Abstract
An anionic amphiphilic dendrimer is reported that possesses increased cytotoxicological potency against prokaryotic cells compared to eukaryotic cells. The half maximal effective concentration (EC50) for the dendrimer against Bacillus subtilis, a Gram-positive bacterial strain, was measured to be 4.1×10−5 M, while that against human umbilical vein endothelial cells (HUVEC) was more than 36x greater at a value of 1.5×10−3 M. EC50 ratios for two commercial amphiphiles, sodium dodecyl sulfate (SDS) and Triton X-100, in addition to a similar synthesized dendritic structure were at most only 3.8x greater. Furthermore, the observed EC50 values appear to be correlated to the critical aggregation constant (CAC) in solution suggesting a mechanism of action for these anionic amphiphilic dendrimers related to their supramolecular structures.
Dendritic macromolecules, due to their structure, unique properties, and precise compositions, are of significant interest1 and are finding uses in an ever-increasing number of medical applications.2 This is especially evident in the drug delivery area where the dendritic structure enables the attachment of a multitude of drugs or targeting moieties as well as the opportunity to control pharmacokinetics through alterations in generation number.3 Our interest lies in the synthesis and evaluation of dendritic macromolecules composed of building blocks that are natural metabolites or known to be biocompatible for ocular tissue repair,4 cartilage tissue engineering,5 and drug delivery.6 In an ongoing effort to expand the biomedical applications of dendrimers and our understandings of the resulting structure-activity relationships, we are investigating anionic dendritic macromolecules as antibacterial agents. Herein, we report the antibacterial activity of an anionic amphiphilic dendrimer and the striking selectivity in its cytotoxicity towards a prokaryotic Gram-positive bacterium compared to a eukaryotic human cell.
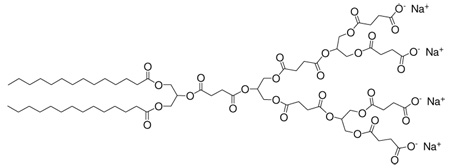
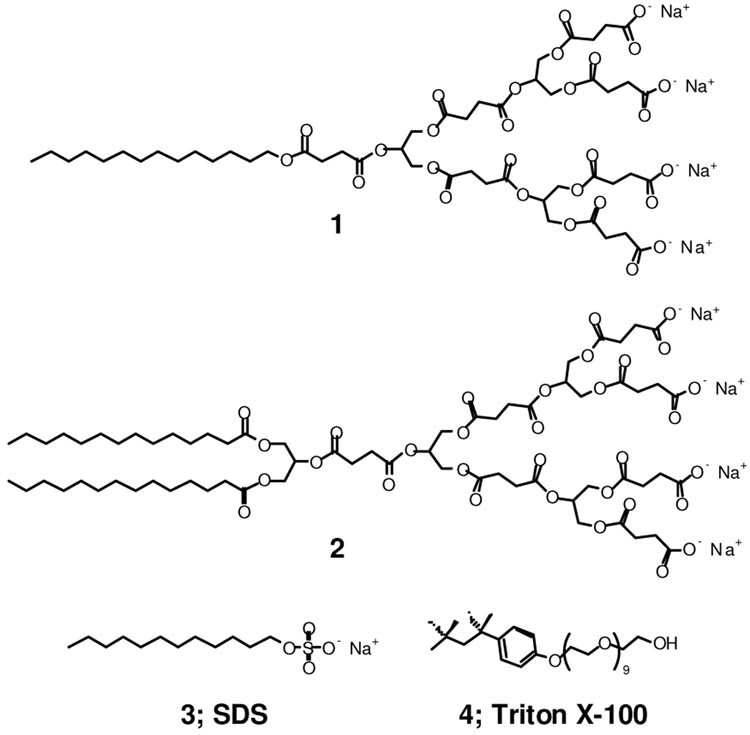
There is a significant global need for new antibacterials and alternative mechanisms of action given the rise in resistance among bacteria.7 Of the various known antibacterial agent classes, amphiphilic compounds act through perturbation and disruption of the prokaryotic membrane.8 We hypothesized that amphiphilic anionic dendrimers may exhibit antibacterial activity with minimal eukaryotic cell cytotoxicity, since dendrimers with terminal anionic charges are generally non-cytotoxic and have low toxicity in zebrafish whole animal development studies.9 On the other hand, cationic dendrimers, some of which have antibacterial properties if the positive charge is properly shielded,10 have repeatedly shown cytotoxicity against a variety of eukaryotic cell lines.3e,11 In addition, there are many reports of linear polycationic agents but only a few descriptions of linear polyanionic antibacterial agents (e.g., sulfonated polystyrene).12 Consequently, we synthesized a series of surface-block anionic amphiphilic dendrimers composed of succinic acid, glycerol, and myristic acid possessing various numbers of acid and alkyl functionalities.13 Based on the physicochemical properties of these amphiphilic anionic dendrimers, we identified two potential candidates, dendrimers 1 and 2 (Figure 1). Both dendrimers were synthesized in 9 steps with an overall yield of 30 and 28%, respectively, for evaluation of antibacterial activity (see SI). Additionally, linear anionic amphiphile sodium dodecyl sulfate (SDS), 3, and neutral-charge amphiphile Triton X-100, 4, were added to the evaluation as positive controls with known antibacterial activity (i.e., disruption of the cytoplamic membrane and protein solublization).8
Figure 1.
