SUMMARY
Purpose
To test the efficacy of the novel candidate anticonvulsant talampanel (GYKI 53773) in a rodent model of hypoxic neonatal seizures. Talampanel is a noncompetitive antagonist of the alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid subtype of the glutamate receptor (AMPAR). We have previously shown that AMPARs play a critical role in the generation of acute seizures and later-life seizure susceptibility in this model of neonatal seizures.
Methods
Seizures were induced in postnatal day (P) 10 Long-Evans rat pups by a 15 min exposure to global hypoxia. Acute seizure activity at P10 and subsequent susceptibility to seizure-induced neuronal injury with a “second-hit” kainite-induced seizure at P30–31 were compared between animals receiving talampanel (1, 5, 7.5, or 10 mg/kg) intraperitoneal (i.p.) versus saline vehicle treatment.
Results
Talampanel treatment suppressed seizures in a dose-dependent manner, with maximal effect at 7.5 and 10 mg/kg. In addition, talampanel treatment 30 min before hypoxia prevented later-life increases in seizure-induced neuronal injury as assessed by in situ DNA nick end-labeling.
Discussion
We have previously demonstrated efficacy of other AMPAR antagonists such as NBQX and topiramate in this model. The present finding shows that the novel agent talampanel, under revaluation as an antiepileptic drug in children and adults, may have clinical potential in the treatment of neonatal seizures, particularly those occurring in the context of hypoxic encephalopathy.
Keywords: Perinatal seizures, Glutamate, Hypoxia/ischemia, Hippocampus, Amygdala, Kainate
Hypoxic encephalopathy is a major cause of neonatal seizures, and can lead to long-term neurologic deficits and epilepsy. The neonatal period has the highest incidence of seizures across the lifespan at 1.8 to 3.5 per 1,000 live births (Hauser & Kurland, 1975; Cowan, 2002; Cowan et al., 2003). The most common cause of neonatal seizures is hypoxic/ischemic encephalopathy (HIE), which occurs during approximately 1–2 per 1,000 live births (Lanska et al., 1995; Ronen et al., 1999; Saliba et al., 1999). Clinical evidence suggests that seizure activity in the setting of HIE may enhance stroke size as evidenced by more exaggerated changes in magnetic resonance spectroscopy (MRS) compared to infants without seizures (Wirrell et al., 2001; Miller et al., 2005). In addition, HIE is associated with a risk of long-term morbidity, including cognitive disorders and epilepsy (Bergamasco et al., 1984; Volpe, 2001; Miller et al., 2005; Scher, 2006). Notably, HIE-related neonatal seizures are often refractory to antiepileptic drug (AED) therapy (Painter et al., 1999; Painter & Alvin, 2001; Sankar & Painter, 2005). The mainstay of treatment remains phenobarbital, benzodiazepines, and phenytoin, and this practice has not changed significantly in the last 60 years, despite little evidence that these drugs are effective in neonatal seizures (Sankar & Painter, 2005).
To date, there have been no new drugs that have been proven effective in clinical trials for suppression of neonatal seizures. We have previously developed a model of hypoxia-induced seizures in the immature rat, in which hypoxia induces early refractory seizures as well as long- term effects, including spontaneous seizures and increased susceptibility to seizure-induced neuronal injury in later life (Jensen et al., 1991). In this model, activation of the alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid subtype of the glutamate receptor (AMPAR) is a critical factor in the epileptogenic effect of hypoxia (Sanchez et al., 2001, 2005). AMPAR antagonists such as NBQX and topiramate effectively suppress the acute and long- term epileptogenic effects of hypoxia in the perinatal rodent (Jensen et al., 1995; Koh & Jensen, 2001; Koh et al., 2004). We have shown that AMPARs are over- expressed on cortical neurons in the neonatal rat and term human, when the susceptibility to hypoxic seizures is highest (Talos et al., 2006a,b). In addition, the AMPAR antagonists topiramate and NBQX do not cause an increase in constitutive apoptotic neuronal death in the immature brain, unlike some conventional AEDs (phenobarbital, phenytoin, diazepam, and valproate), suggesting a better safety profile for use in the neonatal population (Silverstein & Jensen, 2007). Current obstacles to clinical trial development of AMPAR antagonists in the neonatal population include the fact that NBQX is not being developed for clinical use and topiramate is not available in parenteral form. Experimental and human tissue studies suggest that the AMPAR plays a critical role in the generation of neonatal seizures (Talos et al., 2006a,b; Silverstein & Jensen, 2007). Recently, more specific AMPAR antagonists have been investigated for clinical use, and include the novel noncompetitive AMPAR antagonist talampanel (GYKI-53773), which has been shown to be anticonvulsant in experimental models of seizures in the adult brain (Belayev et al., 2001; Jakus et al., 2004). Clinical trials in adults show anticonvulsant efficacy as monotherapy in refractory epilepsy (Langan et al., 2003) and as an add-on for therapy for partial complex seizures (Chappell et al., 2002; Howes & Bell, 2007). Given that talampanel has demonstrated efficacy in adult epilepsy trials, we aimed to examine its potential efficacy for neonatal seizures. As a first step to gather preclinical evidence and to further define a specific role for AMPAR in epileptogenesis in the developing brain, we examined the efficacy of talampanel in a rodent model of neonatal hypoxia-induced seizures.
Materials and Methods
Animals
Seizures were induced in Long-Evans male rats at postnatal (P) day 10. Each litter was divided into three groups: control, hypoxic seizures with vehicle treatment, and hypoxic seizures with talampanel treatment. Animals were maintained in a 12-h light/dark cycle. Pups were returned to their dam following induction of hypoxic seizures or sham, and adult female rats were housed with their pups in cages until pups were weaned at P21. All procedures were undertaken in accordance with the guidelines of the animal care and use committee at Children’s Hospital, Boston.
