Abstract
Transplantation of olfactory ensheathing cells (OECs) into injured spinal cord results in improved functional outcome. Mechanisms suggested to account for this functional improvement include axonal regeneration, remyelination and neuroprotection. OECs transplanted into transected peripheral nerve have been shown to modify peripheral axonal regeneration and functional outcome. However, little is known of the detailed integration of OECs at the transplantation site in peripheral nerve. To address this issue cells populations enriched in OECs were isolated from the olfactory bulbs of adult green fluorescent protein (GFP)-expressing transgenic rats and transplanted into a sciatic nerve crush lesion which transects all axons. Five weeks to six months after transplantation the nerves were studied histologically. GFP-expressing OECs survived in the lesion and distributed longitudinally across the lesion zone. The internodal regions of individual teased fibers distal to the transection site were characterized by GFP expression in the cytoplasmic and nuclear compartments of cells surrounding the axons. Immuno-electron microscopy for GFP indicated that the transplanted OECs formed peripheral type myelin. Immunostaining for sodium channel and Caspr revealed a high density of Nav1.6 at the newly formed nodes of Ranvier which were flanked by paranodal Caspr staining. These results indicate that transplanted OECs extensively integrate into transected peripheral nerve and form myelin on regenerated peripheral nerve fibers, and that nodes of Ranvier of these axons display proper sodium channel organization.
Keywords: Regeneration, olfactory ensheathing cells, peripheral nervous system, transplantation, nodal formation
1. Introduction
Olfactory ensheathing cells (OECs) are specialized glial cells that guide the regeneration of nonmyelinated olfactory axons from the peripheral nasal epithelium through the cribriform plate of the ethmoid bone and into the olfactory bulb. They are located in the olfactory epithelium, olfactory nerve, and the outer layers of the olfactory bulb (Ramon-Cueto and Avila, 1998). The olfactory neuroepithelium undergoes continuous turnover and sends new axons into the olfactory bulb. OECs are thought to play a critical role in guiding the regrowth of olfactory neurons (Devon and Doucette, 1992). Subtypes of OECs have been described as Schwann cell-like (S-type), that can form peripheral myelin, and fibroblast-like (A-type) that can form a cellular channel through which axons can grow (Li et al., 1998; Choi and Raisman, 2006).
Several studies have demonstrated enhanced functional recovery after OEC transplantation into the injured spinal cord (Li et al., 1998; Ramon-Cueto et al., 2000; Plant et al., 2002; Verdu et al., 2003; Sasaki et al., 2004; Garcia-Alias et al., 2005). While the precise mechanism of this functional recovery is not fully understood, several mechanisms have been suggested including remyelination (Devon and Doucette, 1992; Franklin et al., 1996; Imaizumi et al., 1998; Sasaki et al., 2004), long axon tract regeneration (Li et al., 1997; Ramon-Cueto et al., 2000; Imaizumi et al.;2000), axonal sparing (Plant et al., 2002) and plasticity associated with novel polysynaptic pathways (Keyvan-Fouladi et al., 2002; Bareyre et al., 2004). In addition, recruitment of endogenous SCs (Takami et al., 2002; Boyd et al., 2004; Ramer et al., 2004) and remote inhibition of apoptosis of motor cortical neurons (Sasaki et al., 2006a) have been suggested to contribute to improvement in functional outcome in injured spinal cord after OEC transplantation.
OECs have also been considered to enhance repair of peripheral nerve fibers. The rationale is that they may provide a scaffold for the regenerating axons as well as trophic factors and directional cues (Deumens et al., 2006). Transplantation of OECs into axotomized facial nerve has been shown to enhance axonal sprouting (Guntinas-Lichius et al., 2001; Deumens et al., 2006) and to promote the recovery of vibrissae motor performance (Guntinas-Lichius et al., 2002). Choi and Raisman (2005) demonstrated that the rate of eye closure was increased following OEC transplantation in a facial nerve lesion model, but that aberrant nerve branching was not changed.
While a number of recent studies have focused on the potential role of OECs in enhancing functional outcome following peripheral nerve transection, little is known of the detailed integration of OECs into the peripheral nerve injury site. To address this issue OECs from adult green fluorescent protein (GFP)-expressing transgenic rats were transplanted into a sciatic nerve crush lesion, which transects all axons. We report that GFP-expressing OECs survived for at least six months and distributed longitudinally along regenerated axons and remyelinated the axons. Furthermore, the regenerated fibers show reconstructed nodes of Ranvier with proper sodium channel organization, i.e. sodium channel Nav1.6. Thus, transplanted GFP-expressing OECs prepared from adult olfactory bulb are able to remyelinate regenerated peripheral nerve fibers and restore proper nodal structure in injured PNS indicating that they can contribute to local nerve repair.