Structures of the two dendritic anionic amphiphiles under investigation, 1 and 2, and SDS, 3, Triton X-100, 4.
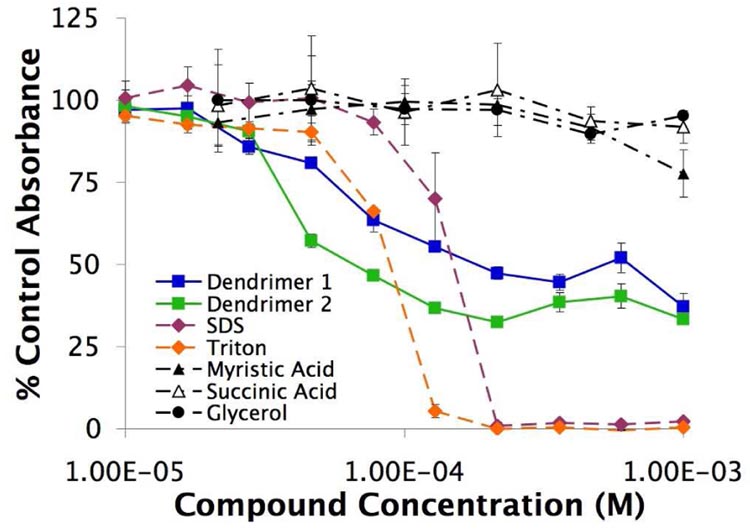
Cytotoxicological experiments were conducted against a wild-type Gram-positive bacterial strain (Bacillus subtilis AG174). Bacteria were cultured until logarithmic growth was achieved and then dilutions were added to LB broth with various concentrations of compounds 1–4 and the constituents of the dendrimers: glycerol, succinic acid, and myristic acid along with an untreated negative control. The turbidity of the wells was monitored for 9 hours and the resulting cytotoxicities are shown in Figure 2. As expected, commercial amphiphiles SDS, 3, and Triton X-100, 4, proved to be toxic while myristic acid, succinic acid, and glycerol were not toxic to the B. subtilis strain over the concentration range tested. Significantly, we observed antibacterial activity for the synthesized anionic amphiphilic dendrimers 1 and 2, though the amplitude of the sigmoidal curve is comparatively compressed. The half maximal effective concentration (EC50) for 1 and 2 are 6.0×10−5 and 4.1×10−5 M, respectively. A partial explanation for this effect was obtained from further kinetic studies which suggested a bacteriostatic mechanism of action that required about 1.5 hours to slow the growth significantly compared to an untreated control.
Figure 2.
