Abstract
Vaginal and laparoscopic hysterectomies have been clearly associated with decreased blood loss, shorter hospital stay, speedier return to normal activities, and fewer abdominal wall infections when compared with abdominal hysterectomies. In this review, the authors outline the 10 steps to a successful laparoscopic hysterectomy.
Key words: Total laparoscopic hysterectomy, Laparoscopic supracervical hysterectomy, Minimally invasive gynecological procedure
Hysterectomy is one of the most commonly performed surgical procedures in the United States, with 570,000 cases performed in 2006.1 Vaginal hysterectomies have been performed successfully for almost 2 centuries, and more recently Reich and colleagues2 introduced the laparoscopic hysterectomy. However, despite the advent of these minimally invasive procedures, abdominal hysterectomy remains the most common surgical approach, with well over half of hysterectomies being performed via a laparotomy.3
Vaginal and laparoscopic hysterectomies have been clearly associated with decreased blood loss, shorter hospital stay, speedier return to normal activities, and fewer abdominal wall infections when compared with abdominal hysterectomies.4–6 In light of these findings, a recent review concluded that vaginal hysterectomy is preferable to abdominal hysterectomy and that a laparoscopic hysterectomy should be attempted when vaginal hysterectomy is not possible.6 The vaginal approach is less expensive, but may be challenging in patients with a history of an adnexal mass, endometriosis, pelvic pain, and prior abdominal surgery, or in patients with a narrow pubic arch or poor vaginal descent.
The relatively slow adaptation of laparoscopic hysterectomy may in part be attributed to inadequate exposure and training during residency.7 In addition, a number of provider barriers have been identified, including insufficient experience and training, lack of hospital equipment, and inadequate support from colleagues. Relatively low reimbursement rates may also curb provider enthusiasm for additional training and incorporation of the laparoscopic hysterectomy into their surgical armamentarium.8
Cervix Removal
Laparoscopic supracervical hysterectomy (LSH) has been associated with a shorter operating time, decreased trauma, and less technical difficulty.9 However, a small proportion of these patients continue to have cyclical bleeding until menopause,10–14 and there is a small risk of developing cervical cancer if the cervix is retained. A retrospective study found that 2.2% of all cervical cancer cases originate from the cervical stump.15 A prospective cohort study of 70 patients undergoing LSH predominantly for endometriosis and pelvic pain found that at 5 years of follow-up, 22.8% required further surgical treatment due to continued vaginal bleeding or pelvic pain.16 In addition, a prospective, randomized trial found higher readmission rates following LSH than after total laparoscopic hysterectomy (TLH). In this relatively small study, there were no statistically significant differences in complications, symptom improvement, or activity limitations.17 Three randomized, controlled trials comparing total to subtotal abdominal hysterectomy found no significant differences in sexual function, urinary tract symptoms, or bowel symptoms.18–20 A recent study with a mean follow-up of 9 years found no significant differences in development of pelvic organ prolapse or other outcomes among patients having a total and subtotal hysterectomy.21
We discuss the available evidence with our patients prior to surgery, and we offer most of them the option of keeping their cervix if they so desire. However, we strongly recommend removing the cervix in patients with a history of abnormal Papanicolaou test results and in patients having a hysterectomy due to pelvic pain or endometriosis.
10 Steps Toward a Successful TLH
1. Preparation and Positioning
Patients are placed in a dorsal lithotomy position with pneumoboots. The arms are tucked at the sides and a foam mattress is situated directly under the patient to prevent sliding during steep Trendelenburg. We keep the table in a low position and have a monitor directly facing each surgeon to promote an ergonomic working environment. The surgeon needs to be familiar with all the equipment in the operating room and should routinely inspect equipment for any malfunction or servicing needs. A partial equipment list is shown in Table 1. In general, it is important for the surgeon to simplify the equipment list as much as possible. This prevents crowding in the operating room and facilitates room turnover and staff familiarity with the equipment being used.
Table 1.