Hypoxia-induced seizures
Long-Evans male rats at postnatal P10 were exposed to global hypoxia for 15 min in an airtight chamber. As per our previously published methods, the oxygen concentration was gradually decreased to 7% for 8 min, 5% for 6 min, and 4% for the final minute (Jensen et al., 1991; Koh et al., 2004). During hypoxia, seizures were video- taped to record seizure activity. The tapes were scored by an investigator, who was blinded to treatment condition for the number of tonic–clonic head and limb movements and cumulative seizure duration during hypoxia. The talampanel/hypoxia group received talampanel by intraperitoneal (i.p.) injection at a dose of 1, 5, 7.5, or 10 mg/kg. Talampanel or vehicle [dimethyl sulfoxide (DMSO) in 0.5% methyl cellulose] was administered 30 min before hypoxia. Litter mate control pups were kept at room air, and the body temperature of all pups were maintained at 32°–34° on a warming blanket. The number of seizure episodes during hypoxia was compared by a one-way analysis of variance (ANOVA).
Kainate-induced seizures
Following induction of hypoxic seizures, animals were permitted to survive until P30–31, when they underwent a “second-hit” challenge of kainate-induced status epilepticus (10 mg/kg i.p.). Seizure activity and severity was recorded over a 3-h period, and latency to seizure onset and maximal severity was determined per our previously described modified Racine scale from 0 to V as follows: 0, no response; I, wet-dog shake and/or behavioral arrest; II, wet-dog shake, staring, pawing, clonic jerks, rearing falling; IV continuous grade III seizures for >30 min; and V, death (Koh et al., 2004). P30–31 rats with prior hypoxic seizures at P10 were compared to their age-matched normoxic littermates. On the basis of our prior studies, we have found that this dose of kainate results in 100% of animals reaching grade IV on this modified Racine scale.
DNA fragmentation by in situ end-labeling histochemistry
We compared neuronal death in hippocampus (areas CA1 and CA3) and amygdala in rats permitted to survive 72-h postkainate-induced seizures. As per our previous studies, neuronal injury was assessed by ISEL staining (Koh & Jensen, 2001; Koh et al., 2004). At 72 h after kainate-induced seizures, rats were killed and then perfused transcardially with 4% paraformaldehyde. Normoxic littermates were also sacrificed at the same age. Each brain was removed, postfixed, cryoprotected in 20% sucrose overnight, and mounted onto a freezing microtome. Three 50-μm–thick coronal sections/per rat at stereotactically identical locations between rats were processed for in situ nick translation using a modification of the protocol developed by Wijsman et al. (Wijsman et al., 1993; Koh et al., 2004). To quantify DNA fragmentation, counts of positively stained cells were compared at a final magnification of ×100. Positively stained cells were counted using a grid reticule over areas CA1, CA3, and the amygdala and the values were compared by a one-way ANOVA. Similar to Koh et al. (2004), counts were made by averaging three fields (4.16 mm2 per region × 3 = 12.5 mm2 total): both left and right hippocampi in CA1, CA3 (−3.0 to −3.3 mm from Bregma and 0–3 mm lateral to midline), and basolateral/basomedial amygdaloid nucleus (−3.0 to −3.3 mm from Bregma and 3–6 mm lateral to midline) on three coronal sections per animal (Paxinos & Watson, 1986) A total of 126 sections from 42 animals were analyzed (three sections per rat and two hippocampi per section).
Assessment of cell death after talampanel administration to normal P10 rats
Because a number of AEDs have been shown to acutely increase constitutive apoptosis in the developing rodent brain following single dosing, we examined whether the optimal dose of talampanel would cause cell death when administered at P10. Normal P10 Long-Evans rats were given one dose of talampanel (i.p.) at 7.5 mg/kg and were sacrificed 24 h after the injection, as described previously (Bittigau et al., 2002). Briefly, each brain was dissected, postfixed, cryoprotected in 20% sucrose overnight, and mounted onto a freezing microtome, and processed for in situ end-labeling as described previously. Counts (cells/12.5 mm2) were made by counting total number of ISEL- positive cells in coronal sections at −2.0 to −2.6 mm from Bregma (Sherwood & Timiras, 1970). Total section counts of talampanel versus vehicle injection were compared with Student’s t test.
Results
Talampanel suppresses hypoxia-induced seizures
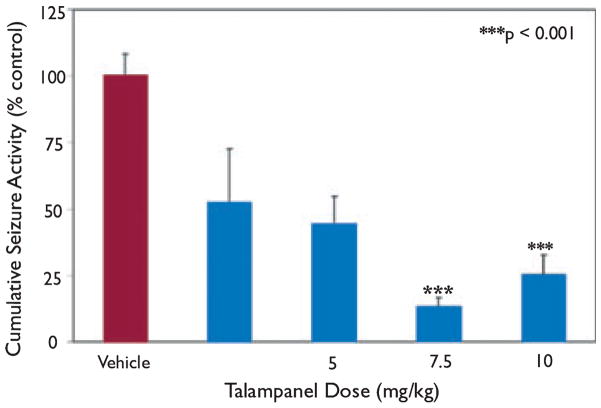
Acute hypoxia-induced seizures were suppressed by talampanel in a dose-related manner within the range of administration from 1–10 mg/kg. Similar to our previously published reports, vehicle-treated rats responded initially to hypoxia with myoclonic jerks, followed by the onset of tonic–clonic head and trunk movement (Jensen et al., 1991; Koh & Jensen, 2001; Koh et al., 2004). To determine treatment efficacy, we compared the number or episodes of tonic–clonic seizures between groups. Compared to vehicle-treated animals, the anticonvulsant activity of talampanel was maximal at 7.5 and 10 mg/kg, where seizures were blocked 74.6% at 10 mg/kg (25.4 ± 7.3, n = 17; p < 0.001) and 86.7% at 7.5 mg/kg (13.4 ± 3.2, n = 17; p < 0.001) (Fig. 1). The effect on time spent in tonic–clonic seizure activity was less at the lower doses of 1 mg/kg (52.6 ± 11.3, n = 7; p = 0.056) and 5 mg/kg (44.28 ± 10.4, n = 17; p = 0.002). There was no difference between groups in the number of myoclonic jerks exhibited during hypoxia. Using the data from all the doses, linear regression analysis of the percentage inhibition of tonic–clonic seizure activity yielded a median effective dose (ED50) of 0.57 mg/kg (SigmaPlot 9.0) (Fig. S1).
