Abstract
6-hydroxydopamine (6-OHDA), a neurotoxic substrate of the dopamine transporter (DAT), is widely used in Parkinson’s disease models. However, the molecular mechanisms underlying 6-OHDA’s selectivity for dopamine neurons and the injurious sequelae that it triggers are not well understood. We tested whether ectopic expression of DAT induces sensitivity to 6-OHDA in non-dopaminergic cortical neurons and evaluated the contribution of Kv channel-dependent apoptosis to the toxicity of this compound in cortical and midbrain dopamine neurons. Cortical neurons expressing DAT accumulated dopamine and were highly vulnerable to 6-OHDA. Pharmacological inhibition of DAT completely blocked this toxicity. We also observed a p38-dependent Kv current surge in DAT-expressing cortical neurons exposed to 6-OHDA, and p38 antagonists and Kv channel blockers were neuroprotective in this model. Thus, DAT-mediated uptake of 6-OHDA recruited the oxidant-induced Kv channel dependent cell death pathway present in cortical neurons. Finally, we report that 6-OHDA also increased Kv currents in cultured midbrain dopamine neurons and this toxicity was blocked with Kv channel antagonists. We conclude that native DAT expression accounts for the dopamine neuron specific toxicity of 6-OHDA. Following uptake, 6-OHDA triggers the oxidant-associated Kv channel-dependent cell death pathway that is conserved in non-dopaminergic cortical neurons and midbrain dopamine neurons.
Keywords: potassium channels, dopamine transporter, 6-hydroxydopamine, neurotoxicity, p38, mitogen activated protein kinase
Parkinson’s disease is characterized by the loss of midbrain dopaminergic neurons. For over 35 years, the catecholamine-derived neurotoxin 6-hydroxydopamine (6-OHDA) has been used to induce dopaminergic cell death in various models of this disorder (Przedborski and Ischiropoulos, 2005). However, at sufficiently high concentrations all catecholamine derivatives, including dopamine and norepinephrine, induce non-selective cell death due to the generation of reactive oxygen species and reactive quinones following auto-oxidation (Heikkila and Cohen, 1972; Rosenberg, 1988; Kumar et al., 1995; Hanrott et al., 2006). As such, the selectivity of 6-OHDA for dopaminergic neurons, under the right experimental circumstances, may be due to the distinct neurochemical properties of this cell type and/or the fact that the catecholaminergic toxin acts as a substrate for the dopamine transporter (DAT), unique to these cells (Blum et al., 2001). Nonetheless, in spite the widespread use of 6-OHDA, the precise molecular mechanism by which this toxin destroys dopaminergic neurons has not been fully delineated, although many mechanisms have been proposed (Blum et al., 2001). Here, we first examined whether DAT expression in non-dopaminergic neurons would be sufficient to render these cells selectively sensitive to 6-OHDA toxicity. In addition, the molecular mechanism of 6-OHDA-induced cell death in this artificial system was later confirmed to exist in dopaminergic neurons, revealing a potentially new neuroprotective strategy for Parkinson’s disease and related disorders.
EXPERIMENTAL PROCEDURES
Green enhanced fluorescent protein, GFP, was from Clontech (Palo Alto, CA). GFP-DAT cDNA was kindly provided by Dr. S. Amara (Pittsburgh, PA, USA). Luciferase, pUHC13-3, was from Dr. H. Bujard (Heidelberg, Germany). Tyrosine hydroxylase antibody was from Chemicon (Temecula, CA, USA). Stromatoxin was purchased from Alomone (Jerusalem, Israel). Freshly prepared 6-OHDA (Sigma Chemical Co., St. Louis, MO, USA) stocks (10 mM) were prepared in 0.15% ascorbate and kept at −80°C until ready to use. Exposure vehicle consisted of minimum essential medium (without phenol red) with 25 mM HEPES and 0.01% bovine serum albumin. Solutions of 100 μM 6-OHDA in the exposure vehicle were prepared immediately (<1 min) prior to use, as they auto-oxidized at neutral pH at a rate of approximately 30% per minute at room temperature, as measured spectrophotometrically (490 nm). No further auto-oxidation products were noted beyond 6–7 minutes. Cortical cultures were prepared from E16 rat embryos as previously described (Hartnett et al., 1997). Cultures were transfected at 18–22 DIV with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA; Pal et al., 2003; ~5% transfection efficiency). Cells were maintained for 