Equipment List for Total Laparoscopic Hysterectomy
| Standard Equipment | Comments/Tips for Usage |
| 0° laparoscope | 5-mm scope most often used |
| Harmonic® Scalpel (Ethicon Endo-Surgery, Somerville, NJ) | |
| Reusable bipolar grasper | Power setting at 50 W |
| 3 5-mm trocars and 1 12-mm trocar | Prefer visual entry |
| Two duckbill graspers | Universal grasping ability |
| RUMI® Uterine Manipulator (CooperSurgical, Trumbull, CT) | Check for all parts prior to start |
| Foley catheter | |
| 14 × 14 cm 0 PDO suture cut in half | |
| LapraTy (Ethicon Endo-Surgery) applicator and clips | |
| Optional Equipment | |
| Diluted vasopressin | Avoid using more than 10 units |
| 5- and 10-mm tenaculum | Helpful for larger uteri |
| Electronic morcellator | |
| 30° laparoscope | Helpful for larger uteri |
| Suction irrigator | |
| Fascia closure device | Not needed for thin patients |
| Triple hook clamps | For vaginal morcellation |
2. Insertion of a Uterine Manipulator
We generally prefer to use the RUMI® Uterine Manipulator (Cooper-Surgical, Trumbull, CT); however, in patients with a very narrow introitus we will use the VCare® Uterine Manipulator/Elevator (ConMed Endosurgery, Utica, NY) because this is easier to insert. We find, however, that the VCare cup is rather shallow, making the delineation of the vaginal fornices challenging in the setting of a long cervix. Occasionally we will have a patient who has never had intercourse or has been on longstanding testosterone treatment due to gender reassignment and in these cases a uterine manipulator is not used. Although placing the RUMI can be challenging, there are several tricks that can simplify this task. First, place a Sims speculum into the vagina, grab the cervix with a tenaculum, and sound the uterus. Then ask for the appropriately sized RUMI tip (6, 8, or 10 cm). The tip can be difficult to attach to the shaft, but dipping the distal end of the shaft in lubricant prior to attaching the tip greatly facilitates this step. The pneumo-occluder then slides over the tip and onto the shaft followed by attachment of the appropriately sized KOH ring (3, 3.5, and 4 cm in width). It is important to choose the correct size because a small ring will not delineate the vaginal fornices and a large ring may increase the risk of a ureteral injury. Next, place a “figure 8” 0 monofilament suture through the anterior lip of the cervix, thread this through the KOH ring, and secure with a hemostat. Insert the tip of the RUMI as far into the cervix as it will go, then release the tenaculum while keeping tension on the cervical stitch. This prevents the uterus from moving cephalad as the tenaculum is removed. After confirming correct placement by palpation or direct visualization (Figure 1), tie the suture to the handle to facilitate specimen removal through the vagina at the end of the case. A Foley catheter is inserted into the bladder and finally the pneumo-occluder is filled with 60 to 100 cc of saline.
Figure 8.
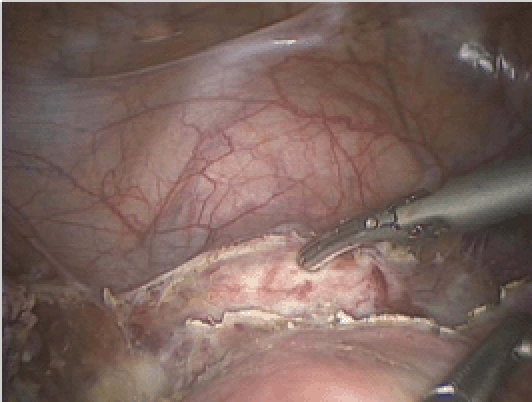
The rim of the KOH ring is seen and felt after mobilizing the bladder.
Figure 1.
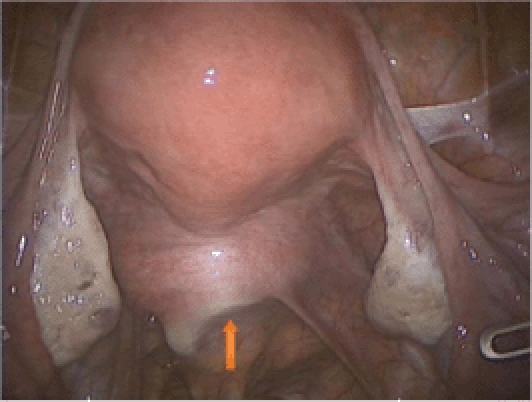
The edge of the KOH colpotomizer is clearly seen (arrow).
3. Abdominal Entry and Trocar Placement
A 5-mm skin incision is made at the deepest part of the umbilicus using a #15 blade.
