Abstract
An efficient method has been developed to synthesize casimiroin (1), a component of the edible fruit of Casimiroa edulis, on a multigram scale in good overall yield. The route was versatile enough to provide an array of compound 1 analogues that were evaluated as QR2 and aromatase inhibitors. In addition, X-ray crystallography studies of QR2 in complex with compound 1 and one of its more potent analogs has provided insight into the mechanism of action of this new series of QR2 inhibitors. The initial biological investigations suggest that compound 1 and its analogues merit further investigation as potential chemopreventive or chemotherapeutic agents.
Introduction
The medicinal values of zapote blanco, an edible fruit of Casimiroa edulis Llave et Lex (Rutaceae), have been proposed for many years. Our continuous efforts to discover chemopreventive agents from natural products led us to perform a study on casimiroin (1), a key constituent of the seeds of zapote blanco, that showed the ability to inhibit mutagenicity induced by 7,12-dimethylbenz[a]anthracene (DMBA) in Salmonella typhimurium strain TM677, as well as inhibition of the formation of DMBA-induced preneoplastic lesions in mouse mammary gland organ culture (MMOC).1 Compound 1, therefore, has the potential to inhibit carcinogenesis. The goals of the present investigation were to devise and execute a practical synthesis of compound 1 that would afford larger quantities for advanced biological testing and also to optimize the potential of compound 1 analogues as inhibitors of QR2 and aromatase (CYP19A1).
Quinone reductase 2 (QR2), a cytosolic FAD-dependent flavoenzyme that catalyzes metabolic reduction of quinones with unexpected co-substrate specificity, is expressed in various organs including heart, liver, skeletal muscle and kidney.2 It has been described as an enzyme of surprises and mysteries,3 and recent studies have revealed that genetic polymorphisms of QR2 are associated with a number of neurological diseases such as Parkinson’s, schizophrenia, and others.4–6 QR2 also has a unique relationship with quinoline-containing antimalarial drugs,7 and it binds melatonin, which has led to its classification as the third melatonin receptor binding site or MT3.8 QR2 shares 49% sequence identity with the NAD(P)H-dependent quinone reductase 1 (QR1), but does not recognize NADH or NAD(P)H as co-substrates. Instead, it catalyses the oxidation of reduced N-alkyl- and N-ribosylnicotinamides by menadione and other quinones. Because of its high similarity in both primary amino acid sequence and tertiary structure to QR1, it is believed that QR2 might also serve as a detoxification enzyme that can produce hydroquinones that are less toxic to cells compared to the parent quinones. However, recent studies have indicated that QR2 may actually transform certain quinone substrates into more highly reactive species that are capable of causing more cellular damage.9–11 Therefore, it is hypothesized that inhibition of QR2 in certain cases may lead to protection of cells against these reactive species. Melatonin, a known anti-oxidant, inhibits QR2 and its X-ray structure in complex with QR2 has been determined.6 Coenzyme Q0, another anti-oxidant, has been shown to be an efficient substrate of the QR2-catalyzed reaction.12 These observations suggest a mysterious link between QR2 and cellular anti-oxidant status. Several QR2 inhibitors have been reported in the literature.13 QR2 is inhibited by a variety of natural polyphenols,12 and the X-ray crystal structure of QR2 in complex with the putative chemopreventive agent resveratrol, a potent inhibitor of the enzyme, has been determined.6,14 These latter observations motivated the present study.
Aromatase, a key cytochrome P450 enzyme, catalyzes the rate-limiting aromatization step in the conversion of androgens (testosterone and and rostenedione) to estrogens (estradiol and estrone). Aromatase inhibitors decrease bioavailable estrogen and have been shown to have considerable antitumor activity in certain breast cancers.15 Such compounds ultimately reduce estradiol receptor stimulation and reduce formation of genotoxic estrogen metabolites.16 Certain nonsteroidal aromatase inhibitors are already in clinical use for the treatment of breast cancer, and several naturally occurring nonsteroidal aromatase inhibitors have shown promising chemopreventive activity.17
In our group, QR2 and aromatase have been targeted for identification of naturally occurring chemopreventive agents.6 Our preliminary observation of the antimutagenic activity of compound 1,1 along with the limited quantities of compound 1 from natural sources, led us to synthesize compound 1 and its analogues for further biological testing. This report discloses a new and improved total synthesis of compound 1, the discovery of its QR2 and aromatase inhibitory activities, and the optimization of these activities resulting in a new series of potent inhibitors of both enzymes. In addition, X-ray crystallography studies of QR2 in complex with compound 1 and a more potent analog are described. These studies have provided insight into the mechanism of QR2 inhibition by compound 1 and related compounds.
Results and Discussion
Chemistry
Although a synthesis of compound 1 has been reported in the literature, it has a month-long critical cyclization step that provides the desired intermediate as a minor product in only 10% yield, resulting in a very poor overall yield (0.67%) over 11 steps.18 The final step involves several recrystallizations followed by sublimation to remove contaminating by-products.
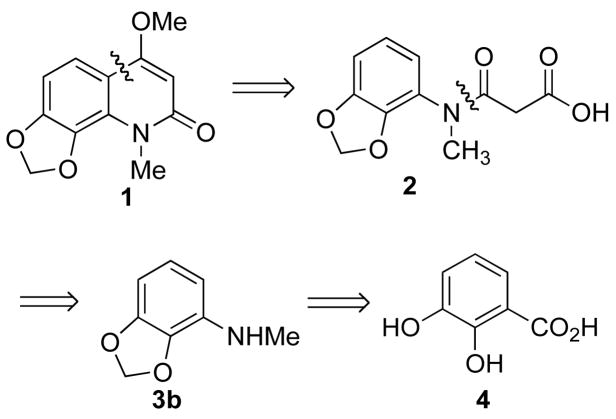
A retrosynthetic analysis of compound 1 is shown in Scheme 1. The key step is the synthesis of intermediate 3b, a degradation product of compound 1, by a Curtius rearrangement. The final product 1 was planned to be made by acid-mediated intramolecular Friedel-Crafts acylation followed by a methylation of an enol.
Scheme 1.
Retrosynthetic Analysis of Compound 1
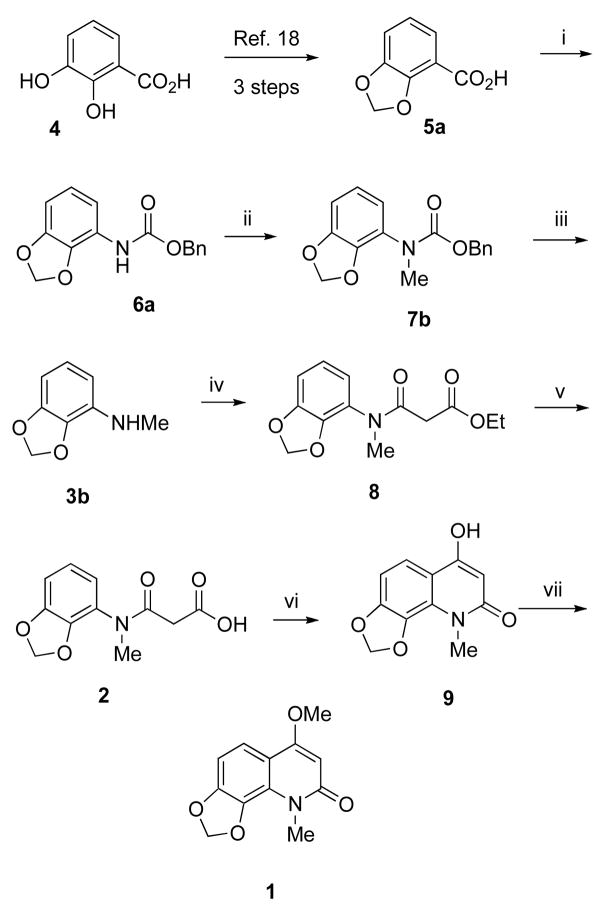
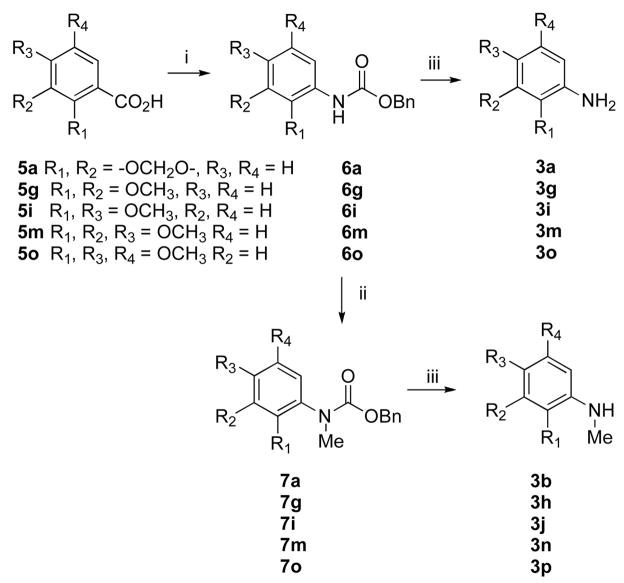
In practice, the present synthesis of compound 1 was executed in a straightforward manner, without any unexpected difficulties, as outlined in Scheme 2. The degradation product 3b of compound 1 was easily prepared in six steps from commercially available 2,3-dihydroxy benzoic acid (4) using a modified literature procedure19 in 27% overall yield. Curtius rearrangement of dioxole benzoic acid 5a using DPPAa and Et3N in refluxing benzene, followed by in situ quenching with benzyl alcohol, produced compound 6a. N-Methylation of 6a using NaH and MeI in THF, followed hydrogenolysis of the benzyl group and decarboxylation, yielded N-methylated amine 3b. Acylation of the secondary amine 3b with diethyl malonate afforded intermediate 8, which was saponified using sodium hydroxide to provide the acid 2. Polyphosphoric acid-mediated cyclization led to intermediate 9. Methylation of 9 with TMS-diazomethane afforded compound 1 as a white powder in ~15% overall yield from commercially available acid 4. The 1H NMR, 13C NMR, IR, mass spectral, and elemental analysis data of the synthetic compound 1 were consistent with those reported for the natural product. Synthetic compound 1 was used for various biological assays to determine its chemopreventive activity. The encouraging biological results motivated the synthesis of additional compound 1 congeners in order to gain insight into the structure-activity relationships and mechanism of action.
Scheme 2a.
aReagents and conditions: (i) (PhO)2PON3, Et3N, PhCH2OH, PhH, 3 h; (ii) NaH, MeI, rt, 2 h; (iii) Pd/C, H2, EtOAc, 30 psi, 1 h; (iv) diethyl malonate, 100–110 °C, 3 h; (v) (a) NaOH, 60 °C, 2 h, (b) HCl; (vi) PPA, 95–100 °C 3 h; (vii) TMSCH2N2, MeOH-Et2O, rt, 24 h
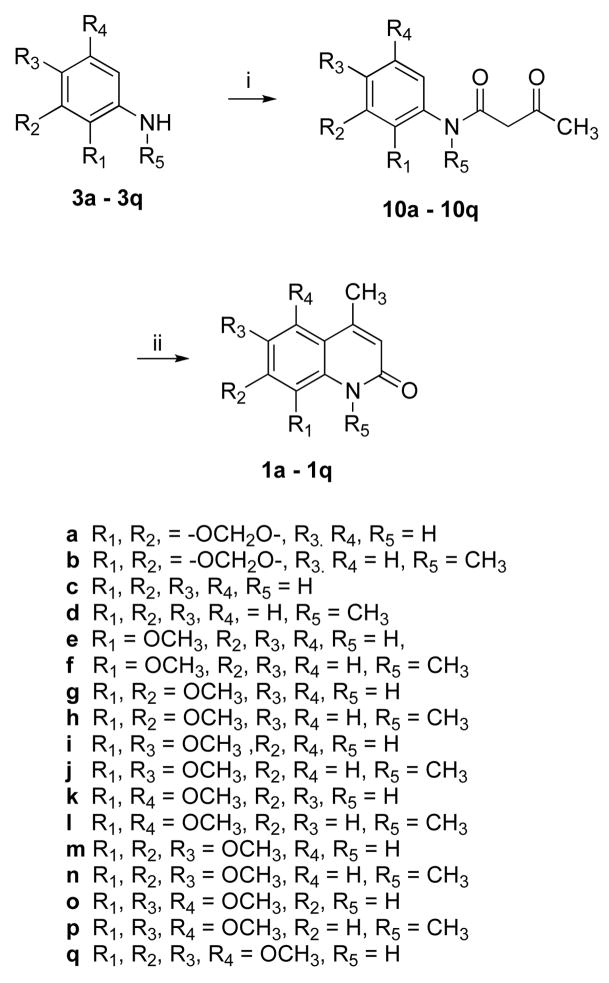
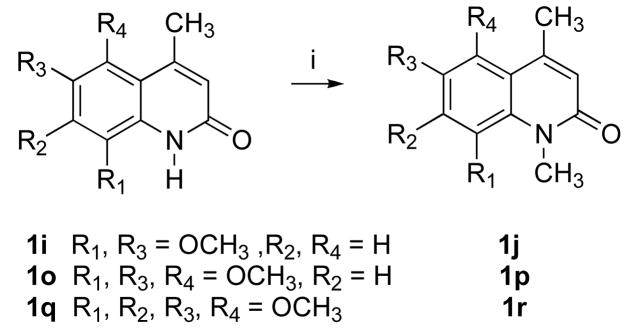
Compound 1 analogues 1a-q (Scheme 3) were synthesized by treating the corresponding amines 3a-q with diketene in refluxing benzene to yield ketoamides 10a-q, followed by a polyphosphoric acid (PPA)-mediated cyclization that afforded very high yields, except for compounds 1j and 1p. PPA-mediated cyclization of compounds 10j and 10p produced quinolinones 1j and 1p, respectively, as minor products. The desired products 1j and 1p were therefore prepared in very high yield by methylating compounds 1i and 1o, respectively, using NaH and MeI in THF, as shown in Scheme 4. Quinolinone 1r was also prepared from 1q in a similar way. Compound 1a was synthesized in 82% yield by treating ketoamide compound 10a with trifluoroacetic acid at room temperature.
Scheme 3a.
aReagents and conditions: (i) diketene, PhH, 95–100 °C, 3h, or CF3CO2H, rt; (ii) PPA, 95–100 °C, 3 h.