24 to 48 hours at 37°C, 5% CO2 prior to recording and toxicity assays. [3H]-Dopamine (DA) uptake assays were performed as described in Prasad and Amara (2001). Uptake was performed for 5 minutes at room temperature in Ringer’s solution containing 1 μM catechol-O-methyltransferase inhibitor Ro 41-0960 (Sigma), and 50 nM 3H-dopamine (60 Ci/mmol; PerkinElmer, Wellesley, MA, USA). Ventral mesencephalic cultures were established from postnatal day 1 rat (Cardozo and Bean, 1995; Hahn et al., 2006). Briefly, dissected tissue was dissociated with 20 U/ml papain (Worthington Biochemicals, Lakewood, NJ, USA) and triturated. The cell suspension was layered onto Basal Medium Eagle solution containing 10 mg/ml trypsin inhibitor 10 mg/ml bovine serum albumin and spun for 8 min at 200 g. The pellet was resuspended and cells plated at a density of 35,000 cells per 31 mm poly-L-lysine-coated glass coverslip. Cultures were fed twice weekly with Basal Medium Eagle with N2 supplement (Gibco, Carlsbad, CA, USA), penicillin/streptomycin, 2% rat serum, 0.6 mM glutamine, 10 mM glucose, and 10 mM HEPES; pH 7.3. Electrophysiological and toxicity experiments were performed at >12 DIV. At this in vitro age, functional expression of the DAT is well established (Valchar and Hanbauer, 1995) and, in our hands, the cells become highly vulnerable to 6-OHDA. We compared the sensitivity of dopaminergic neurons to 6-OHDA toxicity (100 μM, 15 min exposure) at two developmental stages, 8 DIV (n=5) and 15 DIV (n=9). We observed that viability of the younger cells was much grater than in the older cells following the toxin exposure (84.7 ± 15.4% at 8 DIV vs. 44.2 ± 5% at 15 DIV; p<0.01).
Recordings were conducted using the whole-cell configuration of the patch-clamp technique as described previously (McLaughlin et al., 2001). The extracellular solution contained (in mM): 115 NaCl, 2.5 KCl, 2.0 MgCl2, 10 HEPES, 10 D-glucose, pH 7.2, 0.1 mM tetrodotoxin. The electrode solution contained (in mM): 120 K-gluconate, 11 EGTA, 10 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES, 0.22 ATP; pH 7.2 and osmolarity adjusted to 280 mOsm with sucrose. Measurements were obtained under voltage clamp with an Axopatch 200 amplifier (Axon Instruments, Foster City, CA) and pClamp software (Axon Instruments) using 2 MΩ electrodes. Partial compensation (80%) for series resistance was always performed. Currents were filtered at 2 kHz and digitized at 10 kHz (Digidata; Axon Instruments). Potassium currents were evoked with a series of incremental positive voltage steps from a negative holding potential. Steady-state current amplitudes were leak-subtracted (P/N protocol), and normalized to cell capacitance. Capacitive transients were subtracted manually with the amplifier built-in circuits. Transfected cortical neurons were identified by GFP fluorescence. DA neurons were identified by fluorescence by 5,7-dihydroxytryptamine (Silva et al., 1988; Cardozo and Bean, 1995; Hahn et al., 2003). We found that 5,7-dihydroxytryptamine incubation could diminish the neurotoxic consequences of 6-OHDA exposure. As such, 6-OHDA treatments always preceded labeling.
Toxicity assays in cortical neurons were conducted at 48 hours post-transfection in parallel with electrophysiological recordings, but in luciferase co-transfected cells (Boeckman and Aizenman, 1996; Pal et al., 2003), or, in untransfected cells by a lactate dehydrogenase release assay (Hartnett et al., 1997). Cells were rinsed immediately prior to drug treatment. Cells were exposed to either vehicle or 6-OHDA for 15 min at 37°C, 5% CO2. Luciferase activity as an index of cell viability (Boeckman and Aizenman, 1996) was measured using the GeneLux Kit (Perkin Elmer, Boston, MA, USA) in a Victor2 Multilabel Counter (Perkin Elmer). Since we found that the potassium channel blocker tetraethylammonium (TEA) interfered with luciferase expression, toxicity assays involving this drug were quantified by cell counting of GFP-positive neurons. Counts were obtained from 30 fields of a 40X objective per condition, in triplicate. Toxicity in mesencephalic cultures (15 DIV) was determined by cell counts of tyrosine hydroxylase positive cells 24 hours after 6-OHDA treatments. Counts were conducted by determining the total number of TH positive cells in the entire culture dish, with at least 2–3 culture dishes per condition, using a 20X objective.
