Abstract
Astrocytes modulate neuronal activity by releasing chemical transmitters via a process termed gliotransmission. The role of this process in the control of behavior is unknown. Since one outcome of SNARE-dependent gliotransmission is the regulation of extracellular adenosine and because adenosine promotes sleep, we genetically inhibited the release of gliotransmitters and asked if astrocytes play an unsuspected role in sleep regulation. Inhibiting gliotransmission attenuated the accumulation of sleep pressure, assessed by measuring the slow wave activity of the EEG during NREM sleep and prevented cognitive deficits associated with sleep loss. Since the sleep-suppressing effects of the A1 receptor antagonist CPT were prevented following inhibition of gliotransmission and because intracerebroventricular delivery of CPT to wildtype mice mimicked the transgenic phenotype we conclude that astrocytes modulate the accumulation of sleep pressure and its cognitive consequences through a pathway involving A1 receptors.
INTRODUCTION
Although astrocytes are not electrically excitable, they exhibit Ca2+ elevations activated by metabotropic receptors. Natural stimuli such as whisker movement and visual gratings evoke astrocytic Ca2+ signals in the barrel (Wang et al., 2006) and visual cortices (Schummers et al., 2008). Synaptic activation of astrocytes leads to Ca2+ signals and in turn to the release of a number of chemical transmitters from these glia. This process of gliotransmission modulates synaptic activity (Jourdain et al., 2007;Pascual et al., 2005).
Using molecular genetics we have shown that by releasing ATP, astrocytes regulate extracellular adenosine acting on synaptic A1 receptors. Astrocytes release gliotransmitters via many pathways including exocytosis (Jourdain et al., 2007). The exocytotic release of chemical transmitters depends on the formation of a SNARE complex between vesicles and the target membrane (Scales et al., 2000). Conditional astrocyte-selective expression of the SNARE domain of the protein synaptobrevin II prevents both tonic and activity-dependent extracellular accumulation of adenosine that acts on A1 receptors in situ (Pascual et al., 2005). Adenosine is a transmitter involved in the homeostatic drive for sleep following prolonged wakefulness (Porkka-Heiskanen et al., 1997). However, the cellular source and mechanism of action of adenosine in the context of sleep are not well understood (Heller, 2006). Since astrocyte-dependent adenosine accumulation tonically regulates synaptic transmission and can be enhanced in an activity-dependent manner this glial pathway of neuronal modulation is a prime candidate for mediating the progressive changes of the homeostatic drive for sleep. We therefore used astrocyte-specific transgenic mice to determine whether astrocytes contribute to this fundamental behavior. Because astrocytes release a number of transmitters we additionally tested whether observed astrocyte-dependent transgenic phenotypes result from an adenosine deficit by using adenosinergic pharmacological agents in vivo to perform occlusion and mimicry experiments.
RESULTS
Study Design and Rationale
To inhibit the accumulation of astrocyte-derived adenosine we have used the tet-off system (Morozov et al., 2003) to allow conditional expression of a dnSNARE transgene selectively in astrocytes (Pascual et al., 2005). Astrocyte specificity of transgene expression is achieved by using the astrocyte-specific Glial Fibrillary Acidic Protein (GFAP) promoter to drive the expression of tetracycline transactivator (tTA) only in this subset of glia. GFAP.tTA mice were crossed with tetO.dnSNARE mice. The tet-operator (tet.O) drives the expression of dnSNARE and the EGFP reporter. Thus, in bigenic offspring of this mating transgenes are only expressed in GFAP-positive astrocytes (Fig 1A). Conditional suppression of transgene expression is achieved by including doxycycline (Dox) in the diet. Dox binds to tTA preventing it from activating the tet.O promoter. To prevent developmental expression of transgenes we established and maintained all matings in the presence of Dox until weaning. We confirmed that transgenes are not expressed developmentally by monitoring the expression of EGFP during embryonic development and in early post-natal life (Fig. S1).
