Abstract
Purpose of review
WHIM syndrome (WHIM) is a congenital immune deficiency with characteristic clinical features that include: susceptibility to human papilloma virus infection induced warts, condyloma acuminata and carcinomas; neutropenia, B cell lymphopenia and hypogammaglobulinema related recurrent infections; and bone marrow myelokathexis characterized by myeloid hyperplasia and apoptosis. The purpose of this report is to review diagnosis and clinical management, and to highlight new findings about the genetic and biochemical abnormalities that cause WHIM.
Recent findings
Specific mutations identified in WHIM patients include heterozygous C-terminus deletional mutations of portions of the intracellular carboxy terminus of the chemokine receptor, CXCR4. WHIM leukocytes have enhanced responses to SDF1, the cognate ligand of CXCR4. Enhanced activity of CXCR4 delays release of mature neutrophils from the bone marrow resulting in neutropenia and senescence with apoptosis of mature neutrophils retained in the marrow. Recent reports of 2 patients with WHIM who do not have detectable mutations of CXCR4, but whose cells are hyper-responsive to SCF1 raise the possibility that there is more than one genetic basis for WHIM. One patient had low levels of G-protein receptor kinase 3 (GRK3) protein and mRNA, and the functional hyperactivity response to SDF1 was corrected by forced gene transfer mediated excess expression of GRK3, implicating defective GRK3 phosphorylation mediated downregulation of CXCR3 as the cause of WHIM in that patient.
Summary
The main subjects reviewed in this chapter include a detailed characterization of the clinical presentation, diagnosis and treatment of WHIM; advances in understanding the genetic basis of WHIM; and review of new studies which delineate the biochemical consequence of WHIM mutations as resulting in hyperfunction of CXCR4 in response to SDF1.
Keywords: WHIM syndrome, CXCR4, SDF1, myelokathexis, apoptosis, neutropenia, B cell lymphopenia, hypogammaglobulinemia, human papilloma virus, warts, carcinoma, G-protein coupled receptor, G-protein coupled receptor kinase 3
Introduction
WHIM syndrome (WHIM) is an autosomal dominant inheritance immune deficiency that in most kindreds is caused by a gain in function mutation (hyperactivity with failure to down regulate) in CXC chemokine receptor 4 (CXCR4). The name of the syndrome is an acronym derived from major features of the disorder that include, but are not limited to Warts, Hypogammaglobulinemia, recurrent bacterial Infections and Myelokathexis (apoptosis of mature myeloid cells in the marrow) [1-6]. Most, but not all, patients with WHIM are heterozygous carriers of mutations of CXCR4 that cause partial truncations of the carboxyterminal segment of this receptor. When the CXCR4 mutation connection to WHIM was first identified in 2003, it was considered to be the first example of a human disease mediated by dysfunction of a chemokine receptor [7]. In the past two years significant new information has been published about WHIM that provides an increased understanding of CXCR4 signaling. In addition there have been recent reports about clinical problems in patients with WHIM that has added to our understanding of the clinical management of this disorder. These new findings of mechanisms and clinical features of WHIM will be reviewed.
A first step toward understanding the role of CXCR4 in WHIM requires description of structural features of this receptor. CXCR4 is a 352 amino acid member of the G-protein-coupled receptor (GPCR) superfamily, with specificity for the chemokine, stromal cell-derived factor 1 (SDF1; also known as CXCL12) [8-11]. Mice genetically engineered to lack either SDF1 or CXCR4 have similar defects of B-cell lymphopoiesis, bone-marrow myelopoiesis and cardiac septum formation that is associated with a late gestation lethal phenotype [12, 13]. Transcription of CXCR4 is regulated positively by nuclear respiratory factor-1 (NRF-1) and negatively by Ying Yang 1 (YY1) [14-16]. SDF1 binding to CXCR4 leads to activation of pathways that include G-protein mediated and G-protein independent signaling [17-22]. Understanding the regulation of expression and signaling pathways of SDF1 and of its cognate receptor, CXCR4 is likely to be an important area of additional discovery of clinical significance in the next few years. As one example of an area ripe for assessment, we do not know why WHIM patients are particularly susceptible to infection with human papilloma virus (HPV), which in these patients not only causes warts, but also results in condyloma acuminata and dysplastic lesions of the genital-anal mucosa that often progresses to frank carcinoma. Since SDF1 and CXCR4 are present in skin fibroblasts and keratinocytes, respectively, and may affect proliferation of keratinocytes, it is not far-fetched to speculate that HPV infection may impact on regulation of expression or function of SDF1 and/or CXCR4 and that this balance is disturbed in WHIM to favor growth of HPV with proliferation and transformation of epithelium.
Specific mutations of CXCR4 identified in some families with myelokathexis include heterozygous mutations of R334X, S339fs342X, E343X, and G335X, all of which are located in and cause truncations of the intracellular carboxyterminus of CXCR4 [7, 23, 24]. However, as will be discussed in the section on Genetics, there may be other types of genetic lesions affecting CXCR4 signaling that can cause WHIM.
