Abstract
Purpose:
Poxviral vectors have a proven safety record and can be used to incorporate multiple transgenes. Prior clinical trials with poxviral vaccines have shown that immunologic tolerance to self-antigens can be broken. Carcinoembryonic antigen (CEA) and MUC-1 are overexpressed in a substantial proportion of common solid carcinomas. The primary endpoint of this study was vaccine safety, with immunologic and clinical responses as secondary endpoints.
Experimental Design:
We report here a pilot study of 25 patients treated with a poxviral vaccine regimen consisting of the genes for CEA and MUC-1, along with a triad of costimulatory molecules (TRICOM, composed of B7.1, ICAM-1, and LFA-3) engineered into vaccinia (PANVAC-V) as a prime and fowlpox (PANVAC-F) as booster vaccinations.
Results:
The vaccine was well-tolerated. Apart from injection-site reaction, no grade II or greater toxicity was seen in more than 2% of the cycles. Immune responses to MUC-1 and/or CEA were seen following vaccine in 9 of 16 patients tested. A patient with clear cell ovarian cancer and symptomatic ascites had a durable (18-month) clinical response radiographically and biochemically, and one breast cancer patient had a confirmed decrease of > 20% in the size of large liver metastasis.
Conclusions:
This vaccine strategy appears to be safe, is associated with both CD8 and CD4 immune responses, and has shown evidence of clinical activity. Further trials with this agent, either alone or in combination with immunopotentiating and other therapeutic agents, are warranted.
Keywords: Immunotherapy, clinical trial, ovarian cancer, immune monitoring, poxviral vector
Introduction
Carcinoembryonic antigen (CEA) is overexpressed in the vast majority of gastrointestinal cancers and in a substantial proportion of lung, breast, and other types of carcinoma. MUC-1, another tumor-associated antigen (TAA), is overexpressed in the vast majority of gastrointestinal, lung, breast, and ovarian cancers. An effective vaccine that targets both of these TAAs could be a therapeutic agent for a wide array of common solid tumors. Previous clinical trials employing vaccines directed against MUC-1 or CEA individually have shown safety and ability to generate immune responses (1-7).
We have incorporated these 2 TAAs in a poxviral vaccine strategy. Preclinical and clinical studies have shown that immune responses to TAAs encoded by vaccinia plateau after 1 or 2 vaccinations due to neutralizing antibodies (8, 9). However, avipox vectors such as fowlpox are replication-defective and do not make viral coat proteins within mammalian cells. They therefore induce little to no neutralizing antibody response, allowing for a progressively better immune response to TAAs encoded by the vector (4). Poxviral vectors containing TAA have been shown to overcome immunologic tolerance to self-antigens. Two vectors, vaccinia and fowlpox, have been engineered to express both CEA and MUC-1, with a single amino acid substitution in each gene designed to make the gene product more immunogenic (5, 6). The use of agonist epitopes within the TAA has been associated with clinical responses (7, 10, 11). Vectors directed against multiple TAAs may evoke additive or synergistic immune responses and could play an important role in overcoming antigenic escape variance.
These vectors have also been engineered to express a triad of human T-cell costimulatory molecules called TRICOM, which is composed of B7.1, ICAM-1, and LFA-3. Preclinically, TRICOM vectors have been shown to generate higher numbers of TAA-specific T cells and to greatly increase the avidity of those cells (12). These high-avidity T cells can efficiently kill tumor cells, which translates into greater antitumor responses than with the identical vaccine strategy without TRICOM (13). These vaccines are given in a diversified prime-and-boost strategy that has proven to be superior to single vector strategies at generating immune responses, which may translate into improved clinical responses (4, 14, 15). In addition, each vaccine is given with granulocyte-macrophage colony-stimulating factor (GM-CSF), which in previous studies has not been associated with significant toxicity, and has been shown in numerous preclinical and clinical trials to enhance primary immune responses due to enhanced antigen-presenting cell (APC) efficiency (4, 16-19). The dose, route, and schedule of GM-CSF are designed to induce migration of dendritic cells (DCs) to the vaccine site and subsequent maturation of the DCs.
Previous studies have shown that poxviral vaccine strategies can be used safely in patients with advanced cancer, can overcome immunologic tolerance, and have been associated with clinical benefit in some patients (7, 8, 11). Here we report a pilot study of 25 patients treated with a poxviral vaccine consisting of genes for the TAAs CEA and MUC-1, along with TRICOM (designated PANVAC). Patients were vaccinated with PANVAC engineered into recombinant vaccinia (PANVAC-V) as a prime and recombinant fowlpox (PANVAC-F) as multiple booster vaccinations. This represents the first published report of this vaccine. While a corporate-sponsored phase III study1 in patients with advanced pancreatic cancer treated with PANVAC vaccine as second-line therapy failed to improve survival, PANVAC vaccines have not yet been evaluated in a range of carcinomas and, perhaps more importantly, in patients with an expected survival of > 3 months. Results of the study reported here demonstrate the safety of the vaccine and development of both immunological and clinical responses in some patients.
Patients and Methods
Patient selection and trial design
Twenty-five patients with CEA- or MUC-1-expressing metastatic cancers who had progressive disease following standard chemotherapy were enrolled in a pilot trial approved by the National Cancer Institute (NCI) Institutional Review Board and conducted at the NCI. The study was designed to evaluate the safety of this regimen. Because immunologic response was an important secondary endpoint of this trial, with the ELISPOT assay as the readout, all patients after the initial 9 enrolled for safety were required to be HLA-A2-positive. Patients needed to be Zubrod performance status 0 or 1 and have adequate hematological, hepatic, and renal function. In addition, patients were required to have no evidence of an immunocompromised state as defined by nonreactive HIV testing, no diagnosis of altered immune function, no prior radiotherapy to > 50% of nodal groups, no prior splenectomy, and no concurrent steroid use. Prior vaccinia exposure (i.e., smallpox vaccination) was not required. Since all patients were at least 38 years old, each would have had one or more prior smallpox vaccinations.
Exclusion criteria included known allergy to eggs; history of or active skin disorders such as eczema, extensive psoriasis, varicella zoster, impetigo, or burns; history of seizures; serious intercurrent illnesses; noncutaneous malignant process; and close contact with immunocompromised individuals, individuals with the above-mentioned skin conditions, or children under 5 years of age. All patients gave written informed consent in accordance with federal, state, and institutional guidelines and the principles embodied in the Declaration of Helsinki.
Vaccine formulation and treatment plan
Both of the viral vaccine products were manufactured by Therion Biologics Corporation (Cambridge, MA) as part of a Collaborative Research and Development Agreement between Therion and the Laboratory of Tumor Immunology and Biology, NCI. Vaccines were provided by the Cancer Therapy Evaluation Program, NCI. PANVAC-V [Recombinant-Vaccinia-CEA (6D)/MUC-1(L93)/TRICOM] (NSC #727026) was prepared from virus derived from the Wyeth (New York City Board of Health) strain of vaccinia, selected for its favorable toxicity profile. PANVAC-V was constructed by inserting the genes for human CEA, MUC-1, B7.1, ICAM-1, and LFA-3 into the viral genome. PANVAC-F [Recombinant-Fowlpox-CEA (6D)/MUC-1(L93)/TRICOM] (NSC #727027) was constructed by inserting the identical transgenes into the replication-defective avian fowlpox virus. All patients received the same dose and schedule of vaccine. The priming vaccine consisted of 2 × 108 PFU of PANVAC-V administered s.c. The boosting vaccine was given on or about days 15, 29, and 43, then every 28 days while on study. Sargramostim 100 μg was given the day of each vaccine and for 3 consecutive days following. A sterile, nonadherent dressing (i.e., Telfa®) was used to cover the site.