We elevate the deepest part of the umbilicus with a Kocher clamp and insert a Veres needle into the peritoneal cavity. The gas tubing is already connected to the needle to reduce manipulation following insertion. An easy way to confirm intraperitoneal entry is to look for a negative pressure reading on the insufflator. Once intraperitoneal pressure has reached 15 mm Hg, we insert an optical trocar through the umbilicus under direct vision, followed by a complete survey of the abdomen to rule out any visceral injury at the time of entry. The lower quadrant trocar sleeves are placed under direct vision. These trocars are placed lateral to the rectus abdominis muscles, 2 cm above and 2 cm medial to the anterior superior iliac spine. Usually, a 5-mm trocar is placed on the right and a 12-mm trocar on the left. In addition, a 5-mm trocar is placed approximately 8 cm above and parallel to the lower left trocar site. This port will, in most cases, end up being nearly parallel to the umbilical trocar. We find that the 2 ports on the left greatly facilitate suturing and help to maintain an ergonomic position for the surgeon throughout the procedure. The 12-mm port site is ideal for needle passage and specimen retrieval.
4. Hug the Ovaries
The infundibulopelvic (IP) ligament or the utero-ovarian ligament is initially desiccated with a reusable bipolar grasper (Figure 2). It is important to stay close to the ovary (hug the ovary) as this helps to avoid the pelvic sidewall during ovarian removal and the ascending uterine vessel during ovarian conservation. The surgeon should take special care to desiccate the parametrial veins that run between the ovary and the round ligament (Figures 3 and 4) as these can be quite tortuous and tend to bleed if left unattended. The IP ligament or utero-ovarian ligament is then transected close to the ovary using the Harmonic® Scalpel (Ethicon Endo-Surgery, Somerville, NJ). We prefer to use the bipolar grasper prior to cutting with the Harmonic scalpel because bleeding can be encountered despite appropriate use of the Harmonic scalpel, especially in the setting of an enlarged uterus with engorged vascular plexuses. During this step of the procedure, the uterine manipulator is being pushed upwards and to the contralateral side to provide maximal visualization.
Figure 2.
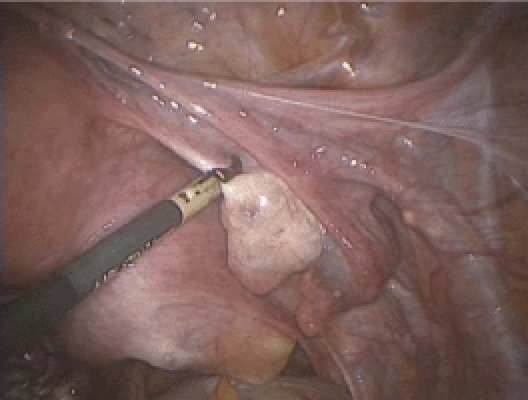
Dessication of the utero-ovarian ligament (ie, hugging the ovary).
Figure 3.
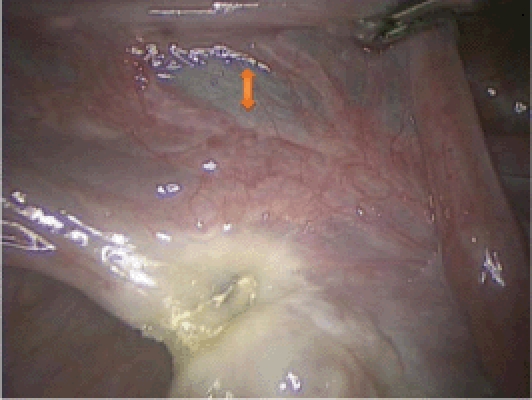
The parametrial venous plexus between the ovary and the round ligament is seen (arrow).
Figure 4.
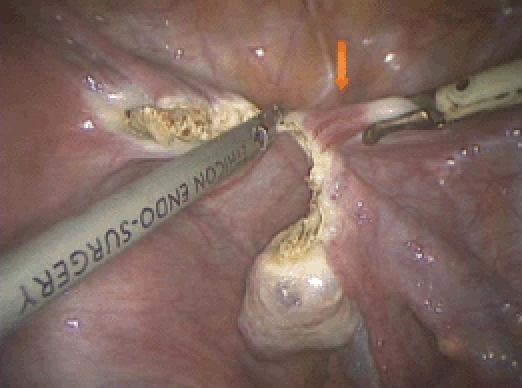
Dissection continues to the round ligament (arrow).
5. Mobilize the Bladder
Transect the round ligament and separate the anterior and posterior leaves of the broad ligament with the Harmonic scalpel (Figures 5 and 6). It is important to find the correct plane; this is where the peritoneum separates easily with gentle manipulation. Next, identify the vesicouterine peritoneal fold and continue the dissection anteriorly, thereby mobilizing the bladder off the lower uterine segment (Figures 7 and 8). It is important to stay in the loose areolar tissue if at all possible. In patients who have had a prior cesarean delivery, this area may be scarred and it is important to stay relatively high on the uterus during the dissection. A reevaluation of the route of dissection is advised if fat is encountered because the fat belongs to the bladder; this may indicate that the dissection is moving too close to the bladder.