Clinical phenotype
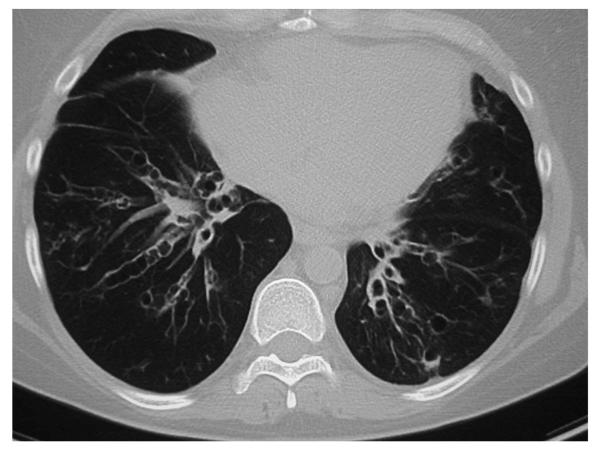
The clinical features of 37 WHIM patients have been reported [1, 3, 6, 7, 24-39]. Pooling information from these diverse reports, the frequency of specific clinical features of WHIM at first presentation for warts, hypogammaglobulinemia, and neutropenia was 78.6% (22/28), 89.6% (26/29), and 91.7% (33/36) of patients, respectively. The clinical features which manifest in a particular patient and the age of initial diagnosis is variable. All patients from early childhood suffered from recurrent infections that include pneumonias, sinusitis, cellulitis, urinary tract infection, thrombophlebitis, omphalitis, osteomyelitis, deep soft tissue abscesses, and skin infections. Common pathogens include, but are not limited to, Haemophilus influenzae, Streptococcus pneumoniae, Klebsiella pneumoniae, Staphylococcus aureus, and Proteus mirabilis. Recurrent pneumonias may in some cases lead to severe bronchiectasis (Figure 1). Those patients who develop bronchiectasis may also suffer from chronic infection with Pseudomonas aerugenosa or Burkhoderia cepacia. The immune deficiency in WHIM is manifested primarily by neutropenia, B cell lymphopenia and hypogammaglobulinemia. As expected, WHIM patients have greater problem with bacterial infections (particularly with encapsulated organisms) than with virus infections. Patients with WHIM who are appropriately managed with granulocyte colony stimulating factor (G-CSF) and periodic administration of intravenous gamma globulin (IVIG) appear to have little problem resolving infections with influenza, parainfluenza, herpes simplex, herpes zoster, and common respiratory viruses. However, there is likely a problem with control of Epstein-Barr Virus, because there are two reports of patients with WHIM syndrome developing EBV-related lymphoproliferative disorders [33, 38].
Figure 1. Severe Bronchiectasis in WHIM Syndrome.
This is a computerized axial tomography radiologic exam of the chest of an adult patient with WHIM syndrome. This patient had frequent pneumonias and sinusitis since childhood, and demonstrates development of a significant degree of bilateral bronchiectasis.
WHIM patients with warts characteristically have numerous warts on hands, feet and trunk. They may also have genital and anal condyloma acuminata, and in female patients significant cervical and vulval dysplasia. Mucosal lesions often progress to frank carcinoma. All of these lesions are associated with human papilloma virus (HPV) infection. The susceptibility to HPV and the resultant degree of pathology from this pathogen is very much out of proportion to the fact that there is not much increase in general susceptibility to virus infection in WHIM. Furthermore, the degree of HPV related disease in WHIM is even highly disproportionate to the level of problems with HPV seen in severe T lymphocyte immunodeficiency disorders such as severe combined immune deficiency where there is a very significant increased susceptibility overall to infection with viruses. Thus, there appears to be an unique and specific failure of immune control of progression of HPV infection in WHIM. It is possible to speculate that the C-terminal truncating mutations of CXCR4 in WHIM which enhance activity of the host CXCR4/SDF1 axis in skin and mucosa may play a role in facilitating HPV infection that is detrimental to host defense.
Hematological findings
The bone marrow of WHIM patients has a marked hyperplasia with many cells demonstrating characteristic abnormalities of cytoplasmic vacuolization, hypersegmented nuclei, and chromatin hypercondensation consistent with apoptosis [3]; a histologic pattern that in the older medical literature has been termed myelokathexis. Paradoxically, this increase of myeloid elements that includes excessive numbers of apoptotic neutrophils in the bone marrow of patients with WHIM is associated with a very significant decrease of absolute counts of neutrophils in the peripheral blood. The clinical characteristics of the neutropenia observed in WHIM patients differs from that in some other types of congenital neutropenia in that acute infection, stimulation with granulocyte- and granulocyte-monocyte-colony stimulating factors (G-CSF, GM-CSF), epinephrine, or glucocorticoids induce a prompt release of neutrophils from the marrow that within hours may reach normal levels [32, 40]. This clinical observation together with the histological features of the bone marrow has suggested that the neutropenia might be a defect in marrow release of neutrophils rather than a production defect, though until recently definitive delineation of mechanism was lacking.
