Abstract
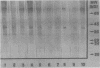
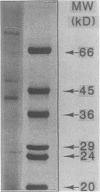
The serologic response to Sporothrix schenckii was investigated in patients with sporotrichosis by solid-phase enzyme-linked immunosorbent assays (ELISAs) and Western immunoblot techniques. A soluble antigen preparation derived from an S. schenckii isolate contained 15 protein staining components ranging in molecular size from 22 to 70 kilodaltons (kDa) by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Sera from 40 patients with sporotrichosis demonstrated Sporothrix immunoglobulin G antibody by ELISA with titers between 128 and 65,200. No sera from 300 healthy individuals or 100 patients with various systemic mycoses other than sporotrichosis had ELISA titers greater than 64. By Western immunoblotting of the antigens separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, sera from 10 patients with cutaneous sporotrichosis reacted with 8 to 10 antigen components (range, 40 to 70 kDa), while sera from 15 patients with extracutaneous sporotrichosis reacted with a greater number of antigen components (15 to 20 bands) over a wider range of molecular sizes (22 to 70 kDa). Antibody to 40- and 70-kDa antigen components was detected by immunoblots in all sera tested from patients with sporotrichosis. Antibody to 22- to 36-kDa antigen components was present in sera from 13 of 15 patients with extracutaneous sporotrichosis, but these lower-molecular-weight components were not detected by sera from patients with cutaneous sporotrichosis. Antibody to these components was not detected by Western blotting in sera from 19 of 20 patients with other fungal diseases or from 30 healthy individuals. Purification of these specific antigen fractions could provide the basis of a sensitive and specific serodiagnostic test to indicate the presence and activity of extracutaneous sporotrichosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asahi M., Lindquist R., Fukuyama K., Apodaca G., Epstein W. L., McKerrow J. H. Purification and characterization of major extracellular proteinases from Trichophyton rubrum. Biochem J. 1985 Nov 15;232(1):139–144. doi: 10.1042/bj2320139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au-Young J. K., Troy F. A., Goldstein E. Serologic analysis of antigen-specific reactivity in patients with systemic candidiasis. Diagn Microbiol Infect Dis. 1985 Sep;3(5):419–432. doi: 10.1016/0732-8893(85)90081-1. [DOI] [PubMed] [Google Scholar]
- Blumer S. O., Kaufman L., Kaplan W., McLaughlin D. W., Kraft D. E. Comparative evaluation of five serological methods for the diagnosis of sporotrichosis. Appl Microbiol. 1973 Jul;26(1):4–8. doi: 10.1128/am.26.1.4-8.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Kumar B. V., Medoff G., Kobayashi G. S., Sieling W. L. Cross-reacting human and rabbit antibodies to antigens of Histoplasma capsulatum, Candida albicans, and Saccharomyces cerevisiae. Infect Immun. 1985 Jun;48(3):806–812. doi: 10.1128/iai.48.3.806-812.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee K. H., Lee J. B., Lee M. G., Song D. H. Detection of circulating antibodies to purified keratinolytic proteinase in sera from guinea pigs infected with Microsporum canis by enzyme-linked immunosorbent assay. Arch Dermatol Res. 1988;280(1):45–49. doi: 10.1007/BF00412688. [DOI] [PubMed] [Google Scholar]
- Matthews R. C., Burnie J. P., Fox A., Woods M., Tabaqchali S. Immunoblot analysis of the serological response in invasive Trichosporon beigelii and Blastoschizomyces capitatus infections. J Clin Microbiol. 1986 Feb;23(2):395–397. doi: 10.1128/jcm.23.2.395-397.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R. C., Burnie J. P., Tabaqchali S. Immunoblot analysis of the serological response in systemic candidosis. Lancet. 1984 Dec 22;2(8417-8418):1415–1418. doi: 10.1016/s0140-6736(84)91618-0. [DOI] [PubMed] [Google Scholar]
- Matthews R., Burnie J. P., Fox A., Tabaqchali S. Immunoblot analysis of serological responses in invasive aspergillosis. J Clin Pathol. 1985 Nov;38(11):1300–1303. doi: 10.1136/jcp.38.11.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor C. G., Ashman L. K. Application of the nitrocellulose transfer technique and alkaline phosphatase conjugated anti-immunoglobulin for determination of the specificity of monoclonal antibodies to protein mixtures. J Immunol Methods. 1982 Oct 29;54(2):267–271. doi: 10.1016/0022-1759(82)90068-0. [DOI] [PubMed] [Google Scholar]
- Odds F. C. Candida albicans proteinase as a virulence factor in the pathogenesis of Candida infections. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Dec;260(4):539–542. doi: 10.1016/s0176-6724(85)80069-9. [DOI] [PubMed] [Google Scholar]
- Scott E. N., Kaufman L., Brown A. C., Muchmore H. G. Serologic studies in the diagnosis and management of meningitis due to Sporothrix schenckii. N Engl J Med. 1987 Oct 8;317(15):935–940. doi: 10.1056/NEJM198710083171505. [DOI] [PubMed] [Google Scholar]
- Strockbine N. A., Largen M. T., Zweibel S. M., Buckley H. R. Identification and molecular weight characterization of antigens from Candida albicans that are recognized by human sera. Infect Immun. 1984 Feb;43(2):715–721. doi: 10.1128/iai.43.2.715-721.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travassos L. R., Gorin P. A., Lloyd K. O. Comparison of the rhamnomannans from the human pathogen Sporothrix schenckii with those from the Ceratocystis species. Infect Immun. 1973 Nov;8(5):685–693. doi: 10.1128/iai.8.5.685-693.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi R., Ko I. J., Matsuda K., Ogawa H. A new keratinolytic proteinase from clinical isolates of Trichophyton mentagrophytes. J Dermatol. 1987 Oct;14(5):506–508. doi: 10.1111/j.1346-8138.1987.tb03616.x. [DOI] [PubMed] [Google Scholar]
- Tsuboi R., Sanada T., Ogawa H. Influence of culture medium pH and proteinase inhibitors on extracellular proteinase activity and cell growth of Sporothrix schenckii. J Clin Microbiol. 1988 Jul;26(7):1431–1433. doi: 10.1128/jcm.26.7.1431-1433.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi R., Sanada T., Takamori K., Ogawa H. Isolation and properties of extracellular proteinases from Sporothrix schenckii. J Bacteriol. 1987 Sep;169(9):4104–4109. doi: 10.1128/jb.169.9.4104-4109.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]