Cytotoxicity of the compounds against Gram-positive B. subtilis. Absorbances were determined by measuring the turbidity of the cell-containing medium and reported as a fraction of untreated bacteria turbidity over the same 9 hour period. (n=3; mean ± SD)
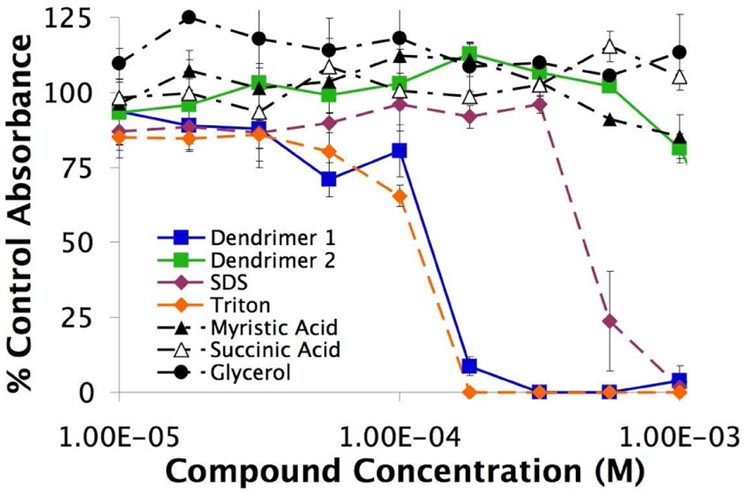
We next examined the eukaryotic cytotoxicity by evaluating all the compounds against a primary cell line of human umbilical vein endothelial cells (HUVECs). Low-passage number HUVEC were equilibrated in a sub-confluent monolayer and challenged with varied concentrations of the compounds for 24 hours. The resultant cell viabilities were determined using a tetrazolium assay (Figure 3). As seen before with the Gram-positive bacteria, glycerol, myristic acid, and succinic acid were not cytotoxic, while both SDS, 3, and Triton X-100, 4, were cytotoxic. Dendrimer 1 also showed cytotoxicity; however, 2 did not show any lethality in the concentration range tested. Subsequent experiments at higher values up to its aqueous solubility limit of 2×10−3 M produced a reduction to around 50 % of the negative untreated control but a complete sigmoidal shape was never obtained and so the EC50 for 2 was estimated to be greater than ≈ 1.5×10−3 M. Importantly, SDS, Triton X-100, and dendrimer 1 affected the viability of both prokaryotes and eukaryotes at similar concentrations with the compounds always having a ratio of eukaryotic: prokaryotic EC50 less than a factor of 3.8, which is non-ideal for an antibacterial compound (Table 1). Dendrimer 2 on the other hand, exhibited a ≥36-fold eukaryotic: prokaryotic EC50 ratio.
Figure 3.
Cytotoxicity of the compounds against HUVEC. Absorbances are calculated as a percentage of the untreated cells over a 24 hour time period. (n=3; mean ± SD)
Table 1.
Experimental properties of the dendritic amphiphiles 1, and 2, as well as SDS and Triton X-100.
| 1 | 2 | SDS | Triton | |
|---|---|---|---|---|
| CAC (M) | 2.0×10−4 | 1.1×10−5 | 1.0×10−3 | 2.4×10−4 |
| HUVEC EC50 (M) | 1.3×10−4 | 1.5×10−3 | 5.4×10−4 | 1.1×10−4 |
| B. Subtilis EC50 (M) | 6.0×10−5 | 4.1×10−5 | 1.4×10−4 | 8.8×10−5 |
| Ratio EC50 | 2.2 | ≥36 | 3.8 | 1.3 |
CMCs for SDS and Triton are from reference 15.
Upon further examination, the cytotoxicity of these compounds appears to be correlated with the formation of supramolecular structures in solution. Amphiphilic dendrimers are known to form a variety of supramolecular structures based on generation number, charge, hydrophilic: hydrophobic ratio, MW, etc. and such structures are actively investigated.14 The critical aggregation concentrations (CAC) for compounds 1 and 2 were measured tensiometrically to be 2.0×10−4 and 1.1×10−5 M, respectively, values similar to their EC50 against B. subtilis and in the case of 1, close to the EC50 against HUVEC as well. However, with 2 there is minimal lethality against HUVEC and there appears to be no correlation between toxicity and CAC in this case (Table 1). We have observed that 2 can form vesicles of ≈100 nm in diameter by TEM. Further experiments are underway to investigate the mechanism of action and supramolecular assemblies for these antibacterial dendrimers, and the resulting eukaryotic:prokaryotic EC50 ratio.