A very significant B cell lymphopenia is a common finding, and many patients may also manifest a decrease in numbers of T lymphocytes. However, the relative proportions of the main T lymphocyte subsets appears normal, and in vitro tests of T lymphocyte proliferation to mitogens are normal [24, 34, 37]. B-lymphocyte counts in patients with WHIM are profoundly depressed with a particularly severe reduction of circulating CD27+ memory B cells that affect both un-switched (IgD+) and switched (IgD-) CD19 B lymphocytes [24]. The significant hypogammaglobulinemia most frequently involves IgG, but may also affect IgM. A likely clinical manifestation of this is the observed recurrent sinusitis, upper respiratory infections and pneumonias with encapsulated bacteria and the development of significant bronchiectasis in a subset of patients (Figure 1). Patients are clearly helped by treatment with IVIG. However, WHIM patients do have significant measureable responses to a clinically beneficial level to active immunizations indicating that the defect in humeral immunity is not complete [7, 24, 34]. More specifically, it has been reported that a WHIM patient immunized with tetanus-toxoid vaccine initially produced normal levels of specific antibody, but serum levels of anti-tetanus-toxoid immunoglobulin became undetectable after a year [24]. This suggests that WHIM patients have normal capacity to produce specific antibody against antigens, but fail to maintain antibody production, which is manifested by a reduction of circulating CD27+ memory B cells.
Diagnosis
The combination of recurrent bacterial infections and recalcitrant warts are a reliable sign of a primary immune deficiency that may include WHIM in the differential diagnosis, but is not itself pathognomonic of WHIM. When initial medical workup of immune function also detects neutropenia, lymphopenia and hypogammaglobulinemia, the diagnosis of WHIM should be strongly considered and the next step should be evaluation of a bone marrow biopsy. If that biopsy shows any hint of myelokathexis, then sequencing of the CXCR4 gene should be performed looking for a characteristic mutation. G-CSF treatment is not only clinically beneficial, but a rapid response within hours to injection of relatively low doses of G-CSF can assist in providing additional supporting evidence for a diagnosis of WHIM. As will be discussed below, there appear to be patients with all the clinical features of WHIM in whom mutations of CXCR4 cannot be found, indicating that there may be other genetic causes of WHIM. Thus, a patient presenting with most features of WHIM including myelokathexis may be given a presumptive diagnosis of WHIM for purposes of clinical management, even if sequencing of CXCR4 finds no mutation.
Clinical diagnosis of WHIM is also made more difficult by the fact that some patients may only manifest a subset of the expected features of this disorder. There are a few patients, usually younger patients, with WHIM who at time of diagnosis may have few or no warts. Conversely, it is important to note that the majority of patients presenting with severe widespread warts, but lacking other clinical features of WHIM likely have other cause for their warts and do not have WHIM. Also, many WHIM patients have a family history suggesting autosomal dominant inheritance of the features of the disorder.
Treatment and Prognosis
The prognosis for WHIM patients depends in part on early recognition of the disorder, with aggressive medical intervention to reduce the frequency of recurrent bacterial infections and to detect and extirpate in the early stages any HPV lesions that appear to be dysplastic or malignant. While both G-CSF and GM-CSF have been used to increase and maintain circulating neutrophil counts in the normal range, G-CSF is probably the preferred and best tolerated agent. The mechanism of action may in part involve, though though likely is not limited to, elevating neutrophil counts in a positive autofeedback loop that involves enhanced release of neutrophil elastase [39, 41-43]. Neutrophil elastase cleaves SDF1 and CXCR4, thus reducing the activity of the SDF1/CXCR4 axis and thus enhancing release of mature neutrophils from the bone marrow to the peripheral blood [44, 45]. Dosing of G-CSF should be determined empirically for each patient, and even then adjustments may be needed periodically (in children as they grow; or in adults because of other factors). Reasonable starting dosing is human recombinant G-CSF at 3 micrograms per kg administered subcutaneously every other day, with a goal to adjust dosing to keep absolute neutrophil count in the circulation at or above 1500 per microliter at the nadir of the alternate day.
With hypogammaglobulinemia, administration of IVIG is effective at decreasing risk of infections [1, 36]. Dosing may be every six weeks with a goal to achieve levels at or above the lower limit of the laboratory reference normal at nadir. It has been reported that the hypogammaglobulinemia may improve following treatment with G-CSF [32]. For that reason, it is important to periodically evaluate the nadir and adjust IVIG dosing. Use of prophylactic antibiotics in WHIM patients has not been evaluated statistically, but it is not unreasonable to extrapolate from studies in other primary immune deficiencies, neutropenias or hypogammaglobulinemic states to support the use of antibiotic prophylaxis. A suggested regimen is daily trimethaprim-sulfamethoxazole to cover a broad range of encapsulated bacteria that include pneumococcus and hemophilus together with anti-staphylococcus activity. There is a risk, particularly in children that in some individuals the trimethaprim-sulfamethoxazole may adversely impact the neutropenia. Extended spectrum oral cephalosporin is an alternative.
