Abstract
Bacterial vaginosis has been appreciated as a unique clinical entity for well over 50 years. Its essential manifestations are well established: a loss of the normal bacterial population of the vagina and their replacement by other species. Investigations into this condition have led to a better understanding of its prevalence and epidemiology. Microbiologic and biochemical studies have exposed the remarkably complex pathophysiologic events that occur with bacterial vaginosis. Several major morbidities accompany this condition. Advances have been made in treatment, including the recent availability of a new therapeutic agent, tinidazole. However, the root cause of the condition is elusive, and as a result managing bacterial vaginosis and its complications is unsatisfactory; moreover, data suggest that therapy now is less successful than in the past. This article brings together the current fund of knowledge about bacterial vaginosis in a way that offers clinicians a realistic view of our capabilities and concerns.
Key words: Bacterial vaginosis, Haemophilus vaginalis, Gardnerella vaginalis, Metronidazole, Tinidazole
The condition known in the 1950s as nonspecific vaginitis (NSV) was studied extensively by Gardner and Dukes.1 They described the associated discharge, pH increase, odor, and granular borders of the squamous cells (clue cells) found by microscopy in the vaginas of patients with this condition. They consistently identified a small, pleomorphic, Gram-negative bacillus in 311 patients with NSV, and in none of 78 normal controls. They named the organism Haemophilus vaginalis, and attributed NSV to its presence. Most importantly, they transmitted the condition to 11 of 15 volunteers via intravaginal inoculation of discharge from patients with NSV, and to only 1 of 13 via intravaginal inoculation of pure H. vaginalis cultures.1,2
In the decades that have followed their work, H vaginalis has been reassigned first to the genus Corynebacterium and then Gardnerella, NSV has been renamed bacterial vaginosis (BV), morbidities and pathophysiologic events associated with BV have been identified, and much has been learned about normal and abnormal vaginal flora. However, the cause of BV remains unknown, and treatments for BV, by all appearances, have become less effective. This article describes the knowledge we have gained relevant to BV, draws conclusions where plausible, and discusses current treatment options and expectations.
Vaginal Flora and Microenvironment
The vagina is a microbiologic battleground. As in all of nature, bacterial species use the weapons available to them to gain dominance and ensure their survival, and benefit or suffer from external influences that affect their environment. The healthy vaginal flora is dominated by Lactobacillus spp that produce hydrogen peroxide (Figure 1); this characteristic eliminates other bacteria unable to synthesize catalase, affording the lactobacilli a tremendous advantage. Hydrogen peroxide producers include L crispatus, L acidophilus, L rhamnosus, and others. Hydrogen peroxide-producing lactobacilli were found in 96% (20/21) of normal healthy vaginas and in only 6% (4/67) of patients with BV; non-hydrogen peroxide-producing lactobacilli were found in only 4% (1/21) of the normals and in 36% (22/67) of those with BV.3 Desirable vaginal lactobacilli are also powerful organic acid producers—providing the normal vaginal pH of < 4.7—using glycogen in the vaginal epithelium as the substrate. They also synthesize bacteriocins, proteins that inhibit other bacterial species. The power of these lactobacilli to dominate their environment is seen in a study in which exponentially growing Escherichia coli were incubated for 2 hours in vaginal fluid from healthy women and women with BV; the normal fluid caused a 100-fold decline in the E coli population, whereas the BV fluid allowed an almost 10-fold increase.4 Although other facultative and anaerobic bacteria, many of which are known pathogens, are always found in the healthy vagina, they are present only in low colony counts.
Figure 1.
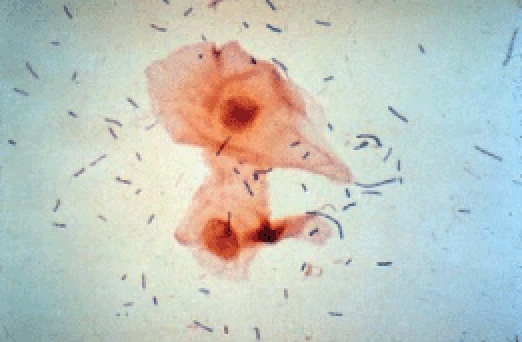
Gram stain of normal vaginal contents (original magnification, ×400). Note predominance of Lactobacillus species that produce hydrogen peroxide, organic acids, and bacteriocins that suppress growth of other species.
The advent of BV is marked by the disappearance of hydrogen peroxide-producing lactobacilli and by a massive growth of anaerobic species. It is not known which of these events occurs first. Is there a factor introduced that causes a die-off of the desirable lactobacilli and the anaerobes then passively occupy the vacant niche, or does an overwhelming influx of anaerobes eliminate the lactobacilli? This basic question about the pathogenesis of BV remains unanswered.
The search for a single organism to explain the pathogenesis of BV has been unrewarding. Although Gardnerella vaginalis is found in almost all women with BV, it is also present in 50% of healthy vaginal flora. Mobiluncus spp, a highly motile curved bacillus, is found only when BV is present, but in only 50% of cases of BV. Atopobium vaginae is a gram-positive anaerobe which, like G vaginalis, is found in the flora of over 95% of BV cases, but also occurs in the vagina of healthy women. Both Mobiluncus spp and A vaginae have high-level resistance to metronidazole, and have been implicated in treatment failures with this agent.5,6 Numerous other anaerobes, particularly Prevotella spp and various anaerobic streptococci, are common participants in BV flora. Perhaps the most enlightening work on BV organisms was provided by Fredricks and colleagues.7 Using nucleic acid amplification techniques for bacterial 16S rDNA, these investigators identified 9 to 17 anaerobic phylotypes (mean, 12.6) per vaginal sample from 27 women with BV, and 58% were novel (previously uncultivated) organisms in such unfamiliar genera as Megasphaera and Sneathia. Moreover, 3 newly recognized species in the order Clostridiales were the most specific for BV. L iners, a non-hydrogen peroxide producer, was found commonly in the BV flora. Using similar techniques, Ferris and colleagues5 concluded that each case of BV has its own unique set of anaerobic species. The role of Mycoplasma spp in BV remains unclear.