2. Results
2.1. Distribution of GFP-expressing OECs transplanted into crushed sciatic nerve lesion
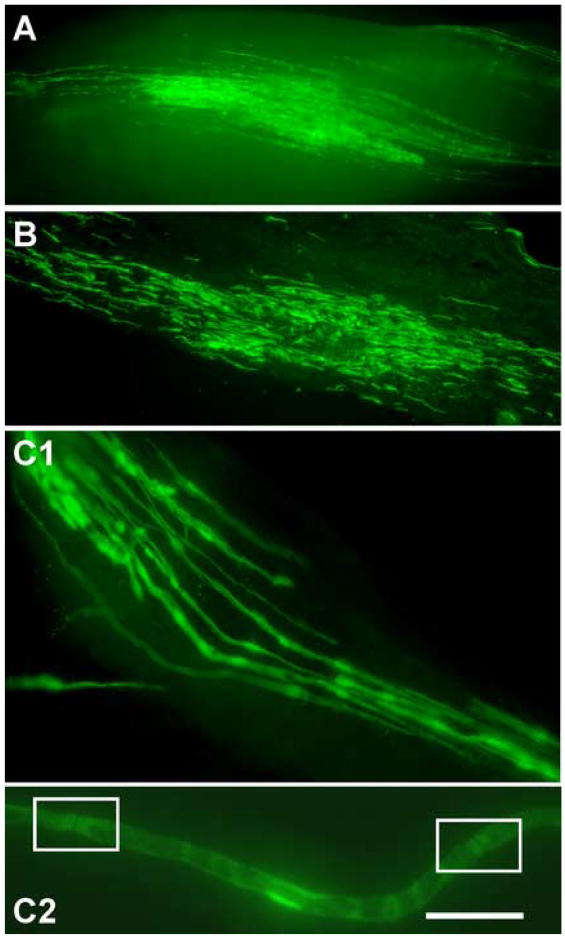
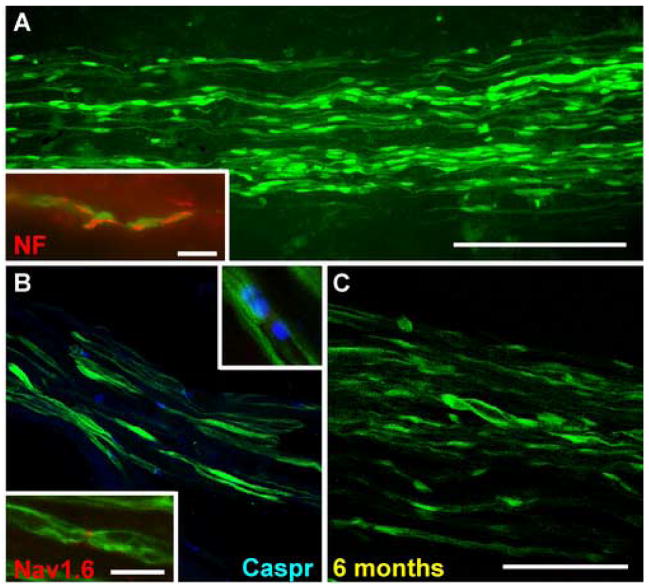
At five weeks post OEC transplantation, whole mount images of live (Fig. 1A) and fixed nerve (Fig. 1B) revealed that GFP-OECs survived in the lesion site and distributed longitudinally along the regenerated axons several millimeters from the injection site. Teased sciatic nerve revealed that the GFP-OECs were associated with individual axons within the lesion site (Fig. 1C). Along the axon, areas of intense fluorescence were detected with dense cytoplasmic regions and nuclei of the GFP-OECs. Within the areas of concentrated fluorescence were breaks where no GFP fluorescence was detected. These regions were determined to be putative nodes of Ranvier (Fig. Fig. 1C2). Confocal images revealed GFP-expressing OECs to be abundant at the lesion site and to distribute longitudinally along the nerve (Fig. 2A). Immunostaining for neurofilament (NF) indicated the close association between the regenerated axon and the GFP-expressing OECs (see inset Fig. 2A). Immunostaining for Caspr and sodium channel indicated the presence of nodes of Ranvier between adjacent green internodes (Fig. 2B), At six months post transplantation, confocal images showed the presence of GFP-OECs indicated by intense green fluorescence (Fig. 2C). This demonstrates the ability of transplanted OECs to survive in the lesion site for at least several months.
Figure 1.