Scheme 4a.
aReagents and conditions: (i) NaH, MeI, THF, 23 °C, 12 h
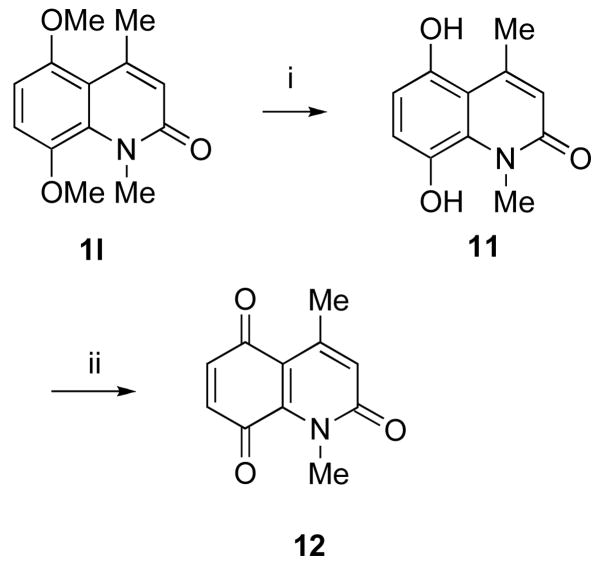
Most of the free amines or mono-methylated amines are commercially available. However, some of them had to be synthesized from the corresponding acids via Curtius rearrangement as shown in Scheme 5. Amines 3l and 3q were synthesized in good yields following literature procedures.20,21 Quinolinone 1l was used to synthesize quinone 12 following a previously reported literature procedure as outlined in Scheme 6.21
Scheme 5a.
aReagents and conditions: (i) (PhO)2PON3, Et3N, PhCH2OH, PhH, 3 h; (ii) NaH. MeI, rt, 2 h; (iii) Pd/C, H2, EtOAc, 30 psi, 1 h.
Scheme 6a.
aReagents and conditions: (i) HBr, heat, 48 h (ii) K2Cr2O7, H2SO4, acetone, 0 °C, 10 min.
Quinone Reductase 2 Inhibitory Activity
QR2 inhibition assays were performed on synthetic compound 1 and its various analogues, as described in the Experimental Section. The IC50 values are reported in Table 1. Synthetic compound 1 was determined to have an IC50 value of 54.1± 6.7 μM. Most of the compound 1 analogues displayed superior inhibitory potency compared with the natural product and were active in the lower micromolar range. In general, N-methylated analogues showed better potency than non-N-methylated quinolinones in inhibiting QR2. Compound 1j, with IC50 1.9 ± 0.2 μM, is comparable in potency with most of the QR2 inhibitors reported in the literature. The replacement of the 6-methoxy group of compound 1 by a methyl group increases the inhibitory activity more than 8-fold (1b, IC50 = 6.2 ± 0.28 μM). Non-N-methylated quinolinone 1a (IC50 = 10.8 ± 2.1 μM) showed a decrease in activity relative to 1b, but still has better potency than the natural product. Complete removal of the dioxole ring from 1a made the resulting compound 1c inactive; however, N-methylated compound 1d showed an IC50 value of 18.2 ± 3.1 μM, an approximately 3-fold improvement of activity relative to compound 1. The presence of an 8-methoxy group (1e, 1f) improved the activity relative to the unsubstituted derivatives (1c, 1d). Quinolinone 1f had an IC50 value 5.8 ± 0.9 μM, which is an approximately 10-fold increase in potency compared with the natural product.
Table 1.
Inhibition of QR2 and Aromatase by Compound 1 and Its Analogues
| Quinolinones | QR2 IC50 (μM) | Aromatase IC50 (μM) | Cytotoxicity IC50 (μM) |
|||
|---|---|---|---|---|---|---|
| Hepa 1c1c7 | MCF7 | LNCaP | LU | |||
| 1 | 54.1 ± 6.7 | 3.92 ± 0.67 | >85.81 | >85.81 | >85.81 | >85.81 |
| 1a | 10.8 ± 2.1 | >98.49 | >98.49 | >98.49 | >98.49 | >98.49 |
| 1b | 6.2 ± 0.8 | 1.25 ± 0.03 | >92.14 | >92.14 | >92.14 | >92.14 |
| 1c | >500 | >125.7 | >125.7 | >125.7 | >125.7 | >125.7 |
| 1d | 18.2 ± 3.1 | >115.6 | >115.6 | >115.6 | >115.6 | >115.6 |
| 1e | 24.1 ± 3.4 | >105.8 | >105.8 | >105.8 | >105.8 | >105.8 |
| 1f | 5.8 ± 0.9 | 2.02 ± 0.1 | >98.48 | >98.48 | >98.48 | >98.48 |
| 1g | 29.3 ± 3.4 | 31.5 ± 3.1 | >91.29 | >91.29 | >91.29 | >91.29 |
| 1h | 9.3 ± 2.3 | 5.76 ± 0.07 | >85.80 | >85.80 | >85.80 | >85.80 |
| 1i | 8.8 ± 1.1 | >91.29 | >91.29 | >91.29 | >91.29 | >91.29 |
| 1j | 1.9 ± 0.2 | 0.96 ± 0.02 | >85.80 | >85.80 | >85.80 | >85.80 |
| 1k | 10.8 ± 1.5 | 26.8 ± 13.77 | >91.22 | >91.22 | >91.22 | >91.22 |
| 1l | 4.1 ± 0.6 | 0.96 ± 0.01 | >85.74 | >85.74 | >85.74 | >85.74 |
| 1m | 10.0 ± 1.4 | 18.47 ± 3.7 | >80.29 | >80.29 | >80.29 | >80.29 |
| 1n | 7.0 ± 1.1 | 2.72 ± 0.02 | >76.01 | >76.01 | >76.01 | >76.01 |
| 1o | 6.0 ± 0.6 | 14.8 ± 0.65 | 34.52 | 42.95 | 45.76 | 35.28 |
| 1p | 10.8 ± 1.3 | 0.10 ± 0.01 | 70.73 | >76.01 | >76.01 | 68.47 |
| 1q | >500 | 5.68 ± 0.24 | >71.66 | >71.66 | >71.66 | >71.66 |
| 1r | >500 | 0.76 ± 0.02 | >68.23 | >68.23 | >68.23 | >68.23 |
| 11 | >500 | >90.4 | NTa | NT | NT | NT |
| 12 | >500 | >98.4 | 54.14 | 60.53 | 58.07 | 49.22 |
| Resveratrol6 | 0.96 +/− 0.13 | NT | NT | NT | NT | NT |
| Melatonin6 | 11.3 +/− 2.1 | NT | N | NT | NT | NT |
| Aminoglutethimide | 0.27 | NT | NT | NT | NT | |
Not tested.
To analyze the effect of methoxy substitutions on the benzene ring, several dimethoxylated (1g-l), trimethoxylated (1m-p), and tetramethoxylated (1q-r) quinolinone derivatives were synthesized and evaluated. It was found that introduction of another methoxy group at the 7 position in 8-methoxylated quinolines (1e, 1d) decreased the inhibitory potency (1g, IC50 29.3 ± 3.4 μM, 1h, IC50 9.3 ± 2.3 μM). In contrast, N-methylated 6,8-dimethoxy derivative 1j (IC50 1.9 ± 0.2 μM) and the non-N-methylated 6,8-dimethoxy compound 1i (IC50 8.8 ± 1.1 μM) have superior potency over other disubstituted analogues. Dimethoxy derivatives 1k (IC50 10.8 ± 1.5 μM), 1l (IC50 4.1 ± 0.6 μM) and trimethoxy derivative 1m (IC50 10.0 ± 1.4 μM) and 1n (IC50 7.0 ± 1.1 μM) revealed a similar pattern but were less potent than the 6,8-dimethoxy derivatives. Surprisingly, a change in pattern was observed for other trimethoxylated series. Non-N-methylated 1o (IC50 6.0 ± 0.6 μM) had better potency than N-methylated 1p (IC50 10.8 ± 1.3 μM). A decrease in inhibitory potency for N-methylated 6,7,8-trimethoxy derivatives relative to 5,6,8-trimethoxy analogues and completely inactive tetramethoxy analogues 1q and 1r suggests that the steric bulk of the consecutive methoxy groups may force them to be pointed out of the planar ring system, making the π-π stacking interaction with FAD unfavorable.
X-Ray Structure Analysis
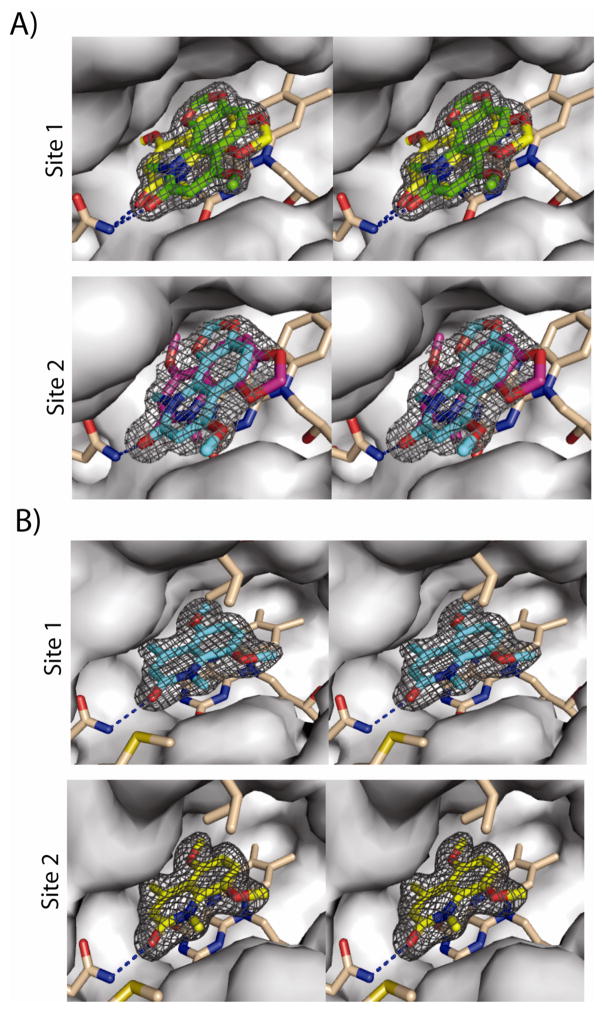
In order to gain insight into the binding mode of this new series of QR2 inhibitors, the X-ray crystal structures of compound 1 and one of the most potent analogs, 1l, from the series in complex with human QR2 were determined. Complete X-ray data sets were collected and processed and the final structural models were refined to 1.84 Å and 1.98 Å for the compound 1 and compound 1l complexes, respectively. The final data collection and refinement statistics are summarized in Table 2. QR2 is a dimer in solution and it crystalizes as a dimer in the asymmetric unit with two nearly equivalent catalytic sites defined as site 1 for chain A and site 2 for chain B.6 Crystals of QR2 in complex with compound 1 produced strong electron density in Fo-Fc difference maps consistent with the natural product being bound in two different orientations within each active site of the dimer (Figure 1a). In contrast, QR2 crystals complexed with compound 1l produced strong electron density for only one orientation of the inhibitor within each active site of the dimer (Figure 1b).
Table 2.
Data Collection and Refinement Statistics for QR2-Inhibitor Complexes
| QR2-Inhibitor Complex | 1 | 1l |
|---|---|---|
| Data Collection | ||
| Space Group | P212121 | P212121 |
| Unit Cell Dimentsions a, b, c (Å) | 56.73, 84.23, 106.31 | 56.34, 83.88, 106.69 |
| Resolution (Å) | 66.08 – 1.85(1.92 – 1.85) | 65.49 – 1.98(2.04 – 1.98) |
| No. Reflections Observed | 241,450 | 197,663 |
| No. Unique Reflections | 44,812 | 35,682 |
| Rmerge (%) | 9.3(62.9)* | 8.4(29.1)* |
| I/σI | 17.8(2.0)* | 21.1(4.5)* |
| % Completeness | 99.9(99.4)* | 99.3(95.2)* |
| Refinement | ||
| Resolution Range | 66.08 – 1.84 | 65.49 – 1.98 |
| No. Refections in Working Set | 42,486 | 33,839 |
| No. Reflections in Test Set | 2,256 | 1,788 |
| Rwork (%) | 18.0 (28.5)* | 18.1 (22.2)* |
| Rfree (%) | 23.1 (35.7)* | 22.7 (33.0)* |
| Average B-factor (Å2) | 22.2 | 26.2 |
Highest resolution data shell is given in parentheses
Figure 1.
X-ray structure and electron density surrounding inhibitors in the active site of human QR2. In each stereoview, QR2 inhibitor complexes depict all FAD and enzyme carbons in grey and beige, oxygen atoms are red and all nitrogen atoms are blue. Hydrogen bonds between Asn161 and the inhibitors are represented as blue dashed lines. (A) QR2 in complex with compound 1. The 2Fo−Fc electron density surrounding the two orientations of compound 1 is contoured at 1s in black. Site 1, compound 1 binds in two orientations in both Site 1 (green and yellow) of chain A (top panel) and Site 2 (blue and magenta) of Chain B (lower panel). The two binding orientations are rotated by ~180° degrees from each other. Each pose was refined at 50% occupancy. The different orientations in the two separate sites are depicted in different colors for clarity. Ile128 and Met154 have been removed for clarity. (B) QR2 in complex with compound 1l binds in one orientation in both Site 1 (blue) of chain A (top panel) and Site 2 (yellow) of Chain B (lower panel). The 2Fo−Fc electron density surrounding compound 1l is contoured at 1s in black.
Two molecules of compound 1 were manually built within the electron density of the QR2-compound 1 active sites, and the model was refined with the occupancy of each molecule to 50%. The two orientations of compound 1 within the active site of a single monomer (site 1) are nearly identical to the orientations within the active site of the second monomer (site 2). The two orientations of compound 1 within a single site are related to each other by a 180° flip. In the first orientation (Figure 1a, color code yellow), the methoxy group of compound 1 points towards Phe178, whereas in the second orientation, it orients towards solvent-exposed surface (Figure 1a, color code green). Despite this difference, the interactions of compound 1 within the QR2 active sites are similar and involve mainly a hydrogen bond between the carbonyl oxygen of compound 1 and the side-chain amide nitrogen of Asn161, and a series of hydrophobic contacts with surrounding side chains and the flavin ring of FAD. These interactions are maintained for both compound 1 and compound 1l.