A number of biochemical and microenvironmental changes have also been described in BV.8–10 The normal vaginal epithelium is covered by a thin layer of mucin. In BV, this presumed protective layer is replaced by a G vaginalis-specific biofilm. β-defensin-1 and -2 mRNA and secretory leukocyte protease inhibitor concentrations are depleted in BV. Interleukins (IL) 1α, 1β, and 1-receptor agonist are increased, and levels of IL-8 (a primary leukotactic cytokine) are depressed. Increases in 70 kD heat-shock protein, lytic enzymes (sialidase, matrix-metalloproteinase 8, phospholipase A2), nitric oxide, and endotoxin are found in the vagina in BV. In aggregate, these events deprive the vagina of normal protective mechanisms and increase destructive and inflammatory influences.
Epidemiology and Clinical Features
BV is a remarkably prevalent condition, occurring in up to 30% of the population. In the evaluation of women aged 14 to 49 years in the 2001–2004 National Health and Nutrition Examination Survey, 29% were positive for BV and the prevalence was 3.13 times greater among African Americans than whites.11 Examination of 1938 young women entering the military revealed BV in 27%; the prevalence in sexually experienced participants was 28% compared with 18% in those who had never had sexual intercourse.12 This study also found a protective effect of oral contraceptive use on the development of BV. In another study, BV developed in 13% (16/120) of virginal adolescent girls over a period of 3 months.13 In a longitudinal, prospective study, Schwebke and Desmond14 followed 96 women without BV but at high risk for sexually transmitted infections (STIs). Of these women, 67 (70%) developed BV within a year. Univariate analysis revealed increased risk for BV from a new sexual partner (relative risk [RR] 1.13; 95% confidence interval [95% CI], 1.02–1.25), and frequency of vaginal intercourse (RR 1.07; 95% CI, 1.01–1.05); condom use was protective (RR 0.80; 95% CI, 0.67–0.98). In multivariate analysis, only sex with a new partner was a significant risk factor (RR 1.74; 95% CI, 1.05–2.87).14 Ness and coworkers15 followed 1193 women with vaginal wet smears every 6 to 12 months for a median of 3 years. They found that 20% of women who were free of BV at a visit were positive for BV at the next visit; multiple sexual partners and history of an STI or BV increased the risk for developing BV. Douching also increased risk for BV in another study of 1200 women (odds ratio [OR] 2.1; RR 1.9; 95% CI, 1.3–3.1) at high risk for STIs,16 but a prospective study of 48 women who historically douched found no difference in vaginal flora between those who stopped douching for 8 weeks and those who continued.17 Women who have sex with women have a high prevalence of BV.18 Yet another study confirmed the protection offered by condoms.19 Smoking, probably through its effect to suppress growth of hydrogen peroxide-producing lactobacilli, increases risk for BV.20
The epidemiology of BV suggests a sexually transmissible agent, but this does not explain the high prevalence of BV in sexually inactive women. Several studies have treated the male partners of women with BV with clindamycin and the nitroimidazole agents typically used for the treatment of BV. These studies all failed to demonstrate a decrease in recurrent BV among the women whose partners were treated.21–23 Thus, if BV is caused by a transmissible agent, it is unlikely to be a clindamycin- or nitroimidazole-susceptible anaerobe. The reason for apparently increased risk of BV among African American women and the protective effect offered by oral contraceptives remains unclear.
The classic symptom of BV is an odor that is usually described as “fishy.” This is caused by the production of amines (including trimethylamine, putrescine, and cadaverine) by the anaerobic bacteria. These amines volatilize increasingly with rising pH, so that patients often note a worsening of this symptom when vaginal alkalinity is enhanced, such as after sex (due to the presence of semen) and during menses (due to the presence of blood). Increased vaginal discharge is a more frequent but less specific symptom of BV. We found these symptoms in 73% and 92% of our symptomatic patients, respectively.24 Perhaps more important, 45% of our patients in this study complained of irritative symptoms (itching, burning, pain) that may have been confused with other causes of vaginitis if symptoms alone were used to guide diagnosis and treatment.24 The unreliability of symptoms for diagnosis was underscored by Klebanoff and colleagues,25 who found complaints of odor and discharge during the preceding 6 months in 58% of patients with BV and in 57% of those without BV. All patients with vaginal symptoms should be examined to confirm the diagnosis. Studies that have used routine screening to identify patients with BV have found that more than 50% of affected individuals are asymptomatic.26
Diagnosis
Over 50 years ago Gardner and Dukes1 described the clinical findings of BV in 1181 patients: (i) vaginal squamous cells with a granular appearance and indistinct borders, (ii) a “disagreeable” odor, (iii) an elevated pH of 5.0 to 5.5, and (iv) a thin, gray, adherent discharge. These findings were later refined, and are now known as the Amsel criteria. Amsel and colleagues26 recommended that a diagnosis of BV be made if 3 of the following 4 findings were present: (a) vaginal pH > 4.5, (b) a thin, homogeneous (“milk-like consistency”) discharge, independent of color and quantity, (c) accentuation of the fishy odor of the discharge with addition of 10% potassium hydroxide (alkalinization) (the whiff test), and (d) clue cells on microscopic examination of vaginal swabbing samples in saline. Subsequent work by Eschenbach and coworkers27 revealed that accuracy of these criteria could be enhanced if a vaginal pH of ≥ 4.7 were used in place of a vaginal pH > 4.5, and if >20% of the vaginal squamous cells were clue cells.
The relative value of each of these diagnostic criteria was examined by Eschenbach and colleagues.27 They found the elevated pH criterion to be the least specific and the whiff test to be least sensitive. Clue cells correlated best with Gram stain results (see below). Their final conclusion was that a vaginal pH ≥ 4.7 correlated best with all other diagnostic criteria. However, a word of caution about this measurement: cervical mucus, which is relatively alkaline, must be avoided in the sampling. A number of researchers have commented on the lack of interobserver reproducibility of the characterization of vaginal discharge, making it the weakest of the 4 criteria. It bears repeating that the Amsel criteria do not call for a greater than normal volume of discharge, only a thinning of the consistency. Finally, the identification of clue cells is best made by examining the edges of the cells. A normal squamous cell has sharp, clear, linear edges, whereas a clue cell has granular, cloudy, rough edges. Stippling over the cystoplasm of a squamous cell does not make it a clue cell (Figure 2). Thomason and colleagues28 studied 310 patients, and determined that the presence of clue cells was the most reliable of the criteria indicating a diagnosis of BV (sensitivity 98%, specificity 94.3%).
