Abstract
Peptide-based catalysts have been applied to the enantioselective syntheses of the title compounds, with this being the first report of the synthesis of an ent-PI5P analogue. The key steps in the synthesis involve asymmetric phosphorylation catalysis. Additional maneuvers were developed with a protecting groups scheme that enabled efficient, streamlined syntheses of these important mediators of biochemical events.
1. Introduction
Phosphatidylinositol (1) and its variously phosphorylated analogues have been identified as tremendously important mediators of biochemical processes. 1 Among the monophosphates, phosphatidylinositol-5-phosphate (PtdIns-5P or “PI5P”, 2) has gained significant attention. Recent reports of cellular processes regulated by this agent are emerging, and its role in biological functions have only started to be appreciated. Among the examples, PI5P has been associated as a regulator of chromatin modification hrough its interaction with the tumor suppressor protein ING2.2 It has also been implicated as a regulator of cell cycle progression,3 as a modulator of gene expression,4 and as an activator of signal transduction pathways.1, 5 Its role in living systems reaches beyond mammalian cells as it has been identified to play a role in the osmotic-stress response in plants.6 Limited investigations of PI5P have identified it as a critical regulator of various cellular functions, however, further biochemical exploration is needed to further our understanding of these processes.
Synthetic chemistry has played a defining role in developing the chemical biology of PtdIns science.7 The synthetic achievements in the field have yielded access to a number of important members of this family of targets.8,9,10,11 The chiral pool has provided a common starting place for many of the synthetic approaches that deliver single-enantiomer compounds. The biomimetic approaches of Prestwich in particular have afforded many members of this class, as well as PI-based probes of PI-dependent biological events.12 The dipalmitoyl-analogue of PI5P (PI5P-DiC16) has been previously synthesized by Prestwich 13 and Falck 14 as the natural enantiomer. Prestwich synthesized the inositol core via a Ferrier rearrangement of methyl α-D-glucopyranoside, while Falck relied on the diastereomeric separation of a camphor ketal intermediate. In addition, Watanabe15 has synthesized the racemic form of PI5P-DiC16 directly from the meso-myo-inositol core.
Our own interest in these compounds stems from the synthetic challenge in developing rapid, streamlined syntheses of these molecules, and difficult-to-access analogs, such that high precision biochemical studies may be undertaken. In particular, we have reported enantioselective synthesis of phosphatidylinositol-3-phosphate, 16 phosphatidylinositol-3,5-bis(phosphate), 17 several deoxygenated analogs of these compounds, and in selected cases, enantiomeric versions that may serve as biological probes. In each of these syntheses, we capitalized on the power of desymmetrization catalysis to enter a chosen enantiomeric series (Scheme 1).
Scheme 1.
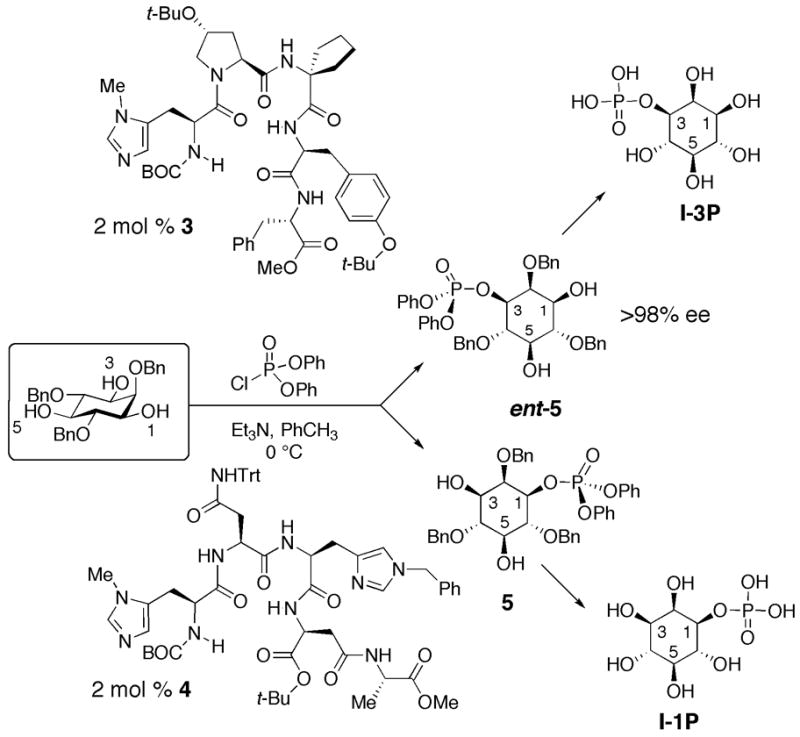
As shown in Scheme 1, catalysts 3 and 4 have emerged as workhorses in our laboratory for the rapid syntheses of compounds in the PtdIns family. Initially, we targeted enantioselective synthesis of inositol-1-phosphate, and its enantiomer inositol-3-phosphate. As shown in Scheme 1, rapid syntheses were indeed possible as a function of the two catalysts we discovered for these purposes.18,19 Herein, we report the application of chiral catalysts 3 and 4 to the enantioselective syntheses of PI5P as well as ent-PI5P. Catalyst-dependent syntheses of these particular phosphatidylinositol-monophosphates have not yet been reported, and thus provide a new entry into each enantiomeric series. Efficient synthetic routes to these compounds could allow for further biochemical investigation of these important cellular messengers in an effort to elucidate their biological roles.
