Abstract
Cystic echinococosis (CE) is a public health problem caused by Echinococcus granulosus. We aimed to determine the efficacy of nitazoxanide (NTZ) and oxfendazole (OXF) against CE in naturally infected sheep. A total of 151 ewes were assigned to the following groups: 15 mg/kg of NTZ weekly for five weeks (NTZ5); two rounds of 15 mg/kg of NTZ a day for five days (NTZ5×2) two weeks apart; 30 mg/kg of OXF a week for 11 weeks (OXF11); 30 mg/kg of OXF plus 15 mg/kg of NTZ a week for 11 weeks (OXF/NTZ); and the control group. OXF11 and OXF/NTZ decreased the number of fertile cysts, increased the number of degenerated cysts, and were more efficacious (49.6–61.2%) against lung cysts and liver cysts (91.8–100%) than any other treatment group. OXF might be an additional strategy for control programs and an optional treatment of human CE after it is licensed.
INTRODUCTION
Cystic echinococosis (CE) is a zoonotic disease caused by the larval stage of Echinococcus granulosus. It is recognized as a major economic and public health problem in many areas worldwide.1–3 The intermediate hosts include sheep, goats, cattle, horses, swine, and, accidentally, humans. The larval stage frequently infects the liver or lungs, 4,5 and the cysts will expand and contain hundreds or thousands of protoscoleces (PSCs). These PSCs can develop into the adult tapeworm upon ingestion by a definitive host (dogs, wolves, or other wild canids). 6,7
Benzimidazole drugs are used to treat CE in humans either prior to or when surgery is not an option because of extreme age, pregnancy, presence of multiple cysts, or cysts are in locations that are surgically risky. 8,9 Albendazole (ABZ) is the drug of choice for perioperative prophylaxis and treatment of inoperable human cases. However prolonged, repeated, high doses of ABZ are often necessary and result in cure rates approaching only 30%. 10,11
Oxfendazole (OXF) has broad-spectrum activity against inhibited larval stages of gastrointestinal roundworms, tapeworms, and lungworms in many animal species. 12 A single dose of OXF at 30 mg/kg treats infection in Taenia solium cysticercosis in pigs. 13 Other studies have demonstrated the efficacy of OXF against CE in animals, specifically sheep and goats, demonstrating efficacies greater than 90% when administered in doses of 30 mg/kg. 4,14 When given to sheep and goats for hydatidosis, OXF is at least as effective as ABZ and is easier to administer. 14 The mean percentage of dead PSCs has been reported to be 99%, 93%, and 68% in sheep treated with OXF daily, weekly, and monthly, respectively.4
Nitazoxanide (NTZ) is another broad-spectrum drug with activity against a wide variety of intestinal parasites in animals and humans. There is in vitro and in vivo (mice) evidence to support the efficacy of NTZ against metacetodes of E. multilocularis15 and E. granulosus.16 Additionally, combined treatment with NTZ and ABZ against E. multilocularis cysts had better efficacy than did ABZ alone. 16
There have been no clinical trials in sheep to determine the efficacy of NTZ or of the combination of OXF and NTZ, although sheep are the most important intermediate host for E. granulosus. Thus, the goals of the present study were to determine and compare the effect of NTZ and OXF given either singly or in combination for treatment of CE in naturally infected sheep.
MATERIALS AND METHODS
Study design
A randomized placebo-controlled trial was carried out to assess the efficacy of NTZ, OXF, and the combination of OXF and NTZ against CE in naturally infected sheep. We randomly selected 151 ewes (mean age = 4 years) from a lot of 800 sheep destined for slaughter; they were identified and assigned to one of the four treatment groups or to the control group. The ewes were slaughtered 4–5 months after initial treatment and lungs and livers were masked after collection and before evaluation. We counted the number of cysts and observed their morphologic characteristics, fertility status, and protoscolex viability to estimate the treatment efficacy. This study was reviewed and approved by the ethical review boards of the Universidad Nacional Mayor de San Marcos, School of Veterinary Medicine.
Animals
Sheep were obtained from the Tupac Amaru, a ranching cooperative located in the Peruvian Central Highlands. Local epidemiology ensured a high probability of selecting infected sheep because this area has a high prevalence (87%) of CE in sheep and humans (9.1%) as reported. 17 A total of 151 Junin-breed ewes (age range = 2–4 years) were randomly selected by applying a systematic sampling procedure in the corral from a lot of 800 sheep destined for slaughter. Each selected animal was then assigned to one of the four treatment groups or the control set until 30 ewes per group was achieved. Animals that were unable to feed by themselves were excluded during the selection process. Each sheep was weighed and identified using numbered ear tags at the beginning of the study. Animal condition was monitored prior to and throughout the experimental period. Sheep were kept in the same corral during the study period and monitored every day for side effects.