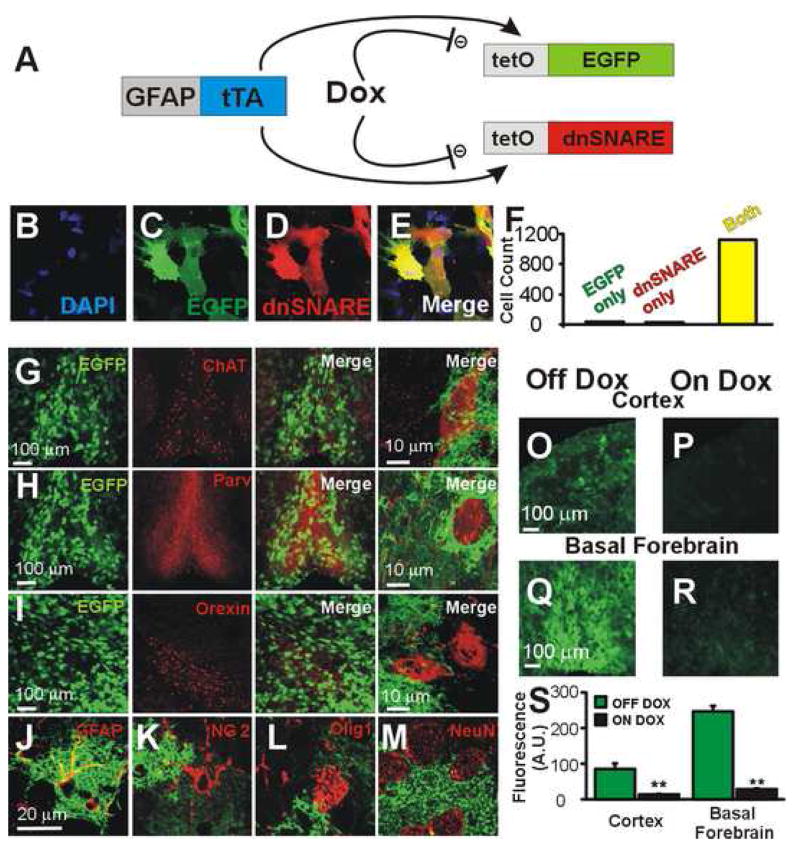
Figure 1. Conditional, astrocytes-specific expression of dnSNARE in brain regions involved in sleep regulation.
(A) Cartoon depicting GFAP promoter driving the expression of dnSNARE and EGFP (reporter) in astrocytes. Dox suppresses expression of both transgenes. (B–F) EGFP is a reliable marker for the dnSNARE, as 97.3% of cultured astrocytes expressing the soluble SNARE domain of Synaptobrevin II (dnSNARE) are EGFP+. In brain sections, astrocytes expressing transgenes (EGFP; green) are in proximity to cholinergic, choline acetyl transferase (ChAT) neurons (red, G) and to noncholinergic, parvalbumin (Parv) positive neurons (red, H) in the basal forebrain and surround orexinergic neurons, (red, I), in the lateral hypothalamus. (J–M) Single optical sections showing that EGFP colocalizes with the astrocytic marker GFAP (J) but not with markers of other glia (NG2, K; Olig1, L) or neurons (NeuN, M). Calibrations for K–M correspond to J. Placing dnSNARE mice on a Dox-containing diet suppresses transgene expression in the cortex (O, P) and the basal forebrain (Q, R). (S) Quantification of O–R (n = 3 animals in each group, **, p < 0.001, unpaired t-test). Differences in EGFP expression between cortex and basal forebrain correlate with relative levels of GFAP expression in these brain regions.
The EGFP reporter was visually detectable in 97.3% of dnSNARE transgene expressing cultured astrocytes (n= 1182 cells, 6 coverslips; Fig. 1B–F) establishing it as a reliable marker for dnSNARE transgene expression. Transgene-positive astrocytes were observed in cortex (Halassa et al., 2007), basal forebrain (Fig. 1, G and H), and lateral hypothalamus (Fig. 1I); brain regions thought to be important for sleep regulation. EGFP+ cells co-localize specifically with the astrocytic marker GFAP, but not with neuronal, NG2 glial, or oligodendroglial markers confirming the astrocytic identity of transgene expressing cells (Fig. 1, J–M). To allow for reversible transgene expression, Dox was removed from the diet at weaning, for a period of two to eight weeks (average of 4 weeks without Dox), to ensure that Dox dissipated from the body (Nakashiba et al., 2008), and comparisons were performed between wildtype and dnSNARE mice assuring that the only variable between these animals was the transgene (Nakashiba et al., 2008). Subsequent addition of Dox suppresses transgene expression within 2–3 weeks (Fig. 1, O–S).
Gliotransmission modulates sleep pressure accumulation
The attenuation of gliotransmission did not significantly impact baseline sleep time or architecture. Both dnSNARE and wildtype mice spent similar periods of time in non-rapid eye movement (NREM) sleep, rapid eye movement (REM) sleep and wakefulness (Fig. S2) and had similar mean 24-hour state-specific EEG spectral profiles (Fig. S3). Nevertheless, we found that sleep pressure was significantly reduced when dnSNARE was expressed in astrocytes. Sleep pressure positively correlates with slow wave activity (SWA); the 0.5–4.0 Hz frequency range of the EEG during NREM sleep (Franken et al., 2001). dnSNARE mice exhibited reduced SWA over the course of the light phase (the normal sleep phase) compared to their wildtype littermates (Fig. 2A).