Early aggressive diagnostic and therapeutic intervention for any suspected infection is essential. Pneumonias are common, and a subset of patients may develop significant bronchiectasis (Figure 1). Patients with extensive bronchiectasis benefit from advice and management by a pulmonary specialist because many of the interventions designed to manage patients with cystic fibrosis can be of benefit to these patients.
Mortality from infection in WHIM appears to be low in closely managed patients that are on G-CSF, IVIG and prophylactic antibiotics, but lethal meningitis and septicemia have been reported. However, cancer is a significant cause of premature mortality in WHIM syndrome. The role of HPV and carcinoma will be discussed just below. Given the small number of reported cases of WHIM syndrome in the literature overall, it is highly significant that there are two cases of EBV B cell lymphomas reported [33, 38] where one patient died from this lymphoproliferative disorder [38].
WHIM patients with significant HPV lesions of mucosal and transitional areas of skin are at very significant risk of invasive destructive and eventually metastatic carcinomas. These HPV related carcinomas are a significant cause of morbidity and premature mortality in WHIM. These lesions may be recurrent; and extraordinary clinical vigilance is required with frequent assessment by a dermatologist and in female patients by a gynecologist. There must be a low threshold for biopsy of any suspect pathology with full surgical removal of any malignant or pre-malignant lesions. The availability of recombinant protein vaccines effective against those HPV serotypes responsible for causing mucosal cancers raises the question of whether early vaccination might help to reduce HPV related malignancies in patients with WHIM. However, the B cell defect in these patients may reduce the effectiveness or duration of protection, and may require periodic re-immunization. This is an issue that should be studied in a prospective clinical protocol.
Genetics
The first report of the heterozygous carriage of a mutation in CXCR4 as the basis of autosomal dominant WHIM was reported in 2003 [7]. Since then results of sequencing have been reported for 26 patients with clinical features of WHIM (Table 1) [1, 3, 6, 7, 24-39], though in two of these patients no specific mutation was identified [25, 46]. One of these mutations R334X was found in 15 patients, the 4 other mutations being represented in only 2-3 each of the remaining patients. It is of note that all of the reported mutations in CXCR4 found in 24 WHIM patients result in a premature stop codon that causes a truncation of the cytoplasmic C-terminus portion of the receptor. The C-terminal region of CXCR4 is known to contain canonical phosphorylation sites that by sequence homology are likely targets of G-protein coupled receptor kinases (GPRKs) involved in ligand-induced GPCR endocytic internalization from the cell surface, a physiological negative feedback mechanism [47]. As will be discussed in greater detail below in the section on Biological Dysfunction, this type of lesion would be predicted to enhance and prolong the function of CXCR4 upon stimulation with SDF1, leading to a dominant gain of function affect as an explanation for the observed autosomal dominant inheritance.
Table 1.
Results extracted from a review of the literature of sequencing CXCR4 in 26 patients with clinical features of WHIM syndrome.
| Site of Mutation in CXCR4 Protein | # of Patients with Mutation |
|---|---|
| R334X | 15 |
| G336X | 2 |
| S338X | 3 |
| S339fs342X | 2 |
| E343X | 2 |
| No mutation | 2 |
Biological dysfunction
SDF binding to CXCR4 with SDF1 triggers a cascade of processes common to GCPRs [48] that includes conformational change in the receptor [49] and the uncoupling from G-proteins that induces a host of downstream effects. The uncoupling of G-proteins is associated with activation of the heterotrimeric Gαβγ-protein complex that mediates signal transduction and induces downstream cellular responses [50]. At the same time negative feedback loops are activated which include induction of GPRK and protein kinase C mediated phosphorylation of the C-terminus cytoplasmic domain of CXCR4. This phosphorylation results in binding of β-arrestins to CXCR4 and interaction with C-terminal sequences, resulting in down regulation through endocytic internalization of receptor and desensitization to ligand stimulation [22, 51, 52].
It is known that ligand blocking antibody to CXCR4 or the specific small molecule inhibitor of CXCR4, AMD3100, induces mobilization of neutrophils from bone marrow [53, 54], suggesting that CXCR4 plays a role in retention of mature neutrophils in the marrow. It has been reported that both neutrophils and T lymphocytes from patients with WHIM that have C-terminal truncations of CXCR4 demonstrate enhanced chemotactic responses to SDF1 [24, 25, 55]. These findings raised the possibility that the enhanced function of WHIM mutant CXCR4 might be responsible for retention of otherwise mature neutrophils in the bone marrow beyond the time when they should be released into the circulation. Recently, this hypothesis was tested in a NOD/SCID mouse human hematopoietic stem cell xenograft model. In that study normal human CD34+ hematopoietic stem cells were forced to express the WHIM R334X mutant CXCR4 by gamma retrovirus mediated gene transfer and transplanted into the NOD/SCID mouse. Neutrophils arising from the R334X CXCR4 transduced human stem cells in the marrow of the NOD/SCID mice, demonstrated significantly decreased release from the xenograft marrow into the circulation than controls, and the WHIM R334X neutrophils retained in the marrow demonstrated increased rates of apoptosis [56]. However, when the normal human CD34+ hematopoietic stem cells forced to express WHIM R334X mutant CXCR4 were instead differentiated in vitro into mature neutrophils in tissue culture there was no enhancement of apoptosis compared to controls. These experiments show that increased number of apoptotic mature myeloid cells in WHIM is secondary to a failure of marrow release of neutrophils. The mature neutrophils in WHIM are instead retained in the marrow past their normal time of release where they progress to senescence and apoptosis in that location rather than in the peripheral tissues or spleen as normally the case.