Treatment groups
The first group received 15 mg/kg body weight (BW) of NTZ once a week for five weeks (NTZ5). There have been no reports of the use of NTZ in sheep and thus we selected one of the highest described nontoxic doses used in humans (approximately 15 mg/kg). 18–20 The second group received two rounds, two weeks apart, of 15 mg/kg BW of NTZ daily for five consecutive days (NTZ5×2). The third group received 30 mg/kg BW of OXF once a week for 11 weeks (OXF11), which was reported to be effective in sheep.4 The fourth group received a combination of 30 mg/kg BW of OXF plus 15 mg/kg BW of NTZ once a week for 11 weeks (OXF/NTZ). In the latter group, OXF was administered one day before NTZ because OXF achieves its peak plasma concentration 24 hours after oral administration; 21 and NTZ reaches its peak within 1–4 hours. 22 The control group received no drug but was handled under similar conditions for 11 weeks to cause the same stress as in the other groups.
Oxfendazole was administered from a bottle of OXF suspension (22.5% solution; Synanthic, Fort Dodge, KS). Nitazoxanide in commercial capsules of 500 mg (Rommers S.A.I.C.F., Buenos Aires, Argentina) was given orally by opening the sheep’s mouths and then giving the animal approximately 30 mL of water. The animals were checked for the next 3–5 minutes to ensure that all of them had swallowed the drugs. The cost of a single 30 mg/kg dose of OXF for an average weight sheep (32 kg) was US $0.26, and a single dose of 15 mg/kg of NTZ for an average weight sheep was US $1.70.
Necropsy procedures and cyst evaluation
Animals were slaughtered 4–5 months after initial treatment in the official abattoir of the Tupac Amaru Cooperative. Lungs and livers were masked with correlative numbers before evaluation. These codes were later matched with the original ones for statistical analysis. All cysts from livers and lungs independently were counted and registered on individual sheets. We randomly sampled up to 10 cysts per organ; if ≤ 10 cysts were present, we evaluated all of them. Cysts were classified according to their macroscopic appearance and hydatid fluid characteristics. For macroscopic evaluation, we used the description reported by Dueger and Gilman4, 23 of normal, calcified, and degenerated cysts. Normal cysts had a white inner layer filled with clear liquid, calcified cysts had a calcareous deposit in the inner wall denoted calcified cysts, and degenerated cysts had a blackened wall filled with turbid or purulent liquid and had a degraded membrane. Hydatid fluid was classified as clear, opaque, yellow, purulent, bloody, or absent in this study.
Fertility was assessed for the fluid of each evaluated hydatid cyst. Hydatid fluid was placed in a 50-mL Falcon tube (BD Biosciences, San Jose, CA) and gravity sedimented for 30 minutes. Sediment of each cyst was observed under a light microscope to look for PSCs. Cysts with PSCs were defined as fertile, otherwise they were infertile and not considered for viability analysis. The cyst fertility percentage was calculated for each group (number of fertile cysts/total cysts). Protoscolex viability was performed using a 0.1% aqueous eosin vital stain for lung and liver cysts separately. 24 Dead PSCs stain red and those that are alive exclude the dye and remain clear. The percentage of live PSCs divided by the total PSCs (live PSCs/total PSCs) was used to calculate the percent protoscolex viability. The PSC viability (%) was estimated for each animal, using all fertile cysts from lungs and liver independently, and then averaged with the results from all individuals in the group to obtain the mean aggregate of PSC viability for each group.
Data analysis
Weight differences of treatment groups as compared with the control group were established by the two-sample mean comparison test at the beginning and at the end of the study. Weight gain was also evaluated by using the same test. The Kruskall-Wallis test was used to estimate the difference in the number of cysts between treatment groups. The chi-square test and the two-sample test of proportion were used to evaluate the presence of cysts with fluid and type of hydatid fluid among the groups. The chi-square test was also used to assess the association of fertility and degenerated cysts with treatment groups, and logistic regression models were used to estimate the odds of having degenerated or fertile cysts taking treatment and organ as the independent variables. Efficacy (%) was estimated as the PSC viability difference of the control group compared with any treated group and divided by the PSC viability of the control group for lung and liver independently. For all the analyses, the significant differences were set to less than 0.05. Percentages are presented with their 95% confidence interval.
RESULTS
This study included 151 Junin bred sheep, whose mean age was four years. Of the original 151 animals, only 121 were examined at the slaughterhouse. Thirty sheep were lost to non-treatment–related causes (other infectious diseases and accidents). These 30 lost animals were equally distributed among the groups, as shown in Table 1. No side effects were observed during this trial. All five treatment groups were similar in weight at the beginning of the study. However, the weight gain was significantly higher (> 3 kg) for sheep treated with OXF alone (OXF11) than in the control group (P < 0.05) (Table 1).
Table 1.
Group-average weight (kg) of sheep at the beginning and at the end of the trial and mean-weight gain (kg) by treatment group*
| Beginning |
Ending |
Weight gain, kg |
||||
|---|---|---|---|---|---|---|
| Treatment group | No. | Mean ± SD | No. | Mean ± SD | Mean ± SD | No. animals lost |
| NTZ5 | 30 | 32.7 ± 5.5a | 23 | 38.3 ± 6.2b | 6.2 ± 7.7d | 7 |
| NTZ5×2 | 30 | 30.7 ± 6.3a | 24 | 37.5 ± 6.1b | 7.3 ± 5.2d | 6 |
| OXF11 | 30 | 32.7 ± 4.9a | 23 | 41.9 ± 7.5† | 9.6 ± 6.2† | 7 |
| OXF/NTZ | 31 | 33.2 ± 5.3a | 26 | 41.8 ± 5.9† | 8.5 ± 6.9d | 5 |
| Control | 30 | 32.6 ± 4.1a | 25 | 38.3 ± 4.0b | 6.0 ± 5.1d | 5 |
NTZ = nitazoxanide; OXF = oxfendazole. Identical superscript letters (a, b, and d) indicate no significant difference (P > 0.05).