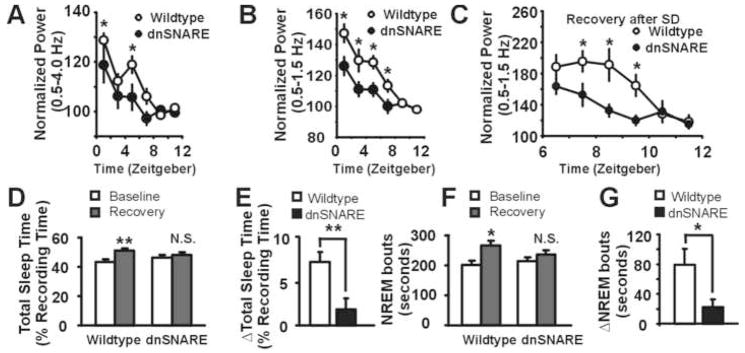
Figure 2. Gliotransmission is essential for sleep pressure accumulation.
(A) SWA (0.5–4.0 Hz) during NREM sleep across the light phase is decreased in the dnSNARE animals (n = 7 animals) compared to their wildtype littermates (n = 8 animals). (ANOVA, p<0.002, F=10.413, posthoc test, *, p < 0.05). (B) low-frequency SWA (0.5–1.5Hz) is reduced across the light phase in the dnSNARE compared to wildtype animals. (ANOVA, p<0.001, F=21.247, posthoc test, *, p <0.05). (C) Following sleep deprivation (SD), low frequency SWA is decreased in the dnSNARE animals (ANOVA, p<0.001, F=7.911, posthoc test, *, p < 0.05). (D) Sleep deprivation increases TST in wildtype (n = 9) but not dnSNARE animals (n = 8) during an 18 hour recovery period compared to a baseline period **, p < 0.001. (E) The increase in total sleep time (TST) after SD over the 18 hours of recovery in the dnSNARE is blunted when directly compared to wildtype animals (unpaired t-test, **, p < 0.01. N.S., non-significant).. (E) Sleep deprivation causes an increase in NREM bout durations in the subsequent 18-hours of recovery in the wildtype animals *, p < 0.05. The increase is not statistically significant in the dnSNARE. (G) When compared directly, the increase in NREM bout duration is blunted in the dnSNARE compared to wildtype animals *, p < 0.05.
Net synaptic potentiation during wakefulness is thought to be a cellular correlate of sleep pressure accumulation, and to be related to the role of sleep in synaptic downscaling (Tononi and Cirelli, 2006). Manipulations targeting synaptic potentiation preferentially affect the slower bands of SWA (0.5–1.5 Hz; low-frequency SWA) (Cirelli et al., 2005). We thus examined changes in the NREM EEG spectrum during the light phase at a finer spectral resolution (Fig. S4) and found a more pronounced difference between the dnSNARE and wildtype mice in low-frequency SWA (Fig. 2B, Fig. S4), consistent with an earlier report of reduced synaptic potentiation in these transgenic animals (Pascual et al., 2005). This effect was independent of the method of data normalization, and was specific to NREM sleep (Fig. S5). The difference between wildtype and dnSNARE was limited to changes in SWA, as there were no significant differences in the power of theta and spindles (Fig. S6). Following sleep deprivation (Fig. S7), wildtype animals exhibited a larger and more prolonged increase in SWA compared to dnSNARE mice (Fig. S8), which was mainly pronounced in the low-frequency range (Fig. 2C).
Increased sleep pressure following sleep deprivation produces a compensatory increase in sleep time (Franken et al., 1991). Previous studies have demonstrated that behavioral recovery following sleep deprivation mainly occurs in the dark phase in C57Bl6/J mice (Kapfhamer et al., 2002). We determined the impact of astrocytic dnSNARE expression on sleep compensation in the 18 hours of recovery. Astrocytic dnSNARE expression reduced the increase in total sleep time (TST) following sleep deprivation (wildtype = 7.53 ± 1.13%, n = 9 mice, dnSNARE = 1.89 ± 1.30%, n = 8 mice, unpaired t-test, p < 0.01) (Fig. 2D–E, Fig. S9). Furthermore, astrocytic dnSNARE expression significantly reduced the increase in NREM bout duration following sleep deprivation, another behavioral measure of the homeostatic response (Fig. 2F–G).
Because astrocytic dnSNARE expression decreases adenosine accumulation and since adenosine has neuroprotective roles in the nervous system we wanted to rule out the possibility that the phenotypes observed in transgenic animals were due to inadvertent neurodegeneration that might have occurred when the source of A1 receptor activation was lost. Thus, we added Dox back to the diet for 2–3 weeks to inhibit transgene expression (Fig 1 O–S) and asked whether dnSNARE animals can revert to a wildtype phenotype. This is a difficult control to perform since antibiotics have been reported to affect some sleep parameters such as SWA (Moulin-Sallanon et al., 2005). Therefore, we closely examined the effect of Dox on wildtype animals, and determined that it had no significant effect on three sleep parameters which we used as markers for reversibility in the dnSNARE animals. Addition of Dox to transgenic animals restores baseline SWA, baseline low-frequency SWA (Fig. S10) as well as the compensatory increase in sleep time following sleep deprivation to wildtype values (Fig. S9, Fig. S10). Reversal of transgenic phenotypes provides compelling evidence that the sleep phenotype we observe in transgene expressing animals results from the reversible gliotransmission-dependent control of sleep pressure.