2. Results and Discussion
Peptide-based asymmetric catalysis provided direct routes to desymmetrized phosphoinositols 5 and ent-5 and we envisioned manipulating these enantiomers to synthesize each PI5P and ent-PI5P, with dioctanoyl side chains, in an optically pure fashion (Figure 2). The synthesis of these phosphatidylinositide derivatives bears inherent challenges due to the decreased reactivity towards phosphorylation of the C5 hydroxyl, in comparison to the C3 hydroxyl. Thus, retrosynthetically the C5 phosphate could be prepared from protected derivative 6, which could be obtained through a selective protection (PG) of the C3 hydroxyl (Figure 2). The glycerol fragment could be installed through the previously developed reaction for these systems of the corresponding phosphoric acid of 7 and alcohol 8 under Mitsunobu-type conditions.16 Inositol derivative 7 can be readily derived from 5 through a previously developed three step sequence to convert the phenyl phosphate ester groups to benzyl phosphate ester groups.16
Figure 2.
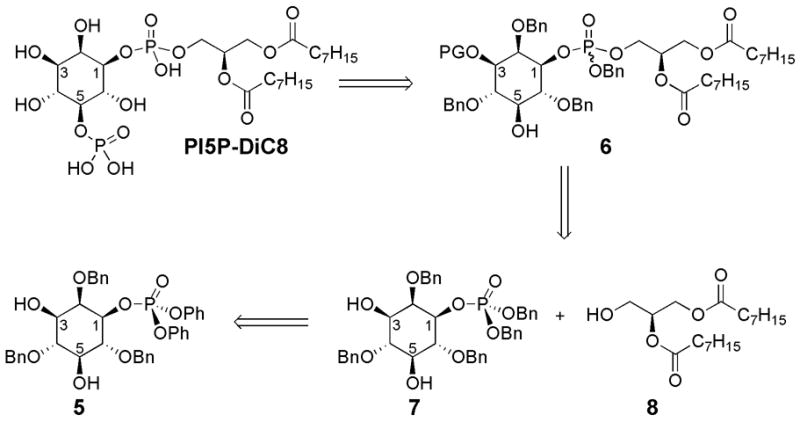
Retrosynthetic analysis for one enantiomeric series of PI5P-DiC8.
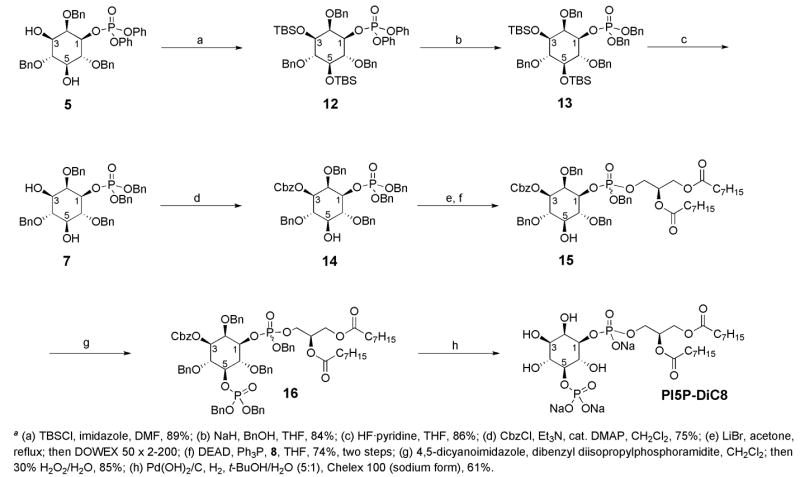
Large-scale production of both 5 and ent-5, utilizing selective catalysts 4 and 3 respectively, provided a suitable starting point for the synthesis of each single-enantiomer PI5P compound (Scheme 1). As previously reported, it was necessary to exchange the phenyl phosphate ester groups to benzyl phosphate ester groups to provide an efficient final deprotection of the desired compounds. Thus the two remaining hydroxyl groups of 5 were protected as the silyl ethers followed by transesterification and subsequent deprotection to provide intermediate 7 (Scheme 2).