Figure 1.
Efficacy of talampanel at blocking acute hypoxia- induced seizures. P10 rat pups were exposed to global hypoxia and acute hypoxia-induced seizures were suppressed by talampanel pretreatment in a dose-dependent manner. Data represent mean number of seizures for talampanel pretreatment group compared to percentage of the mean number of seizures for the paired vehicle pretreatment group. Anticonvulsant activity was maximal at 7.5 and 10 mg/kg, where seizures were blocked 74.6% at 10 mg/kg [25.4 ± 7.3 (±SEM), n = 17; p < 0.001] and 86.7% at 7.5 mg/kg [13.3 ± 3.2 (±SEM), n = 17; p < 0.001].
Talampanel attenuates later-life seizure-induced neuronal injury following hypoxia-induced seizures
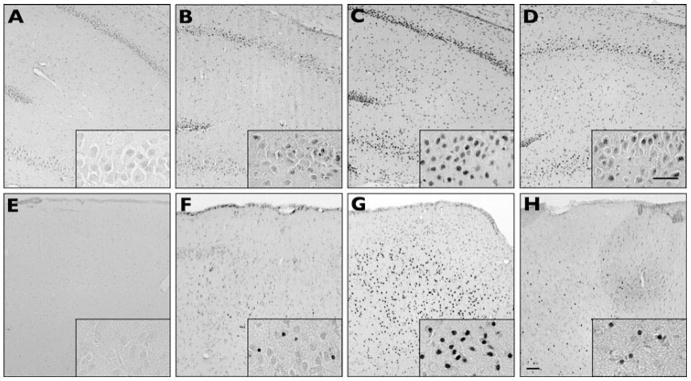
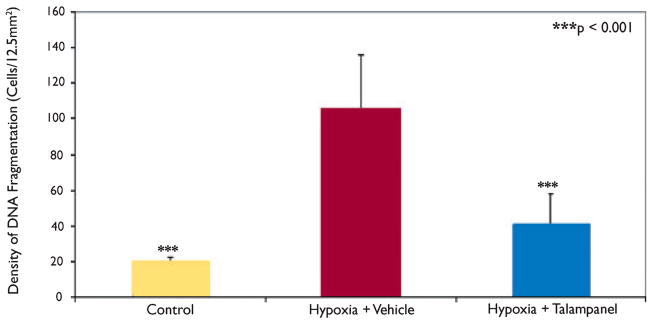
We compared differences in status-induced neuronal injury in rats at P30–31 with prior hypoxic seizures at P10 treated with vehicle or the optimal talampanel dose of 7.5 mg/kg, and naive P30–31 litter mates with no prior hypoxic seizures. Both groups of rats were treated with kainate (10 mg/kg, i.p.) at P30–31, and all animals reached grade IV status epilepticus. Mean latency to onset of seizures was 23.1 ± 2.2 min (±SEM) across all groups, without differences between treatment groups. However, there were differences in the extent of injury between the naive controls, vehicle-, and talampanel-treated hypoxic seizure rats when examined at 72 h after the “second-hit” kainate seizure. The total number of ISEL-positive cells in sections from stereotactically identical regions was compared between naive controls, vehicle-treated, and talampanel- (7.5 mg/kg) treated rats. Naive control litter mate rats showed cell death in hippocampal CA1 and CA3 regions (Figs 2A–D), as well as throughout basal amygdala (basolateral and basomedial amygdaloid nucleus) (Figs 2E–F). Rats pretreated with vehicle prior to hypoxia at P10 showed significantly more (4-fold) combined neuronal injury in amygdala, CA1, and CA3 following kainate-induced seizure at P30–31 (105.8 ± 30.4, n = 14) compared to naïve controls (20.1 ± 2.2 n = 13; p < 0.001) (Fig. 3). Talampanel treatment (7.5 mg/kg) prior to hypoxia at P10 resulted in significant attenuation of cell death in these regions (41.3 ± 16.8, n = 15; p < 0.001) compared to vehicle treatment, representing about a 60% decrease. Notably, the talampanel-treated animals had injury counts comparable to those of naive controls (Fig. 3).
Figure 2.
Talampanel protects against later increases in susceptibility to seizure-induced neuronal injury. At P30–31 (20 days after the P10 hypoxic seizures), kainate was administered i.p. (10 mg/kg) and rats were killed 48 h after seizure induction (P33–34). DNA fragmentation is shown in area CA1 and CA3 of hippocampus (A–D) and the basolateral amygdala (E–H). Naive controls (no hypoxia or kainate-induced seizure) show no appreciable staining in hippocampus (A) or amygdala (E). Control litter mates that did not undergo hypoxia at P10 but did undergo kainate- induced seizures on P30–P31 show less DNA fragmentation in hippocampus (B) and amygdala (F) compared to animals that underwent both hypoxia at P10 and kainate-induced seizures at P30–31 in hippocampus (C) and amygdala (G). Talampanel pretreatment before P10 hypoxia reduced later kainate-induced cell death in hippocampus (D) and amygdala (H). Box insets are higher-magnification views of CA1 hippocampal subfields (A–D) and basolateral amygdala (E–H). Scale bars = 50 μm.