Patients were seen at least monthly while on study. Complete interval histories, physical examinations, blood chemistries, hemograms, and serum tumor markers were obtained. All patients were evaluated for toxicity by the NCI Common Toxicity Criteria version 3 and the vaccinia toxicity grading scale previously published (8). Patients had their first restaging at approximately day 71, with subsequent restaging exams approximately every 56 days thereafter.
Collection of PBMCs
Apheresis was performed twice—prior to vaccine and around day 71. Briefly, 5 × 108–2 × 109 mononuclear cells were obtained by a single-access “4-pass” mononuclear cell procedure on the Haemonetics V50 instrument (Haemonetics Corp., Braintree, MA), during which 2.0 L of whole blood were processed at a flow rate of 70–80 mL/min. At the other monthly intervals, peripheral blood mononuclear cells (PBMCs) from 60 mL of blood were collected in heparinized tubes. The mononuclear fraction of both apheresis packs and tubes was separated by Ficoll-Hypaque density gradient separation, washed 3 times and frozen in 90% heat-inactivated human AB serum and 10% DMSO in liquid nitrogen at a concentration of 1 × 107 cells/mL until assayed.
Generation of T-cell cultures
A modification of the protocol described by Tsang et al. (6) was used to generate CEA-specific T-cell cultures. DCs were prepared using a modification of the procedure described by Sallusto et al. (20). Irradiated (3000 rad) autologous DCs were used as APCs. Autologous nonadherent cells were stimulated in the presence of autologous DCs pulsed with peptides at a concentration of 10 μg/mL at an effector:APC ratio of 10:1. Cultures were maintained for 3 days in medium containing 10% human AB serum, and 4 additional days in the same medium supplemented with 20 U/mL of recombinant human IL-2. After a 7-day in vitro stimulation (IVS) cycle, cells were restimulated as described above for a total of 2 IVS cycles. T cells were assayed at IVS-2.
A MUC-1 specific T-cell line was established from day 70 postvaccination samples from patient 22, as above. For cytokine assay, this T-cell line was used at IVS-3 and autologous DCs were used as APCs. Peptides were used at a concentration of 20 μg/mL. The 48-h culture supernatants were assayed for IFN-γ production.
Tetramer staining
The streptavidin-phycoerythrin (PE)-labeled tetramers used in this study were obtained from Beckman Coulter (Fullerton, CA). All peptides used for tetramer preparation were made by Biosynthesis, Inc. (Lewisville, TX), with a purity of > 90%. CEA-tetramer (YLSGADLNL-tetramer) and HIV Gag-tetramer (SLYNTVATL-tetramer) were used in this study. PBMCs (1 × 106) were stained with 10 μL of tetramer and anti-CD8-FITC antibody (BD Biosciences, San Jose, CA) for 30 min at room temperature in the dark, followed by 2 washes with FACS buffer, then fixed in PBS with 0.5% formaldehyde. Cells were then analyzed using a FACScan and the CELLQuest program (BD Biosciences). Data gathered from 100,000 cells were stored and used to generate results.
Intracellular staining for IFN-γ
Intracellular cytokine (ICC) flow cytometry assays were performed following the method described by Maecker et al. (21). Briefly, PBMCs were thawed and rested overnight in complete RPMI-1640 growth medium (Mediatech, Inc., Herndon, VA) with 10% human AB serum. 2 × 106 PBMCs in 0.2 mL complete RPMI-1640 with 10% human AB serum were plated in 96-well round-bottom plates (Millipore Corporation, Bedford, MA). Cells were stimulated with flu peptide (1 μg/mL), HIV Gag peptide (10 μg/mL), or CAP1-6D peptide (10 μg/mL) for 2 h at 37°C. Anti-CD28 and anti-CD49d antibodies were added to all tubes. Brefeldin A (10 μg/mL) (BD Biosciences) was added and incubated for an additional 4 h at 37°C. Cells were then harvested and stained for IFN-γ-FITC/CD69-PE/CD8-PerCPCy5.5/CD3-APC using a BD FastImmune CD8 ICC detection kit (BD Biosciences). Samples were analyzed in an LSR II with FACSDiVa software (BD Biosciences). Results were expressed in percentage of CD3+/CD8+/CD69+ T cells that were IFN-γ-positive.
ELISPOT assay
Measurement of CD8 immune responses in HLA-A2-positive patients was conducted by performing an ELISPOT assay using C1R-A2 cells as APCs, as previously described (22). ELISPOT measures the frequency of T cells releasing IFN-γ in response to a CEA peptide (CAP1-6D) (YLSGADLNL), a MUC-1 native peptide (ATWGQDVTSV), an HIV gag peptide (SLYNTVATL) and a flu peptide (GILGFVFTL) in pre- and postvaccination PBMCs. A positive response was scored as ≥2 fold increase in IFN-γ secreting cells.
Measurement of CD4 antigen-specific responses
CD4+ T cells (2 × 105/well) were mixed with irradiated APCs in the presence of various concentrations of CEA peptide or CEA protein (AspenBio Pharma, Littleton, CO) in 48-well culture plates. The CD4+ CEA peptide used in this study was described by Kobayashi et al. (23). This CD4+ T-cell epitope was selected from the amino acid sequence of CEA using the algorithm tables from 3 HLA-DR alleles (DRB1*0101, DRB1*0401, and DRB1*0701) described by Southwood et al. (24). The CEA peptide selected (YACFVSNLATGRNNS) was synthesized by Bio-Synthesis, Inc. with purity > 95%. Flu protein and myoglobulin (Sigma, St Louis, MO) were used as controls. Autologous DCs were used as APCs (2 × 104/well). Culture supernatants were collected after 48 h for measurement of IFN-γ using ELISA kits.
Detection of cytokines
Supernatants of T cells stimulated for 24 h with peptide-pulsed autologous Epstein-Barr virus-transformed B cells, in IL-2-free medium at various peptide concentrations, were screened for secretion of IFN-γ using an ELISA kit (BioSource International, Camarillo, CA).
Serological analysis
To detect if antibodies were generated against CA-125, serum was collected from patient 22 prior to the first vaccination and on day 377 following a year of monthly vaccinations. These serum samples were then cryopreserved. Anti-CA-125 antibody (IgG) was quantified in the serum by FACS capture assay, as previously described (7, 25), with the following modifications: Ovarian carcinoma cells SKOV3 (CA-125+; ATCC, Manassas, VA) were preincubated with 10% normal AB serum (Gemini Bioproducts, Sacramento, CA) and then used as the capture cells following fixation and permeabilization (Cytofix/Cytoperm, BD Biosciences). Capture cells were incubated for 60 min with dilutions of patient sera or positive control mAb anti-CA-125 (clone M8072322l; Fitzgerald, Concord, MA). After washing, cells were stained with anti-IgG-FITC. Antibodies were quantified by flow cytometry analysis. Detection limit was 100 ng/mL.