Figure 5.
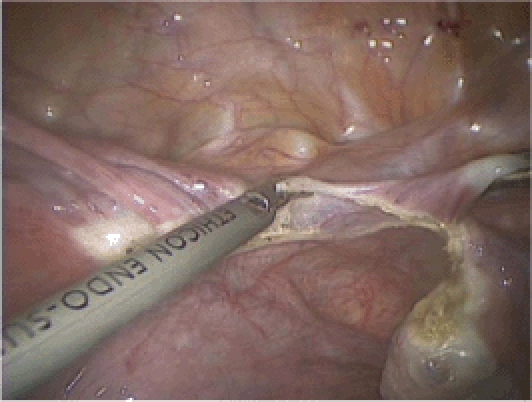
The anterior and posterior leaves of the broad ligament are separated using the Harmonic® Scalpel (Ethicon Endo-Surgery, Somerville, NJ).
Figure 6.
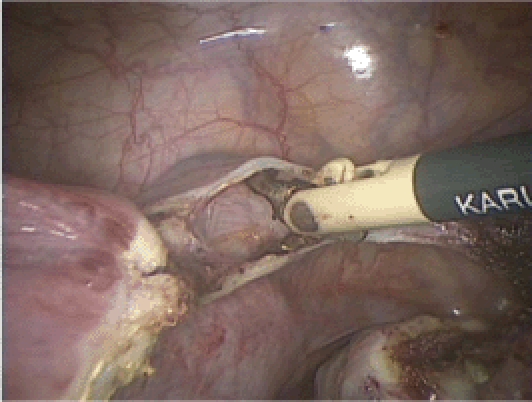
The anterior and posterior leaves of the broad ligament are further separated by using the tip of the Maryland bipolar grasper.
Figure 7.
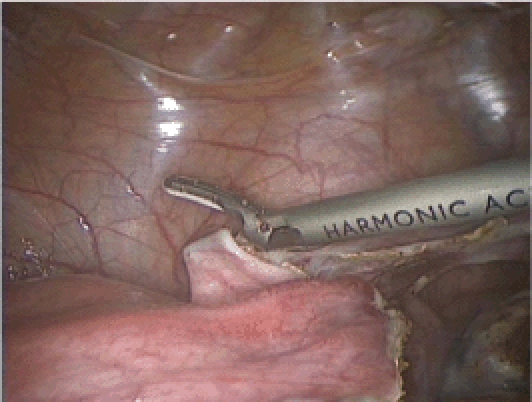
The dissection of the anterior leaf of the broad ligament continues anteriorly, thereby enabling dissection of the bladder from the lower uterine segment.
6. Secure the Uterine Vessels
Due to a wide variety in anatomy and in the course of the uterine vessels, we find it helpful to initially skeletonize them with the Harmonic scalpel. We then desiccate the ascending uterine vessels with the bipolar grasper at the level of internal cervical os (Figures 9 and 10). Note that pushing cephalad with the uterine manipulator helps to move the uterine vessels away from the ureter. Complete desiccation of the vessels can be assessed visually by observing the bubbles coming and going during this process; when the bubbles stop forming the vessel is desiccated and safe to transect with the Harmonic scalpel. We will then usually make 2 cuts with the Harmonic scalpel in an inverted V-shape anterior and medial and posterior and medial to the vascular pedicle. This enables the vascular pedicle to fall out laterally, thereby providing easy and avascular access to the cervical cup (Figure 11). It is important to take the uterine vessels high and then dissect medially to the uterine vessels down to the cup. This averts ureteral injury and provides a healthy vascular pedicle that can be safely desiccated further in the event of bleeding.
Figure 9.
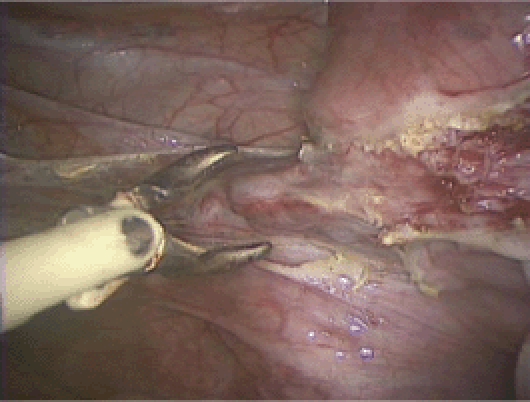
The uterine vessels are skeletonized.