Purinergic gliotransmission stimulates the A1 receptor to modulate sleep homeostasis
Though additional gliotransmitters may be affected by our transgenic manipulation, adenosine is a candidate gliotransmitter that may largely explain the sleep homeostasis phenotype observed in these animals. A number of studies have implicated adenosine in the control of sleep homeostasis. For example, individuals with an adenosine deaminase polymorphism which is expected to result in elevated adenosine, report deeper sleep and exhibit higher SWA (Retey et al., 2005). Thus, a decrease in adenosine in sleep-related brain regions of dnSNARE mice may explain their reduced SWA. We therefore asked whether astrocytic adenosine contributes to the dnSNARE sleep phenotype.
Adenosine levels are decreased at hippocampal synapses by astrocytic dnSNARE expression (Pascual et al., 2005). Because our EEG recordings were sampling synaptic potentials from the cortex, an area of the brain thought to be involved in the homeostatic response to sleep deprivation, we investigated whether A1 receptor-dependent inhibition of intracortical synaptic transmission was reduced in the dnSNARE mice. 8-cyclopentyl-1,3-dimethylxanthine (CPT), an adenosine A1 receptor antagonist led to an enhancement of synaptic transmission in wildtype slices. However, this enhancement was significantly reduced when slices from the dnSNARE animals were examined (Fig. 3, A–C). This was not due to a down-regulation of the A1 receptor, as the response to non-saturating levels of the adenosine receptor agonist 2-chloro-N6-cyclopentyladenosine (CCPA) was similar in slices derived from the two groups of animals (Fig. 3, D–F).
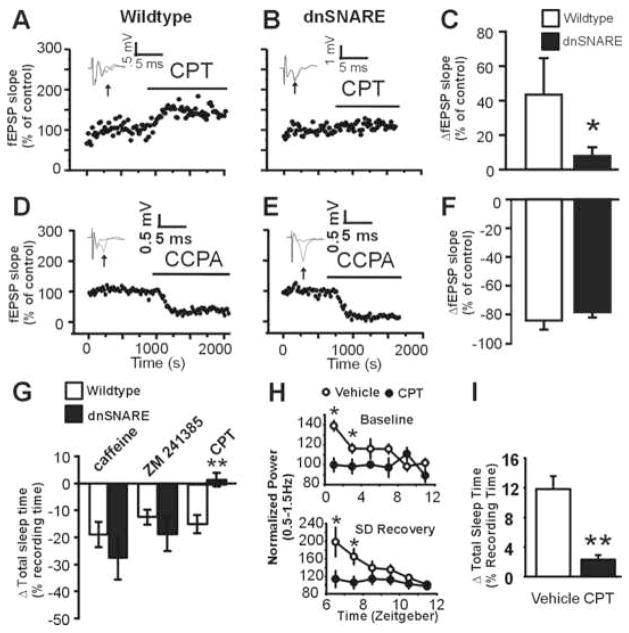
Figure 3. Purinergic gliotransmission stimulates the A1 receptor to modulate sleep homeostasis.
(A) The A1 receptor antagonist CPT (100–200 nM) causes an increase in fEPSP slope in slices from wildtype but not from dnSNARE mice (B). Inset: average of ≥5 fEPSP traces. (C) Average increase in fEPSP slope after CPT application (13 slices, 6 wildtype mice; 18 slices from 6 dnSNARE mice. * p < 0.05, Mann-Whitney test. D–F). The A1 agonist CCPA (500 nM) reduces fEPSP in wildtype (12 slices, 5 animals) and dnSNARE (12 slices, 4 animals, p = 0.44, Student’s t test). (G) Caffeine, ZM 241385 cause equivalent suppression of TST following i.p. injection in wildtype mice (open bars) and dnSNARE mice (closed bars) (Caffeine, t(8) = 0.925, p = 0.38; ZM 241385, t(8) = 0.925, p = 0.38). I.p. injection of CPT suppresses sleep only in wildtype animals (n= 5 animals per group, unpaired student’s t-test, **, p < 0.01). I.c.v. infusion of CPT reduces low-frequency SWA under baseline conditions (top) (ANOVA, p<0.001, F=18, post-hoc test, *, p<0.05) and following sleep deprivation (bottom) (ANOVA, p<0.001, F=16, post-hoc test, *, p < 0.05) (data are normalized to the last 4 hours of the light phase, similar to Fig. 2) (H), and sleep compensation following sleep deprivation was attenuated (unpaired t-test, **, p < 0.01) (I), recapitulating the dnSNARE phenotype. Data in I,H are from 5 vehicle treated animals and 6 CPT treated animals.