Figure 3.
Quantification of cumulative ISEL-positive cells after kainate-induced seizures at P30–31. Rats pretreated with vehicle prior to hypoxia showed significantly more neuronal injury in CA1, CA3, amygdala, and bilateral cortex following kainate-induced seizures at P30–31 [105.75 ± 30.38 (±SEM), n = 14] than did normoxic controls [20.12 ± 2.19 (±SEM), n = 13; p < 0.001] or rats pretreated with talampanel (7.5 mg/kg) prior to hypoxia [41.31 ± 16.75 (±SEM), n = 15; p < 0.001] at P10 (p < 0.001, one-way ANOVA).
Assessment of cell death after talampanel administration to normal P10 rats
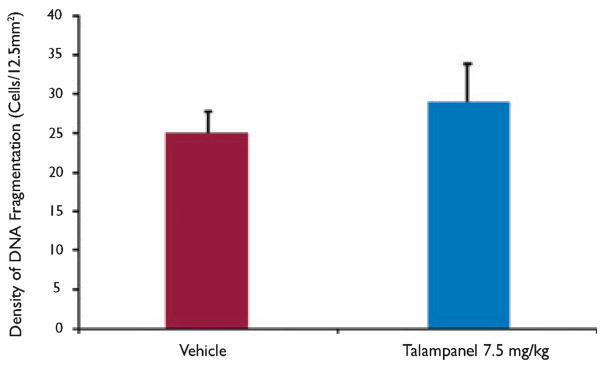
To determine whether the optimal dose of talampanel caused acute cell death at P10, normal P10 male Long- Evans rats were given one dose of talampanel (i.p.) or vehicle at 7.5 mg/kg and perfused 24 h later, at P11. Consistent with prior descriptions of constitutive apoptosis during development (Ikonomidou et al., 1999; Bittigau et al., 2002), there was some scattered baseline cell death in the vehicle-treated P11 rats upon inspection of three stereotactically chosen areas. Over the total coronal section in vehicle-treated pups, 24.9 ± 2.8 (±SEM) ISEL-positive cells were counted per 12.5 mm2 (n = 10 pups) (Fig. 4). No significant difference was observed in the ISEL- positive cell counts in the rodent brain sections following a single dose of talampanel (7.5 mg/kg) [28.8 ± 5.0 (±SEM), n = 10] (Fig. 4). These counts are similar to those reported in our other studies in control groups from animals of this age (Koh & Jensen, 2001).
Figure 4.
Protective doses of talampanel do not alter constitutive apoptosis in the developing rat brain. There was no difference in number of ISEL-positive cells after one dose of talampanel at 7.5 mg/kg compared with vehicle controls at P10. Quantification of ISEL-staining in total coronal section of P10 rats perfused 24 h after one dose of talampanel given at 7.5 mg/kg [28.830 ± 5.003 (±SEM), n = 10] versus vehicle [24.9 ± 2.826 (±SEM), n = 10].
Discussion
We and others have previously shown that AMPARs play a critical role in the induction and potential epileptogenic effects of neonatal seizures. Using our model of hypoxia-induced neonatal seizures in the P10 rat, we show here that the noncompetitive AMPAR antagonist talampanel (GYKI-53773) effectively suppresses seizures in a dose-dependent manner. In addition, early talampanel treatment also prevents the enhanced susceptibility to later-life seizure-induced neuronal injury that has been shown to occur following early life seizures. We also show that therapeutic doses of talampanel do not result in any increase in constitutive apoptosis at P10, consistent with other observations regarding the safety of AMPAR antagonists in the developing brain. Taken together, these findings suggest that the specific AMPAR antagonist talampanel may have potential for treatment of neonatal seizures.
The neonatal period is characterized by increased seizure susceptibility compared to later life. Multiple factors are likely to play a role in the enhanced excitability of the immature brain, including the increase in AMPAR expression (Sanchez et al., 2001; Talos et al., 2006a,b), N-methyl-D-aspartate receptor (NMDAR) expression, the presence of depolarizing γ-aminobutyric acid (GABA) receptors (Dzhala et al., 2005), and increased synaptogenesis (Takashima et al., 1980; Huttenlocher et al., 1982). In accordance with these recent findings, a number of studies have translated this information to therapeutic trials in models of early life seizures and hypoxic/ischemic injury. NMDAR antagonists have been shown to be protective in models of neonatal stroke (Chen et al., 1998; Wen et al., 2004; Vexler et al., 2006), but they do not show efficacy in neonatal seizures (Jensen et al., 1995). In addition, NMDAR antagonists have been reported to increase apoptosis and affect synaptic plasticity in the developing brain (Stafstrom et al., 1997; Ikonomidou et al., 1999), raising a concern for their clinical use in human term infants. As noted previously, GABA receptors are paradoxically depolarizing in early postnatal development, by virtue of high intracellular chloride concentrations compared to the adult (Dzhala et al., 2005; Ben Ari et al., 2007). The chloride importer NKCC1 is highly expressed in developing rat and human brain, making this another potential therapeutic target for treatment of neonatal seizures (Dzhala et al., 2005). Finally, AMPAR expression is increased in immature hippocampus (Sanchez et al., 2001) and cortex (Kumar et al., 2002; Talos et al., 2006a,b) in rodents and humans (Talos et al., 2006a,b). Accordingly, AMPAR antagonists have been shown to be effective in a variety of neonatal seizure and excitotoxicity models. In rodent models, AMPAR antagonists such as NBQX and topiramate have been shown to be effective in both the acute suppression of early life seizures (Jensen et al., 1995; Koh & Jensen, 2001; Cha et al., 2002; Koh et al., 2004) as well as preventing long-term epileptogenic sequelae (Cha et al., 2002; Koh et al., 2004; Suchomelova et al., 2006). AMPAR antagonists also have been demonstrated to be protective in models of hypoxia/ischemia in the developing brain (Follett et al., 2000, 2004; Liu et al., 2002; McCarran & Goldberg, 2007).