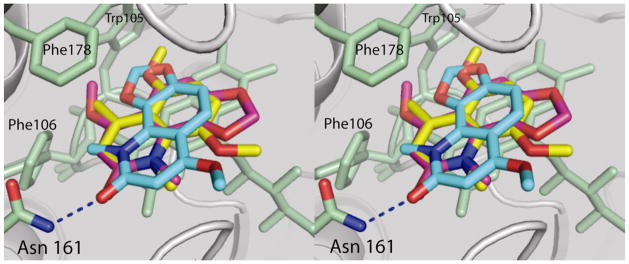
The single orientation of 1l is nearly identical between sites 1 and 2 of the dimer (Figure 1b). A superposition of site 1 from the QR2-compound 1 structure with site 1 of the QR2-1l structure reveals that one of the compound 1 orientations is nearly identical to that of 1l with respect to the quinolinone ring (Figure 2). In general, a careful analysis of the two crystal structures reveal that these two inhibitors, 1 and 1l, bind within the active site and sit parallel to the plane of the isoalloxazine ring of the bound cofactor FAD, occupying very similar positions as menadione in the QR2-menadione complex,22 resveratrol in the QR2-resveratrol complex,13 CB1954 in the QR2-CB1954 complex,23 and melatonin in the QR2-melatonin complex.6 All of these molecules bind deeply within the hydrophobic cleft of QR2, which is surrounded by side chains of Tyr132′, Phe178′, Phe126′, Met164′ and Cys121′ from one side of a QR2 monomer, and Tyr155, Phe106 and the isoalloxazine ring of FAD from the other monomer. The side chain of Trp105 forms the bottom of the hydrophobic cleft and on one end of the cleft reside the side chains of Asn161 and the hydroxyl groups of Tyr155 and Tyr132. These latter two amino acid side chains form a relatively hydrophilic surface at one end of the cleft and also the hydroxyl group of Thr71′ and main-chain carbonyls from Gly68 and Asp117 all point towards the cavity on the other end. The positions of these amino acid residues indicate that both ends have numerous functional groups accessible for hydrogen bonding. The fourth side of this unique 17 Å long and 7 Å wide cavity is accessible to the solvent. Because of the narrow width of this cavity, the enzyme can typically only accommodate molecules that can adopt nearly flat conformations. The crystal structures indicate that the benzene rings of these quinolinones stack with the C ring of FAD, similar to the benzene ring of CB1954. Hydrophobic interactions with Trp105, Gly68, Phe126, and Phe178 help to stabilize inhibitor binding via interactions with the quinolinone ring and carbonyl group. These two inhibitors occupy about two-thirds of the active site.
Figure 2.
Stereoview of a superposition of compound 1 and compound 1l bound within the active sites of human QR2. The two orientations of compound 1 bound within the active sites of chain A (blue) and chain B (magenta) are shown with compound 1l (yellow).
The observation that the weaker inhibitor, compound 1, binds in two orientations within the active site of QR2 whereas the 10-fold more potent inhibitor 1l binds in only one orientation is reminiscent of our seminal observation that weak binding inhibitors of QR2, such as melatonin, tend to bind in multiple oientations whereas their higher affinity analogs, such as iodomelatonin, tend to bind in only one orientation.6 In that study, we found that melatonin, with an IC50 of 11.3 μM, binds in at least three orientations depending on which of three crystal structures of this complex are analyzed, whereas iodomelatonin, with an IC50 of 1.1 μM, binds in only one orientation in the active sites of two independently determined X-ray structures. Our observation that inhibitor binding to QR2 can be a stochastic process dependent on the strength of the interaction has recently been supported by X-ray structures of QR2 in complex with dopamine and andrenochrome, where dopamine was found to bind in two orientations.24
Aromatase Inhibitory Activity
An aromatase inhibition assay was performed on synthetic compound 1 and its analogues, as described in the Experimental Section. The IC50 values of the compounds are reported in Table 1. Synthetic compound 1 was determined to have an IC50 value of 3.9 ± 0.67 μM. The analogues showed good inhibitory potency against aromatase except for a few N-nonmethylated analogues (1a, 1c, 1e, 1i, 11) and two N-methylated analogues (1d and 12). The replacement of the 6-methoxy group of compound 1 by a methyl group increased the inhibitory activity (1b, IC50 = 1.2 ± 0.03 μM). However, removal of dioxole ring made the resulting compounds 1c and 1d inactive. Introduction of an 8-methoxy restored the inhibitory potency of 1f (IC50 2.0 ± 0.1 μM), however, analogue 1e was not active. Interestingly, 7,8-dimethoxy analogue 1g (IC50 31.5 ± 3.1 μM) showed moderate inhibitory activity, but in comparison with the 8-methoxy N-methylated analogue 1f, the activity of 1h was reduced (IC50 5.7 ± 0.07 μM). The 6,8-dimethoxy derivative 1i was found to be inactive, but its congener 1j (IC50 0.96 ± 0.02 μM) showed very potent inhibitory activity in the aromatase assay. All other N-nonmethylated analogues (1k, 1m, 1o, 1q) and N-methylated analogues (1l, 1n, 1p, 1r) showed a similar trend as we have seen for 7,8-dimethoxy analogues (1g, 1h), with the N-methylated inhibitors being more potent. Inhibitory activity for 1j, 1l and 1r are almost the same and the IC50 values are in submicromolar range. 5,6,8-Trimethoxy-N-methylated analogue 1p displayed the best inhibitory potency, with an IC50 value of 0.1 ± 0.004 μM, which compares favorably with the clinically useful aromatase inhibitor aminoglutethimide. These observations show that N-methylated analogues are better aromatase inhibitors and methoxy substitution on the ring makes a significant contribution to the potency.
Cytotoxicity Studies
Ideally, potential chemotherapeutic agents should not be highly cytotoxic. In order to evaluate this aspect, the cytotoxicities of the compounds were evaluated in four different cell lines, and the data are presented in Table 1. The results indicate that the present series of quinolinones are in general not significantly cytotoxic. Those expressing a low degree of cytotoxicity were compounds 1o, 1p, and 12.
Conclusion
An efficient total synthesis of compound 1 and a series of compound 1-based QR2 and aromatase inhibitors have been successfully carried out. Structural modification of the parent natural product resulted in the most active compound 1j vs. QR2 and 1p vs. aromatase. The crystal structures of compound 1 and one of the potent analogues 1l in complex with QR2 provide a detailed description of the enzyme active site that can be utilized in the further design of new QR2 inhibitors.
The present series of compounds inhibits both aromatase and QR2, two distinct targets for the development of chemopreventive agents. As is the case with combination drug therapy, dual targeting through a pleiotropic mechanism offers the potential to enhance efficacy if the two drug targets can function in a synergistic fashion.
Experimental Section
NMR spectra were obtained at 300 MHz (1H) and 75 MHz (13C) in CDCl3 using CHCl3 as internal standard. Flash chromatography was performed with 230–400 mesh silica gel. TLC was carried out using commercially available precoated glass silica gel plates of 2.5 mm thickness. Melting points are uncorrected. Microanalyses were performed at the Purdue Microanalysis Laboratory. All yields refer to yields of isolated compounds. Unless otherwise stated, chemicals and solvents were of reagent grade and used as obtained from commercial sources without further purification. Tetrahydrofuran (THF) was freshly distilled from sodium/benzophenone ketyl radical, acetone from potassium carbonate, dichloromethane from CaH2, benzene from P2O5, and triethylamine from KOH prior to use.
6-Methoxy-9-methyl-[1,3]dioxolo[4,5-h]quinolin-8(9H)-one (1)
A suspension of hydroxyquinolone 9 (300 mg, 1.3 mmol) in ether (1 mL) at 0 °C was treated with excess TMS-diazomethane (2 M in hexanes, 10 mL) and the mixture was stirred for 20 min. Absolute methanol (10 mL) was added dropwise and the mixture was stirred for 6 h. An additional amount of TMS-diazomethane (2 M in hexanes, 5 mL) and absolute methanol (5 mL) were added successively to the mixture and the mixture was stirred for another 12 h. After completion of the reaction, the mixture was treated with a solution of dilute acetic acid (0.5 mL) to decompose the excess diazomethane. Evaporation of the solution and purification by silica gel column chromatography, eluting with an acetone-hexane (1:1) mixture, yielded compound 1 (240 mg, 78%) as a colorless solid: mp 199–201 °C (lit18 mp 202–203 °C). IR (KBr) 3319, 1735, 1645, 1592, 1444, 1465, 1331, 1265, 1232, 1159, 1057, 940 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.51 (d, J = 8.4 Hz, 1 H), 6.74 (d, J = 8.4 Hz, 1 H), 6.01 (s, 2 H), 5.86 (s, 1 H), 3.87 (s, 3 H) 3.80 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 164.0, 162.6, 149.8, 133.5, 126.5, 118.0, 112.9, 104.2, 100.2, 94.5, 55.8, 31.9; EIMS (m/z, relative intensity) 233 (M+, 100), 218 (26), 190 (31), 176 (24), 160 (10), 146 (9), 132 (8), 117 (7), 69 (17); HRMS calcd for C12H11NO4 233.0688, found 233.0692. Anal. (C12H11NO4) C, H, N.
General Procedures for Cyclization
Method A: Using H2SO4
A solution of the acetoacetamide (2 mmol) in concd sulfuric acid (5 mL) was stirred at room temperature for 30–60 min. The reaction mixture was poured on ice (1 g) and neutralized or slightly basified to pH ~8 with 28–30% aqueous ammonia (5–10 mL). The aqueous solution was extracted with chloroform (3 × 50 mL) and the organic extract was dried and evaporated to dryness under reduced pressure. The residue was purified by column chromatography on silica gel, eluting with ethyl ether or ethyl acetate-hexane mixture to produce the pure product in moderate yield.
Method B: Using Polyphosphoric Acid (PPA)
The acetoacetamide (2 mmol) was added to polyphosphoric acid (5 g) at 95–100 °C and the mixture was allowed to stir for 2.5 h at the same temperature, and then poured into ice-water. After polyphosphoric acid had dissolved, the solution was neutralized with 4 N NaOH solution. The deposited solid was collected by filtration or extracted with chloroform and evaporated to dryness. Solid was purified by column chromatography on silica gel, eluting with ethyl ether or ethyl acetate-hexane mixture to yield the pure product in higher yield than obtained with method A.
Method C: Using Trifluoroacetic Acid
A solution of the acetoacetamide (2 mmol) in trifluoroacetic acid (5 mL) was stirred at room temperature for 24 h. The reaction mixture was evaporated to dryness under reduced pressure. The residue was purified by column chromatography on silica gel, eluting with ethyl ether or ethyl acetate-hexane mixture to afford the pure product in good yield. This method was used when isolation of the product was very difficult with methods A and B because of solubility issues.
6-Methyl-[1,3]dioxolo[4,5-h]quinolin-8(9H)-one (1a)
This compound was prepared in 82% yield by following Method C. Colorless solid: mp 285 °C (dec). IR (KBr) 1655, 1645, 1573, 1477, 1435, 1405, 1395, 1292, 1242 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.31 (d, J = 8.7 Hz, 1 H), 6.94 (dd, J = 8.4, 0.9 Hz, 1 H), 6.49 (s, 1 H), 6.19 (d, J = 0.9 Hz, 2 H), 2.51 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 163.8, 152.8, 149.6, 134.2, 121.7, 119.1, 117.6, 116.1, 106.5, 103.3, 19.7; EIMS (m/z, relative intensity) 203 (M+, 100), 174 (17), 161 (6), 146 (35), 117 (27), 90 (11), 69 (23), 57 (27); HRMS calcd for C11H9NO3 203.0582, found 203.0584. Anal. (C11H9NO3) C, H, N.
6,9-Dimethyl-[1,3]dioxolo[4,5-h]quinolin-8(9H)-one (1b)
This compound was prepared in 73% yield by following Method B. Colorless solid: 158–159 °C. IR (KBr) 1652, 1590, 1506, 1416, 1382, 1328, 1261, 1124, 1093, 1050, 1004, 939, 753 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.19 (d, J = 8.4 Hz, 1 H), 6.77 (d, J = 8.4 Hz, 1 H), 6.39 (s, 1 H), 6.01 (s, 2 H), 3.84 (s, 3 H), 2.35 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 162.0, 149.0, 146.3, 133.5, 126.2, 119.5, 118.6, 117.9, 104.1, 100.8, 32.0, 19.4; EIMS (m/z, relative intensity) 217 (M+, 100), 202 (6), 188 (41), 174 (42), 160 (20), 146 (10), 130 (25), 116 (8), 89 (14), 63 (11); HRMS calcd for C12H11NO3 217.0739, found 217.0738. Anal. (C12H11NO3) C, H, N.
4-Methylquinolin-2(1H)-one (1c)
This compound was prepared in 62% yield as a colorless solid by following Method B: mp 214–216 °C (lit25 mp 220–222 °C). IR (KBr) 3300, 2800, 1658, 1635, 1557, 1508, 1492, 1435, 1371, 1261 cm−1; 1H NMR (DMSO-d6, 300 MHz) δ 7.69 (d, J = 8.1 Hz, 1 H), 7.49 (m, 1 H), 7.29 (d, J = 8.1 Hz, 1 H), 7.18 (m, 1 H), 6.39 (s, 1 H), 2.41 (s, 3 H); 13C NMR (DMSO-d6, 75 MHz) δ 161.6, 147.9, 138.6, 130.2, 124.7, 121.6, 120.8, 119.5, 115.4, 18.4; EIMS (m/z, relative intensity) 159 (M+, 44), 130 (100), 103 (9), 89 (5), 77 (11), 63 (6), 51 (7); HRMS calcd for C10H9NO 159.0684, found 159.0683. Anal. (C10H9NO) C, H, N.
1,4-Dimethylquinolin-2(1H)-one (1d)
This compound was prepared in 82% yield as a colorless solid by following Method B: mp 132–134 °C (lit26 mp 130–132 °C). IR (KBr) 1652, 1615, 1592, 1562, 1454, 1370, 1321 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.68 (dd, J = 1.2, 7.8 Hz, 1 H), 7.54 (dd, J = 1.2, 8.4, Hz, 1 H), 7.34 (d, J = 8.4 Hz, 1 H), 7.23 (dd, J = 1.2, 7.8 Hz, 1 H), 6.57 (s, 1 H), 3.67 (s, 3 H), 2.43 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 162.0, 146.3, 139.7, 130.4, 125.1, 121.8, 121.3, 121.0, 114.3 29.1, 18.9; EIMS (m/z, relative intensity) 173 (M+, 82), 144 (100), 130 (71), 115 (15), 103 (12), 89 (7), 77 (17), 63 (8), 51 (9); HRMS calcd for C11H11NO 173.0841, found 173.0841. Anal. (C11H11NO) C, H, N.