In summary, we report the discovery of an anionic amphiphilic dendrimer that possesses Gram-positive antibacterial activity and minimal eukaryotic cell toxicity. This selectivity, as denoted by the lack of overlap in the cytotoxicological curves, is of chemical, biological, and clinical interest as antibacterials such as these would be maximally effective against microbial infections without harming the host. Moreover, dendrimer 2 can be prepared easily in good yield and thus, in the future, may provide a cost-effective route for preparation. Continued efforts in the synthesis of new dendritic macromolecules, characterization of their unique properties, and evaluation in clinically important indications will lead to new solutions for a variety of health care needs.
Supplementary Material
Experimental materials and methods for all procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
The authors would like to thank the in NIH and BU for partial support. We also thank Dr. Tim Gardner.
References
- 1.(a) Tomalia DA, Fréchet JMJ. Dendrimers and Other Dendritic Polymers. Chichester, UK: 2001. ed.; [Google Scholar]; (b) Newkome GR, Moorefield CN, Vögtle F. Dendrimers and Dendrons. Berlin: Wiley-VCH; 2001. p. 623. ed.; [Google Scholar]; (c) Newkome GR, Shreiner CD. Polymer. 2008;49:1–173. [Google Scholar]; (d) Bosman AW, Janssen HM, Meijer EW. Chem. Rev. 1999;99:1665–1688. doi: 10.1021/cr970069y. [DOI] [PubMed] [Google Scholar]
- 2.(a) Lee CC, MacKay JA, Fréchet JMJ, Szoka FC. Nat. Biotech. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]; (b) Svenson S, Tomalia DA. Adv. Drug Del. Rev. 2005;57:2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]; (c) Grinstaff MW. J. Poly. Sci. A. 2008;46:383–400. [Google Scholar]
- 3.(a) Gillies ER, Fréchet JMJ. Drug Discov Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]; (b) Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Fréchet JMJ, Dy EE, Szoka FC. P. Natl. Acad. Sci. USA. 2006;103:16649–16654. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Crampton HL, Simanek EE. Poly. Int. 2007;56:489–496. doi: 10.1002/pi.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Majoros IJ, Myc A, Thomas T, Mehta CB, Baker JR., Jr Biomacromolecules. 2006;7:572–579. doi: 10.1021/bm0506142. [DOI] [PubMed] [Google Scholar]; (e) Gurdag S, Khandare J, Stapels S, Matherly LH, Kannan RM. Bioconjug Chem. 2006;17:275–283. doi: 10.1021/bc0501855. [DOI] [PubMed] [Google Scholar]; (f) Wolinsky JB, Grinstaff MW. Adv. Drug Del. Rev. 2008;60:1037–1055. doi: 10.1016/j.addr.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 4.(a) Carnahan MA, Middleton C, Kim J, Kim T, Grinstaff MW. J. Am. Chem. Soc. 2002;124:5291–5293. doi: 10.1021/ja025576y. [DOI] [PubMed] [Google Scholar]; (b) Velazquez AJ, Carnahan MA, Kristinsson J, Stinnett S, Grinstaff MW, Kim T. Arch Ophthalmol. 2004;122:867–870. doi: 10.1001/archopht.122.6.867. [DOI] [PubMed] [Google Scholar]; (c) Wathier M, Jung PJ, Carnahan MA, Kim T, Grinstaff MW. J. Am. Chem. Soc. 2004;126:12744–12745. doi: 10.1021/ja045870l. [DOI] [PubMed] [Google Scholar]; (d) Wathier M, Johnson SM, Kim T, Grinstaff MW. Bioconjugate Chem. 2006;17:873–876. doi: 10.1021/bc060060f. [DOI] [PubMed] [Google Scholar]; (e) Degoricija L, Johnson CS, Wathier M, Kim T, Grinstaff MW. IOVS. 2007;48 doi: 10.1167/iovs.06-0957. [DOI] [PubMed] [Google Scholar]; (f) Grinstaff MW. Biomaterials. 2007;28:5205–5214. doi: 10.1016/j.biomaterials.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Söntjens S, Nettles DL, Carnahan MA, Setton LA, Grinstaff MW. Biomacromolecules. 2006;7:310–316. doi: 10.1021/bm050663e. [DOI] [PubMed] [Google Scholar]