Figure 2.
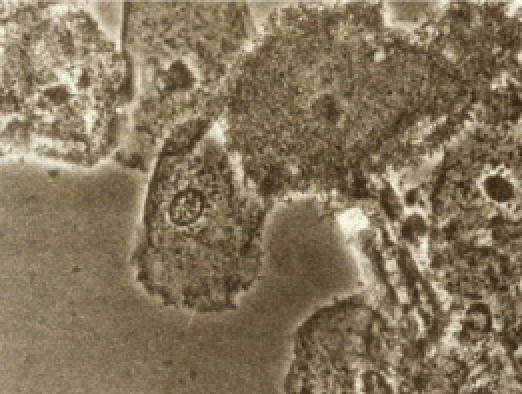
Clue cells in saline (original magnification, ×400). Note the rough, cloudy, irregular borders that define the clue cell in the third and fourth cells from left. The second cell from left has stippling over the cytoplasm, but edges are sharp and linear; this is not a clue cell.
In an effort both to objectivize the diagnosis of BV and to provide for an enduring sample that would be available for later evaluation, Spiegel and coworkers29 presented the use of Gram stain of vaginal swabbing. Their simple scheme of assessing whether the dominant/prevailing bacterial morphotype was long gram-positive bacilli or gram-variable cocci/coccobacilli effectively differentiated between patients without BV (the former) and those with BV (the latter). Nugent and colleagues30 formalized a Gram stain scoring system in which a greater density of Lactobacillus morphotypes lowers the score, a greater density of Gardnerella and Bacteroides morphotypes increases the score, and 1 or 2 points are added for Mobiluncus morphotypes. Scores of 0 to 3 are considered normal, 4 to 6 are intermediate, and 7 to 10 are BV (Table 1). The agreement of Gram stain score and diagnosis by the clinical criteria is imperfect. Gram stain is more sensitive, whereas the Amsel criteria are more specific. Overall concordance between them is 80% to 90%.
Table 1.
| Score | Lactobacillus Morphotypes | Gardnerella and Bacteroides spp Morphotypes | Curved Gram-Variable Rods |
| 0 | 4+ | 0 | 0 |
| 1 | 3+ | 1+ | 1+ or 2+ |
| 2 | 2+ | 2+ | 3+ or 4+ |
| 3 | 1+ | 3+ | |
| 4 | 0 | 4+ |
Morphotypes are scored as the average number seen per oil immersion field. Note that less weight is given to curved gram-variable rods. Total score = lactobacilli + G vaginalis and Bacteroides spp + curved rods.
Quantitation: O, No morphotypes present; 1+, <1 morphotype present; 2+, 1 to 4 morphotypes present; 3+, 5 to 30 morphotypes present; 4+, 30 or more morphotypes present.
Total Interpretation
>0–3 Normal
4–6 Intermediate
7–10 Bacterial Vaginosis
Papanicolaou test diagnosis of BV has a specificity of about 95%; however, the sensitivity may be as low as 50%. Thus, a Papanicolaou test consistent with BV is reliable evidence that BV is present, whereas absence of changes of BV on the Papanicolaou test does not rule out the diagnosis. A number of colorimetric card tests for BV have been developed, based on the presence of an elevated vaginal pH, enzymes, and/or amines associated with the condition. However, none are as accurate as the composite Amsel criteria, they bring additional expense, and they take away the comprehensive information gained by microscopic examination of vaginal swabbing (wet prep).
The criteria to use for the diagnosis of BV in routine clinical practice are often a matter of user preference. Gram stain scoring may be the most accurate approach, but requires a delay of 1 to 2 days to confirm the diagnosis. However, it is relatively easy to determine the dominant bacterial morphotype while examining the wet prep. In the experience of the author, the most practical approach to confirming the diagnosis of BV is to document the presence of 3 or more of the following 4 criteria: (i) >20% of squamous cells examined are clue cells, (ii) the bacterial population in the wet prep is dominated by cocci and coccobacilli, (iii) a vaginal pH ≥ 4.7, and (iv) a positive whiff test.
Morbidities Associated With BV
Symptoms of BV, as troubling as they are, are not the only concern for affected patients. Chief among the other concerns is enhanced susceptibility to other STIs. Studies have found a significant association between BV and human immunodeficiency virus (HIV) infection.31–33 In a prospective study of Kenyan sex workers, the absence of lactobacilli in vaginal cultures was associated with a 2.0-fold increase in HIV acquisition and a 1.7-fold greater risk of developing gonorrhea.34 Another similar study found that BV was associated with a 3.7-fold increase in HIV acquisition over a 2.5-year period.35 Further, women with HIV shed more virus into the cervicovaginal discharge when BV is present.36 Women with BV are also more likely to acquire trichomoniasis and chlamydial cervicitis.37 Another prospective study found that BV conferred a 2.1-fold increased susceptibility to herpes simplex II infection over a 1-year period.38 Schwebke and Desmond39 randomized BV patients to twice-weekly metronidazole gel or observation only (no treatment), and thereafter screened them monthly for STIs for 6 months. They found that suppression of BV with metronidazole prophylaxis did offer protection against Chlamydia infection, but not against other STIs.