8-Methoxy-4-methylquinolin-2(1H)-one (1e)
This compound was prepared in 90% yield as a colorless solid by following Method B: mp 188–190 °C (lit27 187–189 °C). IR (KBr) 3400, 2800, 1649, 1605, 1473, 1463, 1390, 1266 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.33 (brs, 1 H), 7.25 (d, J = 8.1 Hz, 1 H), 6.79 (m, 1 H), 6.97 (d, J = 8.1 Hz, 1 H), 6.53 (s, 1 H), 3.96 (s, 3 H), 2.45 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 161.6, 148.7, 145.5, 128.1, 121.7, 121.2, 120.5, 116.1, 109.7, 55.9, 19.1; EIMS (m/z, relative intensity) 189 (M+, 100), 174 (17), 160 (58), 146 (32), 130 (27), 118 (31), 91 (21), 65 (20), 51 (14); HRMS calcd for C11H11NO2 189.0790, found 189.0787. Anal. (C11H11NO2) C, H, N.
8-Methoxy-1,4-dimethylquinolin-2(1H)-one (1f)
This compound was prepared in 72% yield as a yellowish solid by following Method B: mp 72–74 °C (lit28 mp 69–70.5 °C). IR (KBr) 1657, 1611, 1595, 1459, 1385, 1260 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.36 (d, J = 8.1 Hz, 1 H), 7.32 (m, 1 H), 7.11 (d, J = 8.1 Hz, 1 H), 6.64 (s, 1 H), 3.97 (s, 3 H), 3.95 (s, 3 H), 2.47 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 163.2, 148.6, 145.9, 131.1, 123.4, 122.3, 121.2, 117.4, 113.5, 56.3, 35.2, 19.4; EIMS (m/z, relative intensity) 203 (M+, 80), 188 (100), 173 (27), 160 (51), 144 (43), 130 (45), 103 (7), 69 (13), 55 (12); HRMS calcd for C12H13NO2 203.0946, found 203.0946. Anal. (C12H13NO2) C, H, N.
7,8-Dimethoxy-4-methylquinolin-2(1H)-one (1g)
This compound was prepared in 55% yield as a colorless solid by following Method A: mp 187–189 °C. IR (KBr) 1651, 1620, 1600, 1515, 1469, 1396, 1284, 1104 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.26 (brs, 1 H), 7.28 (d, J = 8.1 Hz, 1 H), 6.77 (d, J = 8.1, Hz, 1 H), 6.61 (s, 1 H), 3.88 (s, 3 H), 3.87 (s, 3 H), 2.35 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 162.3, 152.5, 148.6, 133.6, 132.3, 119.9, 118.6, 115.3, 107.2, 60.8, 56.0, 18.9; EIMS (m/z, relative intensity) 219 (M+, 100), 204 (43), 190 (19), 130 (12), 69 (17), 43 (16); HRMS calcd for C12H13NO3 219.0895, found 219.0895. Anal. (C12H13NO3) C, H, N.
7,8-Dimethoxy-1,4-dimethylquinolin-2(1H)-one (1h)
This compound was prepared in 78% yield as a colorless solid by following Method B: mp 118–120 °C. IR (KBr) 1655, 1589, 1557, 1454, 1387, 1324, 1268, 1057, 795 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.35 (d, J = 9.0 Hz, 1 H), 6.85 (d, J = 9.0, Hz, 1 H), 6.40 (s, 1 H), 3.91 (s, 3 H), 3.87 (s, 3 H), 3.72 (s, 3 H), 2.34 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 163.8, 154.9, 145.9, 137.0, 135.0, 120.9, 118.9, 117.6, 107.0, 61.6, 56.1, 33.8, 19.2; EIMS (m/z, relative intensity) 233 (M+, 61), 218 (93), 202 (35), 175 (100), 130 (20), 118 (18), 77 (22), 51 (17); HRMS calcd for C13H15NO3 233.1052, found 233.1057. Anal. (C13H15NO3) C, H, N.
6,8-Dimethoxy-4-methylquinolin-2(1H)-one (1i)
This compound was prepared in 67% yield as a light brown solid by following Method B: mp 232–234 °C (lit29 mp 231–233 °C). IR (KBr) 1653, 1619, 1605, 1462, 1399, 1380, 1277, 1217 cm−1; 1H NMR (DMSO-d6, 300 MHz) δ 10.5 (brs, 1 H), 6.80 (s, 1 H), 6.72 (s, 1 H), 6.41 (s, 1 H), 3.87 (s, 3 H), 3.81 (s, 3 H), 2.40 (s, 3 H); EIMS (m/z, relative intensity) 219 (M+, 100), 203 (15), 190 (5), 176 (63), 161 (10), 133 (9), 105 (4), 69 (14), 55 (14); HRMS calcd for C12H13NO3 219.0895, found 219.0894. Anal. (C12H13NO3) C, H, N.
6,8-Dimethoxy-1,4-dimethylquinolin-2(1H)-one (1j)
This compound was prepared in 17% yield as a colorless solid by following Method B: mp 157–159 °C. IR (KBr) 1656, 1619, 1595, 1577, 1456, 1394, 1317, 1276, 1166 cm−1; 1H NMR (CDCl3, 300 MHz) δ 6.50 (m, 2 H), 6.43 (s, 1 H), 3.74 (s, 3 H), 3.73 (s, 3 H), 3.72 (s, 3 H), 2.23 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 162.5, 154.5, 149.5, 145.2, 125.7, 123.4, 121.5, 102.6, 98.3, 56.0, 55.2, 34.8, 19.4; EIMS (m/z, relative intensity) 233 (M+, 100), 218 (70), 188 (68), 175 (35), 160 (23), 147 (26), 118 (7), 97 (10), 69 (36); HRMS calcd for C12H13NO3 233.1052, found 233.1048. Anal. (C12H13NO3) C, H, N.
5,8-Dimethoxy-4-methylquinolin-2(1H)-one (1k)
This compound was prepared in 84% yield as a light brown solid by following Method A: mp 187–189 °C (lit30 mp 188–189 °C). IR (KBr) 3400, 2800, 1651, 1613, 1489, 1463, 1441, 1369, 1266 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.09 (brs, 1 H), 6.79 (d, J = 8.7 Hz, 1 H), 6.44 (d, J = 8.7 Hz, 1 H), 6.31 (s, 1 H), 3.84 (s, 3 H), 3.77 (s, 3 H), 2.56 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 161.2, 151.9, 150.3, 139.6, 129.8, 121.4, 111.4, 109.9, 102.1, 56.1, 55.5, 24.5; EIMS (m/z, relative intensity) 219 (M+, 41), 204 (100), 190 (14), 176 (42), 161 (32), 148 (12), 133 (23), 120 (5), 104 (9), 89 (6), 77 (8); HRMS calcd for C12H13NO3 219.0895, found 219.0896. Anal. (C12H13NO3) C, H, N.
5,8-Dimethoxy-1,4-dimethylquinolin-2(1H)-one (1l)
This compound was prepared in 80% yield as a colorless solid by following Method B: mp 122–124 °C (lit20 mp 122–123 °C). IR (KBr) 1644, 1593, 1478, 1459, 1437, 1398, 1263 cm−1; 1H NMR (CDCl3, 300 MHz) δ 6.98 (d, J = 8.7 Hz, 1 H), 6.58 (d, J = 8.7 Hz, 1 H), 6.41 (s, 1 H), 3.80 (s, 3 H), 3.79 (s, 3 H), 3.77 (s, 3 H), 2.55 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 163.8, 152.7, 147.4, 143.2, 133.9, 121.9, 115.4, 104.1, 57.8, 55.8, 36.0, 25.1; EIMS (m/z, relative intensity) 233 (M+, 52), 218 (100), 203 (38), 190 (34), 175 (68), 147 (14), 132 (10), 118 (12), 91 (8), 77 (7); HRMS calcd for C13H15NO3 233.1052, found 233.1056. Anal. (C13H15NO3) C, H, N.
6,7,8-Trimethoxy-4-methylquinolin-2(1H)-one (1m)
This compound was prepared in 70% yield as a colorless solid by following Method B: mp 218–220 °C. IR (KBr) 1647, 1598, 1495, 1461, 1406, 1388, 1378, 1276, 1195, 1120, 1015, 981, 795 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.28 (brs, 1 H), 6.76 (s, 1 H), 6.44 (s, 1 H), 3.97 (s, 3 H), 3.91 (s, 3 H), 3.88 (s, 3 H), 2.39 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 161.8, 149.0, 147.9, 143.7, 139.1, 127.0, 120.5, 115.9, 100.9, 61.3, 61.1, 56.2, 19.2; EIMS (m/z, relative intensity) 249 (M+, 100), 234 (88), 220 (24), 203 (16), 191 (50), 176 (7), 163 (23), 148 (19), 134 (4), 120 (16), 92 (6), 65 (7); HRMS calcd for C13H15NO4 249.1001, found 249.1005. Anal. (C13H15NO4) C, H, N.
6,7,8-Trimethoxy-1,4-dimethylquinolin-2(1H)-one (1n)
This compound was prepared by following Method B in 85% yield as a colorless solid: mp 144–146 °C. IR (KBr) 1655, 1611, 1596, 1554, 1421, 1452, 1392, 1280, 1114, 1045, 915, 795 cm−1; 1H NMR (CDCl3, 300 MHz) δ 6.73 (s, 1 H), 6.38 (s, 1 H), 3.84 (s, 3 H), 3.80 (s, 3 H), 3.75 (s, 3 H), 3.70 (s, 3 H), 2.26 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 162.8, 148.3, 145.5, 145.2, 142.5, 129.4, 120.1, 118.2, 102.0, 61.6, 60.8, 55.8, 33.5, 19.2; EIMS (m/z, relative intensity) 263 (M+, 100), 248 (86), 205 (70), 162 (34), 133 (29), 124 (15), 93 (26), 69 (46); HRMS calcd for C14H17NO4 263.1058, found 263.1160; Anal. (C14H17NO4) C, H, N.
5,6,8-Trimethoxy-4-methylquinolin-2(1H)-one (1o)
This compound was prepared as a colorless solid in 74% yield by following Method B: mp 149–151 °C (lit31 mp 150–152 °C). IR (KBr) 1649, 1605, 1567, 1472, 1381, 1363, 1245, 1203, 1165, 1077, 1050, 1020, 971, 795 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.09 (brs, 1 H), 6.64 (s, 1 H), 6.31 (s, 1 H), 3.83 (s, 3 H), 3.80 (s, 3 H), 3.75 (s, 3 H), 2.53 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 160.7, 148.7, 147.1, 141.4, 140.2, 123.1, 115.0, 99.7, 61.0, 57.0, 56.1, 23.1; EIMS (m/z, relative intensity) 249 (M+, 76), 234 (100), 206 (56), 191 (25), 176 (5), 163 (10), 134 (7), 77 (6), 51 (4); HRMS calcd for C13H15NO4 249.1001, found 249.1004. Anal. (C13H15NO4) C, H, N.
5,6,8-Trimethoxy-1,4-dimethylquinolin-2(1H)-one (1p)
Sodium hydride (60% oil dispersion, 10 mg, 0.25 mmol) was added to a solution of compound 1o (50 mg, 0.2 mmol) in dry DMF (1 mL) and the mixture was stirred at 75–85 °C for 2 h. The reaction mixture was cooled to room temperature and methyl iodide (0.05 mL, 0.76 mmol) was added. After being stirred at 80 °C for 6 h, the reaction mixture was quenched with ice water and extracted with ether. The organic layer was dried and evaporated to dryness under reduced pressure. The crude product was purified by silica gel column chromatography, eluting with ethyl acetate-hexanes (1:1), to yield compound 1p (40 mg, 74%) and 2,5,6,8-tetramethoxy-4-methylquinoline (5 mg, 10%). 1p: yellowish solid, mp 128–130 °C (lit31 mp 130–131°C). IR (KBr) 1657, 1606, 1463, 1362, 1260 cm−1; 1H NMR (CDCl3, 300 MHz) δ 6.78 (s, 1 H), 6.45 (s, 1 H), 3.88 (s, 3 H), 3.82 (s, 3 H), 3.77 (s, 3 H), 3.76 (s, 3 H), 2.57 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 162.9, 147.7, 146.1, 144.9, 141.1, 127.2, 123.6, 118.4, 103.0, 61.3, 57.6, 56.8, 35.8, 23.7; EIMS (m/z, relative intensity) 263 (M+, 100), 248 (43), 233 (26), 220 (47), 205 (40), 190 (12), 176 (11), 162 (10), 134 (8), 91 (7), 69 (15); HRMS calcd for C14H17NO4 263.1158, found 263.1160. Anal. (C14H17NO4) C, H, N.
5,6,7,8-Tetramethoxy-4-methylquinolin-2(1H)-one (1q)
This compound was prepared as a brown solid in 70% yield by following Method B: mp 158–160 °C. IR (KBr) 1653, 1597, 1556, 1483, 1393, 1378, 1259, 1170, 1099, 1073, 1012, 952 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.65 (brs, 1 H), 6.27 (s, 1 H), 3.93 (s, 3 H), 3.85 (s, 3 H), 3.81 (s, 3 H), 3.80 (s, 3 H), 2.52 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 161.6, 148.9, 147.8, 147.6, 141.8, 134.6, 129.2, 121.3, 110.8, 61.2, 23.1; EIMS (m/z, relative intensity) 279 (M+, 88), 264 (100), 249 (6), 236 (45), 221 (37), 206 (20), 178 (29), 135 (6), 79 (4); HRMS calcd for C14H17NO5 279.1107, found 279.1108. Anal. (C14H17NO5) C, H, N.
5,6,7,8-Tetramethoxy-1,4-dimethylquinolin-2(1H)-one (1r)
Yellowish solid, mp 66– 68 °C. IR (KBr) 1655, 1578, 1551, 1458, 1416, 1400, 1387, 1372, 1260, 1089, 1043, 1021 cm−1; 1H NMR (CDCl3, 300 MHz) δ 6.35 (s, 1 H), 3.99 (s, 3 H), 3.85 (s, 3 H), 3.83 (s, 3 H), 3.76 (s, 3 H), 3.68 (s, 3 H), 2.53 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 163.1, 149.8, 148.0, 146.3, 142.0, 138.5, 132.5, 121.6, 113.6, 61.8, 61.4, 61.3, 61.1, 35.2, 23.7; EIMS (m/z, relative intensity) 293 (M+, 100), 278 (90), 263 (21), 248 (40), 220 (38), 192 (28), 164 (7), 131 (4), 123 (11), 93 (10), 84 (15); HRMS calcd for C15H19NO5 293.1263, found 293.1265. Anal. (C14H17NO4) C, H, N.