- 6.Morgan MT, Nakanishi Y, Kroll DJ, Griset AP, Carnahan MA, Wathier M, Oberlies NH, Manikumar G, Wani MC, Grinstaff MW. Cancer Res. 2006;66:11913–11921. doi: 10.1158/0008-5472.CAN-06-2066. [DOI] [PubMed] [Google Scholar]
- 7.(a) MacDougall C, Polk RE. Clin. Microbiol. Rev. 2005;18:638–656. doi: 10.1128/CMR.18.4.638-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mah T-F, O'Toole GA. Trends Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 8.Denyer SP. Int. Biodeterior. Biodegrad. 1995;36:227–245. [Google Scholar]
- 9.Heiden TC, Dengler E, Kao WJ, Heideman W, Peterson RE. Toxicol Appl Pharmacol. 2007;225:70–79. doi: 10.1016/j.taap.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CZ, Beck-Tan NC, Dhurjati P, van Dyk TK, LaRossa RA, Cooper SL. Biomacromolecules. 2000;1:473–480. doi: 10.1021/bm0055495. [DOI] [PubMed] [Google Scholar]
- 11.(a) Hong S, Bielinska AU, Mecke A, Keszler B, Beals JL, Shi X, Balogh L, Orr BG, Baker JR, Jr, Banaszak Holl MM. Bioconjug. Chem. 2004;15:774–782. doi: 10.1021/bc049962b. [DOI] [PubMed] [Google Scholar]; (b) Jevprasesphant R, Penny J, Jalal R, Attwood D, McKeown NB, D'Emanuele A. Int. J. Pharm. 2003;252:263–266. doi: 10.1016/s0378-5173(02)00623-3. [DOI] [PubMed] [Google Scholar]
- 12.(a) Anderson RA, Feathergill KA, Diao X-H, Cooper MD, Kirkpatrick R, et al. J. Androl. 2002;23:426–438. [PubMed] [Google Scholar]; (b) Herold BC, Bourne N, Marcellino D, Kirkpatrick R, Strauss DM, et al. J. Infect. Dis. 2000;181:770–773. doi: 10.1086/315228. [DOI] [PubMed] [Google Scholar]
- 13.Luman NR, Grinstaff MW. Org. Lett. 2005;7:4863–4866. doi: 10.1021/ol051583q. [DOI] [PubMed] [Google Scholar]
- 14.(a) Smith DK, Hirst AR, Love CS, Hardy JG, Brignell SV, Huang B. Prog. Polym. Sci. 2005;30:220–293. [Google Scholar]; (b) Zeng F, Zimmerman SC. Chem. Rev. 1997;97:1681–1712. doi: 10.1021/cr9603892. [DOI] [PubMed] [Google Scholar]; (c) Newkome GR, Moorefield CN. Angew. Chem. Int. Ed. Engl. 1991;30:1178–1180. [Google Scholar]; (d) Hawker CJ, Wooley KL, Fréchet JMJ. J. Chem. Soc. Perkin Trans. I. 1993:1287–1297. [Google Scholar]; (e) Percec V, Cho WD, Ungar G, Yeardley DJP. J. Am. Chem. Soc. 2001;123:1302–1315. [Google Scholar]; (f) van Hest JC, Delnoye DA, Baars MW, van Genderen MH, Meijer EW. Science. 1995;268:1592–1595. doi: 10.1126/science.268.5217.1592. [DOI] [PubMed] [Google Scholar]; (g) Chapman T, Hillyer G, Mahan E, Shaffer K. J. Am. Chem. Soc. 1994;116:11195–11196. [Google Scholar]; (h) van Hest JCM, Baars MWPL, Elissen-Roman C, van Genderen MHP, Meijer EW. Macromolecules. 1995;28:6689–6691. [Google Scholar]; (i) Tomalia DA. Dendrimeric Supramolecular and Supramacromolecular Assemblies. In: Ciferri A, editor. Supramolecular Polymers Second Edition. Boca Raton, FL: CRC Press Taylor & Francis Group; 2005. pp. 187–256. [Google Scholar]
- 15.Helenius A, Simons K. Biochim. Biophys. Acta. 1975;415:29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental materials and methods for all procedures. This material is available free of charge via the Internet at http://pubs.acs.org.