Olfactory ensheathing cell (OEC) transplantation into transected sciatic nerve. Fluorescent images of a living sciatic nerve whole mount taken immediately upon nerve extraction and desheathment at 5 weeks post transplantation (A). B. Comparable whole mount for a fixed nerve. Green fluorescent protein (GFP)-OECs are distributed longitudinally several millimeters from the injection site. Areas of increased fluorescence are the nuclei and dense cytoplasmic regions of the OECs. C1. Higher magnification image of teased, fixed nerve taken immediately upon removal at 5 weeks post transplantation. GFP-OECs associate with individual axons within the lesion site. C2. An individual living axon with putative nodes of Ranvier (boxed) and areas of concentrated fluorescence, which indicate OEC nuclei and cytoplasm.
Scale bar: A=500μm, B = 25μm, C1= 11μm, C2=3.5μm
Figure 2.
Distribution of OECs in injured sciatic nerve A. Confocal images of crushed sciatic nerve at 5 weeks post OEC transplantation demonstrating an abundance of GFP-OECs distributing longitudinally in the injured nerve distal to the transection. Inset. Association of GFP fluorescence with axonal neurofilament staining. B. Caspr immunostaining (blue) demonstrating paranodal regions on regenerated axons associated with GFP-OECs. The upper inset is a higher magnification of an individual nodal region. Immunostaining for sodium channel Nav1.6 indicates a node formation on a regenerated axon (lower inset). C. Survival of GFP-OECs on the regenerated axons 6 months post-crush injury.
Scale bar: A = 200μm, B = 40μm, C = 80μm, D= 13μm, A inset=13μm
2.2. Nav1.6 localizes to nodes of regenerated axons which are remyelinated by OECs
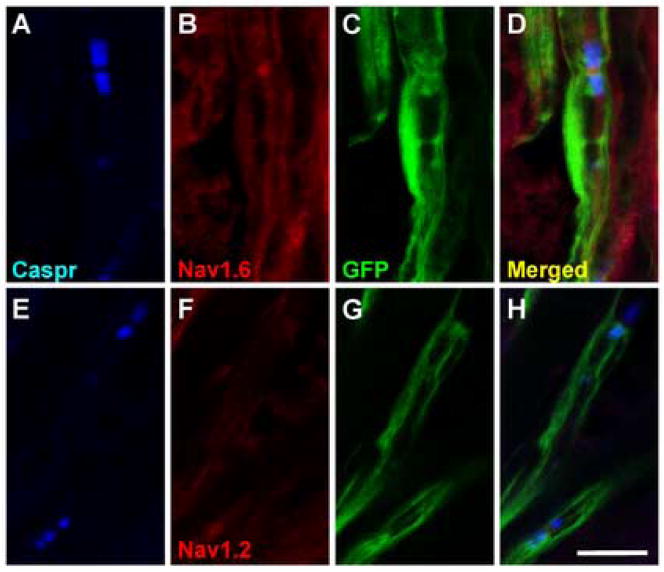
Within the GFP-OEC transplanted sciatic nerves, nodes of Ranvier were identified by flanking paranodal Caspr immunofluorescence (Menegoz et al., 1997; Rios et al., 2000; Sasaki et al. 2006b), which was abundant in these areas (Fig. 2B). At 5 weeks after engraftment of OECs, nodes, identified by Caspr reactivity and associated with adjacent GFP-OECs, could be observed. These areas were characterized by GFP fluorescence in the region of the terminal paranodal loops, as well as within the outer layer of the GFP-OEC sheath (Fig. 2B, inset upper right). These data demonstrate the association of the transplanted OECs with the remyelination of host axons. Intense Nav1.6 staining was observed at the putative nodes of Ranvier positioned between GFP expressing internodes (Fig. 2B inset lower left). We observed Nav1.6 staining at most nodes, whereas detectable Nav1.2 immunostaining was not apparent at any nodes in the sciatic nerve (Fig. 3). This is similar to the CNS remyelinated lesion by transplanted GFP-OECs (Sasaki et al., 2006b). In the PNS, 5 weeks after OEC engraftment, virtually all nodes bounded by GFP-OEC myelin sheaths exhibited Nav1.6 staining (Fig. 3A–D). However, Nav1.2 immunolabeling was not observed at any nodes (Fig. 3E–F). The Nav1.6 labeling was restricted to the nodal domain and was not observed in paranodal or juxtaparanodal regions or beneath the myelin sheath in remyelinated axons. This suggests that the transplanted GFP-OECs are able to contribute to the specific clustering of Nav1.6 channels at the newly formed nodes of Ranvier.
Figure 3.