Scheme 2a.
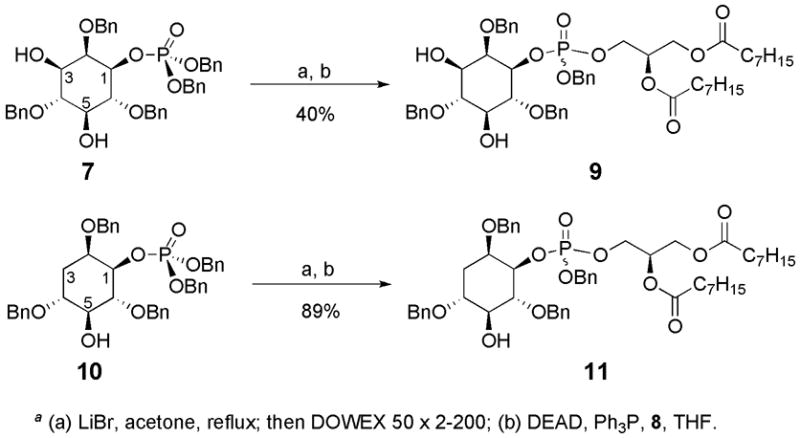
With late stage intermediate 7 in hand, our synthetic plan could either first install the glycerol functionality and then protect the C3 hydroxyl or vice versa. In the examination of the Mitsunobu reaction it was intriguing to recount the reactions of 7 and deoxy-analogue 10, using identical conditions.16 Both analogues underwent monohydrolysis under basic conditions to deliver the corresponding phosphoric acids20 followed by reaction with 8 employing Mitsunobu-type conditions to obtain phosphatidylinositol derivatives 9 and 11 (Scheme 3).16 The yields for these two processes are remarkably different, with the deoxy-compound produced in 89% yield compared to 40% for 9. Further, the purification of 9 was also difficult as a result of poor separation of the DEAD byproduct that is produced in the reaction. The requirement for multiple chromatographic procedures could have contributed to the decreased isolated yield of the Mitsunobu product. Based on this evidence, we suspected that the relatively solvent exposed C3 hydroxyl may be impeding the reaction. Thus, masking this functionality could lead to increased yield of the desired product. As a result, our synthetic strategy evolved in the direction of protecting the C3 hydroxyl followed by subsequent installation of the glycerol fragment.
Scheme 3a.
Two issues guided the choice of protecting group for the C3 hydroxyl. First, it was desirable to have one set of global deprotection conditions as the final synthetic step. Therefore the protecting group to be employed should be removable under previously developed hydrogenolysis conditions. Second, the protection needed to be selective for the C3 hydroxyl over the C5 hydroxyl. Initially we attempted protection of the alcohol as the benzyl ether. We turned our attention towards Dudley’s benzylation conditions, which uses MgO as a mild base. 21 These conditions were chosen with the thought that they should be sufficiently mild in order to preclude phosphate migration. The use of strong bases, such as NaH in the presence of benzyl bromide, was avoided as it was expected to cause racemization of 7 through a migration event. The reaction utilizing Dudley’s reagent was sluggish with the use of toluene at 80 °C and provided only trace amounts of the desired product after 12 hours. Dudley reports the reaction to be more efficient with the use of α,α,α-trifluorotoluene as a solvent, and thus we examined these conditions. These conditions proved to be more reactive, however, even at 80 °C for 3 days with 4 equivalents of both the benzylating reagent and MgO, the desired product was obtained in only 18% yield in a 1:2:1 mixture of benzylation products (3,5-OBn : 3-OBn : 5-OBn). The poor selectivity and yield of the reaction was consistent with our previous experience of low site-selectivity for alkylation reactions when comparing the reactivity of the C1 or C3 hydroxyl with the more hindered C5 hydroxyl.
As previously mentioned, these two positions have shown remarkable differences in reactivity for the phosphorylation reaction.18 Therefore, we envisioned protecting the C3 hydroxyl with a benzyloxycarbonyl, which should provide a more selective reaction as it proceeds through a tetrahedral intermediate, similar to the phosphorylation reaction. In addition, this group is removable under the global hydrogenolysis deprotection conditions. As predicted, the 3-OCbz-inositol derivative 14 was delivered in good yield with no detection of the 5-OCbz regioisomer. This reaction can be catalyzed by either N-methyl-imidazole (NMI), or 4-dimethylaminopyridine (DMAP), and shows a strong dependence on a 1.67:1 molar ratio between benzyl chloroformate and triethylamine. Protected derivative 14 was then subjected to monodebenzylation conditions and subsequent Mitsunobu reaction with glycerol fragment 8 to provide 15 as a mixture of diastereomers at the phosphorus atom. The Mitsunobu reaction with this derivative proceeded in 74% yield, which was a significant increase from the unprotected C3 hydroxyl derivative, which gave only 40% of the desired product under identical conditions. The Cbz protection may have further contributed to the yield of the reaction, as 15 was more easily separated from the many components of the reaction when compared to adduct 9.