A second complication of BV appears to be an enhanced risk of infection after pelvic surgery. Cuff cellulitis following abdominal hysterectomy occurred 3.2 times more often in women with BV in a study of 161 women40; and in 35% (7/20) of those with preoperative BV and 8% (4/50) (P < .1) of those without BV in another study.41 Among 174 women with BV treated preoperatively with metronidazole or placebo for 10 days, pelvic inflammatory disease (PID) after first-trimester pregnancy termination by dilation and curettage procedure (D&C) occurred in 3 patients (3.8%) in the metronidazole group and in 11 (12.2%) in the placebo group (P < .05).42 Another study of postabortal endometritis in 429 women found that preoperative clue cells in the vaginal wet prep was associated with a 5.6-fold (95% CI, 1.8–17.2) increased risk of infection.43 Postoperative fever following major gynecologic surgery was seen in 36% of women with preoperative Gram stain score of 7 to 10, 12% who scored 4 to 6, and 20% of those scoring 0 to 3 (P < .017).44
BV prior to cesarean delivery has been associated with a 6-fold increase in postpartum endometritis,45 and a study has found that placement of a single intravaginal dose of metronidazole prior to cesarean delivery significantly reduces the risk of postcesarean endometritis among women with or without BV (RR 0.42; 95% CI, 0.19–0.92).46
BV probably increases risk for community-acquired PID47–49 and plasma cell (chronic) endometritis,50 and may be causative in some cases of abnormal uterine bleeding.51 BV has also been associated with decreased success of in vitro fertilization procedures,52 and increased risk of cystitis.53
Many studies have found an association between BV during pregnancy and all major adverse pregnancy outcomes (preterm labor, preterm delivery, low birth weight, premature rupture of membranes, postpartum metritis, intra-amniotic infection).54,55 Unfortunately, treatment of BV during pregnancy has been highly inconsistent in prevention of these adverse outcomes.56,57 The management of BV in pregnancy remains an elusive and critically important issue for further investigation, and is likely to be clarified only when a better understanding of the pathogenesis of this condition is reached. Investigation of the potential for genetic influences on the complications of BV has yielded interesting results. For example, Macones and colleagues58 found that the rarer of 2 alleles of a polymorphism in the promoter of the tumor necrosis factor α gene was associated with an increased risk of preterm birth (OR 2.7; 95% CI, 1.7–4.5); in women with this allele and BV the risk of preterm birth was much higher (OR 6.1; 95% CI, 1.9–21.0). It is difficult at present to make recommendations about the management of BV during pregnancy, except to say that women may be safely treated for symptoms with metronidazole at any point during pregnancy.59
Treatment
The understanding that the primary manifestation of BV was an overwhelming overgrowth of anaerobes, rather than Gardnerella alone, provided Pheifer and colleagues60 the opportunity to usher in the modern age of treatment for BV. They showed that the agents used for BV (NSV)—oral doxycycline and ampicillin, and intravaginal sulfonamide—were ineffective, and that 500 mg of oral metronidazole twice daily for 7 days gave an immediate cure rate of 99% (80/81) and persistent cure at 6 weeks of 84% (48/57). Comparable efficacy was shown with oral clindamycin.61 Preparations of intravaginal metronidazole and clindamycin were studied in the 1990s by several authors, and cure rates of 80% to 90% were reported both immediately and 1 month after therapy.62–65
An earmark of BV treatment trials (and all BV studies, for that matter) is the remarkable inconsistency in defining both diagnosis and cure of the disease. Integration of Gram stain into the diagnostic scheme has been tentative—understandably so because its concordance with the clinical criteria is imperfect—and it is not routinely used to make the diagnosis in clinical practice. Some studies used Gram stain as their sole diagnostic test, and others used it for quality control of diagnosis by clinical criteria. Studies employing the clinical criteria used anywhere from 1 to 4 of these diagnostic approaches. Into the 1990s, papers appeared that defined BV as a positive culture for G vaginalis from the vagina. Other studies implicated associations of BV based upon presence or absence of hydrogen peroxide producing lactobacilli or of certain anaerobic organisms. Thus, interpretation of the literature on BV requires some degree of subjectivity based upon the definition used for BV in a given study. Obviously, this is a suboptimal milieu for scientific endeavor.
In 1998, the US Food and Drug Administration (FDA) issued a guidance document entitled Bacterial Vaginosis—Developing Antimicrobial Drugs for Treatment,66 which requires that patients entering studies evaluating drug therapy of BV have a Gram stain score ≥4, clue cells >20% of squamous cells on the wet prep, a vaginal pH ≥ 4.7, a positive whiff test, and a thin homogeneous discharge. Moreover, evaluation of the efficacy of the drug must occur from 21 to 30 days after the first day of treatment, and cure should be defined as a Gram stain score of < 4 and normalization of all 4 of the clinical criteria. Although this document provides much needed standardization, it must be realized that the standard it sets is markedly more stringent and rigorous than has ever been used in past BV studies. Owing to the emphasis of these standards on specificity, some patients with BV will not qualify for therapeutic trials and cure rates from trials using these criteria will not be comparable, even approximately, to those from past trials. For example, in our recent trial of tinidazole for treatment of BV, we found our cure rate based on the FDA guidance document to be 37%, and based on the more traditional normalization of 3 of the 4 clinical criteria to be 57%.67 Among the few other studies that have used the FDA standards, cure rates of 30% to 33% (2% clindamycin vaginal cream, 5 g as a single dose),68 37% (2% clindamycin vaginal cream, 5 g once daily for 5 days),68 35% (500 mg metronidazole + 105 U nystatin in gel, once daily for 5 days),69 and 26% (0.75% metronidazole gel, 5 g once daily for 5 days)69 have been reported. Moreover, as noted above, even application of the older standards for cure does not bring the recent rates into the realm of those from past studies (accepting the inherent inadequacies of comparing results from different studies). BV has thus become a more challenging condition to treat successfully.
Current investigations have shed light on more detailed aspects of treatment of BV. A concern with treatment of BV has always been the dual goals of eradicating anaerobes and providing for regrowth of hydrogen peroxide-producing lactobacilli. Nyirjesy and coworkers70 have shown that regrowth of these lactobacilli is equally likely following topical treatment with either clindamycin or metronidazole, and occurs in about 40% of patients. Although none of the currently available over-thecounter probiotic lactobacillus preparations are known to contain the specialized organisms that dominate the healthy vagina, promising investigations are under way to isolate, store, and deliver them to patients in an effort to enhance the success of therapy. These efforts have been hindered by nuance involving both regrowth and establishment of dominance by these organisms (such as L crispatus, L rhamnosus, and L reuteri). Treatment of BV with topical acidifying agents has been extensively studied; results are highly variable, and this modality is not thought to hold great promise at present.