Nav1.6 at nodes of Ranvier of regenerated sciatic nerve fibers remyelinated by OECs. A. Nav1.6 sodium channel is observed at Caspr-delimited nodes formed on axons associated with GFP expressing cells (A–D). Caspr-delimited nodes formed by GFP-OECs do not exhibit Nav1.2 immunostaining (E–H).
Scale bar = 10 μm.
2.3. Immunoelectron microscopy of GFP-expressing OECs forming myelin
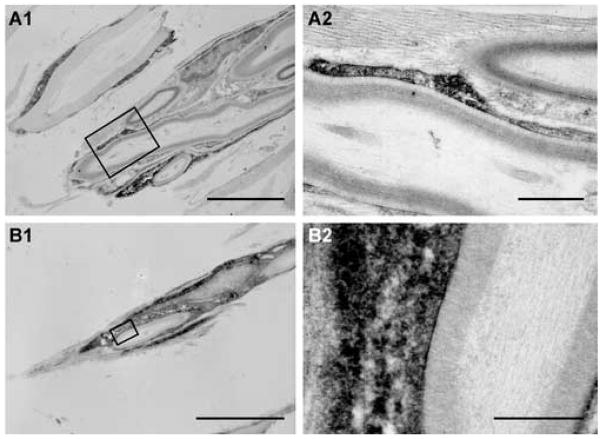
To establish that the OECs derived from GFP rats were indeed responsible for the remyelination, immunoelectron microscopy for GFP-reaction product was performed (Fig. 4). Intense reaction product was observed within the cytoplasm and nuclei of cell profiles surrounding myelinated axons. GFP+ cells were detected in direct contact with host axons. GFP-reaction product was clearly evident in the cytoplasm of most cells that formed the well defined multi-laminate structures characteristic of myelin. In longitudinal sections of myelinated axons, intense reaction product can be seen in the cytoplasm of the myelin-forming cell (Fig. 4A). Large cytoplasmic and nuclear regions (Fig. 4B1) indicate characteristic peripheral pattern of myelination. A limited number of GFP+ cells surrounded axons, but did not form myelin (data not shown). Some unlabeled cells forming peripheral-like myelin could also be detected within transplanted lesions in the same areas as the labeled cells.
Figure 4.
Electron micrographs of anti-GFP immunoperoxidase staining of OECs 7 weeks post transplantation. A1. Reaction product can be seen in the cytoplasm of the GFP-OECs, which have formed myelin around an axon. A2. Higher magnification of boxed area in A1. B1. Another myelinated axon showing intense reaction product in the myelin-forming cell. B2. Higher magnification of boxed area in B1. Scale bar: A1=5μm, A2=1μm, B1=1μm, B2=0.5 μm
3. Discussion
There is much evidence supporting the potential of OECs to form myelin and to have beneficial effects within the context of spinal cord injury. OECs transplanted into spinal cord injury lesions can form myelin with a peripheral-like pattern (Franklin et al., 1996; Imaizumi et al., 1998; Li et al., 1998; Ramon-Cueto et al., 1998; Kato et al., 2000; Sasaki et al., 2004; Sasaki et al., 2006b). The use of GFP-expressing rat OECs for transplantation into a dorsal spinal cord transection (Sasaki et al., 2004) or demyelination (Sasaki et al., 2006b) lesion allowed clear identification of remyelination by engrafted OECs. More recently OECs have been considered to enhance repair of peripheral nerve fibers by possibly providing a scaffold for the regenerating axons as well as trophic factors and directional cues (Deumens et al., 2006). Indeed, transplantation of OECs into axotomized facial nerve was shown to enhance axonal sprouting (Guntinas-Lichius et al., 2001; Deumens et al., 2006), promote recovery of vibrissae motor performance (Guntinas-Lichius et al., 2002) and to increase the rate of eye closure (Choi and Raisman, 2005).
However, details of the morphological integration of OECs transplanted into peripheral nerve are limited. In the present study, we utilized OECs prepared from a GFP transgenic rat which allowed the clear identification of the morphological relationship of the transplanted cells with regenerated axons. We found that the GFP-expressing OECs survive and distribute longitudinally along regenerated axons. Moreover, NF staining revealed the regenerated axons to be surrounded by OECs with GFP fluorescence in their nuclei and cytoplasm. Internodal regions could easily be identified by green fluorescence in the outer mesaxon and cell body even in teased living nerve fibers. Immuno-electron microscopy for GFP firmly established that GFP cells formed peripheral-like myelin on the regenerated axons. At the ultrastructural level over half of the myelinated profiles were associated with GFP+ cells. Thus, the engrafted OECs were able to integrate into and form myelin on regenerated peripheral nerve fibers.