RESULTS
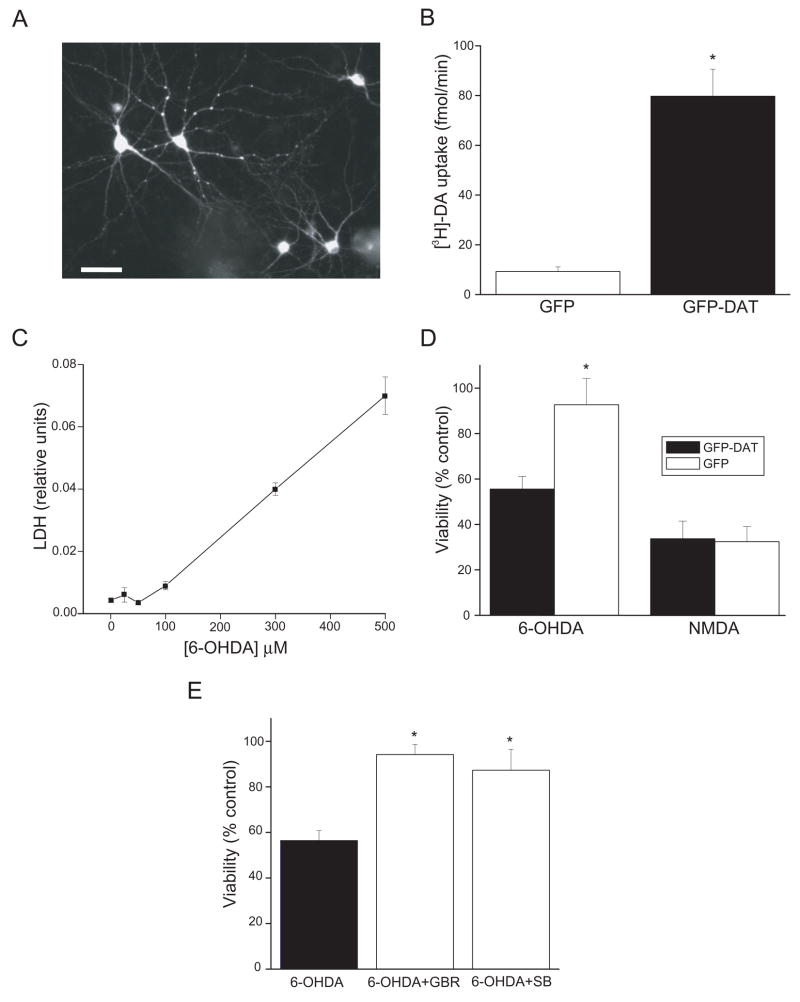
We first provide evidence of a functional separation between dopaminergic neuronal phenotype and DAT function in the toxicity of 6-OHDA. Non-dopaminergic rat cortical neurons in culture were transfected with a plasmid encoding a GFP-DAT fusion protein (Fig. 1A), and its functional expression confirmed by [3H]-dopamine uptake. We observed a significant increase in [3H]-DA uptake in GFP-DAT expressing cells, when compared to neurons transfected with a GFP-only expression vector (Fig. 1B). We then tested whether the catecholaminergic neurotoxin 6-OHDA would preferentially injure the GFP-DAT-expressing cortical neurons. A 15 min exposure to 100 μM 6-OHDA, which was relatively innocuous to untransfected cortical neurons (Fig. 1C), or cortical neurons expressing GFP-only vector (Fig. 1D), was sufficient to kill approximately 50% of the GFP-DAT expressing cortical neurons (Fig. 1D). In contrast, the glutamatergic toxin N-methyl-D-aspartate (200 μM, 15 min) killed neurons equally well, regardless of the expression of GFP-DAT (Fig. 1D). The toxicity of 6-OHDA in GFP-DAT-expressing cortical neurons was dependent on the activity of the transporter as it was completely blocked by the DAT inhibitor GBR12909 (Fig. 1E). This situation is highly reminiscent of the conditions necessary to induce 50% apoptotic cell death in dopaminergic neurons in vitro (Ding et al., 2004). As such, the expression of DAT is necessary and sufficient to render non-dopaminergic neurons selectively susceptible to 6-OHDA toxicity under conditions where the extracellular auto-oxidation of the toxin induces little or no non-specific injury.
Figure 1.