To determine whether the reduction of synaptic A1 activity translated into sleep-related adenosine deficits in vivo, we investigated whether the known sleep-suppressing effects of adenosine receptor antagonists (Huang et al., 2005) were occluded in dnSNARE mice. Astrocytic dnSNARE expression prevented the sleep suppressing effects of CPT (Fig. 3G, Fig. S11) but did not affect the sleep suppressing effects of the A2A antagonist ZM 241385 (Fig. 3G, Fig. S11), or of caffeine (Fig. 3G, Fig. S11), which is known to act via A2A receptors in this behavior (Huang et al., 2005).
To determine if the absence of A1 receptor activation contributes to the dnSNARE sleep homeostasis phenotype, we antagonized the A1 receptor in wildtype mice in vivo via osmotic minipump intracererbroventricular (i.c.v.) infusion of CPT while recording EEG/EMGs. In vivo CPT delivery to wildtype mice recaptured the dnSNARE phenotype. CPT did not affect baseline vigilance states or mean spectral EEG properties (Fig. S12). However, the accumulation of sleep pressure as measured by low-frequency SWA under baseline conditions and following sleep deprivation was blunted in CPT infused animals compared to vehicle controls (Fig. 3H). Further support for the notion that astrocytic adenosine acts through A1 receptors to control sleep homeostasis is provided by the observation that CPT infusion reduced the increase in TST following sleep deprivation (Fig. 3I).
Purinergic gliotransmission contributes to memory impairment following sleep loss
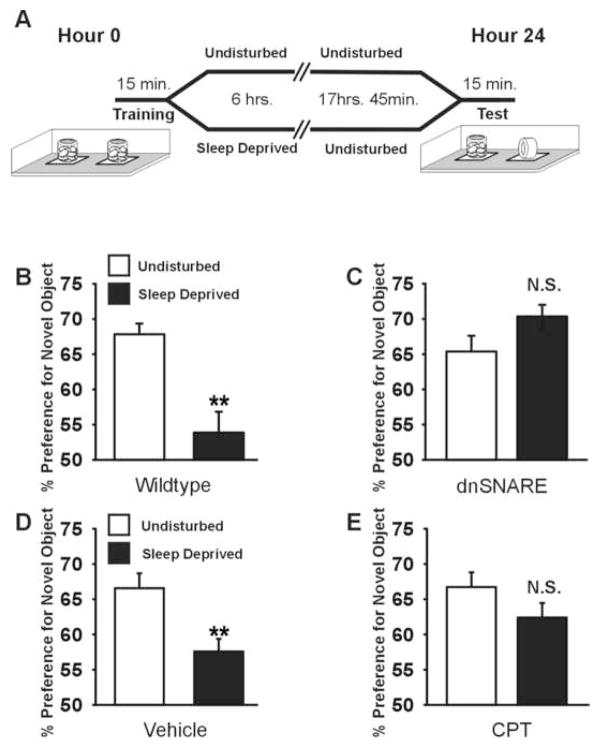
Accumulated sleep pressure caused by prolonged wakefulness can impair cognitive function(Yoo et al., 2007). Because dnSNARE mice accumulated less sleep pressure, we investigated whether their learning exhibited an altered response to prolonged wakefulness compared to wildtype mice. We chose a memory task that is known to be sensitive to the effects of sleep pressure (Palchykova et al., 2006). Novel object recognition (NOR) is a task that uses the spontaneous preference of rodents for novelty to measure recognition memory (Fig. 4A). Both wildtype and dnSNARE mice were able to learn the task equally well when they were trained at the beginning of the light phase and their subsequent sleep was undisturbed (wildtype, n = 16, dnSNARE, n = 16). However, sleep deprivation following training impaired NOR memory in wildtype animals (n = 11; Fig. 4B). Recognition memory in the dnSNARE mice, on the other hand, was unimpaired by sleep deprivation (n = 10, Fig. 4C). This difference is unlikely to be due to sleep deprivation induced alteration in the motivation or attention during the test period because mice were left undisturbed for ~18 hours following sleep deprivation, a period sufficient for behavioral and electrophysiological recovery (Fig. 2C). Furthermore, this difference cannot be attributed to an alteration in task acquisition as dnSNARE and wildtype mice did not differ in exploration time during task acquisition (Fig. S13). dnSNARE mice exhibited neither anxiety-related behaviors nor motor impairment (Fig. S14), which could otherwise prohibit performing the NOR task, and did not exhibit a generalized circuit dysfunction as their contextual fear conditioning memory was similar to wildtype littermates (Fig. S15). Chronic infusion of the A1 receptor antagonist CPT in wildtype mice protected against the memory degrading effects of sleep deprivation, mimicking the dnSNARE phenotype (Fig. 4, D–E).
Figure 4. Purinergic gliotransmission contributes to memory impairment following sleep loss.