Proper sodium channel organization at nodes of Ranvier is critical for appropriate impulse conduction. The kinetically fast and tetrodoxin-sensitive Nav1.6 sodium channel is the dominant sodium channel at mature nodes (Caldwell et al., 2000). The newly constructed nodes of Ranvier on regenerated axons positioned between GFP internodes expressed Nav1.6, but not Nav1.2. This indicates that regenerated axons remyelinated by engrafted OECs establish appropriate mature nodes of Ranvier, a prerequisite for appropriate impulse conduction.
An important consideration in interpreting the results of the present study is the possibility that SC contamination in the OEC preparation might contribute significantly to the population of surviving cells in OEC transplanted lesions. As pointed out by Plant et al. (2002) and further discussed by Boyd et al. (2005), SCs from nerves innervating blood vessels of the olfactory bulb may be a source of SC contamination in OEC preparations. Although p75NGFR and S-100 staining can distinguish between OECs and other cell types within the olfactory bulb, it cannot fully distinguish between OECs and SCs. While we cannot rule out the possibility of minor SC contamination in our OEC cultures from the olfactory bulb, the relative degree of myelination following transplantation of equal numbers of SCs (Radtke et al., 2005) and OECs was similar. Moreover, the extent of remyelination by equal numbers of transplanted genetically marked SCs and OECs was similar in a spinal cord demyelination model (Akiyama et al, 2004). These results suggest that a small contaminant of SCs in the OEC preparation did not account for the remyelination by the transplanted OECs (Akiyama et al., 2004). Importantly, Franklin et al. (1996) used an immortalized OEC line and found in vivo remyelination, which further supports the idea that OECs are able to form myelin under appropriate conditions. However, we emphasize that our preparation of p75NGFR + OECs from the adult olfactory bulb is diverse in culture and includes cells with spindled and flattened shapes. These two cell types within OEC cultures have been subtyped into S-type and A-type, respectively (Li et al., 1998; Choi et al., 2006). While more work is necessary to fully define the subpopulations within OEC cultures prepared from olfactory bulb, transplantation of a relatively acutely prepared culture of OECs from adult olfactory bulb which has therapeutic effects in animal spinal cord injury models results in extensive remyelination of regenerated peripheral nerve fibers.
In summary, transplantation of OECs derived from adult olfactory bulb, myelinate regenerated peripheral nerve fibers and appropriate nodal sodium channels are formed on the regenerated and remyelinated axons. The extent of remyelination was similar to that reported for SC transplantation into the same model system (Radtke et al., 2005) suggesting that a unique population of cells within the olfactory bulb is capable of forming peripheral-like myelin.
4. Methods
4.1. Isolation and Characterization of Donor OECs
Freshly isolated OECs were obtained as reported previously (Sasaki et al., 2006b). Olfactory bulbs were removed from 4 to 8 week-old transgenic rats expressing GFP [“green rat” CZ-004, SD-Tg (Act-EGFP) CZ-004Osb; SLC, Shizuoka, Japan] and the meninges were removed to minimize contamination. The caudal one-third of the bulb was removed and discarded along with as much white matter as possible to isolate the outer nerve layer. Tissue was finely minced with a pair of scalpel blades (#10) on plastic culture dishes and incubated for 25 min in collagenase A (0.75 mg/ml; Roche, Indianapolis, IN), collagenase D (0.75 mg/ml; Roche), and papain (12 U/ml; Worthington, Lakewood, NJ) in calcium-free complete saline solution (CSS) with a trace of cysteine for 25 min at 37°C on a rotary shaker in a CO2 incubator. The tissue suspension was then centrifuged for 7 min at 300 × g. The pellet was resuspended in 2 ml of DMEM (Invitrogen, Carlsbad, CA) with 10% FCS using gentle mechanical trituration with fire-polished pasture pipettes with successively reduced diameters. The volume of media was immediately increased to 20 ml, and undissociated pieces of tissue were allowed to settle for 2 min before transferring the cell suspension to another culture tube and centrifuging as before. Cells were washed twice, resuspended, and preplated for 1 hour in a culture flask at 37°C in a CO2 incubator. Nonadherent cells were gently washed off with DMEM, and the cells were centrifuged and resuspended three times in DMEM. Cells were counted and concentrated to 3.0 × 104 cells/μl just before transplantation. p75NGFR and S100-positive cells were counted in short-term cultures, made from cell suspensions used for transplantation, to assess purity of the cells. Over 95% of the cells were positive for p75NGFR and S100.
