- Martorana A, Martella G, D’Angelo V, Fusco FR, Spadoni F, Bernardi G, Stefani A. Neurotensin effects on N-type calcium currents among rat pallidal neurons: an electrophysiological and immunohistochemical study. Synapse. 2006;60:371–383. doi: 10.1002/syn.20306. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud JC, Gueudet C, Soubrie P. Effects of neurotensin receptor antagonists on the firing rate of rat ventral pallidum neurons. Neuroreport. 2000;11:1437–1441. doi: 10.1097/00001756-200005150-00017. [DOI] [PubMed] [Google Scholar]
- O’Connor WT. Functional neuroanatomy of the ventral striopallidal GABA pathway: new sites of intervention in the treatment of schizophrenia. J Neurosci Methods. 2001;109:31–39. doi: 10.1016/s0165-0270(01)00398-3. [DOI] [PubMed] [Google Scholar]
- Olive MF, Anton B, Micevych P, Evans CJ, Maidment NT. Presynaptic versus postsynaptic localization of mu and delta opioid receptors in dorsal and ventral striatopallidal pathways. J Neurosci. 1997;17:7471–7479. doi: 10.1523/JNEUROSCI.17-19-07471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1986. [Google Scholar]
- Petrie KA, Schmidt D, Bubser M, Fadel J, Carraway RE, Deutch AY. Neurotensin activates GABAergic interneurons in the prefrontal cortex. J Neurosci. 2005;25:1629–1636. doi: 10.1523/JNEUROSCI.3579-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine releases promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Rakovska A, Giovannini MG, Della Corte L, Kalfin R, Bianchi L, Pepeu G. Neurotensin modulation of acetylcholine and GABA release from the rat hippocampus: an in vivo microdialysis study. Neurochem Int. 1998;33:335–340. doi: 10.1016/s0197-0186(98)00036-9. [DOI] [PubMed] [Google Scholar]
- Robledo P, Maldonado R, Koob GF. Neurotensin injected into the nucleus accumbens blocks the psychostimulant effects of cocaine but does not attenuate cocaine self-administration in the rat. Brain Res. 1993;622:105–112. doi: 10.1016/0006-8993(93)90808-z. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Sabol KE, Ricuarte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, DeWit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacol. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Tang XC, McFarland K, Cagle S, Kalivas PW. Cocaine-induced reinstatement requires endogenous stimulation of mu-opioid receptors in the ventral pallidum. J Neurosci. 2005;25:4512–4520. doi: 10.1523/JNEUROSCI.0685-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanganelli S, O’Connor WT, Ferraro L, Bianchi C, Beani L, Ungerstadt U, Fuxe K. Facilitation of GABA release by neurotensin is associated with a reduction of dopamine release in rat nucleus accumbens. Neuroscience. 1994;60:649–657. doi: 10.1016/0306-4522(94)90493-6. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Kalivas PW. Microdialysis and the neurochemistry of addiction. Pharmacol Biochem Behav. 2007 doi: 10.1016/j.pbb.2007.09.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff JD, Bush LG, Gibb JW, Hanson GR. Endogenous neurotensin antagonizes methamphetamine-enhanced dopaminergic activity. Brain Res. 1994;665:237–244. doi: 10.1016/0006-8993(94)91343-9. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Zaborszky L, Alones VE, Heimer L. Evidence for the coexistence of glutamate decarboxylase and Metenkephalin immunoreactivities in axon terminals of rat ventral pallidum. Brain Res. 1985;325:317–321. doi: 10.1016/0006-8993(85)90331-2. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Ventral striatopallidal parts of the basal ganglia in the rat: I. Neurochemical compartmentation as reflected by the distributions of neurotensin and substance P immunoreactivity. J Comp Neurol. 1988;272:516–535. doi: 10.1002/cne.902720406. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. J Comp Neurol. 1990;302:437–446. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]