Figure 10.
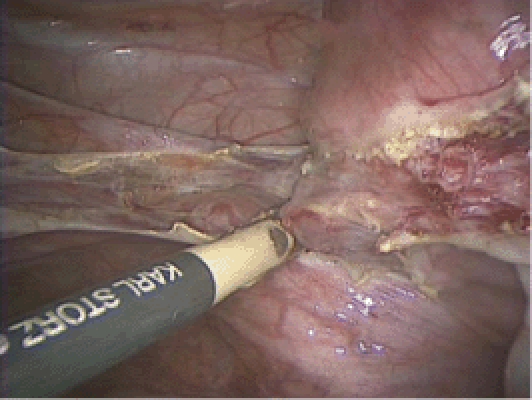
The ascending uterine vessels are coagulated with the bipolar grasper at the level of internal cervical os, staying above the rim of the cervical cup.
Figure 11.
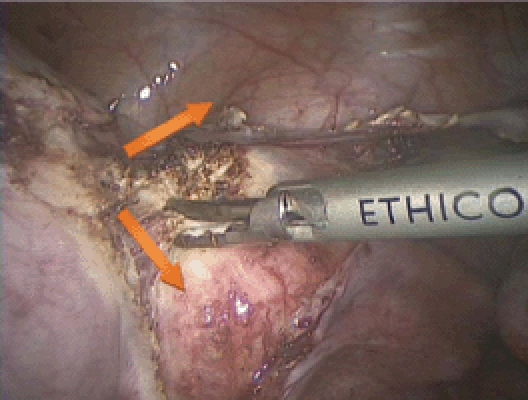
Two incisions are made with the Harmonic® Scalpel (Ethicon Endo-Surgery, Somerville, NJ) medial to the uterine vessels, roughly following an inverted V-shaped pattern. This makes the vascular pedicle fall out laterally and provides avascular access to the cervical ring.
7. Separate the Uterus and Cervix From the Vaginal Apex
Identify the vaginal fornices while pushing cephalad with the uterine manipulator. You will either see the indentation of the KOH colpotomizer or be able to palpate it with a laparoscopic instrument. The Harmonic scalpel is then used to cut circumferentially around the cup. Take care not to direct the Harmonic scalpel directly into the metal because this may result in failure of the device and may even break the active blade (Figures 12 and 13).
Figure 12.
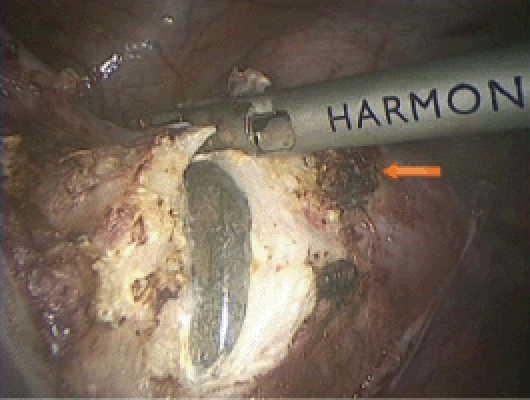
The KOH colpotomizer can be seen. Please note the vascular pedicle lateral to the line of incision (arrow).
Figure 13.
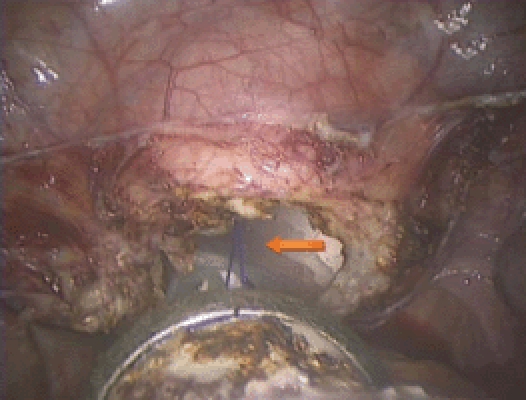
Completion of the colpotomy. The stitch was placed at the beginning of the procedure and helps with vaginal retrieval of the uterus (arrow).