4.2. Crush Lesion and OEC transplantation
Experiments were performed in accordance with National Institutes of Health guidelines for the care and use of laboratory animals. The Veterans Affairs Connecticut Healthcare System Institutional Animal Care and Use Committee approved all animal protocols. Adult wild Sprague Dawley rats (200–225g) were used for this experiment (n=34). The rats were anesthetized with ketamine (75 mg/kg i.p.) and xylazine (10 mg/kg i.p.). The sciatic nerves were exposed near the piriformis tendon and crushed for 20 s with fine forceps (Dumont #5). This procedure transects all of the axons in the nerve, but the epineurium remains intact, so the axons can regenerate into the distal nerve segment. Immediately after the nerve crush, 0.5 μl of a GFP-OEC suspension (3.0 × 104 cells/μl), or DMEM for sham control, was injected just distally to the crush site using a glass pipette (40 μm tip diameter) attached to a 1 μl Hamilton syringe (Radtke et al., 2005).
4.3. Whole mount images of the living nerve
Five weeks after transplantation, animals (n=3) were deeply anesthetized (50 mg/kg sodium pentobarbital, i.p.) and decapitated. Sciatic nerves from transplanted rats were extracted and desheathed with fine forceps. Some samples were put in 4% paraformaldehyde for five minutes and teased with fine forceps. The sections were examined by conventional fluorescence microscopy (Nikon Eclipse 800; Spot RT Color CCD camera; Diagnostic Instruments).
4.4 Immunohistochemistry
Sciatic nerves from the sham control (n=5), GFP-OEC transplanted rats at 5 weeks (n=20) and 6 months (n=3) following transplantation were processed for immunocytochemistry as described previously. Briefly, rats were deeply anesthetized with ketamine/xylazine and perfused transcardially, first with PBS and then with ice-cold 4% paraformaldehyde in 0.14 M Sorensen’s phosphate buffer, pH 7.4. Sciatic nerve were excised and placed in fresh fixative to achieve a total fixation time of 25 min. Tissue was rinsed several times with PBS and cryoprotected in 30% sucrose in PBS overnight at 4°C. Ten micrometer cryosections of the sciatic nerve were cut and mounted on silane-coated glass slides (Sigma). The sections were processed for double immunofluorescent staining for the detection of Nav1.2 or Nav1.6, and Caspr (Black et al., 1999, 2002) or with single immunolabeling for Neurofilament (NF). The primary antibodies used were as follows: polyclonal Nav1.2 (1:100; Alomone Labs, Jerusalem, Israel), polyclonal Nav1.6 (1:100; Alomone Labs), monoclonal NF (1:1000; Sigma) and monoclonal Caspr (1:300) (Rasband et al., 1999) (a generous gift from Dr. M. Rasband, University of Connecticut, Storrs, CT). Secondary antibodies used were as follows: goat anti-rabbit IgG-Cy3 (1:2000; Amersham Biosciences, Piscataway, NJ) and goat anti-mouse IgG-Alexa Fluor 633 or Alexa Fluor 546 (1:1000; Invitrogen, Eugene, OR). The sections were examined by confocal microscopy (Nikon Eclipse E600 microscope; Simple PCI; Compix Imaging Systems, Cranberry Township, PA) and with conventional fluorescence microscopy (Nikon Eclipse 800; Spot RT Color CCD camera; Diagnostic Instruments)
4.5. Immunoelectron microscopy
Seven weeks following transplantation, animals (n=3) were deeply anesthetized (50 mg/kg sodium pentobarbital, i.p.) and perfused transcardially with PBS followed by 4% paraformaldehyde/0.02% glutaraldehyde in PBS. Sciatic nerves were excised, postfixed overnight in 4% paraformaldehyde, and embedded in 3% agar for vibratome sectioning. Free-floating sections (150 μm thick) were incubated in 2% normal goat serum for 30 min and then in rabbit anti-GFP antibody (1:2000; Chemicon) overnight at 4°C. The sections were incubated overnight with an anti-rabbit biotinylated secondary antibody (Sigma, St. Louis, MO) and then incubated for 1 h using a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) following standard protocols. The sections were postfixed with 1% osmium tetroxide for 4 h, dehydrated in graded ethanol solutions and embedded in Epox-812 (Ernest Fullam, Latham, NY). Ultrathin sections were cut at 10 nm and examined on a Philips 100 EM.
Acknowledgments
This work was supported in part by the Department of Veterans Affairs, the NIH, and the National Multiple Sclerosis Society. The Center for Neuroscience and Regeneration Research is a collaboration of the Paralyzed Veterans of America and the United Spinal Association with Yale University. We thank Heather Mallozzi and Margaret Borelli for excellent technical assistance. We also thank Dr. M. Rasband for the generous gift of Caspr antibody.