From 15, the free hydroxyl at C5 of the myo-inositol core was subjected to coupling with dibenzyl diisopropylphosphoramidite under standard conditions to install the phosphate and provide fully protected PI5P-diC8, 16 in excellent yield. Deprotection of 16 employing previously optimized hydrogenolysis conditions with Pearlman’s catalyst (palladium hydroxide on carbon) and Chelex 100 sodium form resin under a hydrogen atmosphere in a water/t-butanol mixture proceeded well to provide PI5P-DiC8 as the sodium salt. On occasion, an unidentified impurity was present in the sample, manifested by a large singlet at 1.91 ppm in the 1H NMR. The presence of this contaminant at this stage has sporadically been a problem in previous synthesis of other phosphoinositide and phosphatidylinositide derivatives. However, exhaustive washing of Pearlman’s catalyst prior to use typically allows the global deprotection to proceed to deliver a pure product. Moreover, size exclusion chromatography proved effective for isolation of analytically pure PI5P-DiC8. In preparative runs, the deprotection sequence may be carried out, with passage of the product through a size exclusion column, to allow for the isolation of analytically pure PI5P-DiC8 in 61% yield, from the fully protected derivative. As a testimony to the robustness of this streamlined synthetic sequence, the entire synthetic procedure was repeated, starting from ent-5, to provide the unnatural enantiomer, ent-PI5P-DiC8, with comparable yields at every step.
3. Conclusion
In summary, we have completed the total syntheses of both the natural and unnatural enantiomers of PI5P with dioctanoyl side chains on the glycerol fragment. Following the catalytic desymmetrization of a central myo-inositol derivative, the key synthetic steps employed are the Mitsunobu coupling reaction and the use of a benzyloxycarbonyl protecting group. The use of the Cbz group provided selective protection of the C3 hydroxyl over the C5 hydroxyl of the inositol ring. The route employed in our synthesis was highly efficient and provided both enantiomers in their optically pure form, thus providing facile access to either a PI5P, or an ent-PI5P analogue (ent-PI5P-DiC8), from a common starting material. PI5P is a newly emerging member of the phospholipid family and its synthesis is essential towards the identification of its role in cellular processes. These syntheses provide rapid, efficient access to these molecules thus enabling the further exploration and identification of novel cellular functions of not only the natural enantiomer, but also the unnatural enantiomer, which could play alternate cellular roles.
4. Experimental
4.1. General
Proton NMR spectra were recorded on 400 or 500 MHz spectrometers. Proton chemical shifts are reported in ppm (δ) relative to internal tetramethylsilane (TMS, δ 0.0 ppm) or with the solvent reference relative to TMS employed as the internal standard (CDCl3, δ 7.26 ppm; CD3OD, δ 3.31 ppm; D2O, δ 4.79 ppm). Data are reported as follows: chemical shift (multiplicity [singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m)], coupling constants [Hz], integration). Carbon NMR spectra were recorded on 400 or 500 MHz spectrometers with complete proton decoupling. Carbon chemical shifts are reported in ppm (δ) relative to TMS with the respective solvent resonance as the internal standard (CDCl3, δ 77.0 ppm) except for D2O in which case a drop of methanol was added as internal standard (CH3OH, δ 49.5 ppm).22 Phosphorous NMR spectra were recorded on 400 (162 MHz) or 500 (202 MHz) spectrometers with complete proton decoupling. Phosphorous chemical shifts are reported in ppm (δ) relative to a 85% H3PO4 external standard. NMR data were collected at 25 °C, unless otherwise indicated. Analytical thin-layer chromatography (TLC) was performed using Silica Gel 60 Å F-254 precoated plates (0.25 mm thickness). TLC Rf values are reported. Visualization was accomplished by irradiation with a UV lamp and/or staining with KMnO4 or ceric ammonium molybdenate (CAM) solutions. Flash column chromatography was performed using Silica Gel 60 Å (32–63 μm).23 Optical rotations were recorded at the sodium D line (path length 1.0 cm). High resolution mass spectra were obtained at institutional providers. The method of ionization is given in parentheses.