Titration of serum antibodies
Anti-vaccinia and anti-fowl pox (IgG) were quantified from the serum of each patient by ELISA essentially as previously described (26). Briefly, Immunlon 4 plates (Dynex Technologies, Chantilly, VA) were coated with vaccinia virus (5 × 104 pfu/well), fowlpox virus (5 × 104 pfu/well), or DPBS (Mediatech, Herndon, VA) and held at 4°C until use. Plates were blocked with 5% BSA in PBS for 1 h at 37°C. The plates were incubated with serum serially diluted from 1:50 to 1:6250, as well as normal human serum or mouse anti-fowlpox antiserum as controls, for 24 h at room temperature. Plates were washed several times with PBS containing 1% BSA and incubated at 37°C for 1h with horseradish peroxidase-conjugated goat antihuman IgG (Fc)-specific antiserum (1:4000) or horseradish peroxidase-conjugated goat anti-mouse IgG (H&L)-specific antiserum (1:4000) for test samples or positive controls respectively. Antibody was detected by a TMB substrate kit (Pierce, Rockford, IL) according to the manufacturer's instructions. The absorbance of each well was read at 450 nm using a Bio-Tek EL310 microplate ELISA reader (Winooski, VT). Vaccinia and fowl pox antibody IgG titers were based on a blanked absorbance of 0.5 and 0.4 respectively.
Fowlpox virus neutralization
Patient serum was diluted 1:50 in DMEM 10% FBS containing 4 × 106 pfu recombinant fowlpox murine B7-1 (rF-mB7-1) and incubated for 1 h at 4°C. Normal human serum with or without rF-mB7-1 was used for controls. MC38 murine colon adenocarcinoma cells (27) (2 × 105) were added to all samples and incubated overnight at 37°C with 5% CO2. Cell surface expression of murine B7-1 was performed as previously described (28). Briefly, cells were stained with a primary PE-labeled anti-murine B7-1antibody (Becton Dickinson) and cell fluorescence was analyzed and compared with isotype-matched controls using a FACScan cytometer (Becton Dickinson).
Results
Baseline characteristics are outlined in Table 1. The median follow-up is 26 months. Patients in this study were heavily pretreated, with 9 of 25 having 3 or more prior chemotherapy regimens. The vaccine was well-tolerated (Supplementary Table S1). Apart from injection-site reaction, grade 2 or greater toxicity attributed to vaccine was seen in < 3% of vaccine cycles. During a flu-like illness that precipitated poor oral intake for 24 h, one patient had a transient witnessed syncope. On subsequent readministration of the vaccine alone on an inpatient basis, no hypotension, presyncope, or other systemic symptoms were observed in this patient.
Table 1.
Patient data
| Patient baseline characteristics Median age 57 (range 35-70) Gender |
No. of patients |
|---|---|
| Female | 8 |
| Male | 17 |
| Performance status | |
| ECOG 0 | 9 |
| ECOG 1 | 16 |
| Prior therapy | |
| Chemotherapy (1 regimen) | 7 |
| Chemotherapy (2 regimens) | 9 |
| Chemotherapy (≥3 regimens) | 9 |
| Radiation | 5 |
| Metastectomy | 9 |
| Primary tumor | |
| Colorectal | 10 |
| Gastric | 3 |
| Pancreatic | 2 |
| Appendiceal | 2 |
| Esophageal adenocarcinoma | 1 |
| Ovarian | 3 |
| Breast | 2 |
| Lung | 2 |
Immune outcomes
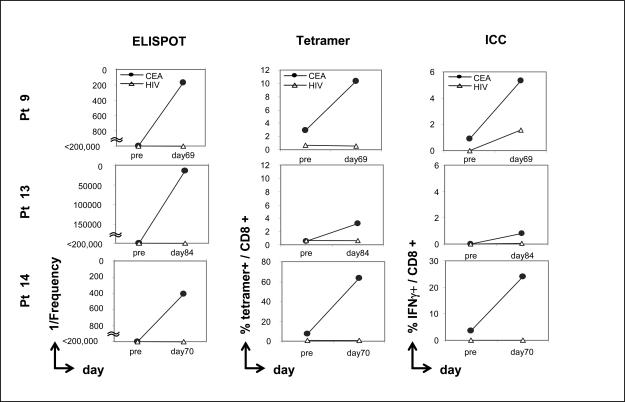
Eight HLA-A2-positive patients who had completed the first 4 vaccinations were analyzed for evidence of CD8-mediated immune response to an HLA-A2-restricted CEA peptide. T-cell responses in those patients were evaluated before vaccine (designated as pre) compared with 1 month after the fourth vaccine (designated post-4; about days 69 to 84), by performing 3 different immune assays:(a) ELISPOT assay for IFN-γ; (b) CEA-tetramer staining; and (c) ICC staining for IFN-γ. In the absence of IVS, PBMCs from all 8 patients showed no CEA-specific CD8 immune responses. After being stimulated in vitro in the presence of the HLA-A2-restricted CEA peptide CAP1-6D for 2 cycles, T cells from 3 of 8 patients demonstrated a substantial increase in postvaccine (but not prevaccine) CEA-specific CD8 immune responses by ELISPOT assay (Fig. 1). Moreover, the percentage of CEA-tetramer-positive T cells, as well as intracellular cytokine staining for IFN-γ-positive cells, was enhanced postvaccination (but not prevaccination) in these 3 patients (Fig. 1 and Supplementary Fig. S1A and B). All samples were negative for responses to an HIV peptide pre- and postvaccination (Fig. 1).
Fig. 1.
Identification of CEA-specific T cells in patients pre- and postvaccination by ELISPOT assay, CEA-MHC-tetramer binding, and intracellular cytokine (ICC) analysis. Effectors were used at IVS-2 (see Patients and Methods). Results are expressed as frequency of IFN-γ-producing cells (ELISPOT assay), % of tetramer binding cells (tetramer binding assay), or % intracellular IFN-γ-positive cells (ICC staining assay), respectively.
ELISPOT assays were also performed on an HLA-A3 gastric cancer patient (patient 1) pre- and postvaccination using a CEA HLA-A3 binding peptide (CAP-7) (6). The results show that the precursor frequency of CEA-specific T cells was < 1/200,000 prevaccination. The precursor frequency of CEA-specific T cells was 1/33,333; 1/85,714; 1/35,294, and 1/54,545 at day 12, day 39, day 69, and day 154 postvaccination, respectively.
We also evaluated CD4 immune responses in 15 patients included in the study, using CEA protein as antigen, by comparing prevaccination and post-4-vaccination (approximately day 70) samples. CEA class II peptides for DRB1*0701 were used in the assay, in addition to CEA protein, for patients with DRB1*0701 allele. CD4+ T cells were isolated from pre- and postvaccination PBMCs and stimulated with autologous DCs pulsed with CEA protein. Flu protein (data not shown) and myoglobin protein were used as positive and negative controls, respectively. Results are shown in Table 2. Six of the 15 patients (patients 8, 9, 13, 15, 19 and 23) with undetectable levels prevaccination showed measurable levels of IFN-γ in response to CEA protein, but not to myoglobin. Patient 22 had a preexisting CD4 response that increased slightly with respect to IFN-γ production postvaccination. All 15 patients had positive immune response to flu protein pre- and postvaccination, with the exception of the prevaccination sample from patient 19. Five patients had the DRB1*0701 allele. A CEA class II peptide for DRB1*0701 was used to detect CD4 immune response in these patients pre- and postvaccination. Three of 5 patients (patients 8, 9, and 14) with undetectable levels prevaccination showed measurable levels of IFN-γ in response to CEA peptide, but not to the negative control HIV class II peptide (Table 2). Two of these 5 patients with positive immune response to the CEA class II peptide postvaccination also had a positive immune response to CEA protein.