Mobiluncus spp are known to be present in the flora in about 50% of cases of BV. These comma-shaped organisms are readily identifiable in a wet prep because of their tendency to spin rapidly around their sagittal axis. They are known to be metronidazole resistant. Nyirjesy and colleagues have shown a better (45%) cure rate with topical clindamycin in 55 patients with Mobiluncus-positive BV compared with topical metronidazole (20%).6 A vaginae is also known to be metronidazole resistant. Austin and colleagues71 studied emergence of resistance in species known to be very commonly involved in the BV flora. Among Prevotella bivia and black-pigmented Prevotella spp, they found essentially no development of resistance during topical metronidazole treatment. However, 7 to 12 days after clindamycin treatment, 51% to 68% of strains were resistant to clindamycin. Counterintuitively, cure rates in these patients were no different at 7 to 12 days, 45 days, or 70 to 90 days after treatment with the 2 agents.
Tinidazole is the first new agent to be approved for treatment of BV in almost 20 years. This second-generation nitroimidazole is also approved for treatment of trichomoniasis, making it the only oral agent approved for both. It has a twice-longer serum half-life than metronidazole, and side effects have been reported at half the frequency as for metronidazole.72,73 Although there have been 21 published clinical trials showing efficacy of tinidazole in treatment of BV, a recent prospective, randomized, double-blind, placebo-controlled US study carried out in 10 geographically diverse centers is notable.67 It is one of the few trials that based study design on the stringent FDA guidelines for conducting therapeutic trials in BV. Two hundred and thirty-five nonpregnant, adult patients with BV were enrolled, with exclusion criteria appropriate to minimize known confounders, and assigned to receive 1 g oral tinidazole once daily for 5 days, 2 g tinidazole once daily for 2 days, or placebo. Compliance was good across the 3 treatment arms (93%, 89%, and 89%, respectively). Cure rates by the FDA guidelines were 37% (28/76), 27% (20/73), and 5% (4/78) (P < .001 for both tinidazole regimens vs placebo) for the 3 treatments, respectively. Adverse events occurred with comparable frequency among tinidazole and placebo recipients, except dysgeusia (altered taste), which occurred significantly more commonly in both tinidazole groups, and nausea, which was more common in the 2 g daily tinidazole group. Overall gastrointestinal side effects were comparable between the tinidazole and placebo groups. Posttreatment vaginal candidiasis occurred at similar rates among the 3 groups. The safety of tinidazole was assured by monitoring vital signs, blood studies, and physical examinations during participation. Tinidazole thus offers a well-tolerated, highly competitive new option for treatment of BV, while requiring less than half as many doses as the currently recommended oral metronidazole regimen.74
Another consideration is that of whom to treat. Few if any clinicians screen every patient for BV; thus the majority of those with asymptomatic BV go undiagnosed and untreated. The existing data are compelling that untreated BV at the time of hysterectomy, surgical pregnancy termination, and cesarean delivery increases risk for pelvic infection after these procedures, although some would argue that the number of these patients studied is not yet adequate to confirm this association. The US Centers for Disease Control and Prevention (CDC) states that an established benefit of therapy for BV is to reduce the risk of infectious complications after abortion or hysterectomy.74 Thus, it seems highly prudent to include testing for BV in the preoperative evaluation of all patients prior to these procedures, and preoperative treatment for those who test positive. If the preoperative interval is very short, a single dose of intravaginal metronidazole has documented efficacy in the setting of cesarean delivery. Because of the consistency of the data showing increased susceptibility to HIV infection and other STIs, it also seems wise to recommend screening of women at risk for STIs for BV, and treatment of those who are positive. CDC describes reduction of risk for STIs as a potential benefit of treatment for BV.74 Women who are symptomatic should be tested for BV and treated if positive.
A final issue is the management of the many patients who fail standard treatment for BV and those who have multiple episodes of recurrent BV. The face value of recent therapeutic trials is that two thirds of BV patients fall into these categories. If the prevalence of BV is indeed close to 30% in the US adult population, then a staggering 20 million women or more fall into these categories. There is currently no recommended therapy. Sobel and colleagues75 gave suppressive 0.75% metronidazole gel versus placebo twice weekly for 16 weeks to 112 women with recurrent BV. In the metronidazole recipients, recurrent BV developed in 26% during therapy and in 59% of those receiving placebo. They conducted a 12-week observation period following the treatment interval, and recurrences for the entire 28 weeks of the study were 51% and 75% for the 2 arms, respectively. It is the undocumented practice of this author to treat women with symptomatic recurrent BV with once-weekly metronidazole, either orally or intravaginally, in 3-month cycles, beginning a new cycle with each postsuppression recurrence. Tinidazole is also an appropriate agent for this application.
Summary
BV is a common condition with an unknown cause. It is associated with substantial morbidities in certain patient populations, including major postoperative infection, enhanced susceptibility to STIs, and adverse pregnancy outcomes. Its symptoms can be devastating to many affected women. Despite the recent availability of a new, effective, and well-tolerated treatment agent, therapeutic success for BV remains inadequate with agents across the board and appears to be progressively declining. We have no scientific direction to guide treatment of millions of women with recurrent BV. The need for a clear understanding of the pathogenesis of BV is acute.
Main Points.
The advent of bacterial vaginosis (BV) is marked by the disappearance of hydrogen peroxide-producing lactobacilli and by a massive growth of anaerobic species. The search for a single organism to explain the pathogenesis of BV has been unrewarding.
BV is a remarkably prevalent condition, occurring in up to 30% of the population.
BV probably increases risk for community-acquired pelvic inflammatory disease and plasma cell (chronic) endometritis, and may be causative in some cases of abnormal uterine bleeding. BV has also been associated with decreased success of in vitro fertilization procedures and increased risk of cystitis.