In related studies, when hematopoietic tissue culture cells or primary human CD34+ cells were forced to express WHIM R334X mutant CXCR4 by gene transfer, the cells exhibited enhanced chemotactic and calcium efflux responses to SDF1, and defects in down regulation related internalization of receptor from the cell surface [57]. All of these findings are consistent with the well established role for the C-terminal cytoplasmic domain of GPCRs, including CXCR4, in regulating ligand mediated desensitization [58] and endocytic internalization/recycling of receptor [7, 24, 57]. Recent detailed biochemical studies suggest that C-terminal truncation of CXCR2 may actually result in enhanced binding of ß-arrestin to regions of the receptor other than the missing C-terminus, but that paradoxically in the absence of the usual additional interactions between ß-arrestin and the missing critical C-terminal regions the result is prolonged activation of the receptor with failure to internalize receptor via endocytosis [55].
Additional evidence connecting a decrease in ligand mediated downregulation of CXCR4 as a cause of WHIM comes from detailed studies of 2 unrelated patients with all of the characteristic clinical features of WHIM who lack detectable mutations in CXCR4 (Table 1). Cells from these patients demonstrate hyperactive responses to SDF1 [25, 46]. Furthermore, in cells from one of these two patients there appeared to be a marked decreased of GRK3 protein and mRNA [25, 46]. Gene transfer mediated expression of GRK3 in this patient’s fibroblasts or leukocytes restored to normal levels the excessive chemotactic response and the ligand mediated internalization of CXCR4 in response to SDF1. This demonstrates that the critical shared biochemical feature common to WHIM patients with C-terminal truncating mutations of CXCR4 and to those WHIM patients who lack detectable mutations in CXCR4 is the gain in function hyperactivity of CXCR4 in response to SDF1. This provides strong supportive evidence that WHIM may be broadly characterized as an immune deficiency disease of functional hyperactivity of CXCR4.
Conclusions
WHIM is an inherited immune deficiency characterized by neutropenia, B cell lymphopenia, myelokathexis, hypogammaglobulinemia, recurrent infections, and a marked susceptibility to human papilloma virus infection with resultant warts, condyloma acuminata and invasive mucosal carcinomas. There is emerging evidence that WHIM syndrome results from functional hyperactivity of CXCR4. The great majority of patients have a heterozygous gain in function C-terminus truncation mutation of CXCR4, though there may be patients manifesting WHIM due to alternate genetic causes of increased activity of CXCR4. The pathophysiologic mechanism by which increased activity of CXCR4 affects B cell number and function is not known, nor is there yet an understanding of how this leads to enhanced susceptibility to HPV infection. Recent studies suggest that enhanced activity of CXCR4 on mature neutrophils in the bone marrow may prevent their release from the marrow, resulting in peripheral blood neutropenia. The normal progression to senescence of excess neutrophils retained in the marrow is likely responsible for the increased numbers of apoptotic neutrophils in the bone marrow observed histologically as myelokathexis. Current clinical management of WHIM includes treatment with G-CSF, IVIG, prophylactic antibiotics, and aggressive surveillance for and surgical extirpation of dysplastic skin and mucosal HPV related lesions. Given that many of the clinical problems affecting WHIM may be a consequence of hyperfunction of CXCR4, we propose that chronic treatment of WHIM patients with a potent inhibitor of CXCR4 function such as Plerixafor (MOBOZIL®; AMD3100) should be studied in an experimental clinical protocol.