Analytical and preparative reverse phase and normal phase HPLC were performed employing a single wavelength UV detector (214 nm or 254 nm). Measurements of enantiomeric excess were carried out via analytical normal phase HPLC equipped with a diode array detector (214 nm and 254 nm) and employing a Chiralcel® OD column at a flow rate of 0.5 mL/min. All reactions were carried out under an argon or nitrogen atmosphere employing oven- and flame-dried glassware. All solvents were distilled from appropriate drying agents prior to use. 7 and ent-7 were synthesized from peptide catalyzed phosphorylation and transesterification reactions.16, 17
4.2. Specific Procedures
Compound 14: To a stirred solution of 7 (190 mg, 0.267 mmol) in CH2Cl2 (0.534 mL) was added 4-(dimethylamino)pyridine (DMAP) (33 mg, 0.267 mmol) followed by triethylamine (0.112 mL, 0.801 mmol). Benzyl chloroformate (0.188 mL, 1.337 mmol) was then added quickly. The reaction solution bubbled and a precipitate formed. The reaction mixture was stirred at room temperature under a nitrogen atmosphere for 12 hours. The reaction was then diluted with CH2Cl2 (50 mL) and the organic layer was washed with saturated NaHCO3 (50 mL) and brine (50 mL). The organic layer was dried (Na2SO4), filtered, and concentrated in vacuo to afford a yellow oil. This crude material was then purified by column chromatography (0–45% ethyl acetate/hexanes) to afford 14 as a clear thick oil (170 mg, 75%). 1H NMR (CDCl3, 500 MHz) δ 7.28-7.12 (m, 30H), 5.04 (ABq, J = 12.1 Hz, 2H), 4.93-4.86 (m, 4H), 4.70 (ABq, J = 11.2 Hz, 2H), 4.64-4.56 (m, 5H), 4.23-4.20 (m, 2H), 3.83 (td, J = 9.5 and 2.3 Hz, 2H), 3.47 (td, J = 9.2 and 2.3, 1H), 2.37 (d, J = 2.4 Hz, 1H); 13C NMR (CDCl3, 500 MHz) δ 154.4, 138.3, 138.2, 138.1, 135.6, 135.6, 135.1, 128.6, 128.6, 128.5, 128.5, 128.5, 128.4, 128.3, 128.2, 127.9, 127.8, 127.8, 127.7, 127.7, 127.5, 79.6, 79.6, 78.9, 77.8, 77.8, 75.3, 75.2, 75.2, 74.4, 74.4, 69.8, 69.5, 69.5, 69.4, 69.3; 31P NMR (CDCl3, 202 Hz) δ –1.7; IR (film, cm−1) 3387, 3089, 3064, 3032, 2946, 2880, 1744, 1495, 1450, 1262, 1013; TLC Rf 0.41 (40% ethyl acetate/hexanes); exact mass calcd for [C49H49O11P]+ requires m/z 85, found 845.3064 (ESI+); [α]D +26.4 (c 1.0, CHCl3).
Compound 15
To a stirred solution of 14 (0.185 g, 0.219 mmol) in 14 mL of acetone (reagent grade) was added LiBr (0.035 g, 0.408 mmol), and the reaction mixture was refluxed for 10 h. It was then cooled to rt and concentrated. The residue was purified by silica gel chromatography eluting with 50% EtOAc/hexanes to 15% CH3OH/CH2Cl2 to give the crude product as a lithium salt. The salt was dissolved in a minimal amount of CH3OH and run through a H+ DOWEX 2 × 200 ion exchange column. Fractions containing the desired product were combined and concentrated to give the crude phosphoric product. The crude residue was then dissolved in THF (1.1 mL), and diacylglycerol 8 (0.151 g, 0.437 mmol) and triphenylphosphine (0.115 g, 0.437 mmol) were added and the reaction was cooled to 0 ºC. DEAD (68 μL, 0.437 mmol) was then added and the reaction mixture was stirred at 0 °C under N2 for 48 h. The mixture was then concentrated under reduced pressure and purified by silica gel chromatography eluting with 040% EtOAc/hexanes to yield 15 as a colorless thick oil (0.165 g, 74%, over two steps). 1H NMR (CDCl3, 400 MHz) δ 7.29-7.15 (m, 25H), 5.07-4.88 (m, 5H), 4.76-4.57 (m, 7H), 4.28-3.81 (m, 8H), 3.50 (m, 1H), 2.48 (s, 1H), 2.17-2.10 (m, 4H), 1.48-1.46 (m, 4H), 1.18-1.15 (m, 16H), 0.79-0.77 (m, 6H); 13C NMR (CDCl3, 400 MHz) δ 173.0, 173.0, 172.6, 154.3, 154.3, 138.2, 138.2, 138.1, 138.0, 137.9, 135.4 135.4, 135.4, 135.3, 135.0, 135.0, 128.7, 128.5, 128.3, 128.2, 128.2, 128.2, 128.0, 127.9, 127.8, 127.8, 127.7, 127.7, 127.6, 127.5, 127.5, 79.5, 79.4, 79.4, 78.9, 78.9, 77.9, 77.9, 77.8, 77.8, 77.8, 77.2, 76.8, 75.3, 75.2, 75.1, 74.4, 69.8, 69.7, 69.6, 69.5, 69.5, 69.2, 69.1, 65.7, 65.6, 65.4, 65.4, 61.5, 61.4, 34.0, 34.0, 33.9, 31.6, 29.0, 28.9, 28.8, 24.7, 24.7, 22.5, 14.0; 31P NMR (CDCl3, 162 Hz) δ −1.7, −1.8; IR (film, cm−1) 3026, 2951, 2925, 2849, 1738, 1456, 1260, 1158, 1113, 1022; TLC Rf 0.21 (30% ethyl acetate/hexanes); exact mass calcd for [C61H78O15P]+ requires m/z 1081.5073, found 1081.5035 (ESI+); [α]D +11.6 (c 1.0, CHCl3).