Despite preclinical data suggesting efficacy of AMPAR antagonists in a variety of models of epilepsy and excitotoxicity in the developing brain, no AMPAR antagonist is available for clinical study in human infants. Both NBQX and topiramate are not available for parenteral administration, which would be a necessity in this population of infants in intensive care with variable gastrointestinal absorption. Talampanel is a selective antagonist for AMPAR, acting in a noncompetitive manner at an allosteric site referred to as the GYKI receptor (Solyom & Tarnawa, 2002). Doses of talampanel in adult trials are in the range of 1–4 mg/kg day, and prior adult mouse models have shown anticonvulsant efficacy at 8–24 mg/kg and neuroprotection against hypoxic/ischemic injury at 12 mg/kg. In this study we report acute seizure suppression in the immature rat brain that is dose dependent, maximal at 7.5 mg/kg, with an ED50 of 0.57 mg/kg. These data are in a similar range to that of the 8.0 mg/kg, i.p., dose of talampanel reported to be neuroprotective in a P7 rat model of excitotoxic injury (Vilagi et al., 2002).
In addition to acute anticonvulsant efficacy, talampanel treatment prior to hypoxic seizures also attenuated the long-term increases in susceptibility to seizure-induced neuronal injury seen in this model. Previous data reveals that adult rats with prior hypoxic seizures show a 2- to 4-fold increase in neuronal injury compared to naive rats. Both NBQX and topiramate administered to rat pups in a pre- or post-treatment paradigm, result in an approximate 60% decrease in the amount of neuronal injury seen after kainate seizures in adulthood in this group of sensitized rats (Koh & Jensen, 2001; Koh et al., 2004). NBQX is an AMPAR and kainate receptor antagonist, with preferential action at AMPARs, and topiramate acts as an AMPAR antagonist but has other potential mechanisms of action, such as GABA agonism, and Ca2+ and Na+ channel block (Shank et al., 2000). Both NBQX and topiramate have been shown to be effective at blocking long-term effects of early life seizures (Jensen et al., 1995; Koh & Jensen, 2001; Cha et al., 2002; Koh et al., 2004). Talampanel is a selective AMPAR antagonist, and early prehypoxia treatment results in a quantitatively similar protective profile (approximately 60% reduction in status-induced cell loss) to these other agents, supporting a specific role for AMPARs in sensitizing neuronal populations to later seizure-induced injury. Given the similarities in the pretreatment results, these data suggest that postseizure treatment trials with talampanel are warranted in this model. Talampanel may not only be effective as an anticonvulsant but, like topiramate, might exhibit antiepileptogenic efficacy in the developing brain. Indeed, we have recently reported that talampanel treatment following hypoxia-induced seizures reverses seizure-induced increases in network and neuronal excitability in hippocampal CA1 neurons in P10 rats (Rakhade et al., 2008).
The pattern of injury following “second hit” seizures has previously been reported to include hippocampal regions (Koh et al., 1999, 2004; Koh & Jensen, 2001; Cha et al., 2002; Stafstrom & Sasaki-Adams, 2003). This susceptibility to injury has been suggested to reflect seizure- induced changes that result in long-term hyperexcitabilty of hippocampal networks. Electrophysiologic recordings of hippocampal neurons reveal both network hyperexcitability and a decrease in GABAergic inhibition on pyramidal neurons in area CA1 (Swann et al., 1989; Brooks-Kayal et al., 1998; Sanchez et al., 2001, 2005; Khazipov et al., 2004; Ben Ari et al., 2007; Rakhade et al., 2008). Here we show that talampanel suppression of acute seizure activity results in protection from these long-term increases seen in the fifth postnatal week of life. Another novel finding is that early life hypoxia-induced seizures alter long-term susceptibility to seizure-induced neuronal injury in the amygdala, and that talampanel also is protective against this long-term change. The observation that there is increased sensitivity to later seizure-induced injury in the amygdala is potentially relevant to clinical data suggesting that early life seizures and asphyxia may predispose to amygdala dysfunction. Early life injury to limbic structures such as the amygdala are thought to increase the risk of mental disorders such as autism and schizophrenia (Daenen et al., 2002; Diergaarde et al., 2004; Shaw et al., 2004).
Recent studies have cautioned against the use of anticonvulsant drugs and certain glutamate receptor antagonists in early postnatal life. NMDARs are essential for normal synaptogenesis and plasticity, and exposure to NMDAR antagonists during development results in increased constitutive apoptosis as well as later-life deficits in neurobehavior and learning (Tandon et al., 1996; Bittigau et al., 2002). A number of the conventional anticonvulsants have also been shown to affect later-life learning (Wirrell, 2005; Kim et al., 2007) and phenobarbital, carbamazepine, valproate, and diazepam have all been reported to increase neuronal apoptosis, even after one administration in early postnatal development in the rodent (Bittigau et al., 2002). Notably, the AMPAR antagonists NBQX and topiramate do not show this increased apoptosis. In addition, AMPAR antagonists appear to have a favorable safety profile in the developing brain, without inducing apoptosis (Bittigau et al., 2002) or affecting later-life learning and behavior (Zhao et al., 2005). Like- wise, we show here that talampanel treatment at P10 with the effective dose does not affect this normal developmental phenomenon of apoptosis. Future studies could address additional potential effects on behavior and learning in this model.