DAT renders non-dopaminergic neurons sensitive to 6-OHDA. A, Micrograph showing the expression of GFP-DAT in transfected cortical neurons (bar, 80 μm). B, Expression of GFP-DAT in cultured cortical neurons leads to functional expression of the transporter as measured by [3H]dopamine (DA) uptake, which is not observed in GFP vector alone-expressing cultures. Values represent the m ± s.e.m. uptake (fmol/min) in three independent experiments (*p < 0.005; t-test). Inset: GFP-DAT expressing neuron. C, Concentration-toxicity relationship for naïve, untransfected cortical neurons exposed to increasing levels of 6-OHDA for 15 min. Toxicity was measured by a lactate dehydrogenase (LDH) release assay (n=4). D, 6-OHDA is toxic to GFP-DAT expressing cortical neurons. GFP-DAT and GFP expressing cortical neurons were exposed to 6-OHDA (100 μM, 15 min) or to NMDA (200 μM, 15 min). Viability was assayed by a luciferase cell survival assay 24 hr. later and expressed as a percent of vehicle-treated control (n=6–10). Note that 6OHDA induced 50% cell death in GFP-DAT expressing neurons but was not lethal to GFP-expressing cells. In contrast, NMDA was equally toxic to both types of neurons (*p<0.001; t-test). E, 6-OHDA toxicity in GFP-DAT expressing cortical neurons is blocked by a DAT uptake inhibitor and by a p38-MAPK antagonist. GFP-DAT expressing cortical neurons were exposed 100 μM 6OHDA (15 min) in the absence or presence of either 10 μM GBR12909 (a DA uptake inhibitor) or 20 μM SB29063 (a p38 inhibitor). Viability was assayed by a luciferase cell survival assay. Note that both these drugs prevented 6OHDA toxicity (n= 4–13; *p<0.01; ANOVA/Dunnet).
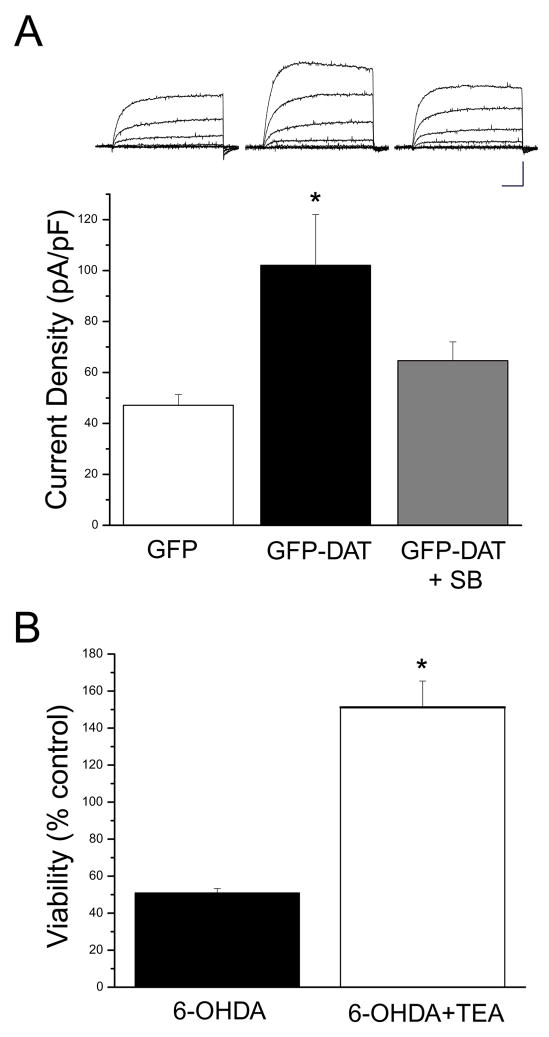
Dopaminergic neurons treated with 6-OHDA undergo caspase-dependent cell death following the phosphorylation of the mitogen-activated protein kinase (MAPK) p38 (Choi et al., 2004). As such, we tested whether the p38 antagonist SB239063 could block 6-OHDA-induced toxicity in GFP-DAT expressing cortical neurons. We observed that SB239063 was indeed very effective in preventing cell death induced by the neurotoxin (Fig. 1E). We have previously reported that oxidative stress in cortical neurons leads to a p38-dependent enhancement of voltage-dependent K+ currents (McLaughlin et al., 2001). This K+ current surge, mediated by the membrane insertion of Kv2.1-encoded K+ channels, is required for apoptosis to occur, and precedes caspase activation (McLaughlin et al., 2001; Pal et al., 2003; Pal et al., 2006). Therefore, we investigated if 6-OHDA activated a similar, p38-dependent pathway in GFP-DAT expressing cortical neurons. A pronounced, toxin-induced K+ current surge was observed in these cells, which was completely suppressed by SB239063 (Fig. 2A). More critically, the K+ channel blocker tetraethylammonium (TEA; Fig. 2B) was neuroprotective against 6-OHDA in GFP-DAT expressing cortical neurons, similar to what we have observed for other oxidant stressors (McLaughlin et al., 2001). These data show that DAT-dependent 6-OHDA toxicity can activate a cell death pathway that is very similar to that triggered by other oxidants in cortical neurons.
Figure 2.