Protected PI5P-DiC8 (16)
To a stirred solution of 15 (0.100 mg, 0.092 mmol) in CH2Cl2 (18.5 mL) was added dibenzyl diisopropylphosphoramidite (311 μL, 0.92 mmol) followed by 4,5-dicyanoimidazole (0.131 g, 1.109 mmol). The reaction was stirred at room temperature under a nitrogen atmosphere for 15 h. The reaction was then cooled to 0 °C and 30% H2O2 (8.6 mL) was added. The reaction was stirred at 0 °C for 1 h at which time the reaction was quenched with saturated Na2SO3 (~70 mL) until no peroxides were detected via starch paper. The reaction was then extracted with DCM (3 × 50 mL) and the organic layers were combined, dried (Na2SO4), filtered, and concentrated under reduced pressure. The resulting residue was then purified by silica gel column chromatography (0–8% ethyl acetate/hexanes) and a second column (70% diethyl ether/hexanes) to afford pure 16 as a thick oil (0.105 g, 85%). 1H NMR (CDCl3, 500 MHz) δ 7.315-7.09 (m, 31H), 6.98-6.94 (m, 4H), 4.98 (s, 2H), 4.94-4.61 (m, 14H), 4.45-4.15 (m, 3H), 4.04-3.72 (m, 6H), 2.17-2.09 (m, 4H), 1.51-1.44 (m, 4H), 1.23-1.15 (m, 16H), 0.82-0.77 (m, 6H); 13C NMR (CDCl3, 500 MHz) δ 173.0, 172.7, 172.6, 154.2, 154.2, 138.0, 138.0, 137.9, 137.8, 135.9, 135.9, 135.8, 135.8, 135.4, 135.4, 135.3, 135.3, 134.9, 134.9, 128.6, 128.6, 128.3, 128.3, 128.2, 128.1, 128.1, 127.9, 127.8, 127.8, 127.7, 127.7, 127.6, 127.6, 127.5, 127.5, 127.4, 127.3, 79.9, 79.8, 78.2, 78.2, 78.2, 78.1, 77.6, 77.5, 77.5, 77.5, 77.2, 76.6, 76.5, 76.3, 76.2, 75.4, 75.3, 74.7, 74.7, 69.9, 69.9, 69.8, 69.8, 69.6, 69.6, 69.3, 69.3, 69.2, 69.2, 69.2, 69.1, 65.8, 65.7, 65.6, 65.5, 61.4, 61.4, 34.0, 34.0, 33.9, 31.6, 29.0, 29.0, 28.9, 24.8, 24.7, 22.6, 14.0; 31P NMR (CDCl3, 202 Hz) δ −1.6, −1.9, −2.0; IR (film, cm−1); TLC Rf 0.37 (30% ethyl acetate/hexanes); exact mass calcd for [C75H91O18P2]+ requires m/z 1341.5675, found 1341.5661 (ESI+); [α]D +8.8 (c 1.0, CHCl3).