In summary, this study supports further investigation of talampanel in the prevention and possibly the long-term effects of neonatal seizures. Here we show that talampanel treatment appears to have similar efficacy to that previously demonstrated for NBQX and topiramate. In addition, talampanel does not alter the normal pattern of apoptosis present in early development. Another finding of note in this study is that early life hypoxia-induced seizures alter susceptibility of amygdala to later-life seizure- induced injury, and this susceptibility to neuronal injury is attenuated by talampanel. A parenteral formulation of talampanel would have excellent potential for translation to the clinic for the indication of neonatal seizures. Given the present data regarding efficacy and safety, additional preclinical trials are justified to evaluate the more clinically relevant postseizure treatment efficacy, as well as additional safety trials to assess more subtle adverse effects on neuromotor development.
Acknowledgments
This work was supported by NIH/NINDS RO1 NS31718 (FEJ) and a grant from the Epilepsy Therapy Development Project/Epilepsy Foundation (FEJ and MF), in addition to core support from the Mental Retardation Research Center Grant (NIH NICHHD P30 HD18655). We also acknowledge the generous gift of talampanel compound and product information from Teva Neuroscience. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Disclosures: Dr. Jensen received a grant from the Epilepsy Therapy Development Project and the Epilepsy Foundation for this work. Dr. Fetell is an employee of TEVA Neuroscience, and provided the compound to Dr. Jensen for study. P. Aujla has no conflicts to disclose.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Plot of mean effective dose for talampanel based on dose-response efficacy of hypoxia-induced seizure suppression at P10. Linear regression analysis of the percentage inhibition of tonic-clonic seizure activity for the doses tested yielded a median effective dose (ED) of 0.57 mg/kg (Sigmaplot 9.0).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Belayev L, Alonso OF, Liu Y, Chappell AS, Zhao W, Ginsberg MD, Busto R. Talampanel, a novel noncompetitive AMPA antagonist, is neuroprotective after traumatic brain injury in rats. J Neurotrauma. 2001;18:1031–1038. doi: 10.1089/08977150152693728. [DOI] [PubMed] [Google Scholar]
- Ben Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Bergamasco B, Penna P, Ferrero P, Gavinelli R. Neonatal hypoxia and epileptic risk: a clinical prospective study. Epilepsia. 1984;25:131–146. doi: 10.1111/j.1528-1157.1984.tb04168.x. [DOI] [PubMed] [Google Scholar]
- Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal A, Jin H, Price M, Dichter MA. Developmental expression of GABA(A) receptor subunit mRNAs in individual hippocampal neurons in vitro and in vivo. J Neurochem. 1998;70:1017–1028. doi: 10.1046/j.1471-4159.1998.70031017.x. [DOI] [PubMed] [Google Scholar]
- Cha BH, Silveira DC, Liu X, Hu Y, Holmes GL. Effect of topiramate following recurrent and prolonged seizures during early development. Epilepsy Res. 2002;51:217–232. doi: 10.1016/s0920-1211(02)00157-2. [DOI] [PubMed] [Google Scholar]
- Chappell AS, Sander JW, Brodie MJ, Chadwick D, Lledo A, Zhang D, Bjerke J, Kiesler GM, Arroyo S. A crossover, add-on trial of talampanel in patients with refractory partial seizures. Neurology. 2002;58:1680–1682. doi: 10.1212/wnl.58.11.1680. [DOI] [PubMed] [Google Scholar]
- Chen HS, Wang YF, Rayudu PV, Edgecomb P, Neill JC, Segal MM, Lipton SA, Jensen FE. Neuroprotective concentrations of the N-methyl-D-aspartate open-channel blocker memantine are effective without cytoplasmic vacuolation following post-ischemic administration and do not block maze learning or long-term potentiation. Neuroscience. 1998;86:1121–1132. doi: 10.1016/s0306-4522(98)00163-8. [DOI] [PubMed] [Google Scholar]
- Cowan LD. The epidemiology of the epilepsies in children. Ment Retard Dev Disabil Res Rev. 2002;8:171–181. doi: 10.1002/mrdd.10035. [DOI] [PubMed] [Google Scholar]
- Cowan F, Rutherford M, Groenendaal F, Eken P, Mercuri E, Bydder GM, Meiners LC, Dubowitz LM, de Vries LS. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361:736–742. doi: 10.1016/S0140-6736(03)12658-X. [DOI] [PubMed] [Google Scholar]
- Daenen EW, Wolterink G, Gerrits MA, van Ree JM. The effects of neonatal lesions in the amygdala or ventral hippocampus on social behaviour later in life. Behav Brain Res. 2002;136:571–582. doi: 10.1016/s0166-4328(02)00223-1. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Gerrits MA, Stuy A, Spruijt BM, van Ree JM. Neonatal amygdala lesions and juvenile isolation in the rat: differential effects on locomotor and social behavior later in life. Behav Neurosci. 2004;118:298–305. doi: 10.1037/0735-7044.118.2.298. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury in developing white matter. J Neurosci. 2000;20:9235–9241. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett PL, Deng W, Dai W, Talos DM, Massillon LJ, Rosenberg PA, Volpe JJ, Jensen FE. Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci. 2004;24:4412–4420. doi: 10.1523/JNEUROSCI.0477-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser WA, Kurland LT. The epidemiology of epilepsy in Rochester, Minnesota, 1935 through 1967. Epilepsia. 1975;16:1–66. doi: 10.1111/j.1528-1157.1975.tb04721.x. [DOI] [PubMed] [Google Scholar]