(A) Novel object recognition paradigm; mice are trained to recognize two identical objects and are either left undisturbed or sleep deprived for six hours following training. At hour 24, mice are tested for the ability to recognize a novel object replacing one of the familiar objects. (B) Sleep deprivation impairs NOR in wildtype mice (t(27) = −4.636; **, p < 0.001) (C) dnSNARE mice are unaffected by the effects of sleep deprivation on NOR memory (t(25) = 1.56, p = 0.132). (D–E) i.c.v. delivery of CPT (t(15 = −1.430; p = 0.173), but not control vehicle (t(15)= −3.251; p < 0.005), into wildtype mice protects against the sleep deprivation-induced memory deficit.
To distinguish between the importance of sleep pressure and sleep per se in regulating recognition memory we performed NOR training at the end of the light phase, when sleep pressure has subsided (Fig. 2A,B), and then sleep deprived the animals for the subsequent six hours during the dark phase. Training wildtype mice at the end of the light phase results in a NOR memory that is insensitive to six hours of sleep deprivation following training (Fig. S16) indicating that sleep immediately following NOR training is not required for the memory per se. These results lead us to conclude that the acute accumulation of sleep pressure can impair memory consolidation.
DISCUSSION
By genetically inhibiting SNARE-dependent release of transmitters from astrocytes, we demonstrate that gliotransmission contributes to sleep homeostasis. Inhibition of gliotransmission reduces SWA, particularly that in the low-frequency range, during the course of the light phase (Fig. 2A, B) and blunts the additional accumulation of low-frequency SWA following sleep deprivation (Fig. 2C). Moreover, this reduction in sleep pressure leads to a reversible reduction in TST following sleep deprivation (Fig. 2D, E; Fig. S9, Fig. S10). Because astrocytes release several transmitters our subsequent pharmacological experiments were critical to determine that the observed transgenic phenotypes involve a decrease in purinergic gliotransmission. In support of previous in situ studies, in vivo behavioral analyses show that astrocytic expression of dnSNARE prevents the A1 antagonist CPT from exerting its normal actions on wakefulness (Fig. 3G). Additionally, i.c.v. administration of CPT mimics the dnSNARE phenotype by preventing the accumulation of sleep pressure (Fig. 3H), and changes in total sleep time following sleep deprivation (Fig. 3I). Although we cannot discount the possibility that additional gliotransmitters also contribute to the sleep phenotypes we observe, our data indicate that astrocytic adenosine acting through A1 receptors contributes to the modulation of sleep pressure, the regulation of sleep homeostasis (Fig. 2, 3) and cognitive deficits following sleep deprivation (Fig. 4). It is important to note that the role of gliotransmission in sleep regulation is likely to be underestimated by our transgenic approach because less than 50% of cortical astrocytes express the dnSNARE transgene (Fellin et al, submitted).
There are two potential concerns about the use of the transgenic system we employed in this study. First, widespread transgene expression might lead to generalized dysfunction of the nervous system. However, we have found that astrocytic dnSNARE expression is highly selective for specific sleep phenotypes. Baseline sleep behavior is unperturbed in the dnSNARE animal (Fig. S2), and the effect on sleep is limited to that of sleep homeostasis. Brain oscillations under baseline conditions are not affected (Fig. S3), arguing strongly against a general circuit deficit in the dnSNARE mouse. The dynamic changes in the NREM sleep spectra are limited to SWA, particularly those in the low frequency range (Fig. S4, S5). Theta and spindle frequencies are unaffected, and the relative waking and REM sleep spectra are also not affected (Fig. S6). Open field behavior, a measure of anxiety, motor learning and memory assessed by the rotarod, contextual fear conditioning, and novel object recognition learning and memory are intact in the dnSNARE animals arguing against a general circuit dysfunction in these mice (Fig. 4, Fig. S14, Fig. S15). Second, dnSNARE expression has the potential to non-specifically interfere with all membrane trafficking pathways in these glial cells. However, we find no perturbation in afferent-induced Ca2+ signals (Fig. S17), and in a separate study we demonstrate that dnSNARE expression does not affect astrocytic resting membrane potential, input resistance or current response to afferent activity (Fellin et al, submitted) indicating that trafficking of receptors, ion-channels, and transporters is not perturbed in these transgenic animals. Thus, in dnSNARE mice astrocytes retain functional integrity when the transgene is expressed.