Value is significantly different from that of the control group (P < 0.05).
SD = standard deviation.
All except one animal (99.2%, 120 of 121) had at least one hydatid cyst in liver, lung, or both organs. The percentage of dual infection (lung and liver) was 86.8% (105 of 121). In the 121 sheep, the total number of cysts were 1,998 (mean = 16.5 cysts/animal), of which 1,414 were pulmonary cysts and 584 were hepatic cysts with a lung-to-liver ratio of 2.4:1. As shown in Table 2 although there were fewer lung and liver cysts in the treatment groups than in the control group, this difference was not statistically significant. Of the 1,998 cysts in the entire group, we only were able to evaluate 1,082 (606 pulmonary cysts and 476 hepatic cysts) because of logistic and laboratory facility constraints in the field.
Table 2.
Total number of cysts and group median by affected organ and treatment group*
| Lung cysts† |
Liver cysts‡ |
Total cysts§ |
||||
|---|---|---|---|---|---|---|
| Treatment group (no.) | Total | Median | Total | Median | Total | Median |
| NTZ5 (23) | 280 | 7 | 120 | 2 | 400 | 11 |
| NTZ5×2 (24) | 413 | 7 | 98 | 3 | 511 | 11 |
| OXF11 (23) | 147 | 4 | 115 | 3 | 262 | 8 |
| OXF/NTZ (26) | 154 | 4 | 91 | 3 | 245 | 8 |
| Control (25) | 420 | 6 | 160 | 4 | 580 | 11 |
NTZ = nitazoxanide; OXF = oxfendazole.
Treatment groups for lung cysts were not statistically different from the control group (P > 0.05).
Treatment groups for liver cysts were not statistically different from the control group (P > 0.05).
Treatment groups for total cysts were not statistically different from the control group (P > 0.05).
Lung cyst evaluation
There were 116 animals with lung cysts, and from those sheep only 30 (25.8%) had fertile cysts at the post-treatment evaluation, most belonging to the control group (n = 11) (Table 3). There was a statistical association of fertile cysts with treatment groups as demonstrated by the chi-square test (P < 0.01), with lower proportion of fertility in the OXF11 group and the combination OXF/NTZ group. We found four sheep with non-viable PSC in lungs: 2 (8.7%) of 23 in the OXF11 group, one (3.8%) of 26 in the OXF/NTZ group, and 1 (4%) of 25 in the control group. Hydatid fluid was pooled from all fertile lung cysts for each animal to obtain individual PSC viability. We found that the OXF11 and OXF/NTZ groups had 20% and 26% of PSC viability, respectively; the control group had 51.6%. Groups treated with NTZ alone had an even higher PSC viability than the control group (Table 3). The efficacies of OXF11 and the combination of OXF/NTZ in reducing PSC viability from lung cysts were 61.2% and 49.6%, respectively. A significantly greater percentage of lung cysts with no fluid was observed in group OXF/NTZ than in the control group (53% versus 37%; P < 0.05).
Table 3.
Number of fertile cysts, proportion of cyst fertility, and average of protoscolex viability (%) by treatment group for lung cysts*
| Treatment group | FC/CF (%)† | No.‡ | Average PSC viability (%)§ |
|---|---|---|---|
| NTZ5 | 21/127 (17) | 6 | 62.2 |
| NTZ5×2 | 14/106 (13)¶ | 3 | 53.7 |
| OXF11 | 6/107 (6)¶ | 4 | 20 |
| OXF/NTZ | 10/105 (9)¶ | 6 | 26.0 |
| Control | 39/155 (25) | 11 | 51.6 |
NTZ = nitazoxanide; OXF = oxfendazole. Result of chi-square test for the association of treatment groups with cyst fertility was statistically significant (P < 0.01).
No. of fertile cysts (FC)/cyst with hydatid fluid (CF) and fertility percentages (%) for each treatment group.
No. of sheep with at least one fertile cyst per treatment group.
Group mean percentage of protoscolex (PSC) viability estimated from total number of animals with fertile cysts.
Proportions of fertile cysts were statistically lower in NTZ5×2, OXF11, and OXF/NTZ groups than in the control group (P < 0.05).