- Howes JF, Bell C. Talampanel. Neurotherapeutics. 2007;4:126–129. doi: 10.1016/j.nurt.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, deCourten C, Garey LJ, Van der Loos H. Synaptogenesis in human visual cortex – evidence for synapse elimination during normal development. Neurosci Lett. 1982;33:247. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova T, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Jakus R, Graf M, Ando RD, Balogh B, Gacsalyi I, Levay G, Kantor S, Bagdy G. Effect of two noncompetitive AMPA receptor antagonists GYKI 52466 and GYKI 53405 on vigilance, behavior and spike-wave discharges in a genetic rat model of absence epilepsy. Brain Res. 2004;1008:236–244. doi: 10.1016/j.brainres.2004.01.087. [DOI] [PubMed] [Google Scholar]
- Jensen FE, Applegate CD, Holtzman D, Belin T, Burchfiel J. Epileptogenic effect of hypoxia in the immature rodent brain. Ann Neurol. 1991;29:629–637. doi: 10.1002/ana.410290610. [DOI] [PubMed] [Google Scholar]
- Jensen FE, Alvarado S, Firkusny IR, Geary C. NBQX blocks the acute and late epileptogenic effects of perinatal hypoxia. Epilepsia. 1995;36:966–972. doi: 10.1111/j.1528-1157.1995.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur J Neurosci. 2004;19:590–600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kondratyev A, Tomita Y, Gale K. Neurodevelopmental impact of antiepileptic drugs and seizures in the immature brain. Epilepsia. 2007;48(Suppl 5):19–26. doi: 10.1111/j.1528-1167.2007.01285.x. [DOI] [PubMed] [Google Scholar]
- Koh S, Jensen FE. Topiramate blocks perinatal hypoxia-induced seizures in rat pups. Ann Neurol. 2001;50:366–372. doi: 10.1002/ana.1122. [DOI] [PubMed] [Google Scholar]
- Koh S, Storey TW, Santos TC, Mian AY, Cole AJ. Early-life seizures in rats increase susceptibility to seizure-induced brain injury in adulthood. Neurology. 1999;53:915–921. doi: 10.1212/wnl.53.5.915. [DOI] [PubMed] [Google Scholar]
- Koh S, Tibayan FD, Simpson J, Jensen FE. NBQX or topiramate treatment following perinatal hypoxia-induced seizures prevents later increases in seizure-induced neuronal injury. Epilepsia. 2004;45:569–575. doi: 10.1111/j.0013-9580.2004.69103.x. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Bacci A, Kharazia V, Huguenard JR. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J Neurosci. 2002;22:3005–3015. doi: 10.1523/JNEUROSCI.22-08-03005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan YM, Lucas R, Jewell H, Toublanc N, Schaefer H, Sander JW, Patsalos PN. Talampanel, a new antiepileptic drug: single- and multiple-dose pharmacokinetics and initial 1-week experience in patients with chronic intractable epilepsy. Epilepsia. 2003;44:46–53. doi: 10.1046/j.1528-1157.2003.128902.x. [DOI] [PubMed] [Google Scholar]
- Lanska MJ, Lanska DJ, Baumann RJ, Kryscio RJ. A population- based study of neonatal seizures in Fayette County, Kentucky. Neurology. 1995;45:724–732. doi: 10.1212/wnl.45.4.724. [DOI] [PubMed] [Google Scholar]
- Liu HN, Giasson BI, Mushynski WE, Almazan G. AMPA receptor-mediated toxicity in oligodendrocyte progenitors involves free radical generation and activation of JNK, calpain and caspase 3. J Neurochem. 2002;82:398–409. doi: 10.1046/j.1471-4159.2002.00981.x. [DOI] [PubMed] [Google Scholar]
- McCarran WJ, Goldberg MP. White matter axon vulnerability to AMPA/kainate receptor-mediated ischemic injury is developmentally regulated. J Neurosci. 2007;27:4220–4229. doi: 10.1523/JNEUROSCI.5542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, Glidden DV, Deming D, Partridge JC, Wu YW, Ashwal S, Ferriero DM. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146:453–460. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Painter MJ, Alvin J. Neonatal seizures. Curr Treat Options Neurol. 2001;3:237–248. doi: 10.1007/s11940-001-0005-x. [DOI] [PubMed] [Google Scholar]
- Painter MJ, Scher MS, Stein AD, Armatti S, Wang Z, Gardiner JC, Paneth N, Minnigh B, Alvin J. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med. 1999;341:485–489. doi: 10.1056/NEJM199908123410704. [DOI] [PubMed] [Google Scholar]
- Rakhade SN, Zhou C, Aujla PK, Fishman R, Sucher NJ, Jensen FE. Early alterations of AMPA receptors mediate synaptic potentiation induced by neonatal seizures. J Neurosci. 2008;28:7979–7990. doi: 10.1523/JNEUROSCI.1734-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. J Pediatr. 1999;134:71–75. doi: 10.1016/s0022-3476(99)70374-4. [DOI] [PubMed] [Google Scholar]
- Saliba RM, Annegers JF, Waller DK, Tyson JE, Mizrahi EM. Incidence of neonatal seizures in Harris County, Texas, 1992–1994. Am J Epidemiol. 1999;150:763–769. doi: 10.1093/oxfordjournals.aje.a010079. [DOI] [PubMed] [Google Scholar]
- Sanchez RM, Koh S, Rio C, Wang C, Lamperti ED, Sharma D, Corfas G, Jensen FE. Decreased glutamate receptor 2 expression and enhanced epileptogenesis in immature rat hippocampus after perinatal hypoxia-induced seizures. J Neurosci. 2001;21:8154–8163. doi: 10.1523/JNEUROSCI.21-20-08154.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez RM, Dai W, Levada RE, Lippman JJ, Jensen FE. AMPA/kainate receptor-mediated downregulation of GABAergic synaptic transmission by calcineurin after seizures in the developing rat brain. J Neurosci. 2005;25:3442–3451. doi: 10.1523/JNEUROSCI.0204-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar R, Painter MJ. Neonatal seizures: after all these years we still love what doesn’t work. Neurology. 2005;64:776–777. doi: 10.1212/01.WNL.0000157320.78071.6D. [DOI] [PubMed] [Google Scholar]