A number of studies aimed at understanding the role of A1 receptors in the regulation of sleep homeostasis have provided conflicting results (Porkka-Heiskanen et al., 1997;Stenberg et al., 2003). However, it is worth noting that in cases where manipulations were performed on animals that were allowed to develop normally, the experimental results agree with our current study by demonstrating that the A1 receptor plays a role in the homeostatic response to sleep deprivation. When A1 receptor antisense oligonucleotides were perfused into the basal forebrain, a transient reduction of the A1 receptor protein resulted, and a transient attenuation of the homeostatic response to sleep deprivation was observed at the level of EEG and behavior (Thakkar et al., 2003). More recently, a conditional knockout of the A1 receptor in mice has been shown to have a phenotype of attenuated sleep pressure accumulation assessed by SWA, not dissimilar to the dnSNARE mouse (Greene & Bjornes, 2008, Purinergic Signalling; S115). In contrast constitutive A1 receptor knockout mice show a modest sleep phenotype (Stenberg et al., 2003). Because the A1 receptor is involved in a number of physiological processes during development (Kimura et al., 2003), it is conceivable that its permanent absence would lead to compensatory responses. Therefore, in agreement with other studies in which developmental compensation was not a concern, our study demonstrates an essential role for the A1 receptor in mammalian sleep homeostasis. Importantly, we provide the cellular origin for adenosine, and point to its critical role in mediating the cognitive consequences of prolonged wakefulness.
Previous studies have concluded that sleep can play a role in memory consolidation (Stickgold et al., 2001). By demonstrating that NOR memory consolidation does not require sleep immediately following learning we do not mean to minimize the possible role of sleep in NOR memory consolidation in general. It should be noted that when mice were sleep deprived, they were allowed to sleep afterwards during the 18 hours prior to the test. Thus, in plastic brain processes that require sleep for consolidation (Frank et al., 2001), it will be important to investigate whether gliotransmission plays a role.
CONCLUSION
Taken together these studies provide the first demonstration that a non-neuronal cell type of the brain, the astrocyte, modulates behavior and provides strong evidence of the important role of A1 receptors in the regulation of sleep homeostasis and the cognitive decline associated with sleep loss. Given that astrocytes express novel receptors (Barres, 2008); these glial cells offer a novel target for the development of therapeutics for sleep and cognitive disorders.
EXPERIMENTAL PROCEDURES
Sleep recording, analysis and comparisons
EEG/EMG implantation surgery was performed as described before (Frank et al., 2002). EEG/EMG signals were conveyed by a light-weight cable, low- and high-pass filtered at 0.3 and 30 Hz and 10 and 100 Hz, respectively (15 LT Bipolar amplifier system, Astro-Med, West Warwick, RI), amplified and sampled at 200 Hz.
Stage scoring of computerized EEG/EMG epochs as rapid-eye-movement [REM], non-REM [NREM] and wake) was done in 4-second epochs by a trained experimenter blind to genotype using (Kissei Comtec America, INC). Epochs containing movement artifacts were included in the state totals, but excluded from subsequent spectral analysis. Following assignment of state scores, data were analyzed as a percentage of the total recording time (1, 2, 12, or 24 hour bins). Spectral analysis was performed using a Fast Fourier Transform (FFT; Hanning Window). EEG slow wave activity (SWA: 0.5–4Hz or low-frequency SWA: 0.5–1.5Hz) was used as a quantitative measure of sleep pressure (Cirelli et al., 2005;Franken et al., 2001). NREM SWA for both recording days was normalized to the last 4 hours of the baseline light phase as described (Franken et al., 2001). These latter analyses were restricted to the light phase, because in agreement with a previous mouse study(Franken et al., 2001), there was too little NREM sleep in most mice during the active (dark) phase for accurate SWA assessments.
In Vivo Pharmacology-acute
Mice prepared for EEG recordings were allowed to recover after surgery and were acclimated as described. Vehicle or drugs; caffeine (10 mg/kg in 0.9% saline), CPT (20mg/kg in DMSO), or ZM 241385 (30mg/kg in DMSO) were i.p. injected at the beginning of the light phase on separate days. At least 48 hours was allowed between vehicle and drug injection in both groups, and at least 2 weeks was allowed between the injection of CPT and ZM 242385. Vigilance states were scored and quantified in the 3 hours following injection as described previously (Huang et al., 2005). Effects of each drug were expressed as the difference in total sleep time after drug injection vs. vehicle injection for each mouse (Fig 3.), and the raw data are presented in Fig. S11.
In Vivo pharmacology-continuous
Micro-osmotic minipumps model 1002 (flow rate 0.25 μL/day) were filled with either CPT or vehicle (50% DMSO in 0.9% saline), secured by the flow moderator, connected to the brain cannula (The Alzet brain infusion kit 3 (DURECT Corporation, Cupertino, CA)) by flexible tubing, and allowed to equilibrate in 0.9% saline for 24 hours at 37° C. Subsequently one minipump was implanted per animal subcutaneously, and the cannula was stereotaxically placed into the left lateral ventricle at the following coordinates (A/P, −0.8 mm; M/L, −1.0 mm; D/V, −2.0 mm). Animals used in NOR studies were allowed to recover for 1 week before subsequent training and testing.