References and recommended reading
Papers of particular interest *, and papers of outstanding interest **, published within the annual period of review have been noted together with commentary to follow:
- 1.Wetzler M, Talpaz M, Kleinerman ES, King A, Huh YO, Gutterman JU, Kurzrock R. A new familial immunodeficiency disorder characterized by severe neutropenia, a defective marrow release mechanism, and hypogammaglobulinemia. Am J Med. 1990;89:663. doi: 10.1016/0002-9343(90)90187-i. [DOI] [PubMed] [Google Scholar]
- 2.Zuelzer WW. “Myelokathexis”--a New Form of Chronic Granulocytopenia. Report of a Case. N Engl J Med. 1964;270:699. doi: 10.1056/NEJM196404022701402. [DOI] [PubMed] [Google Scholar]
- 3.Gorlin RJ, Gelb B, Diaz GA, Lofsness KG, Pittelkow MR, Fenyk JR., Jr. WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. Am J Med Genet. 2000;91:368. [PubMed] [Google Scholar]
- 4.Roland J, Murphy BJ, Ahr B, Robert-Hebmann V, Delauzun V, Nye KE, Devaux C, Biard-Piechaczyk M. Role of the intracellular domains of CXCR4 in SDF-1-mediated signaling. Blood. 2003;101:399. doi: 10.1182/blood-2002-03-0978. [DOI] [PubMed] [Google Scholar]
- 5.Bassan R, Viero P, Minetti B, Comotti B, Barbui T. Myelokathexis: a rare form of chronic benign granulocytopenia. Br J Haematol. 1984;58:115. doi: 10.1111/j.1365-2141.1984.tb06065.x. [DOI] [PubMed] [Google Scholar]
- 6.O’Regan S, Newman AJ, Graham RC. ‘Myelokathexis’. Neutropenia with marrow hyperplasia. Am J Dis Child. 1977;131:655. doi: 10.1001/archpedi.1977.02120190049011. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, Diaz GA. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34:70. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- 8.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 9.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 10.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 11.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145. [PubMed] [Google Scholar]
- 12.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 13.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 14.Moriuchi M, Moriuchi H, Margolis DM, Fauci AS. USF/c-Myc enhances, while Yin-Yang 1 suppresses, the promoter activity of CXCR4, a coreceptor for HIV-1 entry. J Immunol. 1999;162:5986. [PubMed] [Google Scholar]
- 15.Moriuchi M, Moriuchi H, Turner W, Fauci AS. Cloning and analysis of the promoter region of CXCR4, a coreceptor for HIV-1 entry. J Immunol. 1997;159:4322. [PubMed] [Google Scholar]
- 16.Wegner SA, Ehrenberg PK, Chang G, Dayhoff DE, Sleeker AL, Michael NL. Genomic organization and functional characterization of the chemokine receptor CXCR4, a major entry co-receptor for human immunodeficiency virus type 1. J Biol Chem. 1998;273:4754. doi: 10.1074/jbc.273.8.4754. [DOI] [PubMed] [Google Scholar]
- 17.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 18.Vila-Coro AJ, Rodriguez-Frade JM, De Ana A Martin, Moreno-Ortiz MC, Martinez AC, Mellado M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. Faseb J. 1999;13:1699. [PubMed] [Google Scholar]
- 19.Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD. Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci U S A. 2002;99:7478. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 22.Cheng ZJ, Zhao J, Sun Y, Hu W, Wu YL, Cen B, Wu GX, Pei G. beta-arrestin differentially regulates the chemokine receptor CXCR4-mediated signaling and receptor internalization, and this implicates multiple interaction sites between beta-arrestin and CXCR4. J Biol Chem. 2000;275:2479. doi: 10.1074/jbc.275.4.2479. [DOI] [PubMed] [Google Scholar]
- 23.Gulino AV. WHIM syndrome: a genetic disorder of leukocyte trafficking. Curr Opin Allergy Clin Immunol. 2003;3:443. doi: 10.1097/00130832-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Gulino AV, Moratto D, Sozzani S, Cavadini P, Otero K, Tassone L, Imberti L, Pirovano S, Notarangelo LD, Soresina R, Mazzolari E, Nelson DL, Notarangelo LD, Badolato R. Altered leukocyte response to CXCL12 in patients with warts hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome. Blood. 2004;104:444. doi: 10.1182/blood-2003-10-3532. [DOI] [PubMed] [Google Scholar]
- 25.Balabanian K, Lagane B, Pablos JL, Laurent L, Planchenault T, Verola O, Lebbe C, Kerob D, Dupuy A, Hermine O, Nicolas JF, Latger-Cannard V, Bensoussan D, Bordigoni P, Baleux F, Le Deist F, Virelizier JL, Arenzana-Seisdedos F, Bachelerie F. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105:2449. doi: 10.1182/blood-2004-06-2289. [DOI] [PubMed] [Google Scholar]
- 26.Hagan JB, Nguyen PL. WHIM syndrome. Mayo Clin Proc. 2007;82:1031. doi: 10.4065/82.9.1031.A case report of WHIM syndrome with R334X mutation of CXCR4 showing photos of typically characteristic skin warts and marrow myelokathexis..
- 27.Alapi K, Erdos M, Kovacs G, Marodi L. Recurrent CXCR4 sequence variation in a girl with WHIM syndrome. Eur J Haematol. 2007;78:86. doi: 10.1111/j.1600-0609.2006.00779.x.This is the first report of S338X mutation causing WHIM syndrome.