- Scher MS. Neonatal seizure classification: a fetal perspective concerning childhood epilepsy. Epilepsy Res. 2006;70(Suppl 1):S41–S57. doi: 10.1016/j.eplepsyres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Shank RP, Gardocki JF, Streeter AJ, Maryanoff BE. An overview of the preclinical aspects of topiramate: pharmacology, pharmacokinetics, and mechanism of action. Epilepsia. 2000;41(Suppl 1):S3–S9. [PubMed] [Google Scholar]
- Shaw P, Lawrence EJ, Radbourne C, Bramham J, Polkey CE, David AS. The impact of early and late damage to the human amygdala on ‘theory of mind’ reasoning. Brain. 2004;127:1535–1548. doi: 10.1093/brain/awh168. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Timiras PS. A stereotaxic atlas of the developing rat brain. University of California Press; Berkeley: 1970. [Google Scholar]
- Silverstein FS, Jensen FE. Neonatal seizures. Ann Neurol. 2007;62:112–120. doi: 10.1002/ana.21167. [DOI] [PubMed] [Google Scholar]
- Solyom S, Tarnawa I. Non-competitive AMPA antagonists of 2,3-benzodiazepine type. Curr Pharm Des. 2002;8:913–939. doi: 10.2174/1381612024607081. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Sasaki-Adams DM. NMDA-induced seizures in developing rats cause long-term learning impairment and increased seizure susceptibility. Epilepsy Res. 2003;53:129–137. doi: 10.1016/s0920-1211(02)00258-9. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Tandon P, Hori A, Liu Z, Mikati MA, Holmes GL. Acute effects of MK801 on kainic acid-induced seizures in neonatal rats. Epilepsy Res. 1997;26:335–344. doi: 10.1016/s0920-1211(96)00904-7. [DOI] [PubMed] [Google Scholar]
- Suchomelova L, Baldwin RA, Kubova H, Thompson KW, Sankar R, Wasterlain CG. Treatment of experimental status epilepticus in immature rats: dissociation between anticonvulsant and antiepileptogenic effects. Pediatr Res. 2006;59:237–243. doi: 10.1203/01.pdr.0000196333.16608.30. [DOI] [PubMed] [Google Scholar]
- Swann JW, Brady RJ, Martin DL. Postnatal development of GABA-mediated synaptic inhibition in rat hippocampus. Neuroscience. 1989;28:551–561. doi: 10.1016/0306-4522(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Takashima S, Chan F, Becker LE, Armstrong DL. Morphology of the developing visual cortex of the human infant: a quantitative and qualitative Golgi study. J Neuropathol Exp Neurol. 1980;39:487–501. doi: 10.1097/00005072-198007000-00007. [DOI] [PubMed] [Google Scholar]
- Talos DM, Fishman RE, Park H, Folkerth RD, Follett PL, Volpe JJ, Jensen FE. Developmental regulation of alpha-amino- 3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. I. Rodent cerebral white matter and cortex. J Comp Neurol. 2006a;497:42–60. doi: 10.1002/cne.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos DM, Follett PL, Folkerth RD, Fishman RE, Trachtenberg FL, Volpe JJ, Jensen FE. Developmental regulation of alpha- amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor sub- unit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. II. Human cerebral white matter and cortex. J Comp Neurol. 2006b;497:61–77. doi: 10.1002/cne.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon P, Liu Z, Stafstrom CE, Sarkisian M, Werner SJ, Mikati M, Yang Y, Holmes GL. Long-term effects of excitatory amino acid antagonists NBQX and MK-801 on the developing brain. Brain Res Dev Brain Res. 1996;95:256–262. doi: 10.1016/0165-3806(96)00094-6. [DOI] [PubMed] [Google Scholar]
- Vexler ZS, Sharp FR, Feuerstein GZ, Ashwal S, Thoresen M, Yager JY, Ferriero DM. Translational stroke research in the developing brain. Pediatr Neurol. 2006;34:459–463. doi: 10.1016/j.pediatrneurol.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Vilagi I, Takacs J, Gulyas-Kovacs A, Banczerowski-Pelyhe I, Tarnawa I. Protective effect of the antiepileptic drug candidate talampanel against AMPA-induced striatal neurotoxicity in neonatal rats. Brain Res Bull. 2002;59:35–40. doi: 10.1016/s0361-9230(02)00835-3. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurology of the newborn. Saunders; Philadelphia: 2001. [Google Scholar]
- Wen TC, Rogido M, Gressens P, Sola A. A reproducible experimental model of focal cerebral ischemia in the neonatal rat. Brain Res Brain Res Protoc. 2004;13:76–83. doi: 10.1016/j.brainresprot.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Wijsman JH, Jonker RR, Keijzer R, van de Velde CJ, Cornelisse CJ, van Dierendonck JH. A new method to detect apoptosis in paraffin section: in situ end-labeling of fragmented DNA. J Histochem Cytochem. 1993;41:7–12. doi: 10.1177/41.1.7678025. [DOI] [PubMed] [Google Scholar]
- Wirrell EC. Neonatal seizures: to treat or not to treat? Semin Pediatr Neurol. 2005;12:97–105. doi: 10.1016/j.spen.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Wirrell EC, Armstrong EA, Osman LD, Yager JY. Prolonged seizures exacerbate perinatal hypoxic-ischemic brain damage. Pediatr Res. 2001;50:445–454. doi: 10.1203/00006450-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Zhao C, Aviles C, Abel RA, Almli CR, McQuillen P, Pleasure SJ. Hippocampal and visuospatial learning defects in mice with a deletion of frizzled 9, a gene in the Williams syndrome deletion interval. Development. 2005;132:2917–2927. doi: 10.1242/dev.01871. [DOI] [PubMed] [Google Scholar]