Table 2.
CD4 immune response to CEA in patients pre- and postvaccination
| Patient # | Day of sample |
CEA protein | Myoglobin protein |
CEA peptide | HIV peptide |
|---|---|---|---|---|---|
| 6 | Pre | < 15.6 | < 15.6 | NT | NT |
| Day 69 | < 15.6 | < 15.6 | NT | NT | |
| 8 | Pre | < 15.6 | < 15.6 | < 15.6 | < 15.6 |
| Day 69 | 34.5 | < 15.6 | 31.8 | < 15.6 | |
| 9 | Pre | < 15.6 | < 15.6 | < 15.6 | < 15.6 |
| Day 69 | 25.5 | < 15.6 | 26.0 | < 15.6 | |
| 11 | Pre | < 15.6 | < 15.6 | < 15.6 | < 15.6 |
| Day 69 | < 15.6 | < 15.6 | < 15.6 | < 15.6 | |
| 13 | Pre | < 15.6 | < 15.6 | NT | NT |
| Day 84 | 57.2 | < 15.6 | NT | NT | |
| 14 | Pre | < 15.6 | < 15.6 | < 15.6 | < 15.6 |
| Day 70 | < 15.6 | < 15.6 | 42.2 | < 15.6 | |
| 15 | Pre | < 15.6 | < 15.6 | NT | NT |
| Day 44 | 65.5 | < 15.6 | NT | NT | |
| 17 | Pre | < 15.6 | < 15.6 | NT | NT |
| Day 69 | < 15.6 | < 15.6 | NT | NT | |
| 19 | Pre | < 15.6 | < 15.6 | NT | NT |
| Day 69 | 168.0 | < 15.6 | NT | NT | |
| 20 | Pre | < 15.6 | < 15.6 | NT | NT |
| Day 70 | < 15.6 | < 15.6 | NT | NT | |
| 21 | Pre | < 15.6 | < 15.6 | NT | NT |
| Day 71 | < 15.6 | < 15.6 | NT | NT | |
| 22 | Pre | 22.5 | < 15.6 | < 15.6 | < 15.6 |
| Day 69 | 31.5 | < 15.6 | < 15.6 | < 15.6 | |
| 23 | Pre | < 15.6 | < 15.6 | NT | NT |
| Day 71 | 45.0 | < 15.6 | NT | NT | |
| 24 | Pre | < 15.6 | < 15.6 | NT | NT |
| Day 70 | < 15.6 | < 15.6 | NT | NT | |
| 25 | Pre | < 15.6 | < 15.6 | NT | NT |
| Day 70 | < 15.6 | < 15.6 | NT | NT |
Results are expressed in pg/mL of IFN-γ, as assayed by ELISA. CEA protein and CEA class II-restricted peptide were used as antigen. Fresh isolated CD4+ T cells (no IVS) were used as effector. Autologous DCs were used as APCs. Concentrations used were as follows: CEA protein, 50 μg/mL; CEA peptide, 10 μg/mL; myoglobin, 20 μg/mL; HIV peptide, 10 μg/mL. NT: Not tested.
Immune responses pre- and postvaccination to MUC-1 were also evaluated using the ELISPOT assay. Four of 14 patients were positive for the generation of MUC-1 specific T cells postvaccination. All patients were negative (<1 in 200,000) prior to vaccination. Patients 6, 8, 9 and 14, however, had frequencies of MUC-1 specific T cells of 1/20,000, 1/10,000, 1/6,666 and 1/4,000 post- vaccination. A MUC-1 specific T-cell line was also generated from PBMC of patient 22 using the agonist MUC-1 peptide pulsed autologous dendritic cells. This T-cell line was capable of producing 392 pg IFN-γ/ml/106 cells in response to the MUC-1 peptide but not the control PSA peptide.
The results of patient responses to CEA peptide and/or protein and MUC-1 peptide are shown in Supplementary Table S2. Nine of 15 patients were positive for immune responses to either CEA or MUC-1. Eight of 15 patients were positive for CEA, while four of 14 were positive for MUC-1. Six of 14 patients were negative for responses to both antigens and three of 14 patients were positive for responses to both antigens. Patient 22 had a preexisting response to CEA, as shown in Table 2.
Studies were also conducted to determine the immune response to both the vaccinia and the fowlpox vectors pre- and postvaccination. Since all of the patients in the study were over 35 years old, they had at least one prior smallpox vaccination. Thus it is not surprising that the vast majority of patients, i.e., 17 out of 20, had preexisting antibodies to vaccinia. Immune responses to vaccinia went up postvaccination in most patients (Supplementary Table S3). All 20 patients analyzed were negative for antibodies to fowlpox prior to vaccination. The ability to mount an immune response to fowlpox is thus a good indicator of the generalized immune status of patients. Most patients mounted an immune response to fowlpox postvaccination. It is important to point out that none of these patients mounted neutralizing antibodies to fowlpox. There was also no correlation or trend toward the ability of patients to mount immune responses to the antigen in the vaccine and their ability to mount an immune response to fowlpox (Supplementary Table S3).
Clinical outcomes
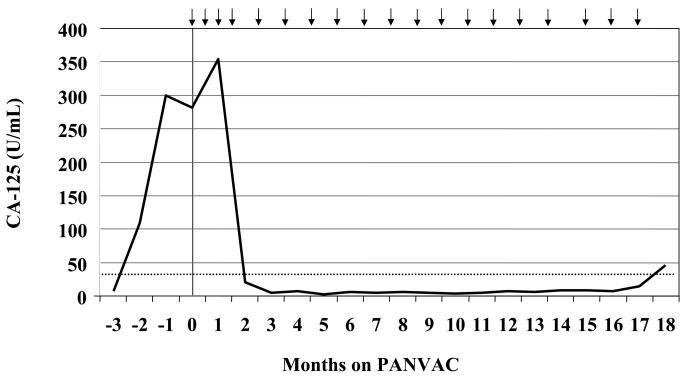
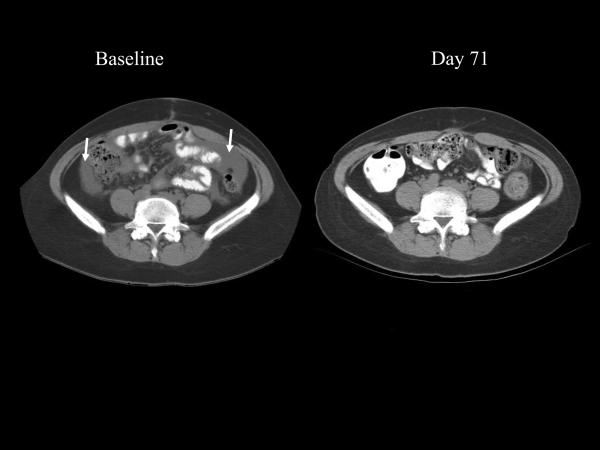
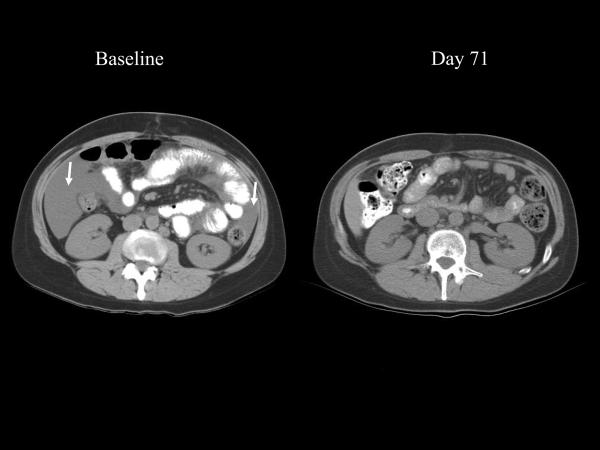
Table 3 describes the clinical outcomes of patients in this study. Patient 22 is a 42-year-old female who initially presented with stage IIIc clear cell ovarian cancer. Prior treatment included standard tumor debulking followed by a clinical trial with carboplatin, paclitaxel, and cetuximab. Within 4 months after chemotherapy the CA-125 began to rise rapidly (Fig. 2), coinciding with the development of ascites associated with substantial gastrointestinal complaints. This patient enrolled on study and by day 16 noted decreased abdominal distension. By the end of the first month of treatment, the early satiety and bloating had substantially improved, the ascites had completely resolved on physical exam, and the patient's weight had decreased by 6 kg. Six weeks into treatment the patient's gastrointestinal symptoms had resolved completely. The first restaging confirmed the absence of ascites and showed a marked improvement in the mesenteric stranding often seen in metastatic ovarian cancer (Fig. 3A and B). Serum from this patient was tested for antibodies specific for CA-125. There was no detectable IgG (any class) in the serum either pre- or postvaccination (day 377). She had evidence of both CEA-specific T cells and MUC-1-specific T cells. During her enrollment on study she remained symptom-free on vaccine, with no recurrent ascites or mesenteric stranding, and prolonged normal CA-125. Eighteen months after initiating vaccine, her CA-125 started to increase. This corresponded with an area of uptake in the sternum and liver seen on PET scan. Although she had no lesions greater than 1 cm on CT scan, she was taken off study. Three months after coming off study, she died with apparent gram-negative sepsis, although this finding is not conclusive. Her remains were autopsied at the NIH and the examination revealed that most areas of tumor had extensive areas of necrosis and/or fibrosis, consistent with her clinical course. There was no evidence of immune-related damage to any normal tissues.