Abbreviations
- OECs
Olfactory ensheathing cells
- SCs
Schwann cells
- GFP
green fluorescent protein
- CNS
central nervous system
- PNS
peripheral nervous system
- CSS
complete saline solution
- DMEM
Dulbecco’s Modified Eagle Medium
- NF
Neurofilament
- PBS
phosphate buffered saline
- EM
electron microscope
- p75NGFR
p75 nerve factor receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama Y, Lankford K, Radtke C, Greer CA, Kocsis JD. Remyelination of spinal cord axons by olfactory ensheathing cells and Schwann cells derived from transgenic rat expressing alkaline phosphates marker gene. Neuron Glia Biol. 2004;1:1–9. doi: 10.1017/S1740925X04000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;10:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Black JA, Cummins TR, Plumpton C, Chen YH, Hormuzdiar W, Clare JJ, Waxman SG. Upregulation of a silent sodium channel after peripheral, but not central nerve injury in DRG neurons. J Neurophysiol. 1999;82:2776–2785. doi: 10.1152/jn.1999.82.5.2776. [DOI] [PubMed] [Google Scholar]
- Black JA, Renganathan M, Waxman SG. Sodium channel Na (v) 1.6 is expressed along nonmyelinated axons and it contributes to conduction. Brain Res. Mol Brain Res. 2002;105(1–2):19–28. doi: 10.1016/s0169-328x(02)00385-6. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Lee J, Skihar V, Doucette R, Kawaja MD. LacZ-expressing olfactory ensheathing cells do not associate with myelinated axons after implantation into the compressed spinal cord. Proc Natl Acad Sci Unit States Am. 2004;17:2162–2166. doi: 10.1073/pnas.0303842101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JG, Doucette R, Kawaja MD. Defining the role of olfactory ensheathing cells in facilitating axon remyelination following damage to the spinal cord. Faseb J. 2005;19:694–703. doi: 10.1096/fj.04-2833rev. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc Natl Acad Sci Unit States Am. 2000;97(10):5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D, Raisman G. Disorganization of the facial nucleus after nerve lesioning and regeneration in the rat: effects of transplanting candidate reparative cells to the site of injury. Neurosurgery. 2005;56:1093–1100. [PubMed] [Google Scholar]
- Deumens R, Koopmans GC, Lemmens M, Mollers S, Honig WM, Steinbusch HW, Brook G, Joosten EA. Neurite outgrowth promoting effects of enriched and mixed OEC/ONF cultures. 2006;397:20–24. doi: 10.1016/j.neulet.2005.11.063. [DOI] [PubMed] [Google Scholar]
- Devon R, Doucette R. Olfactory ensheathing cells myelinate dorsal root ganglion neuritis. Brain Res. 1992;589:175–179. doi: 10.1016/0006-8993(92)91182-e. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Gilson JM, Franceschini IA, Barnett SC. Schwann cell-like myelination following transplantation of an olfactory bulb-ensheathing cell line into areas of demyelination in the adult CNS. Glia. 1996;17(3):217–224. doi: 10.1002/(SICI)1098-1136(199607)17:3<217::AID-GLIA4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Garcia-Alias G, Lopez-Vales R, Fores J, Navarro X, Verdu E. Acute transplantation of olfactory ensheathing cells or Schwann cells promotes recovery after spinal cord injury in the rat. J Neurosci Res. 2005;75:632–641. doi: 10.1002/jnr.20029. [DOI] [PubMed] [Google Scholar]
- Guntinas-Lichius O, Angelov DN, Tomov TL, Dramiga J, Neiss WF, Wewetzer K. Transplantation of olfactory ensheathing cells stimulates the collateral sprouting from axotomized adult rat facial motoneurons. Exp Neurol. 2001;172:70–80. doi: 10.1006/exnr.2001.7774. [DOI] [PubMed] [Google Scholar]
- Guntinas-Lichius O, Wewetzer K, Tomov TL, Azzolin N, Kazemi S, Streppel M, Neiss WF, Angelov DN. Transplantation of olfactory mucosa minimizes axonal branching and promotes the recovery of vibrissae motor performance after facial nerve repair in rats. J Neurosci. 2002;22(16):7121–7131. doi: 10.1523/JNEUROSCI.22-16-07121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Lankford KL, Waxman SG, Greer CA, Kocsis JD. Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in the demyelinated dorsal columns of the rat spinal cord. J Neurosci. 1998;18(16):6176–85. doi: 10.1523/JNEUROSCI.18-16-06176.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Lankford KL, Kocsis JD. Transplantation of olfactory ensheathing cells or Schwann cells restores rapid and secure conduction across the transected spinal cord. Brain Res. 2000;854:70–78. doi: 10.1016/s0006-8993(99)02285-4. [DOI] [PubMed] [Google Scholar]