Liver cyst evaluation
There were 107 sheep with liver cysts, and from those animals 21 (19.6%) had fertile cysts. Only 1 of 23 animals in the OXF11 group had fertile liver cysts with 4% PSC viability. In the OXF/NTZ group, 3 of 26 animals had fertile liver cysts, and none contained viable PSC; the control group showed a PSC viability of 48.9% in 9 of 25 animals (Table 4). We also found three other sheep with no viable PSC; two in the NTZ5 group and one in the control group. The efficacies of the OXF11 and OXF/NTZ groups against liver cysts were 91.8% and 100%, respectively. The NTZ5 group had a PSC viability of 18.5% and an efficacy of 62% for liver cysts (Table 4). Liver cyst fertility was statistically associated with treatment groups with a significantly lower proportion of cyst fertility for the OXF11, NTZ5×2, and OXF/NTZ groups than in the control group (P < 0.05). A significantly greater percentage of liver cysts with no fluid were observed in the OXF11 group than in the controls (72% versus 50%; P < 0.05).
Table 4.
Number of fertile cysts, proportion of cyst fertility and average of protoscolex viability (%) by treatment group for liver cysts*
| Treatment group | FC/CF (%)† | No.‡ | Average PSC viability (%)§ |
|---|---|---|---|
| NTZ5 | 19/83 (23) | 4 | 18.5 |
| NTZ5×2 | 6/78 (8)¶ | 3 | 44.7 |
| OXF11 | 6/108 (6)¶ | 1 | 4 |
| OXF/NTZ | 5/85 (6)¶ | 3 | 0 |
| Control | 26/120 (22) | 9 | 48.9 |
NTZ = nitazoxanide; OXF = oxfendazole. Result of chi-square test for the association of treatment groups with cyst fertility was statistically significant (P < 0.01).
No. of fertile cysts (FC)/cyst with hydatid fluid (CF) and fertility percentages (%) for each treatment group.
No. of sheep with at least one fertile cyst per treatment group.
Group mean percentage of protoscolex (PSC) viability estimated from total number of animals with fertile cysts.
Proportions of fertile cysts were significantly lower in the NTZ5×2, OXF11, and OXF/NTZ groups than the control group (P < 0.05).
Logistic models demonstrated that the OXF11 and OXF/NTZ groups were 81% and 72% less likely to have fertile cysts than the controls after adjusting for organ type (OROXF11 = 0.19, OROXF/NTZ = 0.28, P < 0.05, respectively). The logistic regression analysis also showed that the OXF11 and OXF/NTZ groups were 5.34 and 4.58 times more likely to show degenerated cysts (including calcified cysts) than the control group after adjusting for organ type (OROXF11 = 5.34, OROXF/NTZ = 4.58, P < 0.01) (Table 5, Figures 1 and 2).
Table 5.
Odds ratios for cyst fertility and degenerated cysts among the treatment groups and organs (lung and liver) using logistic regression models*
| Cyst fertility |
Degenerated cysts |
|||
|---|---|---|---|---|
| Treatment group | Adjusted OR | 95% CI | Adjusted OR | 95% CI |
| Control | 1.00 | – | 1.00 | – |
| NTZ5 | 0.73 | 0.19–2.86 | 2.17 | 0.74–6.30 |
| NTZ5×2 | 0.39 | 0.11–1.46 | 1.11 | 0.38–3.52 |
| OXF11 | 0.19 | 0.05–0.73† | 5.34 | 1.89–15.13† |
| OXF/NTZ | 0.28 | 0.09–0.83† | 4.58 | 1.51–13.91† |
| Lung | 1.00 | – | 1.00 | – |
| Liver | 0.91 | 0.58–1.44 | 0.93 | 0.61–1.42 |
OR = odds ratio; CI = confidence interval; NTZ = nitazoxanide; OXF = oxfendazole.
Significantly different from control group (P < 0.05).
Figure 1.
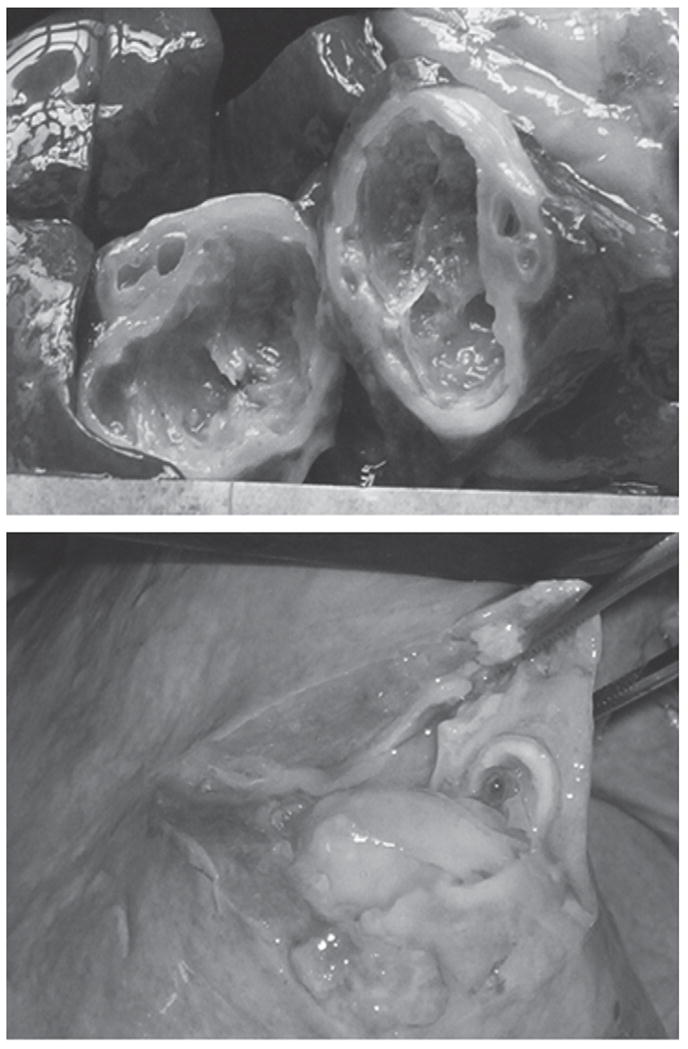
Degenerated hydatid cyst in liver (above) and purulent content of hydatid lung cyst (below) of sheep treated with oxfendazole alone for 11 consecutive weeks at a dose of 30 mg/kg of body weight.