Critical role of Kv channels in 6-OHDA toxicity in GFP-DAT expressing cortical neurons. A, 6-OHDA enhances voltage-dependent K+ currents in GFP-DAT expressing cortical neurons. Bottom, mean current densities in wild type cortical neurons (left; n = 21), GFP-DAT expressing cortical neurons (middle; n = 16) and GFP-DAT expressing cortical neurons pretreated with the p38 inhibitor SB293063 (20 μM, right; n = 10). All cells were treated with 30 μM 6-OHDA for 15 min prior to recording (100 μM of the toxin led to very unstable recordings in GFP-DAT expressing neurons). Currents were evoked by a voltage-step to +5 mV from a holding potential of −70 mV and normalized to cell capacitance (*p<0.05; ANOVA/Dunnet). Top, representative whole-cell recordings from all three treatment groups. Currents were evoked by a series of 15 mV voltage steps to +35 mV from −70 mV. Each set of traces is directly above its corresponding treatment group displayed in the bar graph. Calibration: 5nA, 15ms Note that the 6-OHDA-induced current surge could be blocked by the p38 inhibitor. B, 6-OHDA toxicity in GFP-DAT-expressing cortical neurons can be blocked by a K+ channel inhibitor. GFP-DAT expressing cortical neurons were exposed 100 μM 6OHDA (15 min) in the absence or presence of either 10 mM TEA. TEA treatment continued following toxin exposure. Viability was assayed by cell counting (GFP-positive cells) 24 hr. later as TEA interfered with the luciferase expression assay utilized above. Note TEA protected cells from 6OHDA toxicity (n=3; *p<0.005, t-test).
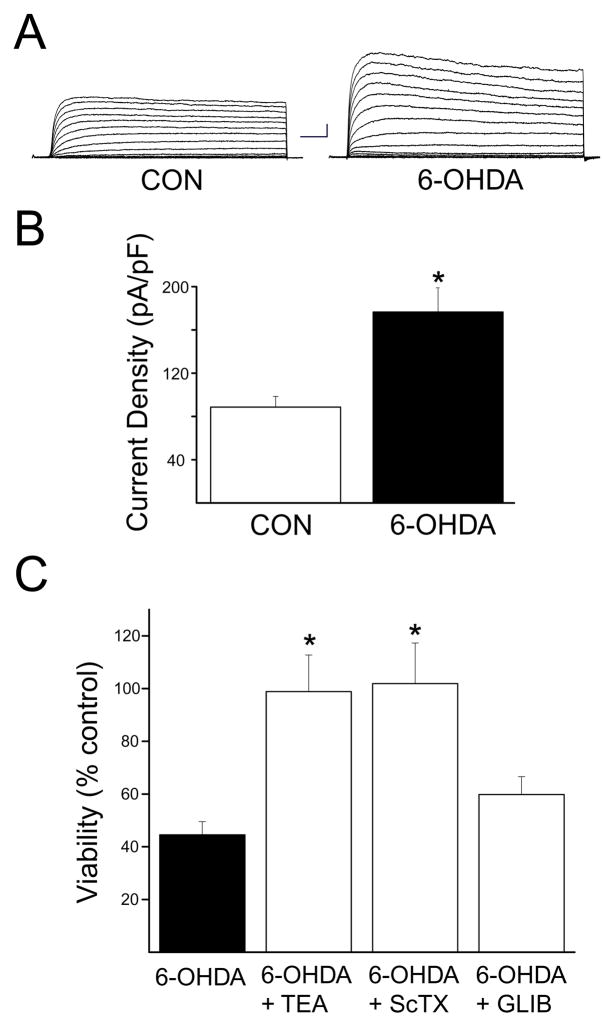
Given these results, we investigated whether 6-OHDA could induce a K+ current surge in dopaminergic neurons, which express DAT endogenously. In addition we tested whether K+ channel antagonists could prevent 6-OHDA toxicity in these cells. Similar to what was observed in GFP-DAT expressing cortical neurons, 6-OHDA induced approximately a 2-fold increase in voltage-dependent K+ currents in dopaminergic neurons obtained from rat mesencephalic cultures (Figs. 3A and B). In addition, this toxin (100 μM, 15 min) produced, approximately, 50% dopaminergic neuronal cell death (Fig. 3C). Importantly, 6-OHDA toxicity to dopaminergic neurons could be completely abrogated by 10 mM TEA (Fig. 3C). We also observed nearly complete neuroprotection by the Kv2.1-selective blocker stromatoxin (ScTX, 100 nM; Shiau et al., 2003; Grishin et al., 2005; Fig. 3C), but not by the K-ATP channel antagonist glibenclamide (1 μM; not shown). These results indicate that 6-OHDA toxicity in dopaminergic cells activates a K+ current surge, reminiscent to what has been reported many cell types undergoing apoptosis (Yu, 2003; Bortner and Cidlowski, 2004). Additionally, blocking K+ channels was neuroprotective, suggesting their critical role in the cell death process.