3-(Benzo[d][1,3]dioxol-4-yl(methyl)amino)-3-oxopropanoic Acid (2)
A mixture of diketo ester 8 (2.0 g, 7.5 mmol) and 5% aqueous sodium hydroxide solution (15 mL) was heated at 60 °C for 2 h. The solution was concentrated to about one-quarter of the original volume and cooled in an ice bath. The mixture was acidified to pH 3.5 by the addition of 2 N HCl. The mixture was extracted with chloroform, dried over Na2SO4 and evaporated to dryness under vacuum to give acid 2 (1.5 g, 85%) as colorless solid: mp 115–117 °C. IR (KBr) 2914, 1737, 1663, 1614, 1486, 1459, 1386, 1362, 1253, 1196, 1054, 1028, 794 cm−1; 1H NMR (CDCl3, 300 MHz) δ 6.83 (m, 2 H), 6.64 (dd, J = 7.8, 1.8 Hz, 1 H), 6.00 (s, 2 H), 3.26 (s, 3 H), 3.21 (s, 2 H); 13C NMR (CDCl3, 75 MHz) δ 170.0, 167.8, 149.1, 142.4, 123.1, 123.0, 119.7, 109.2, 101.9, 36.3, 36.2; EIMS (m/z, relative intensity) 237 (M+, 35), 220 (3), 193 (10), 151 (100), 122 (23), 93 (49), 69 (17), 55 (26); HRMS calcd for C11H11NO5 237.0637, found 237.0644.
General Procedure for CBz Group Deprotection
A mixture of CBz-protected amine (5.0 mmol) and 10% Pd-C catalyst (300 mg) in EtOAc (30 mL) was hydrogenated at 20 psi for 6 h. The suspension was filtered and the filtrate was evaporated. The residue was dissolved in CHCl3 (30 mL) and the solution was extracted with dilute HCl (3 × 50 mL). The combined aqueous extracts were then made basic with concd NH4OH, and the aqueous mixture was again extracted with CHCl3. The combined extracts were dried (Na2SO4) and evaporated to provide amines in high yield, which were pure enough to use for the next step without further purification. A little portion of each compound was taken out and purified by silica gel column chromatography for analysis.
Benzo[d][1,3]dioxol-4-amine (3a)
Yield 92%. Colorless oil. IR (KBr) 3360, 2887, 1654, 1499, 1467, 1358, 1255, 1212, 1212, 1050, 925 cm−1; 1H NMR (CDCl3, 300 MHz) δ 6.70 (m, 1 H), 6.37 (m, 2 H), 5.9 (s, 2 H), 3.37 (brs, 2 H); EIMS (m/z, relative intensity) 137 (M+, 84), 108 (8), 91 (8), 79 (80), 52 (100); HRMS calcd for C7H7NO2 137.0477, found 137.0478.
N-Methylbenzo[d][1,3]dioxol-4-amine (3b)
Yield 95%. Colorless oil. IR (neat) 3401, 2972, 1646, 1474, 1454, 1353, 1275, 1258, 1092, 1052, 926 cm−1; 1H NMR (CDC13, 300 MHz) δ 6.73 (t, J = 8.1 Hz, 1 H), 6.29 (m, 2 H), 5.87 (s, 2 H), 3.58 (brs, 1 H), 2.86 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 146.9, 134.0, 133.6, 122.3, 105.7, 100.4, 98.9, 30.6; positive ion ESIMS (m/z, relative intensity) 152 (MH+, 100), 136 (35), 133 (8), 105 (4); HRMS calcd for C8H9NO2 152.0711, found 152.0712.
2,3-Dimethoxyaniline (3g)
Yield 96%. Colorless oil. IR (neat) 3367, 2938, 1613, 1499, 1478, 1321, 1264, 1228, 1132, 1087, 1052, 1003, 775 cm−1; 1H NMR (CDC13, 300 MHz) δ 6.81 (t, J = 8.4 Hz, 1 H), 6.25 (m, 2 H), 3.84 (m, 2 H), 3.80 (s, 3 H), 3.79 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 152.8, 140.5, 135.7, 124.0, 108.6, 102.0, 59.5, 55.5; positive ion ESIMS (m/z, relative intensity) 153 (M+, 100), 138 (100), 110 (6), 95 (25), 67 (10); HRMS calcd for C8H11NO2 153.0790, found 153.0786.
2,3-Dimethoxy-N-methylaniline (3h)
Yield 93%. Colorless oil. IR (neat) 3417, 1935, 1602, 1514, 1482, 1304, 1263, 1218, 1161, 1110, 1079, 1050, 1003 cm−1; 1H NMR (CDC13, 300 MHz) δ 6.93 (t, J = 8.1 Hz, 1 H), 6.31 (m, 2 H), 4.30 (brs, 1 H), 3.84 (s, 3 H), 3.77 (s, 3 H), 2.82 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 152.2, 143.4, 135.1, 124.3, 124.1, 108.7, 103.7, 101.0, 59.7, 55.6, 30.4; positive ion ESIMS (m/z, relative intensity) 168 (MH+, 100), 166 (13), 152 (32), 137 (8); HRMS calcd for C9H13NO2 + H+ 168.1024, found 168.1025.
2,4-Dimethoxyaniline (3i)
Yield 94%. Colorless solid: mp 34–36 °C (lit32 33–35 °C). IR (neat) 3436, 2939, 2833, 1597, 1515, 1465, 1285, 1240, 1206, 1154, 1035, 919 cm −1; 1H NMR (CDC13, 300 MHz) δ 6.80 (d, J = 8.1 Hz, 1 H), 6.63 (d, J = 2.7 Hz, 1 H), 6.52 (dd, J = 8.1, 2.7 Hz, 1 H), 3.96 (s, 3 H), 3.91 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 152.6, 147.9, 129.5, 114.8, 103.8, 98.9, 55.3, 55.0; EIMS (m/z, relative intensity) 153 (90), 138 (100), 110 (51), 95 (41), 79 (8), 67 (18), 52 (26); HRMS calcd for C8H11NO2 153.0790, found 153.0788.
2,4-Dimethoxy-N-methylaniline (3j)
Colorless oil; IR (neat) 3419, 2935, 2831, 1596, 1520, 1454, 1438, 1286, 1259, 1206, 1153, 1034, 918 cm −1; 1H NMR (300 MHz, CDCl3) δ 6.50 (m, 3 H), 3.80 (s, 3 H), 3.76 (s, 3 H), 2.83 (s, 3 H); 13C NMR (75 MHz, CDCl3) δ 151.4, 147.6, 133.5, 109.0, 103.3, 98.3, 55.2, 54.9, 30.6; EIMS (m/z, relative intensity) 167 (75), 152(100), 124 (27), 109 (28), 93 (7), 80 (6), 66 (7), 52 (9); HRMS calcd for C9H13NO2 167.0946, found 167.0947.
2,3,4-Trimethoxyaniline (3m)
Yield 90%. Colorless oil: IR (neat) 3442, 2937, 1496, 1478, 1424, 1266, 1204, 1128, 1085, 1041, 1012 cm−1; 1H NMR (CDC13, 300 MHz) δ 6.33 (d, J = 8.1 Hz, 1 H), 6.35 (d, J = 8.1 Hz, 1 H), 3.79 (s, 3 H), 3.78 (s, 3 H), 3.69 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 145.7, 142.8, 141.1, 134.2, 109.3, 108.1,60.6, 60.1, 56.5; EIMS (m/z, relative intensity) 183 (M+, 100), 168 (87), 153 (15), 140 (12), 125 (43), 110 (17), 108 (12), 82 (16), 54 (31); HRMS calcd for C9H13NO3 183.0895, found 183.0897.
2,3,4-Trimethoxy-N-methylaniline (3n)
Colorless oil; IR (neat) 3411, 2936, 2830, 1611, 1506, 1486, 1268, 1228, 1203, 1158, 1094, 1026, 937 cm −1; 1H NMR (300 MHz, CDCl3) δ 6.55 (d, J = 8.7 Hz, 1 H), 6.24 (d, J = 8.7 Hz, 1 H), 3.84 (s, 3 H), 3.82 (s, 3 H), 3.75 ( s, 3 H), 2.76 (s, 3 H); 13C NMR (75 MHz, CDCl3) δ 144.8, 142.5, 140.6, 137.8, 108.0, 104.0, 60.6, 60.1, 56.4, 30.7; EIMS (m/z, relative intensity) 197 (91), 182 (100), 167 (29), 152 (10), 139 (20), 136 (6), 124 (16), 110 (5), 96 (15), 68 (22), 53 (8); HRMS calcd for C10H15NO3 197.1052, found 197.1050.
2,4,5-Trimethoxyaniline (3o)
Yield 94%. Colorless solid: mp 84–86 °C. IR (KBr) 3392, 1527, 1468, 1413, 1233, 1204, 1188, 1044, 1027, 824 cm−1; 1H NMR (CDC13, 300 MHz) δ 6.49 (s, 1 H), 6.35 (s, 1 H), 3.78 (s, 3 H), 3.77 (s, 3 H), 3.75 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 143.7, 141.4, 140.7, 128.4, 101.8, 100.0, 57.2, 56.5; EIMS (m/z, relative intensity) 183 (M+, 74), 168 (100), 140 (39), 125 (34), 108 (16), 94 (9), 80 (5), 44 (16); HRMS calcd for C9H13NO3 183.0895, found 183.0894.
2,4,5-Trimethoxy-N-methylaniline (3p)
Yield 91%. Colorless solid: mp 58–60 °C. IR (KBr) 3435, 2942, 1529, 1457, 1449, 1397, 1223, 1210, 1199, 1035, 824 cm−1; 1H NMR (CDC13, 300 MHz) δ 6.51 (s, 1 H), 6.27 (s, 1 H), 3.82 (s, 3 H), 3.78 (s, 3 H), 3.76 (s, 3 H), 2.82 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 144.0, 140.5, 139.7, 133.9, 99.9, 97.4, 57.6, 56.8, 56.2, 30.9; EIMS (m/z, relative intensity) 197 (M+, 74), 182 (100), 166 (8), 154 (31), 139 (35), 123 (31), 109 (14), 82 (12), 69 (11), 53 (12); HRMS calcd for C10H15NO3 197.1052, found 197.1051.
2,3,4,5-Tetramethoxyaniline (3q)
Colorless solid: mp 98–100 °C. (lit20 99–100 °C). IR (KBr) 3360, 2936, 1617, 1499, 1469, 1227, 1124, 1079, 1044, 1007 cm−1; 1H NMR (CDC13, 300 MHz) δ 6.05 (s, 1 H), 3.90 (s, 3 H), 3.76 (s, 3 H), 3.75 (s, 3 H), 3.73 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 149.7, 147.3, 135.7, 134.6, 94.9, 61.3, 61.0, 60.4, 56.0; EIMS (m/z, relative intensity) 213 (60), 198 (100), 183 (13), 168 (10), 155 (17), 140 (22), 112 (11), 84 (10), 68 (5), 53 (6); HRMS calcd for C10H15NO4 213.1001, found 213.0994.
General Procedure to Synthesize Protected Amines from Acids
A mixture of diphenylphosphoryl azide (12 mmol), the required acid (11 mmol) and triethylamine (12 mmol) in benzene (20 mL) was heated under reflux for 2–3 h. After the evolution of N2 had ceased, benzyl alcohol (11 mmol) was added and the mixture was heated at reflux for another 2–5 h. The reaction was monitored by TLC. After cooling, the mixture was washed successively with cold 1 N HC1, aq NaHCO3 solution, and brine, and then dried (Na2SO4). The organic extract was evaporated under reduced pressure and the residue was chromatographed on silica gel, eluting with ethyl ether-hexane (1:1).
Benzyl Benzo[d][1,3]dioxol-4-ylcarbamate (6a)
Yield 75%. Colorless oil. IR (KBr) 3400, 2900, 1735, 1616, 1537, 1450, 1374, 1220, 1101, 1013, 795 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.33 (m, 7 H), 7.17 (m, 1 H), 6.79 (m, 1 H), 6.57 (d, J = 8.4 Hz, 1 H), 5.90 (s, 2 H), 5.19 (s, 2 H); 13C NMR (CDCl3, 75 MHz) δ 153.0, 147.3, 135.8, 129.6, 128.5, 128.3, 128.2, 128.0, 125.2, 121.9, 121.5, 120.0, 113.3, 104.1, 101.1, 67.1; positive ion ESIMS (m/z, relative intensity) 272 (MH+, 20), 264 (6), 102 (100); HRMS calcd for C15H14NO54 272.0923, found 272.0924.
Benzyl 2,3-Dimethoxyphenylcarbamate (6g)
Yield 92%. Colorless oil. IR (KBr) 3422, 2940, 1736, 1607, 1531, 1481, 1459, 1421, 1299, 1232, 1203, 1102, 1043 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.75 (brs, 1 H), 7.33 (m, 5 H), 6.93 (t, J = 8.1 Hz, 1 H), 6.53 (m, 1 H), 5.11 (s, 2 H), 3.73 (s, 6 H); 13C NMR (CDCl3, 75 MHz) δ 152.9, 151.8, 136.7, 135.8, 131.8, 128.3, 128.1, 123.9, 110.6, 106.4, 66.7, 60.3, 55.4; EIMS (m/z, relative intensity) 287 (M+, 19), 243 (19), 228 (52), 213 (3), 179 (11), 164 (7), 136 (8), 91 (100), 77 (8), 65 (21); HRMS calcd for C16H17NO4 287.1158, found 287.1160.
Benzyl 2,4-Dimethoxyphenylcarbamate (6i)
Yield 95%. Colorless semisolid. IR (KBr) 3400, 2900, 1728, 1618, 1531, 1492, 1417, 1208, 1158, 1037 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.98 (brs, 1 H), 7.35 (m, 5 H), 7.06 (s, 1 H), 6.46 (m, 2 H), 5.17 (s, 2 H), 3.78 (s, 3 H), 3.76 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 155.8, 153.3, 148.9, 136.2, 128.5, 128.2, 120.9, 119.8, 119.0, 103.7, 98.6, 66.7, 55.5, 55.4; EIMS (m/z, relative intensity) 287 (M+, 19), 243 (9), 179 (30), 152 (100), 136 (22), 124 (57), 109 (17), 91 (88), 79 (16), 65 (23); HRMS calcd for C16H17NO4 287.1158, found 287.1156.