PI5P-DiC8
Compound 16 (0.095 g, 0.071 mmol) was dissolved in t-BuOH/H2O (5:1) (6 mL) and Chelex (Na form) resin was added till a thick slurry. Palladium hydroxide on carbon (0.190 g) was added and the chamber evacuated. The solution was placed under 1 atm of H2 and stirred for 18 hours. The reaction mixture was filtered over a celite pad and the celite was washed with ethanol (20 mL), ethanol/water (1:1) (15 mL), and H2O (15 mL). The filtrate was filtered through a 0.22 μm filter and concentrated under reduced pressure (no heating). The resulting solid was dissolved in a minimal amount of water and run through a Sephadex G-10 size exclusion column eluting with water. Fractions containing pure product were combined and lyophilized. The solid was dissolved in a minimal amount of water and run through a Chelex (Na form) ion exchange column to fully form the desired sodium salt. Fractions containing the desired product were combined and lyophilized to yield the sodium salt of PI5P-DiC8 as a white fluffy solid (0.032 g, 61% yield). 1H NMR (D2O, 500 MHz) δ 5.32 (q, J = 7.7 Hz, 1H), 4.43 (dd, J = 12.2 and 1.5 Hz, 1H), 4.27 (dd, J = 12.1 and 8.2 Hz, 1H), 4.22 (t, J = 2.6 Hz, 1H), 4.08-4.03 (m, 3H), 3.93-3.85 (m, 2H), 3.80 (t, J = 9.3 Hz, 1H), 3.62 (dd, J = 10.0 and 2.4 Hz, 1H), 2.48-2.30 (m, 4H), 1.64-1.58 (m, 4H), 1.37-1.25 (m, 16H), 0.89-0.85 (m, 6H); 13C NMR (D2O, 500 MHz) δ 175.8, 175.7, 79.7, 79.7, 76.6, 76.5, 72.5, 71.7, 71.5, 71.5, 71.2, 63.9, 34.8, 34.8, 32.4, 29.7, 29.6, 29.6, 29.5, 25.5, 25.4, 23.2, 23.2, 14.5, 14.4; 31P NMR (D2O, 202 Hz) δ 4.3, −0.6; exact mass calcd for [C25H49O16P2]+ requires m/z 667.2496, found 667.2476 (ESI+); [α]D +2.75 (c 2.0, H2O, pH 9).
ent- PI5P-DiC8
Synthesis and spectral data identical to I5P-DiC8 α] −8.75 (c 2.0, H2O, pH 8).24 D
Figure 1.
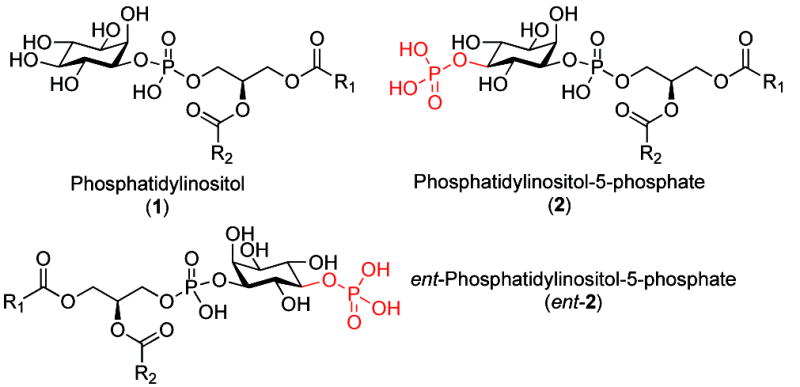
Phosphatidylinositol and its 5-phosphorylated variants.
Acknowledgments
We thank the National Institutes of General Medical Sciences of the National Institutes of Health for support (GM-068649).
Footnotes
Dedicated to Professor John F. Hartwig on the Occasion of his winning the Tetrahedron Young Investigator Award.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2–3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
References and Notes
- 1.Pendaries C, Tronchere H, Racaud-Sultan C, Gaits-Iacovoni F, Coronas S. Adv Enz Reg. 2005;45:201–14. doi: 10.1016/j.advenzreg.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 2.(a) Huang W, Zhang HL, Davrazou F, Kutateladze TG, Shi XB. J Am Chem Soc. 2007;129:6498–506. doi: 10.1021/ja070195b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jones DR, Divecha N. Linking lipids to chromatin. Curr Opin Gen Devel. 2004;14:196–02. doi: 10.1016/j.gde.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Clarke JH, Letcher AJ, D’Santos CS, Halstead JR, Irvine RF. Biochem J. 2001;357:905–10. doi: 10.1042/0264-6021:3570905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez-Venegas R, Sadder M, Hlavacka A, Baluska F, Xia YN, Lu GQ, Firsov A, Sarath G, Moriyama H, Dubrovsky JG, Avramova Z. Proc Nat Acad Sci (USA) 2006;103:6049–054. doi: 10.1073/pnas.0600944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pendaries C, Tronchere H, Arbibe L, Mounier J, Gozani O, Cantley L, Fry MJ, Gaits-Iacovoni F, Sansonetti PJ, Payrastre B. Embo J. 2006;25:1024–034. doi: 10.1038/sj.emboj.7601001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meijer HJ, Berrie CP, Iurisci C, Divecha N, Musgrave A, Munnik T. Biochem J. 2001;360:491–98. doi: 10.1042/0264-6021:3600491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prestwich GD. Chem Biol. 2004;11:619–37. doi: 10.1016/j.chembiol.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 8.For syntheses of PIP-compounds with unsaturated side chains, see: Kubiak RJ, Bruzik KS. J Org Chem. 2003;68:960–68. doi: 10.1021/jo0206418.Gaffney PRJ, Reese CB. J Chem Soc, Perkin Trans 1. 2001:192–05.Watanabe Y, Nakatomi M. Tetrahedron. 1999;55:9743–754.