Table 3.
Clinical outcomes
| Pt # | Age | Gender | Cancer | Performance status (ECOG) |
Prior chemos |
PFS¤ | OS† (mos.) |
|---|---|---|---|---|---|---|---|
| 1 | 62 | F | Gastric | 0 | 1 | 5 | 21 |
| 2 | 57 | F | Breast | 0 | 4 | 2 | 27 |
| 3 | 35 | M | Colon | 1 | 7 | 2 | 19 |
| 4* | 40 | F | Gastric | 1 | 1 | 6 | 22 |
| 5 | 60 | F | Pancreatic | 1 | 1 | 2 | 18 |
| 6* | 57 | M | Colon | 0 | 2 | 30+ | 30+ |
| 7* | 56 | F | Appendiceal | 1 | 2 | 29+ | 29+ |
| 8 | 67 | F | Breast | 1 | 3 | 6 | 25+ |
| 9 | 50 | M | Colon | 0 | 2 | 2 | 15 |
| 10* | 61 | F | Gastric | 0 | 2 | 4 | 18 |
| 11* | 61 | M | Colon | 0 | 3 | 3 | 22+ |
| 12 | 48 | F | Colon | 1 | 3 | 2 | 14 |
| 13 | 52 | F | Colon | 1 | 3 | 4 | 23+ |
| 14 | 65 | F | Pancreatic | 0 | 2 | 2 | 13 |
| 15* | 53 | M | Appendiceal | 1 | 5 | 2 | 22+ |
| 16 | 63 | F | Lung | 1 | 7 | 2 | 12 |
| 17 | 67 | M | Colon | 1 | 3 | 2 | 11 |
| 18 | 56 | F | Lung | 1 | 2 | 1 | N/A |
| 19 | 66 | M | Esophageal adenocarcinoma | 1 | 1 | 4 | 20+ |
| 20 | 38 | F | Colon | 1 | 1 | 2 | 18 |
| 21 | 70 | M | Colon | 0 | 2 | 2 | 12 |
| 22 | 42 | F | Ovarian | 1 | 1 | 18 | 21 |
| 23* | 40 | F | Rectal | 1 | 1 | 9 | 17+ |
| 24 | 57 | F | Ovarian | 1 | 2 | 2 | 6 |
| 25* | 53 | F | Ovarian | 0 | 2 | 19+ | 19+ |
Patient had no radiographic evidence of disease at baseline.
PFS, progression free survival. Plus sign in this column indicates patient remains on trial.
OS, overall survival. Plus sign in this column indicates patient alive at time of last contact.
Fig. 2.
Serum CA-125 levels from a 42-year-old patient (# 22) with platinum-refractory clear cell ovarian cancer who received PANVAC-V on day 1, followed by multiple boosts with PANVAC-F (vaccinations designated by arrows). The CA-125 level decreased from a peak of 351 U/mL to less than 10 U/mL out to 18 months on study.
Fig. 3.
Representative sections (A and B) from a CT scan on baseline and day 71 after initiation of vaccination for patient 22 (ovarian cancer). Baseline study reveals ascites (arrows) and mesenteric stranding, both of which are absent at day 71 and all subsequent restagings.
Patient 8 is a 67-year-old female who presented with metastatic breast cancer and multiple bulky liver metastases. In the 14 months prior to trial she was treated with a series of hormonal therapies, but nonetheless had a steady increase in CA27.29 from 81 U/mL to 883 U/mL (doubling about every 3 months). The 5 index lesions identified on CT at baseline were measured per RECIST guidelines. The sum of unidimensional measure of these lesions was 12 cm. At her first restaging, this sum had decreased by 21%, and immune responses were seen to both CEA and MUC-1 (Table 2 and Supplementary Table S2). By her second restaging (day 127), index lesions had decreased by 24% from baseline, however at the third restaging, they had returned to baseline. These restagings were accompanied by a decrease in CA27.29 from 657 U/mL on study to 435 U/mL then 441 U/mL before rising as high as 1160 U/mL when she came off study.
A number of patients had prolonged survival after coming off trial, and several patients had somewhat unexpected clinical responses to subsequent therapies. In light of previous studies showing enhanced effects of chemotherapy following treatment with vaccine (29-32), we thought it prudent, where possible, to document clinical responses following vaccine therapy: (A) Patient 1, who had metastatic gastric cancer previously treated with 3 cycles of capecitabine, oxaliplatin, and epirubicin, which were poorly tolerated, was on trial for 5 months before coming off for development of symptomatic ascites. As described above, it was demonstrated that she developed a 6-fold increase in CEA-specific T cells postvaccination. Following protocol, patient 1 received fluorouracil, leucovorin, and bevacizumab, which led to decreased ascites and a prolonged stable course. She survived for 21 months following initiation of trial. (B) Patient 8 (breast cancer) had a positive response to single-agent capecitabine following vaccine, with a decrease in CA27.29 from 1679 to 421 and a decrease in CEA from 22.1 U/mL to 8 U/mL. Upon progression, she began taking paclitaxel and bevacizumab and has remained on this regimen for 15 months, during which time she has had a decrease in CA27.29 from 1839 U/mL to 76 U/mL. (C) Patient 13 (colon cancer) had a remarkable decrease in CEA from 297 U/mL to 7 U/mL on 5-fluorouracil, leucovorin, oxaliplatin, and bevacizumab following vaccine. (D) Patient 10 had a similar decrease in CEA from 118.7 U/mL to 50 U/mL on 5-fluorouracil, leucovorin, oxaliplatin, and bevacizumab following vaccine; however, chemotherapy had to be discontinued. This patient's CEA continued to decline off chemotherapy to a nadir of 25 U/mL.