Benzyl 2,3,4-Trimethoxyphenylcarbamate (6m)
Yield 73%. Colorless solid: mp 53– 55 °C. IR (KBr) 3400, 2900, 1731, 1599, 1525, 1495, 1459, 1412, 1211, 1102, 1023 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.75 (d, J = 9.0 Hz, 1 H), 7.35 (m, 5 H), 6.61 (d, J = 9.0 Hz, 1 H), 5.17 (s, 2 H), 3.87 (s, 3 H), 3.83 (s, 3 H), 3.80 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 153.3, 149.0, 142.4, 141.7, 136.0, 128.5, 128.3, 125.1, 112.9, 107.0, 66.8, 61.0, 60.8, 56.1; EIMS (m/z, relative intensity) 317 (M+, 20), 273 (8), 258 (10), 182 (65), 167 (29), 123 (27), 91 (100), 77 (2), 65 (19); HRMS calcd for C17H19NO5 317.1263, found 317.1261.
Benzyl 2,4,5-Trimethoxyphenylcarbamate (6o)
Yield 90%. Colorless solid: mp 99–100 °C. IR (KBr) 3400, 2900, 1728, 1614, 1531, 1457, 1456, 1407, 1202, 1137, 1036 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.81 (brs, 1 H), 7.35 (m, 5 H), 7.11 (s, 1 H), 6.50 (s, 1 H), 5.16 (s, 2 H), 3.82 (s, 6 H), 3.78 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 153.3, 144.5, 143.0, 141.5, 136.1, 128.5, 128.28, 120.5, 103.9, 97.8, 66.8, 56.59, 56.50, 56.3; EIMS (m/z, relative intensity) 317 (M+, 44), 258 (6), 209 (8), 182 (22), 123 (33), 91 (100), 65 (14); HRMS calcd for C17H19NO5 317.1263, found 317.1265.
General Procedure for N-Methylation
A mixture of NaH (12 mmol, 60% dispersion in oil, washed twice with n-hexane) and mono-protected amine (8 mmol) in DMF (10 mL) was stirred at room temperature for 30 min. A solution of CH3I (24 mmol) was added and the resulting mixture was stirred at room temperature for 2 h. Ice water (20 g) was added to this mixture and the resulting mixture was extracted with CHC13 (3 × 100 mL). The combined extracts were washed with brine (50 mL), dried (Na2SO4), and evaporated. The residue was purified by column chromatography on silica gel, eluting with a 9:1 mixture of petroleum ether and ethyl acetate. Evaporation of the solvents gave methylated carbamate in very high yield.
Benzyl Benzo[d][1,3]dioxol-4-yl(methyl)carbamate (7a)
Yield 94%. Colorless oil. IR (KBr) 1712, 1633, 1492, 1459, 1388, 1345, 1301, 1252, 1152, 1102, 1059, 795 cm−1; 1H NMR (CDC13, 300 MHz) δ 7.26 (m, 5 H), 6.75 (m, 3 H), 5.86 (brs, 2 H), 5.16 (brs, 2 H), 3.26 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 155.0, 148.4, 141.9, 136.4, 128.2, 127.7, 125.3, 121.3, 120.1, 106.8, 101.0, 67.2, 36.7; positive ion ESIMS (m/z, relative intensity) 308 (MH+, 35), 285 (100), 242 (68); HRMS calcd for C16H15NO4 + Na+ 308.0899, found 308.0906.
Benzyl 2,3-Dimethoxyphenyl(methyl)carbamate (7g)
Yield 89%. Colorless solid: mp 92–94 °C (lit33 mp 92–93 °C). IR (KBr) 1707, 1587, 1488, 1479, 1389, 1316, 1265, 1157, 1075, 794 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.22 (m, 5 H), 6.99 (t, J = 8.4 Hz, 1 H), 6.81 (m, 2 H), 5.12 (s, 2 H), 3.83 (s, 3 H), 3.73 (s, 3 H), 3.20 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 155.7, 153.3, 145.2, 136.7, 128.2, 127.6, 127.5, 123.5, 120.6, 111.4, 67.0, 60.6, 55.8, 37.5; positive ion ESIMS (m/z, relative intensity) 324 (MNa+, 49), 283 (18), 279 (35), 278 (8), 277 (60), 244 (7), 243 (10); HRMS calcd for C17H19NO4 + Na+ 324.1212, found 324.1208.
Benzyl 2,4-Dimethoxyphenyl(methyl)carbamate (7i)
Yield 90%. Colorless oil. IR (KBr) 1705, 1609, 1587, 1513, 1455, 1389, 1352, 1209, 1159, 1033 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.22 (m, 6 H), 6.40 (m, 2 H), 5.11 (s, 2 H), 3.77 (s, 3 H), 3.66 (s, 3 H), 3.14 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 159.7, 155.8, 155.7, 137.2, 128.9, 128.1, 127.9, 127.4, 127.2, 125.0, 103.9, 99.2, 66.7, 55.4, 55.3, 37.5; EIMS (m/z, relative intensity) 301 (M+, 22), 166 (33), 151 (42), 91 (100), 65 (11); HRMS calcd for C16H19NO4 301.1314, found 301.1315.
Benzyl Methyl(2,3,4-trimethoxyphenyl)carbamate (7m)
Yield 92%. Colorless oil. IR (KBr) 1708, 1598, 1497, 1471, 1418, 1390, 1352, 1212, 1155, 1094 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.35 (m, 5 H), 6.86 (d, J = 8.7 Hz, 1 H), 6.61 (d, J = 8.7 Hz, 1 H), 5.11 (s, 2 H), 3.84 (s, 6 H), 3.79 (s, 3 H), 3.21 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 155.9, 152.8, 149.7, 142.4, 136.6, 129.4, 128.0, 127.3, 122.7, 106.3, 66.8, 60.7, 55.8, 37.9; EIMS (m/z, relative intensity) 331 (M+, 9), 196 (100), 181 (45), 166 (10), 147 (12), 91 (82), 77 (2), 65 (13); HRMS calcd for C18H21NO5 331.1420, found 331.1424.
Benzyl Methyl(2,4,5-trimethoxyphenyl)carbamate (7o)
Yield 85%. Colorless solid: mp 98–99 °C. IR (KBr) 1704, 1608, 1515, 1455, 1386, 1330, 1206, 1148, 1036 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.76 (m, 5 H), 7.14 (s, 1 H), 6.98 (s, 1 H), 5.56 (s, 2 H), 4.33 (s, 3 H), 4.23 (s, 3 H), 4.16 (s, 3 H), 3.64 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 155.9, 148.9, 148.4, 142.3, 136.8, 128.1, 127.9, 127.6, 127.3, 127.0, 123.1, 112.4, 97.6, 66.5, 56.1, 55.9, 37.2; EIMS (m/z, relative intensity) 331 (M+, 73), 196 (45), 181 (42), 166 (22), 137 (10), 91 (100), 65 (15); HRMS calcd for C18H21NO5 331.1420, found 331.1421.
Ethyl 3-(Benzo[d][1,3]dioxol-4-yl(methyl)amino)-3-oxopropanoate (8)
A mixture of amine 3b (1.51 g, 10.0 mmol) and excess diethyl malonate (15 mL) was heated at 110 °C for 3 h while argon gas was bubbled through the solution. The excess diethyl malonate and ethanol produced during the reaction were removed under reduced pressure. The crude product was purified by silica gel column chromatography, eluting with 30% ethyl acetate in hexanes, to afford carbamate 8 (2.1g, 82%) as a colorless oil. IR (neat) 3459, 2971, 1741, 1672, 1631, 1460, 1375, 1253, 1096, 1055, 928, 795 cm−1; 1H NMR (CDCl3, 300 MHz) δ 6.75 (m, 2 H), 6.62 (dd, J = 7.8, 1.8 Hz, 1 H), 5.92 (s, 2 H), 4.00 (q, J = 7.2 Hz, 2 H), 3.19 (s, 2 H), 3.15 (s, 3 H), 1.11 (t, J = 8.4 Hz, 3 H); 13C NMR (CDCl3, 75 MHz) δ 167.2, 165.9, 148.6, 142.5, 125.0, 122.2, 120.6, 108.2, 101.4, 60.9, 41.1, 35.8, 13.8; EIMS (m/z, relative intensity) 265 (M+, 35), 220 (10), 178 (9), 151 (100), 120 (5), 93 (12), 77 (3), 65 (20); HRMS calcd for C13H15NO5 + Na+ 288.0848, found 288.0847.
6-Hydroxy-9-methyl-[1,3]dioxolo[4,5-h]quinolin-8(9H)-one (9)
The acid 2 (500 mg, 2.1 mmol) was added to polyphosphoric acid (5 g) at 95–100 °C and the mixture was allowed to stir for 3 h at the same temperature and then poured into ice-water. After polyphosphoric acid had dissolved, a white solid product appeared in the flask. The solid hydroxyquinolone 9 (400 mg, 88%) was collected by filtration, washed with ether and dried under vacuum. The solid was used for the next step without further purification: mp 310 °C (dec, lit18 mp 310–315 °C). 1H NMR (CF3CO2D, 300 MHz) δ 8.06 (d, J = 8.4 Hz, 1 H), 7.29 (d, J = 8.4 Hz, 1 H), 6.72 (s, 1 H), 6.27 (s, 2 H), 4.28 (s, 3 H); EIMS (m/z, relative intensity) 219 (M+, 100), 190 (27), 149 (22), 120 (15), 93 (29), 69 (27), 46 (8); HRMS calcd for C11H9NO4 219.0532, found 219.038.
General Procedure for the Synthesis of Amides (10a-g)
Diketene (10.0 mmol) was added to a magnetically stirred solution of the substituted anilines (3a-g, 11.0 mmol) in benzene (10 mL) and the mixture was heated at 100 °C for 90–120 min. The reaction mixtures were monitored by TLC. After completion of the reactions, the solutions were evaporated under reduced pressure and the residues were chromatographed on silica gel, eluting with ethyl ether-hexane (1:1).
N-(Benzo[d][1,3]dioxol-4-yl)-3-oxobutanamide (10a)
Yield 68%. Colorless solid: mp 64–66 °C. IR (KBr) 3300, 2800, 1716, 1668, 1636, 1542, 1419, 1451, 1358, 1254, 1178, 1158, 1068, 1022, 934, 794 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.10 (brs, 1 H), 7.54 (d, J = 8.4 Hz, 1 H), 6.77 (m, 1 H), 6.59 (d, J = 7.8 Hz, 1 H), 5.93 (s, 2 H), 3.57 (s, 2 H), 2.29 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 205.1, 163.3, 147.5, 137.3, 121.8, 121.0, 114.9, 105.1, 101.3, 49.2, 31.2; EIMS (m/z, relative intensity) 221 (M+, 14), 163 (33), 137 (100), 109 (8), 79 (17), 52 (13); HRMS calcd for C11H11NO4 221.0688, found 221.0690.
N-(Benzo[d][1,3]dioxol-4-yl)-N-methyl-3-oxobutanamide (10b)
Yield 70%. Colorless oil. IR (neat) 1721, 1662, 1630, 1604, 1487, 1459, 1425, 1377, 1358, 1253, 1194, 1090, 1055, 1032, 927 cm−1; 1H NMR (CDCl3, 300 MHz) δ 6.78 (m, 2 H), 6.63 (m, 1 H), 5.96 (s, 2 H), 3.30 (s, 2 H), 3.21 (s, 3 H), 2.11 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 202.1, 166.9, 148.8, 142.6, 125.3, 122.4, 120.6, 108.5, 101.5, 49.6, 35.9, 30.0; EIMS (m/z, relative intensity) 235 (M+, 31), 178 (4), 151 (100), 122 (12), 93 (23), 65 (10); HRMS calcd for C12H13NO4 235.0845, found 235.0846.
3-Oxo-N-phenylbutanamide (10c)
Yield 80%, colorless solid: mp 82–84 °C (lit34 mp 84–86 °C). IR (KBr) 3300, 1716, 1661, 1599, 1544, 1444, 1410, 1314, 1231, 1157, 1029, 755 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.16 (brs, 1 H), 7.50 (m, 2 H), 7.26 (m, 2 H), 7.06 (m, 1 H), 3.52 (s, 2 H), 2.25 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 204.9, 163.7, 137.4, 128.8, 124.4, 120.0, 49.8, 31.0; EIMS (m/z, relative intensity) 177 (M+, 8), 119 (18), 93 (100), 66 (28), 58 (12), 43 (11); HRMS calcd for C10H11NO2 177.0790, found 177.0794.
N-Methyl-3-oxo-N-phenylbutanamide (10d)
Yield 85%, colorless oil. IR (neat) 1721, 1655, 1595, 1496, 1427, 1379, 1358, 1343, 1302, 1162, 1123 776 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.37 (m, 3 H), 7.13 (m, 2 H), 3.24 (s, 5 H), 2.04 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 202.2, 166.5, 143.4, 129.8, 129.5, 128.1, 127.1, 49.8, 37.2, 30.2; positive ion ESIMS (m/z, relative intensity) 382 (2M+, 100), 192 (MH+, 63), 122 (30), 93 (100), 66 (28), 58 (12), 43 (11); HRMS calcd for C11H13NO2 + H+ 192.1024, found 192.1023.
N-(2-Methoxyphenyl)-3-oxobutanamide (10e)
Yield 70%, yellow solid: mp 82–84 °C (lit35 83–84 °C). IR (KBr) 3300, 1714, 1677, 1600, 1537, 1488, 1461, 1435, 1336, 1253, 1159, 750 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.20 (brs, 1 H), 8.27 (d, J = 8.1 Hz, 1 H), 7.01 (m, 1 H), 6.96 (m, 1 H), 6.84 (m, 1 H), 3.83 (s, 3 H), 3.54 (s, 2 H), 2.25 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 204.3, 163.2, 148.1, 127.1, 123.9, 120.7, 119.9, 109.9, 55.6, 50.6, 30.8; EIMS (m/z, relative intensity) 207 (M+, 47), 149 (41), 108 (100), 92 (7), 80 (35), 43 (52); HRMS calcd for C12H13NO3 207.0895, found 207.0891.
N-(2-Methoxyphenyl)-N-methyl-3-oxobutanamide (10f)
Yield 75%, colorless oil. IR (neat) 1720, 1655, 1510, 1433, 1464, 1357, 1379, 1279, 1240, 1108, 795 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.42 (m, 1 H), 7.26 (m, 1 H), 7.02 (m, 2 H), 3.93 (s, 3 H), 3.31 (s, 3 H), 3.29 (s, 2 H), 2.42 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 202.3, 167.4, 154.6, 131.5, 129.7, 128.8, 121.0, 111.8, 55.2, 49.6, 36.0, 29.7; EIMS (m/z, relative intensity) 221 (M+, 10), 190 (21), 122 (100), 94 (27), 65 (15), 43 (46); HRMS calcd for C12H15NO3 221.1052, found 221.1050.