- 28.Tarzi MD, Jenner M, Hattotuwa K, Faruqi AZ, Diaz GA, Longhurst HJ. Sporadic case of warts, hypogammaglobulinemia, immunodeficiency, and myelokathexis syndrome. J Allergy Clin Immunol. 2005;116:1101. doi: 10.1016/j.jaci.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 29.Taniuchi S, Masuda M, Fujii Y, Izawa K, Kanegane H, Kobayashi Y. The role of a mutation of the CXCR4 gene in WHIM syndrome. Haematologica. 2005;90:1271. [PubMed] [Google Scholar]
- 30.Mentzer WC, Jr., Johnston RB, Jr., Baehner RL, Nathan DG. An unusual form of chronic neutropenia in a father and daughter with hypogammaglobulinaemia. Br J Haematol. 1977;36:313. doi: 10.1111/j.1365-2141.1977.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 31.Christ MJ, Dillon CA. Myelokathexis in a mother and infant: a second case suggesting dominant inheritance. Mil Med. 1997;162:827. [PubMed] [Google Scholar]
- 32.Hord JD, Whitlock JA, Gay JC, Lukens JN. Clinical features of myelokathexis and treatment with hematopoietic cytokines: a case report of two patients and review of the literature. J Pediatr Hematol Oncol. 1997;19:443. doi: 10.1097/00043426-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Chae KM, Ertle JO, Tharp MD. B-cell lymphoma in a patient with WHIM syndrome. J Am Acad Dermatol. 2001;44:124. doi: 10.1067/mjd.2001.111337. [DOI] [PubMed] [Google Scholar]
- 34.Taniuchi S, Yamamoto A, Fujiwara T, Hasui M, Tsuji S, Kobayashi Y. Dizygotic twin sisters with myelokathexis: mechanism of its neutropenia. Am J Hematol. 1999;62:106. doi: 10.1002/(sici)1096-8652(199910)62:2<106::aid-ajh8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 35.Aprikyan AA, Liles WC, Park JR, Jonas M, Chi EY, Dale DC. Myelokathexis, a congenital disorder of severe neutropenia characterized by accelerated apoptosis and defective expression of bcl-x in neutrophil precursors. Blood. 2000;95:320. [PubMed] [Google Scholar]
- 36.Goddard EA, Hughes EJ, Beatty DW. A case of immunodeficiency characterized by neutropenia, hypogammaglobulinaemia, recurrent infections and warts. Clin Lab Haematol. 1994;16:297. doi: 10.1111/j.1365-2257.1994.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 37.Arai J, Wakiguchi H, Hisakawa H, Kubota H, Kurashige T. A variant of myelokathexis with hypogammaglobulinemia: lymphocytes as well as neutrophils may reverse in response to infections. Pediatr Hematol Oncol. 2000;17:171. doi: 10.1080/088800100276532. [DOI] [PubMed] [Google Scholar]
- 38.Imashuku S, Miyagawa A, Chiyonobu T, Ishida H, Yoshihara T, Teramura T, Kuriyama K, Imamura T, Hibi S, Morimoto A, Todo S. Epstein-Barr virus-associated T-lymphoproliferative disease with hemophagocytic syndrome, followed by fatal intestinal B lymphoma in a young adult female with WHIM syndrome. Warts, hypogammaglobulinemia, infections, and myelokathexis. Ann Hematol. 2002;81:470. doi: 10.1007/s00277-002-0489-9. [DOI] [PubMed] [Google Scholar]
- 39.Hess U, Ganser A, Schnurch HG, Seipelt G, Ottmann OG, Falk S, Schulz G, Hoelzer D. Myelokathexis treated with recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) Br J Haematol. 1992;80:254. doi: 10.1111/j.1365-2141.1992.tb08910.x. [DOI] [PubMed] [Google Scholar]
- 40.Cernelc P, Andoljsek D, Mlakar U, Pretnar J, Modic M, Zupan IP, Zver S. Effects of molgramostim, filgrastim and lenograstim in the treatment of myelokathexis. Pflugers Arch. 2000;440:R81. [PubMed] [Google Scholar]
- 41.Wetzler M, Talpaz M, Kellagher MJ, Gutterman JU, Kurzrock R. Myelokathexis: normalization of neutrophil counts and morphology by GM-CSF. Jama. 1992;267:2179. [PubMed] [Google Scholar]
- 42.Weston B, Axtell RA, Todd RF, 3rd, Vincent M, Balazovich KJ, Suchard SJ, Boxer LA. Clinical and biologic effects of granulocyte colony stimulating factor in the treatment of myelokathexis. J Pediatr. 1991;118:229. doi: 10.1016/s0022-3476(05)80488-3. [DOI] [PubMed] [Google Scholar]
- 43.Bohinjec J, Andoljsek D. Neutrophil-releasing activity of recombinant human granulocyte-macrophage colony stimulating factor in myelokathexis. Br J Haematol. 1992;82:169. doi: 10.1111/j.1365-2141.1992.tb04609.x. [DOI] [PubMed] [Google Scholar]
- 44.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 45.Valenzuela-Fernandez A, Planchenault T, Baleux F, Staropoli I, Le-Barillec K, Leduc D, Delaunay T, Lazarini F, Virelizier JL, Chignard M, Pidard D, Arenzana-Seisdedos F. Leukocyte elastase negatively regulates Stromal cell-derived factor-1 (SDF-1)/CXCR4 binding and functions by amino-terminal processing of SDF-1 and CXCR4. J Biol Chem. 2002;277:15677. doi: 10.1074/jbc.M111388200. [DOI] [PubMed] [Google Scholar]
- 46.Balabanian K, Levoye A, Klemm L, Lagane B, Hermine O, Harriague J, Baleux F, Arenzana-Seisdedos F, Bachelerie F. Leukocyte analysis from WHIM syndrome patients reveals a pivotal role for GRK3 in CXCR4 signaling. J Clin Invest. 2008;118:1074. doi: 10.1172/JCI33187.This manuscript provides compelling evidence that GPCR kinase-3 (GRK3) specifically regulates SDF1 mediated internalization and desensitization of CXCR4. Cells from two patients with WHIM syndrome who lack mutations of CXCR4 have excessive activity of CXCR4 that is corrected by gene transfer mediated expression of GRK3. One of the patient’s cells demonstrates decreased amounts of GRK3 protein and mRNA.