Figure 3.
Kv channels mediate 6-OHDA toxicity in dopaminergic neurons. A, 6-OHDA enhances voltage-dependent K+ currents in mesencephalic dopaminergic neurons. Currents were evoked from 13 DIV dopaminergic cells in mesencephalic cultures by a series of 10 mV voltage steps to +80 mV from a holding voltage of −80 mV under control conditions and following a 15 min treatment with 30 μM 6-OHDA. B, Mean ± s.e.m. current densities under control (n =10) and 6-OHDA treatment (n = 6) conditions. Currents were evoked by a voltage step to +10 mV from a holding potential of −80 mV and normalized to cell capacitance (*p<0.001; t-test). (g) 6-OHDA toxicity in dopaminergic neurons can be blocked by TEA and stromatoxin, but not glibenclamide. Mesencephalic cultures (15 DIV) were treated with 100 μM 6OHDA (15 min) in the absence or presence of 10 mM TEA, 100 nM stromatoxin (ScTX), or 1 μM glibenclamide (GLIB). TEA, ScTX and GLB treatments continued following toxin exposure. Cultures were fixed and immunostained for the presence of tyrosine hydroxylase (TH) 24 hours later. TH positive cells were then counted to assay DA cell survival. Note TEA and STR, but not GLIB, protected cells from 6-OHDA toxicity (n=3–9; *p<0.01; ANOVA/Dunnet). TEA, STR, and GLIB alone did not affect their viability (not shown).
DISCUSSION
The selectivity of 6-OHDA for catecholaminergic neurons, and, especially, dopaminergic neurons, has been the subject of much debate due to the fact that this substance, and indeed all catecholaminergic-derived compounds, can auto-oxidize in physiological solutions to generate highly reactive quinones, as well as free radicals (Heikkila and Cohen, 1972; Rosenberg, 1988; Kumar et al., 1995; Hanrott et al., 2006). Nonetheless, as early as 1970, it was suggested that 6-OHDA could selectively damage dopaminergic neurons in vivo due to the long-term depletion of central dopamine following intracerebral injections of the toxin (Uretski and Iversen, 1970). The sparing of 6-OHDA-treated cultured dopaminergic neurons by DAT inhibitors provided unequivocal evidence that, at least under certain conditions, the selectivity of the toxin could be accounted by cellular uptake systems (Cerruti et al., 1993). The results presented in this study corroborate this finding and indicate that under conditions where extracellular auto oxidation of 6-OHDA is not sufficient to induce non-selective injury (Hanrott et al., 2006), the presence of DAT activity can render the catecholaminergic derivative toxic, even in neurons not having an otherwise dopaminergic phenotype. As such, DAT expression is not only sufficient, but a decisive component for the manifestation of selective 6-OHDA toxicity.
Cellular K+ efflux is a requisite step for apoptosis to proceed in a variety cell death paradigms (Yu, 2003; Bortner and Cidlowski, 2004). The enhancement of potassium currents during the apoptotic process leads to a decrease in the concentration of this cation in the cytoplasm, which acts as a permissive cell death signal (Yu, 2003; Bortner and Cidlowski, 2004). Our group has demonstrated that the K+ efflux accompanying oxidant-induced apoptosis is mediated by an enhancement of voltage-gated K+ currents (McLaughlin et al., 2001). This phenomenon is triggered by the oxidative liberation of intraneuronal Zn2+, which leads to a p38-mediated exocytotic insertion of Kv2.1-encoded channels (Aizenman et al., 2000; McLaughlin et al., 2001; Pal et al., 2003; Pal et al., 2006). Recently, however, it was demonstrated that activation of ATP-sensitive potassium channels (K-ATP) was critical for the selective vulnerability of nigral dopaminergic neurons to the mitochondrial complex I inhibitors rotenone and 1-methyl-4-phenylpyridinium (Liss et al., 2005). However, the molecular link between K-ATP channel activation and cell death pathways was not established, nor was it demonstrated whether these channels were also associated with 6-OHDA-induced toxicity. The lack of neuroprotection by the K-ATP channel blocker glibenclamide observed in our studies indicates that these channels are not directly involved in the neurotoxicity induced by 6-OHDA in mesencephalic cultures. Nonetheless, it is entirely plausible that different K+ channels are able to provide various exit routes for the cation, even in the same cell type, depending on the injurious stimulus. While metabolically-sensitive K-ATP channels may be ideally suited to detect alterations in mitochondrial function by complex 1 inhibitors (Liss et al., 2005), voltage-gated channels like Kv2.1, which are directly linked to oxidative signaling processes (Pal et al., 2004), mediate 6-OHDA toxicity, and possibly the toxicity of other oxidants. It is noteworthy that the general term “oxidative stress” is commonly and intimately associated with nigral cell death in the Parkinson’s disease literature (Abou-Sleiman et al., 2006). The neuroprotective actions of ScTX, a Kv2.1-selective blocker, observed here, suggest that this channel mediates K+ efflux in dopaminergic neurons undergoing apoptosis, just like it does in cortical cells (Pal et al., 2003). Interestingly, Kv2.1 has recently been shown to be a major binding partner of DAT (Maiya et al., 2006). Our data strongly indicates that K+ channels may provide novel therapeutic targets for neuroprotection in Parkinson’s disease and related disorders.