N-(2,3-Dimethoxyphenyl)-3-oxobutanamide (10g)
Yield 67%, colorless oil. IR (neat) 3300, 1712, 1681, 1603, 1539, 1478, 1463, 1422, 1297, 1260, 1090, 1002, 794 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.47 (brs, 1 H), 7.92 (d, J = 8.1 Hz, 1 H), 6.99 (dd, J = 8.1, 16.5 Hz, 1 H), 6.63 (d, J = 8.1 Hz, 1 H), 3.88 (s, 3 H), 3.83 (s, 3 H), 3.59 (s, 2 H), 2.29 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 204.4, 163.4, 152.0, 137.8, 131.9, 124.0, 112.6, 107.7, 60.7, 55.7, 50.3, 31.0; EIMS (m/z, relative intensity) 237 (M+, 68), 206 (8), 153 (51), 138 (100), 95 (18), 43 (99); HRMS calcd for C12H15NO4 237.1001, found 237.1009.
N-(2,3-Dimethoxyphenyl)-N-methyl-3-oxobutanamide (10h)
Yield 70%, colorless oil. IR (neat) 1721, 1656, 1587, 1478, 1429, 1378, 1356, 1317, 1267, 1234, 1148, 1096, 1051, 1003, 794 cm−1; 1H NMR (CDCl3, 300 MHz) δ 6.98 (m, 1 H), 6.85 (m, 1 H), 6.63 (d, J = 8.1 Hz, 1 H), 3.79 (s, 3 H), 3.74 (s, 5 H), 3.15 (s, 3 H), 2.20 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 202.2, 167.1, 153.3, 136.6, 128.0, 124.1, 120.6, 112.2, 60.7, 55.7, 49.1, 36.5, 29.8; positive ion ESIMS (m/z, relative intensity) 274 (MNa+, 68), 252 (MH+), 180 (100); HRMS calcd for C13H17NO4 + Na+ 274.1055, found 274.1054.
N-(2,4-Dimethoxyphenyl)-3-oxobutanamide (10i)
Yield 80%, colorless solid: mp 92–94 °C. IR (KBr) 3300, 1713, 1667, 1615, 1603, 1535, 1459, 1416, 1302, 1262, 1282, 1208, 1158, 1128, 1030, 750 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.00 (brs, 1 H), 8.15 (d, J = 8.7 Hz, 1 H), 6.44 (d, J = 8.7 Hz, 1 H), 6.40 (brs, 1 H), 3.84 (s, 3 H), 3.75 (s, 3 H), 3.54 (s, 3 H), 2.29 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 204.6, 162.8, 156.6, 149.7, 120.9, 120.8, 103.5, 98.6, 55.8, 55.5, 50.6, 31.0; EIMS (m/z, relative intensity) 237 (M+, 67), 179 (55), 153 (40), 138 (100), 110 (12), 93 (5), 52 (4); HRMS calcd for C12H15NO4 237.1001, found 237.1001.
N-(2,4-Dimethoxyphenyl)-N-methyl-3-oxobutanamide (10j)
Yield 80%, colorless oil. IR (neat) 1720, 1655, 1609, 1586, 1513, 1462, 1421, 1379, 1317, 1283, 1210, 1162, 1113, 1031, 795 cm−1; 1H NMR (CDCl3, 300 MHz) δ 6.99 (d, J = 1.5 Hz, 1 H), 6.42 (m, 2 H), 3.74 (s, 3 H), 3.72 (s, 3 H), 3.09 (s, 5 H), 2.05 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 202.4, 167.8, 160.5, 155.5, 129.1, 124.7, 104.4, 99.3, 55.4, 55.2, 49.6, 36.1, 29.7; EIMS (m/z, relative intensity) 251 (M+, 5), 220 (3), 167 (65), 152 (100), 136 (7), 124 (23), 109 (12), 98 (22), 56 (9); HRMS calcd for C13H17NO4 251.1158, found 251.1161.
N-(2,5-Dimethoxyphenyl)-3-oxobutanamide (10k)
Yield 94%, colorless solid: mp 70–72 °C (lit36 mp 67–68 °C). IR (KBr) 3300, 2800, 1715, 1676, 1600, 1537, 1486, 1465, 1430, 1361, 1279, 1221, 1159, 1046, 795 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.20 (brs, 1 H), 7.97 (d, J = 3.0 Hz, 1 H), 6.72 (d, J = 9.0 Hz, 1 H), 6.50 (dd, J = 9.0, 3.0 Hz, 1 H), 3.76 (s, 3 H), 3.68 (s, 3 H), 3.51 (s, 2 H), 2.22 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 204.2, 163.3, 153.4, 142.3, 127.8, 110.7, 108.4, 106.3, 56.1, 55.5, 50.4, 30.7; positive ion ESIMS (m/z, relative intensity) 260 (MNa+, 100); HRMS calcd for C12H15NO4 + Na+ 260.0899, found 260.0900.
N-(2,5-Dimethoxyphenyl)-N-methyl-3-oxobutanamide (10l)
Yield 80%, colorless solid: mp 69–71 °C (lit20 mp 68.5–69 °C). IR (KBr) 1720, 1658, 1611, 1508, 1464, 1424, 1376, 1310, 1274, 1219, 1181, 1107, 1041, 728 cm−1; 1H NMR (CDCl3, 300 MHz) δ 6.81 (m, 2 H), 6.67 (m, 1 H), 3.70 (s, 3 H), 3.69 (s, 3 H), 3.12 (m, 5 H), 2.08 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 202.3, 167.3, 153.6, 148.7, 132.0, 114.6, 114.2, 112.5, 55.6, 49.5, 35.9, 29.7; positive ion ESIMS (m/z, relative intensity) 274 (MNa+, 100), HRMS calcd for C13H17NO4 + Na+ 274.1055, found 274.1056.
3-Oxo-N-(2,3,4-trimethoxyphenyl)butanamide (10m)
Yield 85%, colorless solid: mp 86–88 °C. IR (KBr) 3300, 2800, 1711, 1674, 1601, 1535, 1494, 1462, 1412, 1360, 1272, 1164, 1095, 1060, 1010, 795 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.28 (brs, 1 H), 7.86 (d, J = 9.0 Hz, 1 H), 6.53 (d, J = 9.0 Hz, 1 H), 3.86 (s, 3 H), 3.80 (s, 3 H), 3.77 (s, 3 H), 3.51 (s, 2 H), 2.21 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 204.5, 163.1, 149.6, 143.1, 141.5, 125.0, 114.7, 106.6, 60.9, 60.6, 55.8, 49.8, 30.8; EIMS (m/z, relative intensity) 267 (M+, 20), 209 (100), 183 (83), 168 (49), 153 (7), 140 (11), 123 (19), 108 (12), 95 (4), 80 (12), 69 (8); HRMS calcd for C13H17NO5 267.1107, found 267.1111.
N-Methyl-3-oxo-N-(2,3,4-trimethoxyphenyl)butanamide (10n)
Yield 78%, colorless oil. IR (neat) 1712, 1633, 1492, 1459, 1388, 1345, 1301, 1252, 1152, 1102, 1059, 795 cm−1; 1H NMR (CDCl3, 300 MHz) δ 6.69 (d, J = 9.0 Hz, 1 H), 6.51 (d, J = 9.0 Hz, 1 H), 3.73 (s, 3 H), 3.70 (s, 3 H), 3.69 (s, 3 H), 3.04 (m, 5 H), 1.96 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 202.0, 167.3, 153.6, 149.0, 142.2, 129.2, 122.6, 106.6, 60.8, 60.5, 55.6, 49.1, 36.5, 29.6; positive ion ESIMS (m/z, relative intensity) 282 (MH+, 100), 251 (3), 198 (21), 183 (2); HRMS calcd for C14H19NO5 + H 282.1343, found 282.1341.
3-Oxo-N-(2,4,5-trimethoxyphenyl)butanamide (10o)
Yield 67%. Colorless solid: mp 90–92 °C (lit37 mp 92–93 °C). IR (KBr) 3300, 2800, 1714, 1668, 1534, 1465, 1405, 1342, 1219, 1203, 1031, 750 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.02 (brs, 1 H), 7.97 (s, 1 H), 6.46 (s, 1 H), 3.78 (s, 3 H), 3.76 (s, 3 H), 3.73 (s, 3 H), 3.49 (s, 2 H), 2.23 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 204.2, 162.9, 145.0, 142.3, 120.2, 105.4, 97.3, 56.5, 56.2, 50.3, 30.8; EIMS (m/z, relative intensity) 267 (M+, 64), 252 (5), 209 (44), 194 (32), 168 (100), 140 (28), 108 (13), 69 (15); HRMS calcd for C13H17NO5 267.1107, found 267.1106.
N-Methyl-3-oxo-N-(2,4,5-trimethoxyphenyl)butanamide (10p)
Yield 81%. Colorless oil. IR (neat) 1731, 1599, 1525, 1495, 1459, 1412, 1270, 1240, 1211, 1102, 1089, 1023, 795 cm−1; 1H NMR (CDCl3, 300 MHz) δ 6.61 (s, 1 H), 6.46 (s, 1 H), 3.82 (s, 3 H), 3.71 (s, 6 H), 3.13 (s, 2 H), 3.08 (s, 3 H), 2.04 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 202.5, 167.7, 149.5, 148.8, 142.8, 123.0, 112.2, 97.3, 56.4, 56.0, 55.8, 49.5, 36.0, 29.8; EIMS (m/z, relative intensity) 281 (M+, 37), 250 (10), 209 (12), 197 (33), 182 (100), 166 (12), 154 (17), 123 (10), 98 (11), 69 (26); HRMS calcd for C14H19NO5 281.1263, found 281.1261.
3-Oxo-N-(2,3,4,5-tetramethoxyphenyl)butanamide (10q)
Yield 77%. Colorless oil. IR (neat) 3500, 2800, 1710, 1682, 1604, 1532, 1486, 1464, 1410, 1364, 1219, 1129, 1098, 1006 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.4 (brs, 1 H), 7.73 (s, 1 H), 3.86 (s, 3 H), 3.85 (s, 6 H), 3.82 (s, 3 H), 3.77 (s, 3 H), 3.54 (s, 2 H), 2.04 (s, 3 H); 13C NMR (CDCl3, 75 MHz) δ 204.5, 163.2, 149.0, 146.3, 138.6, 136.1, 126.9, 99.2, 61.2, 61.1, 61.0, 51.0, 49.9, 31.0; EIMS (m/z, relative intensity) 297 (M+, 47), 282 (10), 239 (22), 198 (100), 183 (21), 155 (27), 140 (17), 127 (5), 69 (16); HRMS calcd for C14H19NO6 297.1212, found 297.1210.
Expression and Purification of Human QR2 Gene
Human QR2 was purified from 3 liters of E. coli BL21(DE3) grown in Luria Bertani medium supplement with 100 μg/mL ampicillin (Fisher Scientific, Pittsburg, PA) following our previously reported procedure.6 Freshly prepared and non-frozen QR2 was used to set up crystallization experiments. For kinetic assays and inhibitor studies, QR2, previously stored at − 80 °C, was thawed on ice and then adjusted to the desired concentration by diluting in storage buffer without glycerol.
Steady-State Kinetic Assays and IC50 Value Determination
The activity of QR2 under steady-state conditions was evaluated on SpectraMax Plus 384 UV/Vis spectrophotometer thermostated at 23 °C by monitoring the increase in absorbance of the product formazan at 610 nm which is produced from MTT. The assays were run in 96 well plates at a final assay volume of 200 μL. Each assay mixture contained 12 nM QR2, 17.5 μM NMeH, and 200 μM MTT in a reaction buffer containing 100 mM NaCl, 0.1% Triton-X100 (Fisher Biotech 93004), and 0.6 mg/mL BSA (Sigma A3059). The reactions were initiated by the addition of QR2. The initial slopes of the reaction (ΔAbs/Δtime) were used to calculate the initial rates using a molar extinction coefficient of 11,300 mM−1 cm−1 for MTT.
IC50 values were determined at 23 °C in 96 well plates using an assay volume of 200 μL and the assay conditions described above. Inhibitor concentrations ranged from 0.4 μM to 500 μM, and 11 different inhibitor concentrations were chosen for each IC50 value determination. Assays at each inhibitor concentration were performed in triplicate, and the average rate values and standard deviation at each rate were used to determine the IC50 values. The resulting data were used to calculate the percent inhibition values (%I) versus the non-inhibited control which consisted of reaction mixture with no inhibitor. The percent inhibition data were then plotted as a function of inhibitor concentration ([I]) and the data were fit via non-linear regression to the following equation: %I = %Imax/(1+(IC50/[I])) describing a simple binding isotherm. Data were fit using the Enzyme Kinetics Module of program SigmaPlot from SPSS Scientific. IC50 values are reported along with standard errors.
QR2 Crystallization and X-ray Structure Determination
Crystals of the QR2 apoenzyme were grown according to our previously reported protocols.6 The resulting crystals were soaked overnight with inhibitor in a solution that consisted of 1 μl of a 10 mM inhibitor solution (dissolved in 100% DMSO) that was mixed into 9 μL of the mother liquor solution that contained 1.339 M ammonium sulfate, 0.1 M bis-Tris, pH 6.7, 0.1 M NaCl, 5 mM DTT and 12 μM FAD. Crystals were then retrieved with a nylon loop which was then used to swipe the crystals through the well solution supplemented with 30% glycerol. The crystals were immediately flash-frozen by plunging into liquid nitrogen. Crystals were stored in liquid nitrogen in Dewar flasks until X-ray data collection.
X-Ray diffraction data were collected at the SER-CAT beamline 22-BM located at the Advanced Photon Source (APS) at Argonne National Laboratories. Flash-frozen crystals were mounted on a goniostat under a stream of dry N2 at 100 K. X-ray data were collected on either a MAR 225 mm or MAR315 CCD detector. Individual data sets were processed and scaled in the P212121 space group using HKL2000. Initial phases for the QR2-compound complex’s were determined via molecular replacement using the program Phaser in the CCP4 suite. Model building and structural refinement were performed using the programs WinCoot and Refmac from the CCP4 suite. The atomic coordinates and structure factors have been deposited in the Protein Data bank (www.pdb.org)38 under PDB codes XXXX for the QR2-compound 1 complex and XXXX for the 1l complex.