Three patients who were without radiographic evidence of disease at initiation of trial remain without evidence of disease 18 months or more since being enrolled. Their clinical course is as follows: (A) Patient 6 was originally diagnosed with a 6 × 4-cm invasive, moderately differentiated Duke's C adenocarcinoma with 4 of 22 lymph nodes positive. He underwent adjuvant fluorouracil and leucovorin chemotherapy for 6 months, followed by irinotecan chemotherapy for 4 months. Twenty months later he was found to have a liver lesion, which was resected. He subsequently enrolled on study and has remained on study for 30+ months. (B) Patient 7 was found to have a pelvic mass and pleural effusion. She underwent a total abdominal hysterectomy and bilateral oophorectomy. Pathology revealed metastatic, poorly differentiated signet cell cancer of the appendix, with omental and ovarian involvement. She then underwent a right hemicolectomy, which confirmed a poorly differentiated T4N1M1 cancer. She underwent adjuvant chemotherapy with 6 cycles of fluorourical, leucovorin, and oxaliplatin, followed by 2 more cycles with fluorourical and leucovorin, then 1 month of capecitabine. One month after stopping capecitabine, she was enrolled on study and has remained on study for 29+ months. (C) Patient 25 was originally diagnosed with a stage III capillary serous-appearing ovarian carcinoma and underwent debulking followed by 6 cycles of carboplatin and paclitaxel. A second-look operation revealed microscopic residual disease, and the patient underwent 4 cycles of intraperitoneal cisplatin chemotherapy. During that time her CA-125 dropped from 15 U/mL to 6 U/mL. She enrolled on study 4 months later and has remained on study for 19+ months. Her CA-125 has remained at or below 6 U/mL while on study.
Discussion
The study reported here was the first NCI-sponsored study of PANVAC-VF and the first trial of its use other than in patients with endstage pancreatic cancer (33). This trial demonstrates the safety of this vaccine and provides evidence of clinical benefit in some patients. In a small trial with a variety of tumor types, as reported here, it is difficult to determine the true level of vaccine activity. The majority of patients with multiple prior chemotherapy regimens and advanced metastatic disease had progressive disease at first restaging. For patients who presented with no evaluable disease, the median time to progression was 6 months (range, 2 to 27+ months). However, several patients did have clear evidence of clinical benefit. Breast cancer that metastasizes to the liver is generally very aggressive and relatively resistant to treatment (34, 35). Thus, stabilization or shrinkage in liver metastasis suggests clinical activity. Although patient 8 (breast cancer) did not meet RECIST response criteria in the 5 bulky index lesions in her liver, if one assumes that her lesions were spheres, a decrease of 24% would equate to a decrease of over 50% in tumor volume. Furthermore, during the 6-month period of stable disease, she had no symptoms from either disease or treatment.
There is even clearer evidence of antitumor activity from the vaccine in patient 22, who had clear cell ovarian cancer, which is associated with poor prognosis and is largely unresponsive to systemic therapy. She was also platinum refractory, with a rapidly rising CA-125 within 4 months following chemotherapy (the Gynecologic Oncology Group recommends using the tumor marker CA-125 as a formal response criterion following therapy of ovarian cancer) (36-38). Her ascites and associated symptoms completely resolved within the first 6 weeks of vaccine therapy, and her CA-125 went from 284 U/mL on study to 351 U/mL, then dropped within normal limits by 2 months, where it remained until 18 months on study (Fig. 2). During the patient's time on trial, 3 retroperitoneal lymph nodes detected on CT scan were 1.5–2.1 cm, but given her dramatic clinical response she remained on trial, and the size of all affected lymph nodes subsequently decreased to < 1 cm. The increased lymph node size may have been a reaction to vaccine (the patient also had inguinal adenopathy that transiently increased following each injection in alternating thighs), or may represent an influx of TAA-specific T cells into lymph nodes involved with tumor. A PET scan done about day 180 demonstrated uptake in 2 lesions, corresponding to 2 retroperitoneal lymph nodes on CT. These had standardized uptake values of 4.6 (1-cm lymph node) and 2.9 (1.6-cm lymph node)—a level of activity that can be seen with an inflammatory lymph node (39). On autopsy, lymph nodes taken from those areas showed fibrosis consistant with a previous inflammatory process. Other studies have identified lymph nodes that increased in size following vaccination and later decreased (39, 40). These findings should be taken into consideration by investigators conducting immunotherapy trials who consider removing a patient from trial based solely on an enlarging lymph node, when the patient has an otherwise improving clinical picture.
A previous corporate-sponsored clinical trial employing PANVAC in patients with second-line pancreatic cancer showed no clinical benefit compared with standard treatment options. Patients with advanced pancreatic cancer have a median overall survival of < 3 months (41, 42). To date, numerous randomized clinical trials employing a variety of chemotherapeutic agents or combinations of agents have failed to significantly increase survival in this patient population (43). Preclinical and clinical data have provided evidence that cancer vaccines are most effective in patients with either early-stage disease or low tumor burden, and when given prior to or in combination with conventional chemotherapy, hormone therapy, or radiation. Thus, poor results in a phase III trial in patients with metastatic pancreatic cancer who have failed frontline treatment is more a failure of clinical trial design than of potential vaccine efficacy in other disease settings.
A unique and intriguing aspect of vaccine therapy is its ability to initiate a dynamic process of host immune response that may be exploited in subsequent therapies. Several clinical studies have provided evidence of this phenomenon. In a phase I study at the Dana-Farber Cancer Institute (30), 17 patients with advanced-stage progressive cancer received a plasmid/microparticle vaccine directed against cytochrome P4501B1, which is overexpressed on most tumors. Ten of 11 patients who failed to develop immunity to the vaccine progressed on subsequent therapies. In contrast, 5 patients who did develop immunity to vaccine unexpectedly showed marked responses to salvage therapy administered on progression. In most cases, salvage therapy lasted at least a year.
This phenomenon was also exemplified in a follow-up study from patients who received a vaccine (sipuleucel-T) or placebo for advanced prostate cancer (44). After progressing on study, patients who received docetaxel chemotherapy were followed. There was a striking and statistically significant increase in overall survival with docetaxel treatment in patients having had prior vaccine (n = 51) vs. placebo (n = 31) (32). The median survival was 34.5 months for patients who received vaccine followed by docetaxel. In contrast, the median survival was 25.4 months for patients who received placebo and subsequent treatment with docetaxel, a 9.1-month difference (P = 0.023, HR 1.9). These groups appeared to be well balanced based on their baseline prognostic factors, using an independently validated predictive nomogram (45).
It is tempting to speculate that chemotherapy can augment immune responses through a variety of mechanisms. These could include destruction or decreased function of regulatory elements within the immune system (e.g., regulatory T cells), apoptosis of tumor cells in a way that stimulates the immune system, a decrease in immune regulatory substances elaborated by tumor cells, and alteration of the phenotypic characteristics of tumor cells, making them more amenable to immune-mediated recognition and destruction. Indeed, these mechanisms have been demonstrated in a variety of preclinical studies (46-52).