Acknowledgments
Supported by NIH grants NS43277 (E.A.), HL080632 (E.S.L.), and NS053050 (E.S.L.).
Abbreviations
- DA
dopamine
- DAT
dopamine transporter
- DIV
days in vitro
- GFP
green fluorescent protein
- 6-OHDA
6-hydroxydopmaine
- Kv
voltage-dependent potassium channel
- ScTX
stromatoxin
- TEA
tetraethylammonium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- Aizenman E, Stout AK, Hartnett KA, Dineley KE, McLaughlin B, Reynolds IJ. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J Neurochem. 2000;75:1878–88. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- Blum D, Torch S, Lambeng N, Nissou MF, Benabid AL, Sadoul R, Verna JM. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol. 2001;64:135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Boeckman FA, Aizenman E. Pharmacological properties of acquired excitotoxicity in Chinese hamster ovary cells transfected with N-methyl-D-aspartate receptor subunits. J Pharmacol Exp Ther. 1996;279:515–523. [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. The role of apoptotic volume decrease and ionic homeostasis in the activation and repression of apoptosis. Pflugers Arch. 2004;448:313–318. doi: 10.1007/s00424-004-1266-5. [DOI] [PubMed] [Google Scholar]
- Cardozo DL, Bean BP. Voltage-dependent calcium channels in rat midbrain dopamine neurons: modulation by dopamine and GABAB receptors. J Neurophysiol. 1995;74:1137–48. doi: 10.1152/jn.1995.74.3.1137. [DOI] [PubMed] [Google Scholar]
- Cerruti C, Drian MJ, Kamenka JM, Privat A. Protection by BTCP of cultured dopaminergic neurons exposed to neurotoxins. Brain Res. 1993;617:138–142. doi: 10.1016/0006-8993(93)90624-v. [DOI] [PubMed] [Google Scholar]
- Choi WS, Eom DS, Han BS, Kim WK, Han BH, Choi EJ, Oh TH, Markelonis GJ, Cho JW, Oh YJ. Phosphorylation of p38 MAPK induced by oxidative stress is linked to activation of both caspase-8- and -9-mediated apoptotic pathways in dopaminergic neurons. J Biol Chem. 2004;279:20451–20460. doi: 10.1074/jbc.M311164200. [DOI] [PubMed] [Google Scholar]
- Ding YM, Jaumotte JD, Signore AP, Zigmond MJ. Effects of 6-hydroxydopamine on primary cultures of substantia nigra: specific damage to dopamine neurons and the impact of glial cell line-derived neurotrophic factor. J Neurochem. 2004;89:776–787. doi: 10.1111/j.1471-4159.2004.02415.x. [DOI] [PubMed] [Google Scholar]
- Grishin A, Ford H, Wang J, Li H, Salvador-Recatala V, Levitan ES, Zaks-Makhina E. Attenuation of apoptosis in enterocytes by blockade of potassium channels. Am J Physiol Gastrointest Liver Physiol. 2005;289:G815–G821. doi: 10.1152/ajpgi.00001.2005. [DOI] [PubMed] [Google Scholar]
- Hahn J, Tse TE, Levitan ES. Long-term K+ channel-mediated dampening of dopamine neuron excitability by the antipsychotic drug haloperidol. J Neurosci. 2003;23:10859–10866. doi: 10.1523/JNEUROSCI.23-34-10859.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J, Kullmann PHM, Horn JP, Levitan ES. D2 Autoreceptors Chronically Enhance Dopamine Neuron Pacemaker Activity. J Neurosci. 2006 doi: 10.1523/JNEUROSCI.4976-05.2006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrott K, Gudmunsen L, O’Neill MJ, Wonnacott S. 6-hydroxydopamine-induced apoptosis is mediated via extracellular auto-oxidation and caspase 3-dependent activation of protein kinase Cδ. J Biol Chem. 2006;281:5373–5382. doi: 10.1074/jbc.M511560200. [DOI] [PubMed] [Google Scholar]
- Hartnett KA, Stout AK, Rajdev S, Rosenberg PA, Reynolds IJ, Aizenman E. NMDA receptor-mediated neurotoxicity: a paradoxical requirement for extracellular Mg2+ in Na+/Ca2+ -free solutions in rat cortical neurons in vitro. J Neurochem. 1997;68:1836–1845. doi: 10.1046/j.1471-4159.1997.68051836.x. [DOI] [PubMed] [Google Scholar]