8. Removal of the Uterus
Pull the uterus into the vagina if it fits. The uterus can remain there to maintain pneumoperitoneum during suturing. Alternatively, the uterus is removed and a glove with a pair of 4 × 4 sponges is placed into the vagina to maintain pneumoperitoneum (Figure 14). If the uterus is too large to fit through the vagina, it can be carefully morcellated transvaginally by using a 10-blade scalpel and triple hooks for retraction. In patients with limited vaginal access, the uterus can be morcellated using an electronic morcellator. It is important to keep the tip of the morcellator in clear view at all times.
Figure 14.
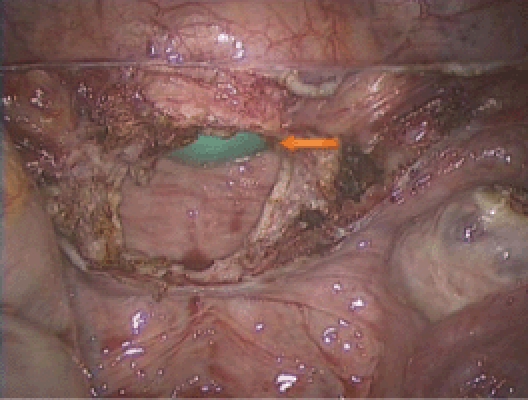
A glove with 2 4 × 4 sponges is seen in the vagina (arrow) and is used to maintain pneumoperitoneum prior to and during vaginal cuff suturing.
9. Vaginal Cuff Closure
We use one half of a 14 × 14 cm 0 Quill™ SRS suture (Angiotech, Vancouver, BC, Canada) that has been cut in the middle with a LapraTy clip (Ethicon Endo-Surgery) secured at the distal end.22 Closure begins at the distal angle of the vaginal cuff and proceeds in a running fashion, making sure to include the vaginal mucosa and the pubocervical and rectovaginal fascia (Figures 15–17). Each bite should be approximately 1 cm in thickness—this can be easily underestimated due to the magnification of the laparoscope. A LapraTy is then placed at the end of suture and the needle is cut free and removed through the 12-mm port. The pelvis can now be irrigated and hemostasis at all sites is assured.
Figure 15.
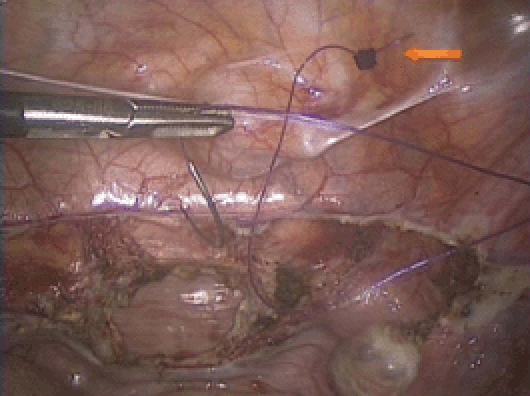
Vaginal cuff closure. 0 Quill™ SRS suture (Angiotech, Vancouver, BC, Canada) with a LapraTy (Ethicon Endo-Surgery, Somerville, NJ) at the end (arrow). Please note the orientation of the needle during insertion. The smiley-face configuration facilitates easy loading on the needle driver.
Figure 17.
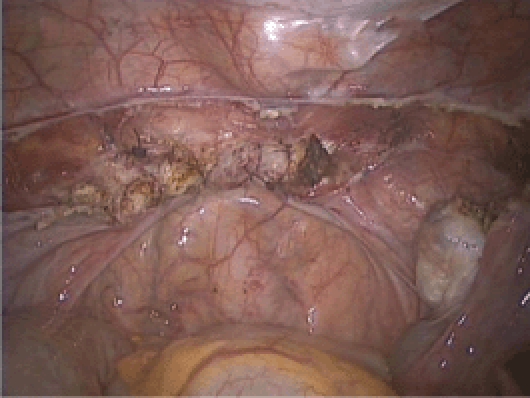
Completion of the vaginal cuff closure.
10. Port Site Closure
The fascia at the 12-mm incision in the left lower quadrant is closed using 0 vicryl sutures with a fascia closure device. The skin is closed with 4-0 monocryl suture in a continuous subcutaneous fashion. The 5-mm incisions are closed with Dermabond® (Ethicon Endo-Surgery). We then inject 20 cc of 0.5% Marcaine® (Sanofi, Markham, ON, Canada) at all incision sites to reduce immediate postoperative pain.23 We do not perform cystoscopy routinely, but in select cases cystoscopy is performed after vaginal closure to check ureteral patency and for any signs of bladder injury. The patient is given 5 cc of Indigo carmine intravenously 5 minutes prior to closure. Please note that a normal cystoscopy does not exclude a delayed thermal injury to either the ureters or the bladder.