Tumor antigen-associated T-cell effector function was monitored in a selected number of vaccinated patients included in this study by using ELISPOT, peptide-MHC tetramer binding, and intracellular cytokine flow cytometry assays, all of which have been recommended for immune monitoring to detect antigen-specific CD8-positive T cells in cancer immunotherapy trials by a workshop sponsored by the Society for Biological Therapy (53). The ELISPOT is a functional assay that detects individual T cells that secrete cytokines such as IFN-γ upon stimulation with a specific antigen in an MHC-restricted manner. Peptide-MHC tetramer assay measures the binding of peptide-MHC tetramers to antigen-specific T cells via the T-cell receptor. Intracellular cytokine assay quantifies functional antigen-specific T cells and determines the phenotype of T cells secreting the cytokine using multicolor flow cytometry. In the study reported here, by using T cells following 2 CEA peptide restimulation cycles, all 3 assays detected CEA-specific CD8 T cells in the blood of 3 of 8 postvaccination samples and 0 of 8 prevaccination samples.
It should be pointed out that the ELISPOT assay for CEA employed the agonist epitope, which is also present in the TRICOM vaccine. We have previously shown, however, that T cells generated using the agonist peptide will recognize the native CEA peptide. More importantly, we have also previously shown that CTL generated against the agonist CEA peptide were capable of lysing human cells that endogenously express CEA (54, 55). The ELISPOT assay for the MUC-1 peptide used the native MUC-1 epitope.
In addition, CEA-specific CD4 responses were detected in 8 of 16 patients analyzed, as measured by IFN-γ production by CD4 T cells after stimulation with CEA protein or peptide. Although absolute levels of IFN-γ were low without IVS (22 to 168 pg/mL), they were similar to the flu protein control (36 to 130 pg/mL). These results demonstrate that both CD8 and CD4 CEA-specific T-cell responses were increased postvaccination. A previous trial with CEA-TRICOM vaccines demonstrated significant generation of CEA-specific immune responses in the majority of patients treated, without the need for in vitro stimulation of the patients' PBMCs (7). One possible explanation is that the patients in this trial had more prior chemotherapy, especially given recent advances in chemotherapy options for metastatic colorectal cancer. This is important, as it has previously been shown that the number of prior chemotherapy regimens correlates inversely with the ability of the patient to mount an immune response (56).
It is possible that time to progression in advanced metastatic disease will not reflect the true clinical benefit of an active immunotherapy. One recently published phase III clinical trial showed no significantly improved time to progression, but did show statistically significant and clinically meaningful improvement in overall survival in patients with metastatic cancer (44). If long-term, effective antitumor memory is achieved, any subsequent therapy could not only have direct antitumor activity, but could further activate the primed immune system with dying cells. Furthermore, nonspecific tumor-directed cytotoxic therapy (e.g., chemotherapy or radiation therapy) could target T-regulatory cells and thus change the balance to a more active antitumor immune response. Finally, subsequent therapy could lead to alteration of the phenotypic characteristics of tumor cells, making them more amenable to immune-mediated recognition and killing.
This trial demonstrates that PANVAC-VF is safe and is associated with the generation of CD8 and CD4 antigen-specific immune responses postvaccination. These immune responses were seen in more than half of patients tested. Furthermore, this trial provides early evidence of clinical benefit. Based on the encouraging clinical course of several patients in this trial, we have initiated a pilot study for ovarian cancer patients and breast cancer patients to gain more information on which to base a large clinical endpoint study.
Supplementary Material
Acknowledgments
The authors acknowledge the Intramural Research Program of the Center for Cancer Research, NCI, NIH for their support of this study. We also express appreciation to the professionals at the NIH Clinical Center Blood Bank for their part in obtaining apheresis from study patients, and to the medical oncology fellows at the NCI for their attention to patient care. We also wish to thank Theresa Ferrara and Carolyn Smith for help with patient samples and data. Finally, we thank Bonnie L. Casey and Debra Weingarten for their editorial assistance in the preparation of this manuscript.
Footnotes
Data from this study were presented in part as an oral presentation at the 42nd Annual Meeting of the American Society of Clinical Oncology, June 4, 2006, Atlanta, GA.
References
- 1.Ramanathan RK, Lee KM, McKolanis J, et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54:254–64. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wierecky J, Mueller M, Brossart P. Dendritic cell-based cancer immunotherapy targeting MUC-1. Cancer Immunol Immunother. 2006;55:63–7. doi: 10.1007/s00262-005-0673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pantuck AJ, van Ophoven A, Gitlitz BJ, et al. Phase I trial of antigen-specific gene therapy using a recombinant vaccinia virus encoding MUC-1 and IL-2 in MUC-1-positive patients with advanced prostate cancer. J Immunother. 2004;27:240–53. doi: 10.1097/00002371-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Marshall JL, Hoyer RJ, Toomey MA, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–73. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 5.Tsang KY, Palena C, Gulley J, Arlen P, Schlom J. A human cytotoxic T-lymphocyte epitope and its agonist epitope from the nonvariable number of tandem repeat sequence of MUC-1. Clin Cancer Res. 2004;10:2139–49. doi: 10.1158/1078-0432.ccr-1011-03. [DOI] [PubMed] [Google Scholar]
- 6.Tsang KY, Zaremba S, Nieroda CA, Zhu MZ, Hamilton JM, Schlom J. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995;87:982–90. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 7.Marshall JL, Gulley JL, Arlen PM, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–31. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 8.Gulley J, Chen AP, Dahut W, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53:109–17. doi: 10.1002/pros.10130. [DOI] [PubMed] [Google Scholar]
- 9.Hodge JW, McLaughlin JP, Kantor JA, Schlom J. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine. 1997;15:759–68. doi: 10.1016/s0264-410x(96)00238-1. [DOI] [PubMed] [Google Scholar]
- 10.Fong L, Hou Y, Rivas A, et al. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci U S A. 2001;98:8809–14. doi: 10.1073/pnas.141226398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulley J, Todd N, Dahut W, Schlom J, Arlen P. A phase II study of PROSTVAC-VF vaccine, and the role of GM-CSF, in patients (pts) with metastatic androgen insensitive prostate cancer (AIPC) [abstract] J Clin Oncol. 2005;23:A2504. [Google Scholar]
- 12.Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. J Immunol. 2005;174:5994–6004. doi: 10.4049/jimmunol.174.10.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MG, Schlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–7. [PubMed] [Google Scholar]
- 14.Kaufman H, Wang W, Manola J, et al. Phase II prime/boost vaccination using poxviruses expressing PSA in hormone dependent prostate cancer:follow-up clinical results from ECOG 7897 [abstract] J Clin Oncol. 2005;23:A4501. [Google Scholar]
- 15.Kaufman HL, Wang W, Manola J, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–32. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 16.Morrissey PJ, Bressler L, Park LS, Alpert A, Gillis S. Granulocyte-macrophage colony-stimulating factor augments the primary antibody response by enhancing the function of antigen-presenting cells. J Immunol. 1987;139:1113–9. [PubMed] [Google Scholar]