- Heikkila R, Cohen G. Further studies on the generation of hydrogen peroxide by 6-hydroxydopamine. Potentiation by ascorbic acid. Mol Pharmacol. 1972;8:241–248. [PubMed] [Google Scholar]
- Kumar R, Agarwal ML, Seth PK. Free radical-generated neurotoxicity of 6-hydroxydopamine. J Neurochem. 1995;64:1703–1707. doi: 10.1046/j.1471-4159.1995.64041703.x. [DOI] [PubMed] [Google Scholar]
- Liss B, Haeckel O, Wildmann J, Miki T, Seino S, Roeper J. K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nat Neurosci. 2005;8:1742–1751. doi: 10.1038/nn1570. [DOI] [PubMed] [Google Scholar]
- Maiya R, Ponomarev I, Linse KD, Harris RA, Mayfield RD. Defining the dopamine transporter proteome by convergent biochemical and in silico analyses. Genes Brain Behav. 2006 doi: 10.1111/j.1601-183X.2006.00236.x. in press. [DOI] [PubMed] [Google Scholar]
- McLaughlin BA, Pal S, Tran MP, Parsons AA, Barone FC, Erhardt JA, Aizenman E. p38 activation is required upstream of potassium current enhancement and caspase cleavage in thiol oxidant-induced neuronal apoptosis. J Neurosci. 2001;21:3303–3311. doi: 10.1523/JNEUROSCI.21-10-03303.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J Neurosci. 2003;23:4798–4802. doi: 10.1523/JNEUROSCI.23-12-04798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, He K, Aizenman E. Nitrosative stress and potassium channel-mediated neuronal apoptosis: is zinc the link? Pflug Archiv. 2004;448:296–303. doi: 10.1007/s00424-004-1256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Takimoto K, Aizenman E, Levitan ES. Apoptotic surface delivery of K+ channels. Cell Death Differ. 2006;13:661–667. doi: 10.1038/sj.cdd.4401792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad BM, Amara SG. The dopamine transporter in mesencephalic cultures is refractory to physiological changes in membrane voltage. J Neurosci. 2001;21:7561–7567. doi: 10.1523/JNEUROSCI.21-19-07561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Ischiropoulos H. Reactive oxygen and nitrogen species: weapons of neuronal destruction in models of Parkinson’s disease. Antiox Redox Signal. 2005;7:685–693. doi: 10.1089/ars.2005.7.685. [DOI] [PubMed] [Google Scholar]
- Rosenberg PA. Catecholamine toxicity in cerebral cortex in dissociated cell culture. J Neurosci. 1988;8:2887–2894. doi: 10.1523/JNEUROSCI.08-08-02887.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau YS, Huang PT, Liou HH, Liaw YC, Shiau YY, Lou KL. Structural basis of binding and inhibition of novel tarantula toxins in mammalian voltage-dependent potassium channels. Chem Res Toxicol. 2003;16:1217–1225. doi: 10.1021/tx0341097. [DOI] [PubMed] [Google Scholar]
- Silva NL, Mariani AP, Harrison NL, Barker JL. 5,7-dihydroxytryptamine identifies living dopaminergic neurons in mesencephalic cultures. Proc Natl Acad Sci USA. 1988;85:7346–7350. doi: 10.1073/pnas.85.19.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uretsky NJ, Iverson LL. Effects of 6-hydroxydopamine on catecholamine containing neurons in the rat brain. J Neurochem. 1970;17:269–278. doi: 10.1111/j.1471-4159.1970.tb02210.x. [DOI] [PubMed] [Google Scholar]
- Valchar M, Hanbauer I. Rat mesencephalic neuronal cells cultured for different periods as a model of dopamine transporter ontogenesis. Mol Neurobiol. 1995;11:111–119. doi: 10.1007/BF02740689. [DOI] [PubMed] [Google Scholar]
- Yu SP. Regulation and critical role of potassium homeostasis in apoptosis. Prog Neurobiol. 2003;70:363–386. doi: 10.1016/s0301-0082(03)00090-x. [DOI] [PubMed] [Google Scholar]