Aromatase Assay
Aromatase activity was assayed as previously reported, with the necessary modifications to assay in a 384-well plate. Briefly, the test compound (3.5 μL) was preincubated with 30 μL of NADPH regenerating system (2.6 mM NADP+, 7.6 mM glucose 6-phosphate, 0.8 U/ml glucose-6-phosphate dehydrogenase, 13.9 mM MgCl2, and 1 mg/mL albumin in 50 mM potassium phosphate buffer, pH 7.4) for 10 min at 37 °C. The enzyme and substrate mixture (33 μL of 80 μL/mL CYP19 enzyme, BD Biosciences), 0.4 μM dibenzylfluorescein, 4 mg/mL albumin in 50 mM potassium phosphate, pH 7.4, and the plate were incubated for 30 min at 37 °C before quenching with 25 μL of 2 N NaOH. After termination of the reaction and shaking for 5 min, the plate was further incubated for 2 h at 37 °C. This enhances the ratio of signal to background. Fluorescence was measured at 485 nm (excitation) and 530 nm (emission). IC50 values and dose-response curves were based on three independent experiments performed in duplicate using five concentrations of test substance (Table 1).
Cytotoxicity Testing
LNCaP (prostate), MCF7 (breast), and Hepa 1c1c7 (mouse liver) cell lines were acquired from ATCC (Manassas, VA). The LU lung cancer cell line was provided by the Department of Surgical Oncology, University of Illinois Chicago, Chicago, IL. Evaluation of cytotoxic potential was performed as described previously.39–41 In brief, using 96-well plates, cells were seeded at a density of 5.0 × 104 cells/mL, and treated with a 10 μL solution of each compound in 10% DMSO to yield final concentrations ranging from 0–20 μg/mL. A zero day control plate was incubated for 30 min at 37 °C. All other plates were incubated for 72 h. After incubation, cells were fixed by the addition of 50 μL cold 50% aqueous trichloroacetic acid, incubated at 4 °C for 30 min, washed with tap water, and air-dried. Cells were then stained with 100 μL of 0.4% sulforhodamine B in 1% acetic acid for 30 min at 23 °C. After washing with 1% acetic acid (4x) to remove excess dye and air-drying, bound dye was solubilized by the addition of 200 μL 10 mM Tris base (pH 10). Finally, the plates were shaken for 5 min, and absorption at 515 nm was determined with a Beckman Coulter ELx800 plate reader. All determinations were performed in triplicate, and the 50% inhibitory (IC50) values of active samples were assessed by linear regression analyses of dose-response data.
Supplementary Material
Acknowledgments
This work was supported by program project grant P01 CA48112 awarded by the National Cancer Institute. Some of this research was conducted in a facility constructed with the financial support of a Research facilities Improvement program grant C06-14499 from the National Institutes of Health.
Footnotes
Abbreviations: DPPA, diphenylphosphoryl azide; TMS, trimethylsilyl; FAD, flavin adenine dinucleotide; HRMS, high-resolution mass spectrometry; EIMS, electron-impact mass spectrometry; DMF, dimethylformamide; ESIMS, electrospray-ionization mass spectrometry; MTT, (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; BSA, bovine serum albumin; DMSO, dimethylsulfoxide; SER-CAT, Southeast Regional Collaborative Access Team; CCD, charge-coupled device; CCP4, Collaborative Computational Project Number 4; NADPH, the reduced form of nicotinamide adenine dinucleotide phosphate (NADP+).
Supporting Information Available: Results from elemental analyses of intermediates and target compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ito A, Shamon LA, Yu B, Mata-Greenwood E, Lee SK, van Breemen RB, Mehta RG, Farnsworth NR, Fong HHS, Pezzuto JM, Kinghorn AD. Antimutagenic Constituents of Casimiroa edulis with Potential Cancer Chemopreventive Activity. J Agric Food Chem. 1998;46:3509–3516. [Google Scholar]
- 2.Jaiswal AK. Human NAD(P)H-Quinone Oxidoreductase(2) - Gene Structure, Activity, and Tissue-Specific Expression. J Biol Chem. 1994;269:14502–14508. [PubMed] [Google Scholar]
- 3.Vella F, Gilles FA, Delagrange P, Boutin JA. NRH:Quinone Reductase 2: An Enzyme of Surprises and Mysteries. Biochem Pharmacol. 2005;71:1–12. doi: 10.1016/j.bcp.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Harada S, Fujii C, Hayashi A, Ohkoshi N. An Association between Idiopathic Parkinson’s Disease and Polymorphisms of Phase II Detoxification Enzymes: Glutathione S-transferase M1 and Quinone Oxidoreductase 1 and 2. Biochem Biophys Res Commun. 2001;288:887–892. doi: 10.1006/bbrc.2001.5868. [DOI] [PubMed] [Google Scholar]
- 5.Harada S, Tachikawa H, Kawanishi Y. A Possible Association between an Insertion/Deletion Polymorphism of the NQO2 Gene and Schizophrenia. Psychiatr Genet. 2003;13:205–209. doi: 10.1097/00041444-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Calamini B, Santarsiero BD, Boutin JA, Mesecar AD. Kinetic, Thermodynamic and X-Ray Structural Insights into the Interaction of Melatonin and Analogues with Quinone Reductase 2. Biochem J. 2008;413:81–91. doi: 10.1042/BJ20071373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ware RW, Hinkley LA, Hardeman KP, Jenks MG. Preparation of Quinolines and Quinazolines as Inhibitors of Quinone Reductase. 2006;2 2006. [Google Scholar]
- 8.Mailliet F, Ferry G, Vella F, Thiam K, Delagrange P, Boutin JA. Organs from Mice Deleted for NRH:Quinone Oxidoreductase 2 Are Deprived of the Melatonin Binding Site MT3. FEBS Lett. 2004;578:116–120. doi: 10.1016/j.febslet.2004.10.083. [DOI] [PubMed] [Google Scholar]
- 9.Celli CM, Tran N, Knox R, Jaiswal AK. NRH:Quinone Oxidoreductase 2 (NQO2) Catalyzes Metabolic Activation of Quinones and Anti-tumor Drugs. Biochem Pharmacol. 2006;72:366–376. doi: 10.1016/j.bcp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Jamieson D, Tung AT, Knox RJ, Boddy AV. Reduction of Mitomycin C Is Catalysed by Human Recombinant NRH:Quinone Oxidoreductase 2 Using Reduced Nicotinamide Adenine Dinucleotide As an Electron Donating Co-factor. Br J Cancer. 2006;95:1229–1233. doi: 10.1038/sj.bjc.6603414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knox RJ, Jenkins TC, Hobbs SM, Chen S, Melton RG, Burke PJ. Bioactivation of 5-(Aziridin-1-yl)-2,4-dinitrobenzamide (CB 1954) by Human NAD(P)H Quinone Oxidoreductase 2: a Novel Co-Substrate-Mediated Antitumor Prodrug Therapy. Cancer Res. 2000;60:4179–4186. [PubMed] [Google Scholar]
- 12.Boutin JA, Chatelain-Egger F, Vella F, Delagrange P, Ferry G. Quinone Reductase 2 Substrate Specificity and Inhibition Pharmacology. Chem Biol Interact. 2005;151:213–228. doi: 10.1016/j.cbi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Buryanovskyy L, Fu Y, Boyd M, Ma YL, Hsieh TC, Wu JM, Zhang ZT. Crystal Structure of Quinone Reductase 2 in Complex with Resveratrol. Biochemistry. 2004;43:11417–11426. doi: 10.1021/bi049162o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boutin JA, Audinot V, Ferry G, Delagrange P. Molecular Tools to Study Melatonin Pathways and Actions. Trends in Pharmacol Sci. 2005;26:412–419. doi: 10.1016/j.tips.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Fabian CJ, Kimler BF, Mayo MS, Grizzle WE, Masood S, Ursin G. Clinical Approaches to Discovering and Testing New Breast Cancer Prevention Drugs. In: Kelloff GJ, Hawk ET, Sigman CC, editors. Cancer Chemoprevention. Vol. 2. Humana Press, Inc.; Totowa, N. J.: 2005. pp. 213–237. [Google Scholar]
- 16.Strasser-Weippl K, Goss PE. Counteracting Estrogen as Breast Cancer Prevention. In: Kelloff GJ, Hawk ET, Sigman CC, editors. Cancer Chemoprevention. Vol. 2. Humana Press, Inc.; Totowa, N. J.: 2005. pp. 249–264. [Google Scholar]
- 17.Maiti A, Cuendet M, Croy VL, Endringer DC, Pezzuto JM, Cushman M. Synthesis and Biological Evaluation of (+/−)-Abyssinone II and Its Analogues as Aromatase Inhibitors for Chemoprevention of Breast Cancer. J Med Chem. 2007;50:2799–2806. doi: 10.1021/jm070109i. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein B, Hylton TA. Heterocyclic Compounds–V. The Synthesis of Casimiroin. Tetrahedron. 1964;20:1725–1728. [Google Scholar]
- 19.Ple PA, Green TP, Hennequin LF, Curwen J, Fennel M, Allen J, Lambert-van der Brempt S, Costello G. Discovery of a New Class of Anilinoquinazoline Inhibitors with High Affinity and Specificity for the Tyrosine Kinase Domain of c-Src. J Med Chem. 2004;47:871–887. doi: 10.1021/jm030317k. [DOI] [PubMed] [Google Scholar]
- 20.Perez JM, Vidal L, Grande MT, Menendez JC, Avendano C. Regioselectivity of the Diels-Alder Reactions of 2,5,8(1h)-Quinolinetriones. Tetrahedron. 1994;50:7923–7932. [Google Scholar]
- 21.Su TL, Kohler B, Chou TC, Chun MW, Watanabe KA. Synthesis of the Acridone Alkaloids Glyfoline and Congeners - Structure Activity Relationship Studies of Cytotoxic Acridones. J Med Chem. 1992;35:2703–2710. doi: 10.1021/jm00092a022. [DOI] [PubMed] [Google Scholar]
- 22.Foster CE, Bianchet MA, Talalay P, Zhao QJ, Amzel LM. Crystal Structure of Human Quinone Reductase Type 2, a Metalloflavoprotein. Biochemistry. 1999;38:9881–9886. doi: 10.1021/bi990799v. [DOI] [PubMed] [Google Scholar]
- 23.Fu Y, Buryanovskyy L, Zhang ZT. Crystal Structure of Quinone Reductase 2 in Complex with Cancer Prodrug CB1954. Biochem Biophys Res Commun. 2004;336:332–338. doi: 10.1016/j.bbrc.2005.08.081. [DOI] [PubMed] [Google Scholar]
- 24.Fu Y, Buryanovskyy L, Zhang ZT. Quinone Reductase 2 Is a Catechol Quinone Reductase. J Biol Chem. 2008;283:23829–23835. doi: 10.1074/jbc.M801371200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kazuko S, Kazuhisa F, Seitaro S, Masatomo H. Studies on Tertiary Amine Oxides. LXVI. Reactions of Quinoline 1-Oxide Derivatives with Tosyl Chloride in the Presence of Triethylamine. Chem Pharm Bull. 1980;28:493–499. [Google Scholar]
- 26.Knorr L. Synthetic Experiments with Acetoacetic Ester. Justus Liebigs Ann Chem. 1886;236:69–115. [Google Scholar]
- 27.Forbis RM, Rinehart KL. Nybomycin. VII. Preparative Routes to Nybomycin and Deoxynybomycin. J Am Chem Soc. 1973;95:5003–5013. doi: 10.1021/ja00796a037. [DOI] [PubMed] [Google Scholar]
- 28.Nadzan AM, Rinehart KL. Nybomycin. 9. Synthetic and Biosynthetic Incorporation of N-15 as a Means of Assigning C-13 Nuclear Magnetic-Resonance Spectrum of Nybomycin. J Am Chem Soc. 1977;99:4647–4654. doi: 10.1021/ja00456a021. [DOI] [PubMed] [Google Scholar]
- 29.Uray G, Niederreiter KS, Belaj F, Fabian WMF. Long-Wavelength-Absorbing and -Emitting Carbostyrils with High Fluorescence Quantum Yields. Helv Chim Acta. 1999;82:1408–1417. [Google Scholar]
- 30.Avendano C, Delacuesta E, Gesto C. A Comparative-Study of Synthetic Approaches to 1-Methyl-2,5,8(1h)-Quinolinetrione and 4-Methyl-2,5,8(1h)-Quinolinetrione. Synthesis-Stuttgart. 1991:727–730. [Google Scholar]
- 31.Yoshiyasu K, Shinsuke N, Masaro S, Takanabu Y, Akinori K. Synthesis of 2-(1H)Quinolinonequinones using Oxidative Demethylation with Cerium (IV) Ammonium Nitrate. Heterocycles. 1993;36:1909–1924. [Google Scholar]
- 32.Mitchell H, Leblanc Y. Amination of Arenes with Electron-Deficient Azodicarboxylates. J Org Chem. 1994;59:682–687. [Google Scholar]
- 33.Kamikawa T, Hanaoka Y, Fujie S, Saito K, Yamagiwa Y, Fukuhara K, Kubo I. SRS-A Antagonist Pyranoquinolone Alkaloids from East African Fagara Plants and Their Synthesis. Bioorg Med Chem. 1996;4:1317–1320. doi: 10.1016/0968-0896(96)00110-1. [DOI] [PubMed] [Google Scholar]
- 34.Williams JW, Krynitsky JA. Acetoacetanilide. Org Synth. 1941;21:4–5. [Google Scholar]
- 35.Forbis RM, Rinehart KL. Nybomycin. IV. Total Synthesis of Deoxynybomycin. J Am Chem Soc. 1970;92:6995–6996. doi: 10.1021/ja00726a061. [DOI] [PubMed] [Google Scholar]
- 36.Kaslow CE, Sommer NB. Substituted Lepidines. J Am Chem Soc. 1946;68:644–647. [Google Scholar]
- 37.Kitahara Y, Nakahara S, Shimizu M, Yonezawa T, Kubo A. Synthesis of 2(1H)-Quinolinonequinones and 2-Alkoxyquinolinequinones using Oxidative Demethylation with Cerium (IV) Ammonium Nitrate. Heterocycles. 1993;36:1909–1924. [Google Scholar]
- 38.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang HJ, Ma CY, Van Hung N, Cuong NM, Tan GT, Santarsiero BD, Mesecar AD, Soejarto DD, Pezzuto JM, Fong HHS. Miliusanes, a Class of Cytotoxic Agents from Miliusa sinensis. J Med Chem. 2006;49:693–708. doi: 10.1021/jm0509492. [DOI] [PubMed] [Google Scholar]
- 40.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 41.Likhitwitayawuid K, Angerhofer CK, Cordell GA, Pezzuto JM, Ruangrungsi N. Traditional Medicinal-Plants of Thailand .20. Cytotoxic and Antimalarial Bisbenzylisoquinoline Alkaloids from Stephania-Erecta. J Nat Prod. 1993;56:30–38. doi: 10.1021/np50091a005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.