Abstract
Our recent studies showed that maintenance of protein tyrosine phosphorylation by PTP inhibition enhanced cell growth, clonogenic survival, and mutagenesis after a single low-level Cr(VI) exposure, thereby suggesting that tyrosine phosphorylation-dependent signaling may govern inappropriate survival in human lung fibroblasts (HLFs). Our goal is to identify specific phospho-tyrosine regulator(s)/downstream effectors involved in enhanced survival after Cr(VI) exposure and PTP inhibition. Phosphotyrosine profiling array showed that PTP inhibition following Cr(VI) exposure increased tyrosine phosphorylation of specific proteins, such as FGR and ABL, which are upstream regulators of both Erk and Akt pathways. To explore the roles of these pathways in the PTP-induced increase in clonogenic survival after Cr(VI) exposure, we examined the effect of combined Akt1 and Erk1/2 knockdown via siRNA technology. Akt1 and/or Erk1/2 silencing had no effect on the PTP inhibitor-induced increase in survival following Cr(VI) exposure, suggesting the presence of non-Akt/non-Erk-mediated survival signaling. Interestingly, geldanamycin, an HSP90 inhibitor and non-specific Raf inhibitor, abrogated the PTP inhibitor-mediated increase in survival following Cr(VI) exposure and abolished the expression/activity of c-Raf and activity of Mek. These findings prompted us to explore upstream regulators of Erk, i.e., Ras, c-Raf and Mek for their potential roles in clonogenic survival. GW5074, a specific c-Raf kinase inhibitor did not alter the effect of the PTP inhibitor but decreased Cr(VI)-mediated clonogenic lethality, potentially though Mek hyperactivation. A genetic approach with a c/a Mek1 mutant also showed that Mek activity was not directly associated with the PTP inhibitor effect. Finally, a genetic approach with d/n or c/a Ras and c-Raf mutants, showed that Ras and c-Raf activities play a substantive role in enhancing clonogenic survival by PTP inhibition following Cr(VI) insult. In conclusion, these studies highlight a novel pro-survival mechanism for clonogenic survival in the face of genotoxic stress in the presence of PTP inhibition via an Erk/Mek-independent and Ras/c-Raf-dependent regulation in normal human lung fibroblasts.
Keywords: PTP inhibitor, sodium orthovanadate, hexavalent chromium [Cr(VI)], genotoxic stress, clonogenic lethality, cell survival, Ras/Raf/Mek/Erk pathway
1. Introduction
In the United States, lung cancer is the leading cause of cancer death [1]. Patients with early stage disease can be effectively treated with surgery, but most patients present at diagnosis with advanced stage, which is essentially incurable since systematic chemotherapy has poor long-term outcomes in these patients. Even after surgery, 50% of operated patients will develop metastatic disease [1]. All these facts emphasize the need for new early detection tools and for more effective therapies for lung cancer. Characterization of cellular and molecular alterations in normal human cells upon genotoxin exposure may be applicable to targeting early oncogenesis in the clinical setting. Indeed, studies on the molecular basis of carcinogenesis show promise in the development of targeted agents that inhibit the development of cancer.
Upon exposure to a genotoxin (i.e., hexavalent chromium [Cr(VI)]) cells undergo apoptosis, growth arrest, and cell cycle checkpoint arrest depending on the extent of the damage. Cellular survival in the face of genotoxic stress may produce an intrinsically death-resistant phenotype; such a selective growth advantage may allow for the emergence of transformed cells. Many of the early, transforming events that occur in carcinogenesis are only now becoming better understood. There are numerous reports that dysregulated protein tyrosine phosphorylation is responsible for the maintenance of proliferative signals and is involved in the early stages of neoplasia [2, 3]. While protein tyrosine kinases catalyze the addition of phosphate, PTPs catalyze the removal (for review, see [4]).