Figure 2.
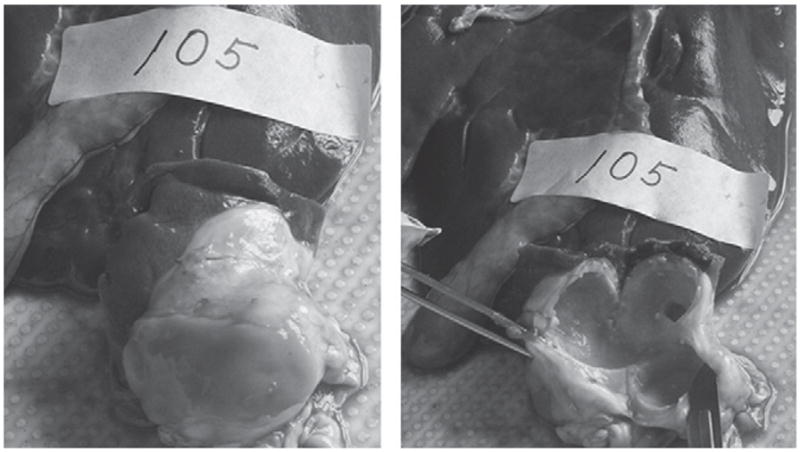
Liver hydatid cyst of a sheep treated with oxfendazole (30 mg/kg) and nitazoxanide (15 mg/kg) weekly for 11 consecutive weeks. Shown are an abscess in the liver (right) and the cavity of the liver hydatid cyst (left).
DISCUSSION
Sheep with CE treated with OXF alone (OXF11) or in combination with NTZ (OXF/NTZ) showed a decrease in the number of fertile cysts and an increase in the number of degenerated cysts. OXF11 and OXF/NTZ were more efficacious in killing protoscoleces in a range of 49.6% to 61.2% for lung cysts and between 91.8% and 100% for liver cysts than any other treatment group and control. In contrast, NTZ when used alone was not effective against hydatid cysts. In our study, lung cysts were more resistant to OXF than liver cysts. From a production standpoint, sheep treated with OXF alone gained more than 3 kg than controls.
No side effects were detected in any of the treatment groups, which suggested that the dose levels of NTZ and OXF used in this trial are safe in sheep. The OXF dose of 30 mg/kg was selected on the basis of its efficacy against Taenia solium cysticercosis in pigs 13 and treatment of sheep for hydatid cysts. 4,14 However, the 15 mg/kg BW dose of NTZ was selected on the basis of the highest described nontoxic dose used in humans 18–20 and a trial performed in pigs. 25 It is crucial to point out that the conclusions of our study are limited to ruminants because OXF blood levels may be different in monogastric animals. The half-life and the area under the curve are likely to be much shorter in humans, in the same way as multigastric animals have a much longer albendazole/albendazole sulfoxide half-life. However, our results and a previous one4 open the possibility of evaluating OXF as a candidate for human hydatidosis, but it will need to wait until it is proven safe and efficacious for humans.
Chemotherapy in sheep might be an interesting addition to other control measures for elimination of CE, such as dog praziquantel-treatment, education, livestock veterinary inspection, and sheep vaccination. 26 Treatment of dogs will stop new infections of sheep but will not affect the reservoir of cysts present in previously infected sheep. 27 Conversely, sheep hydatidosis can be prevented by using a highly effective vaccine 28 but to date it has not been used in any national control program because of cost and logistics. It has been also estimated that infection acquired before sheep vaccination can still be transmitted to dogs, and it would take up to 15 years before all cyst reservoir (infected sheep) disappear from a specific herd. 27 Oxfendazole might, indirectly, decrease the number of dogs infected or decrease the worms in a susceptible dog by killing protoscoleces from lung and liver cysts in already infected sheep. The long half-life of OXF and its prolonged presence in sera (up to seven days after administration) make this drug one of the best candidates for treatment of hydatidosis in sheep. 21,29,30 Intermediate host interventions, such as vaccination and treatment with OXF, would reduce the time for reaching the attack and consolidation phases within a control program.