Novel Object recognition task
This task uses differential level exploration between familiar and unfamiliar (or novel) objects as a behavioral measure for recognition memory based on the animal’s preference for novelty. One week prior to training, mice were individually housed and handled for one minute a day for three days. Mice were habituated to the experimental arena (a rectangular open field (60 cm × 50 cm × 26 cm)) by allowing exploration for 15 minutes in the absence of objects. During training, mice were placed in the experimental arena in the presence of two identical objects and allowed to explore for 15 min. After a retention interval of 24 h, mice were placed again in the arena where one of the objects had been replaced by a novel one and were allowed to explore for 15 min. Training and testing was videotaped and analyzed by an experimenter blind to the genotype or drug treatment. Preference for the novel object was expressed as the percent time spent exploring the novel object relative to the total time spent exploring both objects. Statistical analysis was performed using Student’s t-test.
Additional methodology concerning transgenic animal production and maintenance, histological analyses, sleep data analysis, slice electrophysiology, and behavioral tests are described in detail in the supplementary methods.
Supplementary Material
Acknowledgments
We are grateful to the NINDS, NIA and NIMH for providing funding for this work and to members of the Haydon, Frank and Abel laboratories for thoughtful discussions
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Barres BA. The Mystery and Magic of Glia: A Perspective on Their Roles in Health and Disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Cirelli C, Huber R, Gopalakrishnan A, Southard TL, Tononi G. Locus ceruleus control of slow-wave homeostasis. Journal of Neuroscience. 2005;25:4503–4511. doi: 10.1523/JNEUROSCI.4845-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 4.Frank MG, Stryker MP, Tecott LH. Sleep and sleep homeostasis in mice lacking the 5-HT2c receptor. Neuropsychopharmacology. 2002;27:869–873. doi: 10.1016/S0893-133X(02)00353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. Journal of Neuroscience. 2001;21:2610–2621. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franken P, Dijk DJ, Tobler I, Borbely AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–R208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 7.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. Journal of Neuroscience. 2007;27:6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heller HC. A global rather than local role for adenosine in sleep homeostasis. Sleep. 2006;29:1382–1383. doi: 10.1093/sleep/29.11.1382. [DOI] [PubMed] [Google Scholar]
- 9.Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 10.Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- 11.Kapfhamer D, Valladares O, Sun Y, Nolan PM, Rux JJ, Arnold SE, Veasey SC, Bucan M. Mutations in Rab3a alter circadian period and homeostatic response to sleep loss in the mouse. Nat Genet. 2002;32:290–295. doi: 10.1038/ng991. [DOI] [PubMed] [Google Scholar]
- 12.Kimura M, Saitoh N, Takahashi T. Adenosine A(1) receptor-mediated presynaptic inhibition at the calyx of Held of immature rats. J Physiol. 2003;553:415–426. doi: 10.1113/jphysiol.2003.048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morozov A, Kellendonk C, Simpson E, Tronche F. Using conditional mutagenesis to study the brain. Biol Psychiatry. 2003;54:1125–1133. doi: 10.1016/s0006-3223(03)00467-0. [DOI] [PubMed] [Google Scholar]
- 14.Moulin-Sallanon M, Millet P, Rousset C, Zimmer L, Debilly G, Petit JM, Cespuglio R, Magistretti P, Ibanez V. Chloramphenicol decreases brain glucose utilization and modifies the sleep-wake cycle architecture in rats. Journal of Neurochemistry. 2005;93:1623–1632. doi: 10.1111/j.1471-4159.2005.03167.x. [DOI] [PubMed] [Google Scholar]
- 15.Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- 16.Palchykova S, Winsky-Sommerer R, Meerlo P, Durr R, Tobler I. Sleep deprivation impairs object recognition in mice. Neurobiol Learn Mem. 2006;85:263–271. doi: 10.1016/j.nlm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 18.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Retey JV, Adam M, Honegger E, Khatami R, Luhmann UF, Jung HH, Berger W, Landolt HP. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc Natl Acad Sci U S A. 2005;102:15676–15681. doi: 10.1073/pnas.0505414102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scales SJ, Chen YA, Yoo BY, Patel SM, Doung YC, Scheller RH. SNAREs contribute to the specificity of membrane fusion. Neuron. 2000;26:457–464. doi: 10.1016/s0896-6273(00)81177-0. [DOI] [PubMed] [Google Scholar]
- 21.Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- 22.Stenberg D, Litonius E, Halldner L, Johansson B, Fredholm BB, Porkka-Heiskanen T. Sleep and its homeostatic regulation in mice lacking the adenosine A1 receptor. J Sleep Res. 2003;12:283–290. doi: 10.1046/j.0962-1105.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 23.Stickgold R, Hobson JA, Fosse R, Fosse M. Sleep, learning, and dreams: off-line memory reprocessing. Science. 2001;294:1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- 24.Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. Journal of Neuroscience. 2003;23:4278–4287. doi: 10.1523/JNEUROSCI.23-10-04278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca(2+) signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- 27.Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–392. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.