Postoperative Management
We give our patients 30 mg of ketorolac tromethamine intravenously at the end of surgery and every 6 hours after that for a total of 4 doses. Oral oxycodone/acetaminophen per os is used for breakthrough pain. Postoperative nausea and vomiting risk factors are evaluated24 and patients are given the appropriate prophylactic and therapeutic antiemetics, such as dexamethasone, ondansetron, and a hyoscine hydrobromide patch.25 Patients either go home the day of surgery or the following morning. We are prospectively evaluating recovery and resumption of normal activities following our laparoscopic hysterectomy cases and we have found that patients resume their normal activities on average within 2 to 3 weeks following surgery.
Summary
Total laparoscopic hysterectomy is a safe and effective procedure for women needing a hysterectomy. We enjoy a high operative volume and perform approximately 200 laparoscopic hysterectomy cases annually with a conversion rate of 1 in every 400 cases. The 10 steps described herein are not meant to be an absolute truth, but rather a true and tested method that has served us well to safely accomplish this procedure.
Additional Practical Tips for Challenging Cases
If the uterus is large and requires manipulation with a tenaculum, consider injecting dilute vasopressin subserosally prior to applying traction to the uterus. This can reduce bleeding associated with pulling and tearing of the uterine serosa.
In cases with poor exposure, we routinely use sutures to retract organs away from the surgical field. A redundant sigmoid can be retracted by taking a series of bites with a 0 prolene suture through the epiploica and pulling the suture through the lower quadrant port. The port is removed to get the sutures out and then reinserted. The sutures are then secured to the skin with a hemostat. Take care to include a number of epiploica to avoid tearing.
Alternatively, ovaries or other structures can be tacked away using a 6-inch suture (0 Quill PDO or 0 vicryl) with a LapraTy on the end. The needle is passed through the structure and then a bite is taken through the inside of the anterior abdominal wall. This end of the suture is then secured with another LapraTy and the needle is cut away and removed.
If access to the uterine vessels is difficult, take the uterine vessels up high initially to secure the blood supply to the upper uterus and then gradually work down, staying medially to the vessels.
Maintain exposure at all times-do not dig yourself into a hole-always be ready to deal with a sudden onset of bleeding.
The combination of a prior cesarean delivery and a large uterus is a set up for bladder injury-stay high on the vesicouterine peritoneum, respect any fat that you see, and watch out for air in the Foley balloon.
In severely distorted anatomy consider entering the retroperitoneum sooner rather than later. The easiest starting point is usually at the round ligament.
Figure 16.
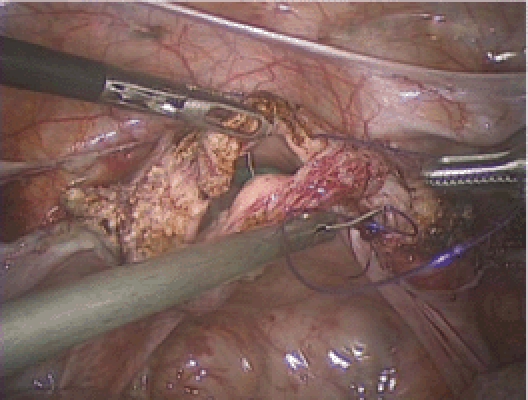
Vaginal cuff closure. It is important to take full thickness bites here.