It was not clear how protoscolex death occurs with OXF therapy in this study. We suspect that OXF may damage the cyst wall, which enables the host immune response to be mounted against the cyst and its contents as described. 31 Infiltration of cells (neutrophils, eosinophils, and plasma cells) may lead to changes in cyst fluid and later degeneration, 14 altering the parasite-host equilibrium. 32,33 However, the degenerated/calcified cysts observed in the control group may be explained by the natural history of cyst involution that is also driven by individual immune responses. 34
Sheep treated with OXF alone gained 3 kg more than those sheep in the control group, an additional advantage that should be taken into account for cost-effectiveness analysis. The additional weight gain in treated animals is probably caused by the broad spectrum of activity against nematodes and tapeworms that OXF exhibits. 21 For instance, OXF has been reported to be effective against Moniezia sp. and Dyctiocaulus sp. in ruminants, 35–37 parasites that are commonly found in the Central Peruvian Highlands. Blanton and others 14 also observed an increase in weight in sheep and goats treated with 30 mg/kg of OXF twice a week for one month.
The CE prevalence of 99.2% (120 of 121) is higher than those in other studies in the Peruvian Highlands with rates of 87% 17 and 77% 23 in sheep. Our study also confirms the preponderance of lung lesions (lung-to-liver ratio = 2.4:1), as noted in studies in the Peruvian Andes. 17,23 This finding is not well explained because the liver has been reported to work as the main filter for oncospheres. 38 However, pulmonary shunting caused by high altitude may enable more oncospheres to reach the lung. Human infections at high altitude in Peru has more lung cysts than liver cysts in contrast to a preponderance of cysts in the liver compared with the lung in other South American countries (Argentina, Chile, and Uruguay) endemic for Echinococcus with ratios between 1:3 and 1:13. 39,40
One of the study limitations might be the definition of viable cyst, actually the desire long term, dead or inactive. We calculated protoscolex viability as a surrogate of cyst viability as many other investigators have done. However, the absence of living protoscoleces in a cyst does not necessarily mean the cyst is dead or inactive. It could also mean that the germinal layer is not producing protoscoleces at that time. Therefore, for a cyst to be non-viable, one would need to demonstrate that the cyst is incapable of re-establishing the infection for a specific period of time, or to show histologically the complete degeneration of the membrane. In the same context, we evaluated the animals only 4–8 weeks after treatment. Thus, we do not know whether the effect of the drugs is permanent or whether parasites that have survived treatment will reactivate the cyst in the future. A trial that evaluated PZQ and ABZ in sheep waited up to seven months before slaughtering the animals to enable any residual live parasites to recover. 41
There was a bias in our study to evaluate proportionally more liver cysts than pulmonary cysts because we restricted our study to 10 randomly selected cysts/organ. There were often many more lung cysts not evaluated because of their increased number compared with liver cysts. For this reason, we performed the analysis independently, lung and liver, to avoid any missinterpretation of the findings.
Certainly most, if not all, of the effect against hydatid cysts is attributable to OXF in this trial. We demonstrate that OXF may be a successful agent that can be added to current control measures to interrupt transmission of E. granulosus. The study also demonstrates the potential for this agent to be evaluated for treatment of human CE. Although OXF was highly effective, the addition of NTZ to the treatment regimen had no effect on efficacy.
Acknowledgments
We thank Sarah Marcus for help in English editing and Sari Cece for support.
Financial support: This study was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health Tropical Medicine Research Centers Program grant “New Tools to Understand and Control Endemic Parasites” (1 P01 AI51976), the Infectious Diseases Training Program in Peru (3 D43 TW006581), and the ICIDR (U01 AI35894).
References
- 1.Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckert J, Conraths FJ, Tackmann K. Echinococcosis: an emerging or re-emerging zoonosis? Int J Parasitol. 2000;30:1283–1294. doi: 10.1016/s0020-7519(00)00130-2. [DOI] [PubMed] [Google Scholar]
- 3.Schantz PM. Parasitic zoonoses in perspective. Int J Parasitol. 1991;21:161–170. doi: 10.1016/0020-7519(91)90006-s. [DOI] [PubMed] [Google Scholar]