- 47.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 48.Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, Virelizier JL, Baggiolini M, Sykes BD, Clark-Lewis I. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. Embo J. 1997;16:6996. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang X, Shen J, Cui M, Shen L, Luo X, Ling K, Pei G, Jiang H, Chen K. Molecular dynamics simulations on SDF-1alpha: binding with CXCR4 receptor. Biophys J. 2003;84:171. doi: 10.1016/S0006-3495(03)74840-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007;1768:952. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orsini MJ, Parent JL, Mundell SJ, Marchese A, Benovic JL. Trafficking of the HIV coreceptor CXCR4. Role of arrestins and identification of residues in the c-terminal tail that mediate receptor internalization. J Biol Chem. 1999;274:31076. doi: 10.1074/jbc.274.43.31076. [DOI] [PubMed] [Google Scholar]
- 52.Haribabu B, Richardson RM, Fisher I, Sozzani S, Peiper SC, Horuk R, Ali H, Snyderman R. Regulation of human chemokine receptors CXCR4. Ro phosphorylation in desensitization and internalization. J Biol Chem. 1997;272:28726. doi: 10.1074/jbc.272.45.28726. [DOI] [PubMed] [Google Scholar]
- 53.Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, Gonzalo JA, Henson PM, Worthen GS. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood. 2004;104:565. doi: 10.1182/blood-2003-10-3638. [DOI] [PubMed] [Google Scholar]
- 54.Hendrix CW, Collier AC, Lederman MM, Schols D, Pollard RB, Brown S, Jackson JB, Coombs RW, Glesby MJ, Flexner CW, Bridger GJ, Badel K, MacFarland RT, Henson GW, Calandra G. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J Acquir Immune Defic Syndr. 2004;37:1253. doi: 10.1097/01.qai.0000137371.80695.ef. [DOI] [PubMed] [Google Scholar]
- 55.Lagane B, Chow KY, Balabanian K, Levoye A, Harriague J, Planchenault T, Baleux F, Gunera-Saad N, Arenzana-Seisdedos F, Bachelerie F. CXCR4 dimerization and {beta}-arrestin-mediated signaling account for the enhanced chemotaxis to CXCL12 in WHIM syndrome. Blood. 2008;112:34. doi: 10.1182/blood-2007-07-102103.This provides detailed biochemical analysis showing that augmented responsiveness of WHIM leukocytes is in part accounted for by enhanced beta arrestin 2-dependent signaling downstream of the truncated CXCR4 receptor. They demonstrate that mutant CXCR4 spontaneously forms heterodimers with wild-type CXCR4 leading to enhanced functional interactions between beta arrestin 2 that paradoxically prolong activity rather than downregulate activity of the receptor when C-terminal regions are missing.
- 56.Kawai T, Choi U, Cardwell L, DeRavin SS, Naumann N, Whiting-Theobald NL, Linton GF, Moon J, Murphy PM, Malech HL. WHIM syndrome myelokathexis reproduced in the NOD/SCID mouse xenotransplant model engrafted with healthy human stem cells transduced with C-terminus-truncated CXCR4. Blood. 2007;109:78. doi: 10.1182/blood-2006-05-025296.This is the first time that the myelokathexis of WHIM has been reproduced in an animal model. It is a NOD/SCID mouse marrow xenograft model using a human hematopoietic stem cell xenograft forced to express the R338X mutant CXCR4. The model demonstrates both the neutopenia and excessive apoptosis of matured myeloid elements in the marrow.
- 57.Kawai T, Choi U, Whiting-Theobald NL, Linton GF, Brenner S, Sechler JM, Murphy PM, Malech HL. Enhanced function with decreased internalization of carboxy-terminus truncated CXCR4 responsible for WHIM syndrome. Exp Hematol. 2005;33:460. doi: 10.1016/j.exphem.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Futahashi Y, Komano J, Urano E, Aoki T, Hamatake M, Miyauchi K, Yoshida T, Koyanagi Y, Matsuda Z, Yamamoto N. Separate elements are required for ligand-dependent and -independent internalization of metastatic potentiator CXCR4. Cancer Sci. 2007;98:373. doi: 10.1111/j.1349-7006.2007.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]