The most practical approach to confirming the diagnosis of BV is to document the presence of 3 or more of the following 4 criteria: (i) > 20% of squamous cells examined are clue cells, (ii) the bacterial population in the wet prep is dominated by cocci and coccobacilli, (iii) a vaginal pH ≥ 4.7, and (iv) a positive whiff test.
Tinidazole is the first new agent to be approved for treatment of BV in almost 20 years. This second-generation nitroimidazole is also approved for treatment of trichomoniasis, making it the only oral agent approved for both. It has a twice-longer serum half-life than metronidazole, and side effects have been reported at half the frequency as for metronidazole.
Footnotes
Dr. Livengood is a consultant for Mission Pharmaceutical.
References
- 1.Gardner HL, Dukes CD. Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified “nonspecific” vaginitis. Am J Obstet Gynecol. 1955;69:962. [PubMed] [Google Scholar]
- 2.Criswell BS, Ludwig CL, Gardner HL, et al. Vaginitis by inoculation from culture. Obstet Gynecol. 1969;33:195. [PubMed] [Google Scholar]
- 3.Eschenbach DA, Davick PR, Williams BL, et al. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 1989;27:251–256. doi: 10.1128/jcm.27.2.251-256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valore EV, Wiley DJ, Ganz T. Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infect Immun. 2006;74:5693–5702. doi: 10.1128/IAI.00524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferris MJ, Norori J, Zozaya-Hinchliffe M, Martin DH. Cultivation-independent analysis of changes in bacterial vaginosis flora following metronidazole treatment. J Clin Microbiol. 2007;45:1016–1018. doi: 10.1128/JCM.02085-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyirjesy P, McIntosh MJ, Steinmetz JI, et al. The effects of intravaginal clindamycin and metronidazole therapy on vaginal mobiluncus morphotypes in patients with bacterial vaginosis. Sex Transm Dis. 2007;34:197–202. doi: 10.1097/01.olq.0000235152.98601.d7. [DOI] [PubMed] [Google Scholar]
- 7.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 8.Swidsinski A, Mendling W, Loening-Baucke V, et al. Adherent biofilms in bacterial vaginosis. Obstet Gynecol. 2005;106:1013–1023. doi: 10.1097/01.AOG.0000183594.45524.d2. [DOI] [PubMed] [Google Scholar]
- 9.Genç MR, Karasşahin E, Onderdonk AB, et al. Association between vaginal 70-kD heat shock protein, interleukin-1 receptor agonist, and microbial flora in mid trimester pregnant women. Am J Obstet Gynecol. 2005;192:916–921. doi: 10.1016/j.ajog.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Cauci S, Guaschino S, De Aloysio D, et al. Interrelationships of interleukin-8 with interleukin-1beta and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol Hum Reprod. 2003;9:53–58. doi: 10.1093/molehr/gag003. [DOI] [PubMed] [Google Scholar]
- 11.Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol. 2007;109:114–120. doi: 10.1097/01.AOG.0000247627.84791.91. [DOI] [PubMed] [Google Scholar]
- 12.Yen S, Shafer MA, Moncada J, et al. Bacterial vaginosis in sexually experienced and non-sexually experienced young women entering the military. Obstet Gynecol. 2003;102:927–933. doi: 10.1016/s0029-7844(03)00858-5. [DOI] [PubMed] [Google Scholar]
- 13.Bump RC, Buesching WJ., 3rd Bacterial vaginosis in virginal and sexually active adolescent females: evidence against exclusive sexual transmission. Am J Obstet Gynecol. 1988;158:935–939. doi: 10.1016/0002-9378(88)90097-x. [DOI] [PubMed] [Google Scholar]
- 14.Schwebke JR, Desmond R. Risk factors for bacterial vaginosis in women at high risk for sexually transmitted diseases. Sex Transm Dis. 2005;32:654–658. doi: 10.1097/01.olq.0000175396.10304.62. [DOI] [PubMed] [Google Scholar]
- 15.Ness RB, Kip KE, Soper DE, et al. Variability of bacterial vaginosis over 6- to 12-month intervals. Sex Transm Dis. 2006;33:381–385. doi: 10.1097/01.olq.0000204748.89222.33. [DOI] [PubMed] [Google Scholar]
- 16.Ness RB, Hillier SL, Richter HE, et al. Douching in relation to bacterial vaginosis, lactobacilli, and facultative bacteria in the vagina. Obstet Gynecol. 2002;100:765. doi: 10.1016/s0029-7844(02)02184-1. [DOI] [PubMed] [Google Scholar]
- 17.Klebanoff MA, Andrews WW, Yu KF, et al. A pilot study of vaginal flora changes with randomization to cessation of douching. Sex Transm Dis. 2006;33:610–613. doi: 10.1097/01.olq.0000216050.41305.c1. [DOI] [PubMed] [Google Scholar]
- 18.Marrazzo JM, Koutsky LA, Eschenbach DA, et al. Characterization of vaginal flora and bacterial vaginosis in women who have sex with women. J Infect Dis. 2002;185:1307–1313. doi: 10.1086/339884. [DOI] [PubMed] [Google Scholar]
- 19.Baeten JM, Nyange PM, Richardson BA, et al. Hormonal contraception and risk for sexually transmitted disease acquisition: results from a prospective study. Am J Obstet Gynecol. 2001;185:380–385. doi: 10.1067/mob.2001.115862. [DOI] [PubMed] [Google Scholar]
- 20.Mijac VD, Dukić SV, Opavski NZ, et al. Hydrogen peroxide producing lactobacilli in women with vaginal infections. Eur J Obstet Gynecol Reprod Biol. 2006;129:69–76. doi: 10.1016/j.ejogrb.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 21.Colli E, Landoni M, Parazzini F, et al. Treatment of male partners and recurrence of bacterial vaginosis: a randomised trial. Genitourin Med. 1997;73:267–270. doi: 10.1136/sti.73.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vejtorp M, Bollerup AC, Vejtorp L, et al. Bacterial vaginosis: a double-blind randomized trial of the effect of treatment of the sexual partner. Br J Obstet Gynaecol. 1988;95:920–926. doi: 10.1111/j.1471-0528.1988.tb06581.x. [DOI] [PubMed] [Google Scholar]