- 4.Dueger EL, Moro PL, Gilman RH. Oxfendazole treatment of sheep with naturally acquired hydatid disease. Antimicrob Agents Chemother. 1999;43:2263–2267. doi: 10.1128/aac.43.9.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris DL, Clarkson MJ, Stallbaumer MF, Pritchard J, Jones RS, Chinnery JB. Albendazole treatment of pulmonary hydatid cysts in naturally infected sheep: a study with relevance to the treatment of hydatid cysts in man. Thorax. 1985;40:453–458. doi: 10.1136/thx.40.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pawlowski ZS, Eckert J, Vuitton DA, Ammann R, Kern P, Craig PS, Dar FK, De Rosa F, Filice C, Gottstein B, Grimm F, Macpherson CN, Sato N, Todorov T, Uchino J, von Sinner W, Wen H. Echinococcosis in humans: clinical aspects, diagnosis and treatment. In: Gemmell JE, Mal JR, Roberts MG, Meslin F, Pawlowski Z, editors. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Paris: World Health Organization; 2001. pp. 20–66. [Google Scholar]
- 7.Thompson R, McManus DP. Aetiology: parasites and life-cycles. In: Eckert J, Gemmell MA, Meslin F, Pawlowski Z, editors. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Paris: World Health Organization; 2001. pp. 1–19. [Google Scholar]
- 8.Pawlowski Z, Eckert J, Vuitton DA, Ammann R, Kem P, Craig PS, Diaz KF, De Rosa F, Filice C, Gottstein B, Grimm F, Macpherson CN, Sato N, Todorov T, Uchino J, von Sinner W, Wen H. Echinococcosis in humans: clinical aspects, diagnosis and treatment. In: Eckert J, Gemmell MA, Meslin F, Pawlowski Z, editors. WHO/OIE Manual on Echinococcosis in Humans and Animals: a Public Health Problem of Global Concern. Paris: World Health Organization; 2002. pp. 20–71. [Google Scholar]
- 9.Mohamed AE, Yasawy MI, Al Karawi MA. Combined albendazole and praziquantel versus albendazole alone in the treatment of hydatid disease. Hepatogastroenterology. 1998;45:1690–1694. [PubMed] [Google Scholar]
- 10.Horton RJ. Albendazole in treatment of human cystic echinococcosis: 12 years of experience. Acta Trop. 1997;64:79–93. doi: 10.1016/s0001-706x(96)00640-7. [DOI] [PubMed] [Google Scholar]
- 11.Horton RJ. Chemotherapy of Echinococcus infection in man with albendazole. Trans R Soc Trop Med Hyg. 1989;83:97–102. doi: 10.1016/0035-9203(89)90724-4. [DOI] [PubMed] [Google Scholar]
- 12.Marriner SE, Bogan JA. Pharmacokinetics of oxfendazole in sheep. Am J Vet Res. 1981;42:1143–1145. [PubMed] [Google Scholar]
- 13.Gonzalez AE, Falcon N, Gavidia C, Garcia HH, Tsang VC, Bernal T, Romero M, Gilman RH. Treatment of porcine cysticercosis with oxfendazole: a dose-response trial. Vet Rec. 1997;141:420–422. doi: 10.1136/vr.141.16.420. [DOI] [PubMed] [Google Scholar]
- 14.Blanton RE, Wachira TM, Zeyhle EE, Njoroge EM, Magambo JK, Schantz PM. Oxfendazole treatment for cystic hydatid disease in naturally infected animals. Antimicrob Agents Chemother. 1998;42:601–605. doi: 10.1128/aac.42.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stettler M, Fink R, Walker M, Gottstein B, Geary TG, Rossignol JF, Hemphill A. In vitro parasiticidal effect of nitazoxanide against Echinococcus multilocularis metacestodes. Antimicrob Agents Chemother. 2003;47:467–474. doi: 10.1128/AAC.47.2.467-474.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker M, Rossignol JF, Torgerson P, Hemphill A. In vitro effects of nitazoxanide on Echinococcus granulosus protoscoleces and metacestodes. J Antimicrob Chemother. 2004;54:609–616. doi: 10.1093/jac/dkh386. [DOI] [PubMed] [Google Scholar]
- 17.Moro PL, McDonald J, Gilman RH, Silva B, Verastegui M, Malqui V, Lescano G, Falcon N, Montes G, Bazalar H. Epidemiology of Echinococcus granulosus infection in the central Peruvian Andes. Bull World Health Organ. 1997;75:553–561. [PMC free article] [PubMed] [Google Scholar]
- 18.Stockis A, De Bruyn S, Gengler C, Rosillon D. Nitazoxanide pharmacokinetics and tolerability in man during 7 days dosing with 0.5 g and 1 g b.i.d. Int J Clin Pharmacol Ther. 2002;40:221–227. doi: 10.5414/cpp40221. [DOI] [PubMed] [Google Scholar]
- 19.Davila-Gutierrez CE, Vasquez C, Trujillo-Hernandez B, Huerta M. Nitazoxanide compared with quinfamide and mebendazole in the treatment of helminthic infections and intestinal protozoa in children. Am J Trop Med Hyg. 2002;66:251–254. doi: 10.4269/ajtmh.2002.66.251. [DOI] [PubMed] [Google Scholar]
- 20.Rossignol JF, Abaza H, Friedman H. Successful treatment of human fascioliasis with nitazoxanide. Trans R Soc Trop Med Hyg. 1998;92:103–104. doi: 10.1016/s0035-9203(98)90974-9. [DOI] [PubMed] [Google Scholar]
- 21.Soraci AL, Mestorino N, Errecalde JO. Some pharmacokinetic parameters of oxfendazole in sheep. Vet Res Commun. 1997;21:283–287. doi: 10.1023/a:1005843010696. [DOI] [PubMed] [Google Scholar]