- 9.For representative syntheses of PI3P-compounds with saturated side chains, see: Morisaki N, Morita K, Nishikawa A, Nakatsu N, Fukui Y, Hashimoto Y, Shirai R. Tetrahedron. 2000;56:2603–614.Falck JR, Krishna UM, Capdevila JH. Bioorg Med Chem Lett. 2000;10:1711–713. doi: 10.1016/s0960-894x(00)00315-2.Painter GF, Grove SJA, Gilbert IH, Holmes AB, Raithby PR, Hill ML, Hawkins PT, Stephens LR. J Chem Soc, Perkins Trans 1. 1999:923–36.Chen J, Feng L, Prestwich GD. J Org Chem. 1998;63:6511–522. doi: 10.1021/jo972046p.Wang DS, Chen CS. J Org Chem. 1996;61:5905–910.Bruzik KS, Kubiak RJ. Tetrahedron Lett. 1995;36:2415–418.
- 10.For representative syntheses of PI3,5P2-compounds with saturated side chains, see: Falck JR, Krishna UM, Katipally KR, Capderila JH, Ulug ET. Tetrahedron Lett. 2000;41:4271–275.Han F, Hayashi M, Watanabe Y. Chem Lett. 2003;32:724–25.Han F, Hayashi M, Watanabe Y. Eur J Org Chem. 2004:558–66.Nishikawa A, Saito S, Hashimoto Y, Koga K, Shirai R. Tetrahedron Lett. 2001;42:9195–198.
- 11.For representative syntheses of PI-compounds, see: Watanabe Y, Kiyosawa Y, Hyodo S, Hayashi M. Tetrahedron Lett. 2005;46:281–84.
- 12.Xu Y, Lee SA, Kutateladze TG, Sprissa D, Shisheva A, Prestwich GD. J Am Chem Soc. 2006;128:885–97. doi: 10.1021/ja0554716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng J, Prestwich GD. Tetrahedron Lett. 1998;39:3965–968. [Google Scholar]
- 14.Falck JR, Krishna UM, Katipally KR, Capdevila JH, Ulug ET. Tetrahedron Lett. 2000;41:4271. [Google Scholar]
- 15.Watanabe Y, Ishikawa H. Tetrahedron Lett. 2000;41:8509. [Google Scholar]
- 16.Sculimbrene BR, Xu Y, Miller SJ. J Am Chem Soc. 2004;126:13182–3183. doi: 10.1021/ja0466098. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Sculimbrene BR, Miller SJ. J Org Chem. 2006;71:4919–928. doi: 10.1021/jo060702s. [DOI] [PubMed] [Google Scholar]
- 18.(a) Sculimbrene BR, Miller SJ. J Am Chem Soc. 2001;123:10125–0126. doi: 10.1021/ja016779+. [DOI] [PubMed] [Google Scholar]; (b) Sculimbrene BR, Morgan AJ, Miller SJ. J Am Chem Soc. 2002;124:11653–1656. doi: 10.1021/ja027402m. [DOI] [PubMed] [Google Scholar]; (c) Sculimbrene BR, Morgan AJ, Miller SJ. Chem Commun. 2003:1781–785. doi: 10.1039/b304015c. [DOI] [PubMed] [Google Scholar]
- 19.For related strategies to various inositol-poly(phosphates), see: Morgan AJ, Komiya S, Xu Y, Miller SJ. J Org Chem. 2006;71:6923–931. doi: 10.1021/jo0610816.
- 20.Mahmoodi NO. Phosphorus, Sulfur, Silicon. 2002;177:2887–893. [Google Scholar]
- 21.Poon KWC, Dudley GB. J Org Chem. 2006;71:3923–3927. doi: 10.1021/jo0602773. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb HE, Kotlyar V, Nudelman A. J Org Chem. 1997;62:7512–515. doi: 10.1021/jo971176v. [DOI] [PubMed] [Google Scholar]
- 23.Still WC, Kahn M, Mitra J. J Org Chem. 1978;43:2923. [Google Scholar]
- 24.We note that the specific rotations of our samples are not exactly opposite in magnitude. This fact is consistent with observations in the literature that report strongly pH-dependent values for optical rotations for phosphoinositides and inositol phosphates. Similarly, the adjustment of the solutions of the samples to exactly the identical pH is difficult, due in part to difficulties in achieving identical ionization states for the samples. See Ozaki S, Kondo Y, Shiotani N, Ogasawara T, Watanabe Y. J Chem Soc, Perkin Trans 1. 1992:729–37.Mayr GW, Dietrich W. FEBS Lett. 1987;213:278–82. doi: 10.1016/0014-5793(87)81505-3.