- 23.Vutyavanich T, Pongsuthirak P, Vannareumol P, et al. A randomized double-blind trial of tinidazole treatment of the sexual partners of females with bacterial vaginosis. Obstet Gynecol. 1993;82:550–554. [PubMed] [Google Scholar]
- 24.Livengood CH , 3rd, Thomason JL, Hill GB. Bacterial vaginosis: diagnostic and pathogenetic findings during topical clindamycin therapy. Am J Obstet Gynecol. 1990;163:515–520. doi: 10.1016/0002-9378(90)91187-h. [DOI] [PubMed] [Google Scholar]
- 25.Klebanoff MA, Schwebke JR, Zhang J, et al. Vulvovaginal symptoms in women with bacterial vaginosis. Obstet Gynecol. 2004;104:267–272. doi: 10.1097/01.AOG.0000134783.98382.b0. [DOI] [PubMed] [Google Scholar]
- 26.Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 27.Eschenbach DA, Hillier S, Critchlow C, et al. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol. 1988;158:819–828. doi: 10.1016/0002-9378(88)90078-6. [DOI] [PubMed] [Google Scholar]
- 28.Thomason JL, Gelbart SM, Anderson RJ, et al. Statistical evaluation of diagnostic criteria for bacterial vaginosis. Am J Obstet Gynecol. 1990;162:155–160. doi: 10.1016/0002-9378(90)90839-y. [DOI] [PubMed] [Google Scholar]
- 29.Spiegel CA, Amsel R, Holmes KK. Diagnosis of bacterial vaginosis by direct gram stain of vaginal fluid. J Clin Microbiol. 1983;18:170–177. doi: 10.1128/jcm.18.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen CR, Duerr A, Pruithithada N, et al. Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand. AIDS. 1995;9:1093–1097. doi: 10.1097/00002030-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Sewankambo N, Gray RH, Wawer MJ, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–550. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 33.Royce R, Thorp J, Granados A, et al. Bacterial vaginosis associated with HIV infection in pregnant women from North Carolina. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:382–386. doi: 10.1097/00042560-199904010-00009. [DOI] [PubMed] [Google Scholar]
- 34.Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 35.Taha TE, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12:1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Cu-Uvin S, Hogan JW, Caliendo AM, et al. Association between bacterial vaginosis and expression of human immunodeficiency virus type 1 RNA in the female genital tract. Clin Infect Dis. 2001;33:894–896. doi: 10.1086/322613. [DOI] [PubMed] [Google Scholar]
- 37.Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663–668. doi: 10.1086/367658. [DOI] [PubMed] [Google Scholar]
- 38.Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319–325. doi: 10.1086/375819. [DOI] [PubMed] [Google Scholar]
- 39.Schwebke JR, Desmond R. A randomized trial of metronidazole in asymptomatic bacterial vaginosis to prevent the acquisition of sexually transmitted diseases. Am J Obstet Gynecol. 2007;196:517.e1–517.e6. doi: 10.1016/j.ajog.2007.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1016–1021. doi: 10.1016/0002-9378(90)91115-s. discussion 1021–1023. [DOI] [PubMed] [Google Scholar]
- 41.Larsson PG, Platz-Christensen JJ, Forsum U, Påhlson. Clue cells in predicting infections after abdominal hysterectomy. Obstet Gynecol. 1991;77:450–452. [PubMed] [Google Scholar]
- 42.Larsson PG, Platz-Christensen JJ, Thejls H, et al. Incidence of pelvic inflammatory disease after first-trimester legal abortion in women with bacterial vaginosis after treatment with metronidazole: a double-blind, randomized study. Am J Obstet Gynecol. 1992;166:100–103. doi: 10.1016/0002-9378(92)91838-2. [DOI] [PubMed] [Google Scholar]
- 43.Hamark B, Forssman L. Postabortal endometritis in chlamydia-negative women—association with preoperative clinical signs of infection. Gynecol Obstet Invest. 1991;31:102–105. doi: 10.1159/000293111. [DOI] [PubMed] [Google Scholar]
- 44.Lin L, Song J, Kimber N, et al. The role of bacterial vaginosis in infection after major gynecologic surgery. Infect Dis Obstet Gynecol. 1999;7:169–174. doi: 10.1002/(SICI)1098-0997(1999)7:3<169::AID-IDOG10>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. Bacterial vaginosis as a risk factor for post-cesarean endometritis. Obstet Gynecol. 1990;75:52–58. [PubMed] [Google Scholar]
- 46.Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98:745–750. doi: 10.1016/s0029-7844(01)01517-4. [DOI] [PubMed] [Google Scholar]
- 47.Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol. 2005;162:585–590. doi: 10.1093/aje/kwi243. [DOI] [PubMed] [Google Scholar]
- 48.Peipert JF, Montagno AB, Cooper AS, Sung CJ. Bacterial vaginosis as a risk factor for upper genital tract infection. Am J Obstet Gynecol. 1997;177:1184–1187. doi: 10.1016/s0002-9378(97)70038-3. [DOI] [PubMed] [Google Scholar]
- 49.Haggerty CL, Hillier SL, Bass DC, Ness RB PID Evaluation and Clinical Health study investigators, authors. Bacterial vaginosis and anaerobic bacteria are associated with endometritis. Clin Infect Dis. 2004;39:990–995. doi: 10.1086/423963. [DOI] [PubMed] [Google Scholar]
- 50.Korn AP, Bolan G, Padian N, et al. Plasma cell endometritis in women with symptomatic bacterial vaginosis. Obstet Gynecol. 1995;85:387–390. doi: 10.1016/0029-7844(94)00400-8. [DOI] [PubMed] [Google Scholar]