- 22.Parashar A, Arya R. Nitazoxanide. Indian Pediatr. 2005;42:1161–1165. [PubMed] [Google Scholar]
- 23.Dueger EL, Gilman RH. Prevalence, intensity, and fertility of ovine cystic echinococcosis in the central Peruvian Andes. Trans R Soc Trop Med Hyg. 2001;95:379–383. doi: 10.1016/s0035-9203(01)90188-9. [DOI] [PubMed] [Google Scholar]
- 24.Himonas C, Antoniadou-Sotiriadou K, Papadopoulos E. Hydatidosis of food animals in Greece: prevalence of cysts containing viable protoscoleces. J Helminthol. 1994;68:311–313. doi: 10.1017/s0022149x00001541. [DOI] [PubMed] [Google Scholar]
- 25.Theodos CM, Griffiths JK, D’Onfro J, Fairfield A, Tzipori S. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob Agents Chemother. 1998;42:1959–1965. doi: 10.1128/aac.42.8.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craig PS, Larrieu E. Control of cystic echinococcosis/hydatidosis: 1863–2002. Adv Parasitol. 2006;61:443–508. doi: 10.1016/S0065-308X(05)61011-1. [DOI] [PubMed] [Google Scholar]
- 27.Heath D, Yang W, Li T, Xiao Y, Chen X, Huang Y, Yang Y, Wang Q, Qiu J. Control of hydatidosis. Parasitol Int. 2006;55(Suppl):S247–S252. doi: 10.1016/j.parint.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 28.Lightowlers MW, Lawrence SB, Gauci CG, Young J, Ralston MJ, Maas D, Health DD. Vaccination against hydatidosis using a defined recombinant antigen. Parasite Immunol. 1996;18:457–462. doi: 10.1111/j.1365-3024.1996.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 29.Lanusse CE, Gascon LH, Prichard RK. Comparative plasma disposition kinetics of albendazole, fenbendazole, oxfendazole and their metabolites in adult sheep. J Vet Pharmacol Ther. 1995;18:196–203. doi: 10.1111/j.1365-2885.1995.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 30.Todorov T, Vutova K, Donev S, Ivanov A, Katzarov K, Takov D. The types and timing of the degenerative changes seen in the cysts during and after benzimidazole treatment of cystic echinococcosis. Ann Trop Med Parasitol. 2005;99:649–659. doi: 10.1179/136485905X65125. [DOI] [PubMed] [Google Scholar]
- 31.Urrea-Paris MA, Moreno MJ, Casado N, Rodriguez-Caabeiro F. Relationship between the efficacy of praziquantel treatment and the cystic differentiation in vivo of Echinococcus granulosus metacestode. Parasitol Res. 2002;88:26–31. doi: 10.1007/s004360100468. [DOI] [PubMed] [Google Scholar]
- 32.Teggi A, Lastilla MG, De Rosa F. Therapy of human hydatid disease with mebendazole and albendazole. Antimicrob Agents Chemother. 1993;37:1679–1684. doi: 10.1128/aac.37.8.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menezes da Silva A. Hydatid cyst of the liver-criteria for the selection of appropriate treatment. Acta Trop. 2003;85:237–242. doi: 10.1016/s0001-706x(02)00271-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Li J, McManus DP. Concepts in immunology and diagnosis of hydatid disease. Clin Microbiol Rev. 2003;16:18–36. doi: 10.1128/CMR.16.1.18-36.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kern P. Echinococcus granulosus infection: clinical presentation, medical treatment and outcome. Langenbecks Arch Surg. 2003;388:413–420. doi: 10.1007/s00423-003-0418-y. [DOI] [PubMed] [Google Scholar]
- 36.Reinemeyer CR, Courtney CH. Fármacos contra cestodos y trematodos. In: Adams HR, editor. Farmacología y Terapéutica Veterinaria. Zaragosa, Spain: Editorial Acribia; 2003. pp. 1049–1061. [Google Scholar]
- 37.Hemphill A, Mueller J, Esposito M. Nitazoxanide, a broad-spectrum thiazolide anti-infective agent for the treatment of gastrointestinal infections. Expert Opin Pharmacother. 2006;7:953–964. doi: 10.1517/14656566.7.7.953. [DOI] [PubMed] [Google Scholar]
- 38.García JL, Álvarez AI, Redondo PA, Prieto JG. Estudio de la fertilidad y viabilidad de quistes hidatídicos ovinos. Rev Esp Salud Publica. 1997;71:445–449. [PubMed] [Google Scholar]
- 39.Moro PL, Bonifacio N, Gilman RH, Lopera L, Silva B, Takumoto R, Verastegui M, Cabrera L. Field diagnosis of Echinococcus granulosus infection among intermediate and definitive hosts in an endemic focus of human cystic echinococcosis. Trans R Soc Trop Med Hyg. 1999;93:611–615. doi: 10.1016/s0035-9203(99)90068-8. [DOI] [PubMed] [Google Scholar]
- 40.Purriel P, Schantz PM, Beovide H, Mendoza G. Human echinococcosis (hydatidosis) in Uruguay: a comparison of indices of morbidity and mortality, 1962–71. Bull World Health Organ. 1973;49:395–402. [PMC free article] [PubMed] [Google Scholar]
- 41.Morris DL, Richards KS, Clarkson MJ, Taylor DH. Comparison of albendazole and praziquantel therapy of Echinococcus granulosus in naturally infected sheep. Vet Parasitol. 1990;36:83–90. doi: 10.1016/0304-4017(90)90096-t. [DOI] [PubMed] [Google Scholar]