- 17.Kass E, Panicali DL, Mazzara G, Schlom J, Greiner JW. Granulocyte/macrophage-colony stimulating factor produced by recombinant avian poxviruses enriches the regional lymph nodes with antigen-presenting cells and acts as an immunoadjuvant. Cancer Res. 2001;61:206–14. [PubMed] [Google Scholar]
- 18.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwak LW, Young HA, Pennington RW, Weeks SD. Vaccination with syngeneic, lymphoma-derived immunoglobulin idiotype combined with granulocyte/macrophage colony-stimulating factor primes mice for a protective T-cell response. Proc Natl Acad Sci U S A. 1996;93:10972–7. doi: 10.1073/pnas.93.20.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maecker HT, Moon J, Bhatia S, et al. Impact of cryopreservation on tetramer, cytokine flow cytometry, and ELISPOT. BMC Immunol. 2005;6:17. doi: 10.1186/1471-2172-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arlen P, Tsang KY, Marshall JL, et al. The use of a rapid ELISPOT assay to analyze peptide-specific immune responses in carcinoma patients to peptide vs. recombinant poxvirus vaccines. Cancer Immunol Immunother. 2000;49:517–29. doi: 10.1007/s002620000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi H, Omiya R, Ruiz M, et al. Identification of an antigenic epitope for helper T lymphocytes from carcinoembryonic antigen. Clin Cancer Res. 2002;8:3219–25. [PubMed] [Google Scholar]
- 24.Southwood S, Sidney J, Kondo A, et al. Several common HLA-DR types share largely overlapping peptide binding repertoires. J Immunol. 1998;160:3363–73. [PubMed] [Google Scholar]
- 25.Hodge JW, Grosenbach DW, Aarts WM, Poole DJ, Schlom J. Vaccine therapy of established tumors in the absence of autoimmunity. Clin Cancer Res. 2003;9:1837–49. [PubMed] [Google Scholar]
- 26.Grosenbach DW, Barrientos JC, Schlom J, Hodge JW. Synergy of vaccine strategies to amplify antigen-specific immune responses and antitumor effects. Cancer Res. 2001;61:4497–505. [PubMed] [Google Scholar]
- 27.Robbins PF, Kantor JA, Salgaller M, Hand PH, Fernsten PD, Schlom J. Transduction and expression of the human carcinoembryonic antigen gene in a murine colon carcinoma cell line. Cancer Res. 1991;51:3657–62. [PubMed] [Google Scholar]
- 28.Hodge JW, Rad AN, Grosenbach DW, et al. Enhanced activation of T cells by dendritic cells engineered to hyperexpress a triad of costimulatory molecules. J Natl Cancer Inst. 2000;92:1228–39. doi: 10.1093/jnci/92.15.1228. [DOI] [PubMed] [Google Scholar]
- 29.Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12:878–87. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 30.Gribben JG, Ryan DP, Boyajian R, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res. 2005;11:4430–6. doi: 10.1158/1078-0432.CCR-04-2111. [DOI] [PubMed] [Google Scholar]
- 31.Arlen PM, Gulley JL, Parker C, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–9. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrylak D. Defining the optimal role of immunotherapy and chemotherapy: advanced prostate cancer patients who receive sipuleucel-T (PROVENGE) followed by docetaxel derive greatest survival benefit [abstract]; Chemotherapy Foundation Symposium 14th Annual Meeting; November 2006. [Google Scholar]
- 33.Madan RA, Arlen PM, Gulley JL. PANVAC-VF:poxviral-based vaccine therapy targeting CEA and MUC1 in carcinoma. Expert Opin Biol Ther. 2007;7:543–54. doi: 10.1517/14712598.7.4.543. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto N, Watanabe T, Katsumata N, et al. Construction and validation of a practical prognostic index for patients with metastatic breast cancer. J Clin Oncol. 1998;16:2401–8. doi: 10.1200/JCO.1998.16.7.2401. [DOI] [PubMed] [Google Scholar]
- 35.Selzner M, Morse MA, Vredenburgh JJ, Meyers WC, Clavien PA. Liver metastases from breast cancer: long-term survival after curative resection. Surgery. 2000;127:383–9. doi: 10.1067/msy.2000.103883. [DOI] [PubMed] [Google Scholar]
- 36.Rustin GJ, Quinn M, Thigpen T, et al. Re: New guidelines to evaluate the response to treatment in solid tumors (ovarian cancer) J Natl Cancer Inst. 2004;96:487–8. doi: 10.1093/jnci/djh081. [DOI] [PubMed] [Google Scholar]
- 37.Rustin GJ. Use of CA-125 to assess response to new agents in ovarian cancer trials. J Clin Oncol. 2003;21:187–93. doi: 10.1200/JCO.2003.01.223. [DOI] [PubMed] [Google Scholar]
- 38.Rustin GJ, Nelstrop AE, McClean P, et al. Defining response of ovarian carcinoma to initial chemotherapy according to serum CA 125. J Clin Oncol. 1996;14:1545–51. doi: 10.1200/JCO.1996.14.5.1545. [DOI] [PubMed] [Google Scholar]
- 39.Jones RL, Cunningham D, Cook G, Ell PJ. Tumour vaccine associated lymphadenopathy and false positive positron emission tomography scan changes. Br J Radiol. 2004;77:74–5. doi: 10.1259/bjr/19323466. [DOI] [PubMed] [Google Scholar]
- 40.Loveland BE, Zhao A, White S, et al. Mannan-MUC1-pulsed dendritic cell immunotherapy: a phase I trial in patients with adenocarcinoma. Clin Cancer Res. 2006;12:869–77. doi: 10.1158/1078-0432.CCR-05-1574. [DOI] [PubMed] [Google Scholar]
- 41.Schuetz T, Marshall J, Kaufman H, Safran H, Panicali D. Two phase I studies of prime-boost vaccinations with vaccinia-fowlpox vaccines expressing CEA, MUC-1, and TRICOM costimulatory molecules (B7.1/ICAM-1/LFA-3) in patients with advanced pancreatic cancer [abstract] J Clin Oncol. 2004;22(14S):2564. [Google Scholar]
- 42.Schuetz T, Kaufman H, Marshall J, Safran H. Extended survival in second-line pancreatic cancer after therapeutic vaccination [abstract] J Clin Oncol. 2005;23(16S):2576. [Google Scholar]
- 43.Eckel F, Schneider G, Schmid R. Pancreatic cancer: a review of recent advances. Expert Opin Investig Drugs. 2006;15:1395–410. doi: 10.1517/13543784.15.11.1395. [DOI] [PubMed] [Google Scholar]
- 44.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–94. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 45.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 46.Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–47. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 47.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–94. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 48.Machiels JP, Reilly RT, Emens LA, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer research. 2001;61:3689–97. [PubMed] [Google Scholar]
- 49.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–8. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 50.Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gulley JL, Madan RA, Arlen PM. Enhancing efficacy of therapeutic vaccinations by combination with other modalities. Vaccine. 2007;25(Suppl 2):B89–96. doi: 10.1016/j.vaccine.2007.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keilholz U, Weber J, Finke JH, et al. Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the Society for Biological Therapy. J Immunother. 2002;25:97–138. doi: 10.1097/00002371-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Salazar E, Zaremba S, Arlen PM, Tsang KY, Schlom J. Agonist peptide from a cytotoxic T-lymphocyte epitope of human carcinoembryonic antigen stimulates production of Tc1-type cytokines and increases tyrosine phosphorylation more efficiently than cognate peptide. Int J Cancer. 2000;85:829–38. doi: 10.1002/(sici)1097-0215(20000315)85:6<829::aid-ijc16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 55.Zaremba S, Barzaga E, Zhu M, Soares N, Tsang KY, Schlom J. Identification of an enhancer agonist cytotoxic T lymphocyte peptide from human carcinoembryonic antigen. Cancer Res. 1997;57:4570–7. [PubMed] [Google Scholar]
- 56.von Mehren M, Arlen P, Gulley J, et al. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res. 2001;7:1181–91. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.