- 51.Larsson PG, Bergman B, Forsum U, Påhlson C. Treatment of bacterial vaginosis in women with vaginal bleeding complications or discharge and harboring Mobiluncus. Gynecol Obstet Invest. 1990;29:296–300. doi: 10.1159/000293339. [DOI] [PubMed] [Google Scholar]
- 52.Eckert LO, Moore DE, Patton DL, et al. Relationship of vaginal bacteria and inflammation with conception and early pregnancy loss following in-vitro fertilization. Infect Dis Obstet Gynecol. 2003;11:11–17. doi: 10.1155/S1064744903000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harmanli OH, Cheng GY, Nyirjesy P, et al. Urinary tract infections in women with bacterial vaginosis. Obstet Gynecol. 2000;95:710–712. doi: 10.1016/s0029-7844(99)00632-8. [DOI] [PubMed] [Google Scholar]
- 54.Kurki T, Sivonen A, Renkonen OV, et al. Bacterial vaginosis in early pregnancy and pregnancy outcome. Obstet Gynecol. 1992;80:173–177. [PubMed] [Google Scholar]
- 55.Gravett MG, Nelson HP, DeRouen T, et al. Independent associations of bacterial vaginosis and Chlamydia trachomatis infection with adverse pregnancy outcomes. JAMA. 1986;256:1899–1903. [PubMed] [Google Scholar]
- 56.Kekki M, Kurki T, Pelkonen J, et al. Vaginal clindamycin in preventing preterm birth and peripartal infections in asymptomatic women with bacterial vaginosis: a randomized, controlled trial. Obstet Gynecol. 2001;97:643–648. doi: 10.1016/s0029-7844(01)01321-7. [DOI] [PubMed] [Google Scholar]
- 57.McDonald HM, Brocklehurst P, Gordon A. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev. 2007;1 doi: 10.1002/14651858.CD000262.pub3. CD000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macones GA, Parry S, Elkousy M, et al. A polymorphism in the promoter region of TNF and bacterial vaginosis: preliminary evidence of gene-environment interaction in the etiology of spontaneous preterm birth. Am J Obstet Gynecol. 2004;190:1504–1508. doi: 10.1016/j.ajog.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Struthers BJ. Metronidazole appears not to be a human teratogen: review of literature. Infect Dis Obstet Gynecol. 1997;5:326–335. doi: 10.1155/S1064744997000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pheifer TA, Forsyth PS, Durfee MA, et al. Nonspecific vaginitis: role of Haemophilus vaginalis and treatment with metronidazole. N Engl J Med. 1978;298:1429–1434. doi: 10.1056/NEJM197806292982601. [DOI] [PubMed] [Google Scholar]
- 61.Greaves WL, Chungafung J, Morris B, et al. Clindamycin versus metronidazole in the treatment of bacterial vaginosis. Obstet Gynecol. 1988;72:799–802. [PubMed] [Google Scholar]
- 62.Hillier SL, Lipinski C, Briselden AM, Eschenbach DA. Efficacy of intravaginal 0.75% metronidazole gel for the treatment of bacterial vaginosis. Obstet Gynecol. 1993;81:963–967. [PubMed] [Google Scholar]
- 63.Livengood CH , 3rd, McGregor JA, Soper DE, et al. Bacterial vaginosis: efficacy and safety of intravaginal metronidazole treatment. Am J Obstet Gynecol. 1994;170:759–764. doi: 10.1016/s0002-9378(94)70278-0. [DOI] [PubMed] [Google Scholar]
- 64.Livengood CH , 3rd, Thomason JL, Hill GB. Bacterial vaginosis: treatment with topical intravaginal clindamycin phosphate. Obstet Gynecol. 1990;76:118–123. [PubMed] [Google Scholar]
- 65.Hillier SL, Lipinski C, Briselden AM, Eschenbach DA. Efficacy of intravaginal 0.75% metronidazole gel for the treatment of bacterial vaginosis. Obstet Gynecol. 1993;81:963–967. [PubMed] [Google Scholar]
- 66.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), authors [Accessed February 14, 2009];Guidance for Industry: Bacterial Vaginosis—Developing Antimicrobial Drugs for Treatment. 1998 Jul; Draft guidance.
- 67.Livengood CH , 3rd, Ferris DG, Wiesenfeld HC, et al. Effectiveness of two tinidazole regimens in treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2007;110:302–309. doi: 10.1097/01.AOG.0000275282.60506.3d. [DOI] [PubMed] [Google Scholar]
- 68.Clindesse Vaginal Cream [package insert] Bridgeton, MO: TherRx Corporation; [Google Scholar]
- 69.Thomas KK, Sanchez S, Garcia PJ, Holmes KK. Why do different criteria for ‘cure’ yield different conclusions in comparing two treatments for bacterial vaginosis? Sex Transm Dis. 2005;32:526–530. doi: 10.1097/01.olq.0000175293.46256.eb. [DOI] [PubMed] [Google Scholar]
- 70.Nyirjesy P, McIntosh MJ, Gattermeir DJ, et al. The effects of intravaginal clindamycin and metronidazole therapy on vaginal lactobacilli in patients with bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1277–1282. doi: 10.1016/j.ajog.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 71.Austin MN, Beigi RH, Meyn LA, Hillier SL. Microbiologic response to treatment of bacterial vaginosis with topical clindamycin or metronidazole. J Clin Microbiol. 2005;43:4492–4497. doi: 10.1128/JCM.43.9.4492-4497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Welling PG, Monro AM. The pharmacokinetics of metronidazole and tinidazole in man. Arzneimittelforschung. 1972;22:2128–2132. [PubMed] [Google Scholar]
- 73.Mohanty KC, Deighton R. Comparison of 2 g single dose of metronidazole, nimorazole, and tinidazole in the treatment of vaginitis associated with Gardnerella vaginalis. J Antimicrob Chemother. 1987;19:393–399. doi: 10.1093/jac/19.3.393. [DOI] [PubMed] [Google Scholar]
- 74. [Accessed March 2, 2009];Sexually Transmitted Diseases Treatment Guidelines 2006. 2007 Apr 12; US Centers for Disease Control and Prevention Web site. http://www.cdc.gov/STD/treatment/2006/toc.htm.
- 75.Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283–1289. doi: 10.1016/j.ajog.2005.11.041. [DOI] [PubMed] [Google Scholar]