- Kato T, Honmou O, Uede T, Hashi K, Kocsis JD. Transplantation of human olfactory ensheathing cells elicits remyelination of demyelinated rat spinal cord. Glia. 2000;30:209–218. doi: 10.1002/(sici)1098-1136(200005)30:3<209::aid-glia1>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyvan-Fouladi N, Li Y, Raisman G. How do transplanted olfactory ensheathing cells restore function? Brain Res Brain Res Rev. 2002;40:325–327. doi: 10.1016/s0165-0173(02)00215-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science. 1997;277:2000–2002. doi: 10.1126/science.277.5334.2000. [DOI] [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G. Regeneration of adult rat corticospinal axons induced by transplanted olfactory ensheathing cells. J Neurosci. 1998;18(24):10514–10524. doi: 10.1523/JNEUROSCI.18-24-10514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegoz M, Gaspar P, LeBert M, Galvez T, Burgava F, Palfrey C, Ezan P, Arnos F, Girault JA. Paranodin, a glycoprotein of neuronal paranodal members. Neuron. 1997;19:319–331. doi: 10.1016/s0896-6273(00)80942-3. [DOI] [PubMed] [Google Scholar]
- Plant GW, Currier PF, Cuervo EP, Bates ML, Pressman Y, Bunge MB, Wood PM. Purified adult ensheathing glia fail to myelinate axons under culture conditions that enable Schwann cells to form myelin. J Neurosci. 2002;22:6083–6091. doi: 10.1523/JNEUROSCI.22-14-06083.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke C, Akiyama Y, Lankford KL, Vogt PM, Krause DS, Kocsis JD. Integration of engrafted Schwann cells into injured peripheral nerve: axonal association and nodal formation on regenerated axons. Neurosci Lett. 2005;387(2):85–89. doi: 10.1016/j.neulet.2005.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer LM, Au E, Richter MW, Liu J, Tetzlaff W, Roskams AJ. Peripheral olfactory ensheathing cells reduce scar and cavity formation and promote regeneration after spinal cord injury. J Comp Neurol. 2004;473(1):1–15. doi: 10.1002/cne.20049. [DOI] [PubMed] [Google Scholar]
- Ramon-Cueto A, Avila J. Olfactory ensheathing glia: properties and function. Brain Res Bull. 1998;46(3):175–187. doi: 10.1016/s0361-9230(97)00463-2. [DOI] [PubMed] [Google Scholar]
- Ramon-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. 1998;18(10):3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25:425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Peles E, Trimmer JS, Levinson SR, Lux SE, Shrager P. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J Neurosci. 1999;19:7516–7528. doi: 10.1523/JNEUROSCI.19-17-07516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios JC, Melendez-Vasquez CV, Einheber S, Lustig M, Grumet M, Hemperly J, Peles E, Salzer JL. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J Neurosci. 2000;20:8354–8364. doi: 10.1523/JNEUROSCI.20-22-08354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Lankford KL, Zemedkun M, Kocsis JD. Identified olfactory ensheathing cells transplanted into the transected dorsal funiculus bridge the lesion and form myelin. J Neurosci. 2004;24:8485–8493. doi: 10.1523/JNEUROSCI.1998-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Hains BC, Lankford KL, Waxman SG, Kocsis JD. Protection of corticospinal tract neurons after dorsal spinal cord transection and engraftment of olfactory ensheathing cells. Glia. 2006a;53:352–359. doi: 10.1002/glia.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Black JA, Lankford KL, Tokuno HA, Waxman SG, Kocsis JD. Molecular reconstruction of nodes of Ranvier after remyelination by transplanted olfactory ensheathing cells in the demyelinated spinal cord. J Neurosci. 2006b;26(6):1803–1812. doi: 10.1523/JNEUROSCI.3611-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami T, Oudega M, Bater ML, Wood PM, Kleitman N, Bunge MB. Schwann ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci. 2002;22:6670–6681. doi: 10.1523/JNEUROSCI.22-15-06670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdu E, Garcia-Alias G, Fores J, Lopez-Vales R, Navarro X. Olfactory ensheathing cells transplanted in lesioned spinal cord prevent loss of spinal cord parenchyma and promote functional recovery. Glia. 2003;42:275–286. doi: 10.1002/glia.10217. [DOI] [PubMed] [Google Scholar]