References
- 1.DeFrances CJ, Lucas CA, Buie VC, et al. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008;5:1–20. [PubMed] [Google Scholar]
- 2.Reich H, DeCaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213–216. [Google Scholar]
- 3.Wu JM, Wechter ME, Geller EJ, et al. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110:1091–1095. doi: 10.1097/01.AOG.0000285997.38553.4b. [DOI] [PubMed] [Google Scholar]
- 4.Clayton RD. Hysterectomy. Best Pract Res Clin Obstet Gynaecol. 2006;20:73–87. doi: 10.1016/j.bpobgyn.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Johnson N, Barlow D, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2006;2 doi: 10.1002/14651858.CD003677.pub3. CD003677. [DOI] [PubMed] [Google Scholar]
- 6.Johnson N, Barlow D, Lethaby A, et al. Methods of hysterectomy: systematic review and meta-analysis of randomised controlled trials. BMJ. 2005;330:1478. doi: 10.1136/bmj.330.7506.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reich H. Total laparoscopic hysterectomy: indications, techniques and outcomes. Curr Opin Obstet Gynecol. 2007;19:337–344. doi: 10.1097/GCO.0b013e328216f99a. [DOI] [PubMed] [Google Scholar]
- 8.Englund M, Robson S. Why has the acceptance of laparoscopic hysterectomy been slow? Results of an anonymous survey of Australian gynecologists. J Minim Invasive Gynecol. 2007;14:724–728. doi: 10.1016/j.jmig.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Lyons TL. Laparoscopic supracervical hysterectomy. Obstet Gynecol Clin North Am. 2000;27:441–450. doi: 10.1016/s0889-8545(00)80034-0. [DOI] [PubMed] [Google Scholar]
- 10.Van der Stege JG, van Beek JJ. Problems related to the cervical stump at follow-up in laparoscopic supracervical hysterectomy. JSLS. 1999;3:5–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Planas MV. Cervicectomy following supravaginal hysterectomy. Am J Obstet Gynecol. 1960;79:480–485. doi: 10.1016/0002-9378(60)90247-7. [DOI] [PubMed] [Google Scholar]
- 12.Härkki-Siren P, Sjöberg J, Kurki T. Major complications of laparoscopy: a follow-up Finnish study. Obstet Gynecol. 1999;94:94–98. [PubMed] [Google Scholar]
- 13.Nisolle M, Donnez J. Subtotal hysterectomy in patients with endometriosis-an option. Fertil Steril. 1997;67:1185–1187. doi: 10.1016/s0015-0282(97)90054-6. [DOI] [PubMed] [Google Scholar]
- 14.Harris WJ, Daniell JF. Early complications of laparoscopic hysterectomy. Obstet Gynecol Surv. 1996;51:559–567. doi: 10.1097/00006254-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Hellström AC, Sigurjonson T, Pettersson F. Carcinoma of the cervical stump. The radiumhemmet series 1959–1987. Treatment and prognosis. Acta Obstet Gynecol Scand. 2001;80:152–157. [PubMed] [Google Scholar]
- 16.Okaro EO, Jones KD, Sutton C. Long term outcome following laparoscopic supracervical hysterectomy. BJOG. 2001;108:1017–1020. doi: 10.1111/j.1471-0528.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- 17.Morelli M, Noia R, Chiodo D, et al. Laparoscopic supracervical hysterectomy versus laparoscopic total hysterectomy: a prospective randomized study [in Italian] Minerva Ginecol. 2007;59:1–10. [PubMed] [Google Scholar]
- 18.Thakar R, Ayers S, Clarkson P, et al. Outcomes after total versus subtotal abdominal hysterectomy. N Engl J Med. 2002;347:1318–1325. doi: 10.1056/NEJMoa013336. [DOI] [PubMed] [Google Scholar]
- 19.Learman LA, Summitt RL , Jr, Varner RE, et al. A randomized comparison of total or supracervical hysterectomy: surgical complications and clinical outcomes. Obstet Gynecol. 2003;102:453–462. doi: 10.1016/s0029-7844(03)00664-1. [DOI] [PubMed] [Google Scholar]
- 20.Gimbel H, Zobbe V, Andersen BM, et al. Randomised controlled trial of total compared with subtotal hysterectomy with one-year follow up results. BJOG. 2004;110:1088–1098. [PubMed] [Google Scholar]
- 21.Thakar R, Ayers S, Srivastava R, Manyonda I. Removing the cervix at hysterectomy: an unnecessary intervention? Obstet Gynecol. 2008;112:1262–1269. doi: 10.1097/AOG.0b013e31818f3bf5. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg JA, Einarsson JI. The use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy. J Minim Invasive Gynecol. 2008;15:621–623. doi: 10.1016/j.jmig.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Einarsson JI, Sun J, Orav J, Young AE. Local analgesia in laparoscopy: a randomized trial. Obstet Gynecol. 2004;104:1335–1339. doi: 10.1097/01.AOG.0000146283.90934.fd. [DOI] [PubMed] [Google Scholar]
- 24.Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg. 2006;102:1884–1898. doi: 10.1213/01.ANE.0000219597.16143.4D. [DOI] [PubMed] [Google Scholar]
- 25.Einarsson JI, Audbergsson BO, Thorsteinsson A. Scopolamine for prevention of postoperative nausea in gynecologic laparoscopy, a randomized trial. J Minim Invasive Gynecol. 2008;15:26–31. doi: 10.1016/j.jmig.2007.08.616. [DOI] [PubMed] [Google Scholar]