Signaling pathways that regulate cell survival and proliferation are altered in the process of carcinogenesis. One of the intracellular signal transduction pathways that drives tumorigenesis and cancer progression is the Ras/Raf/Mek/Erk pathway. This signal transduction cascade regulates fundamental cellular processes including cell proliferation and survival, differentiation, and apoptosis. These specific cell fates are dependent upon the duration and intensity of activation of the individual components in the signaling cascade, as well as on the cell lineage-specific substrates [5–7]. The Ras/Raf/Mek/Erk pathway interacts with other mitogenic pathways (i.e., PI3K/Akt) to determine cell fate after extracellular stimuli. Maintenance of cell survival and growth is achieved in part through the continuous progression of cell cycle and consequent proliferation. All components in the Ras/Raf/Mek/Erk cascade have been shown to be involved in cell cycle progression, cell survival and proliferation.
Our recent study [8] showed that maintenance of protein tyrosine phosphorylation by PTP inhibition was associated with increased cell proliferation, clonogenic survival, and mutagenesis after a single Cr(VI) exposure in human lung fibroblasts. Notably, PTP inhibition increased Cr(VI)-induced forward mutations at the HPRT locus in two mammalian cell lines, which was coincident with enhanced clonogenic survival, suggesting regulators of tyrosine phosphorylation may determine cell survival/death as an initial event after Cr(VI) insult. The goal of the current study was to identify specific phospho-tyrosine regulator(s)/downstream effectors involved in enhanced survival after Cr(VI) exposure and PTP inhibition. Here we report that both Ras and c-Raf activities play an important role in the increase of clonogenic survival in the presence of PTP inhibition following Cr(VI) insult in normal human lung fibroblasts.
2. Materials and methods
2.1 Cell Culture and Chromium Treatment
Human lung fibroblasts (HLFs, ATCC, Manassas, VA) were maintained and treated with sodium chromate (Na2CrO4.4H2O) ( J.T. Baker Chemical Company, Philipsburg, NJ) in the absence or presence of the PTP inhibitor, sodium orthovanadate (SOV, Na3VO4) (Sigma-Aldrich, St. Louis, MO) as we have previously described [8]. U0126, geldanamycin (GA), and GW5074 were from BioMol (Plymouth Meeting, PA). Unless otherwise specified, all chemicals were from Sigma (Sigma-Aldrich, St. Loius, MO) and were of the highest purity available.
2.2 Phosphotyrosine Profiling Array
To explore the effect of PTP inhibition on protein tyrosine phosphorylation after Cr(VI) exposure, TranSignal Phosphotyrosine Profiling Arrays (Panomics, Fremont, CA) were utilized according to the manufacturer’s protocol. Briefly, total protein was isolated from HLFs treated with 1 μM Cr in the absence or presence of 10 μM SOV for 24 hrs. One milligram of the respective protein lysate was incubated with the membrane array overnight. After washing the membranes, tyrosine-phosphorylated proteins were detected with a biotin-conjugated anti-phosphotyrosine antibody and streptavidin-HRP. Chemiluminescence images from membranes exposed to Hyperfilm ECL were analyzed with a Personal Densitometer SI and ImageQuant software (GE Healthcare, Piscataway, NJ). The array contains 38 SH2 domain-containing phosphotyrosine proteins spotted in duplicate along with positive orientation markers on the bottom and the right edges of the membrane. These positive markers were used as a normalization index across the multiple membranes.
2.3 Transfection
All transient transfections were performed with the Amaxa Nucleofector system (Amaxa, Gaithersburg, MD) according to the manufacturer’s protocol. In all transfection experiments, pmaxGFP (Amaxa) was used as a transfection control and transfection efficiency was approximately 70–80% as assessed by fluorescence microscopy. Briefly, adherent HLFs were serum starved for 4 hrs, collected by trypsinization, and approximately 1.8 × 106 cells suspended in nucleofection solution R were mixed with the indicated amount of siRNA or plasmid, and then transfected using the T20 pulsing parameter. Cells were transferred into culture wells or dishes containing prewarmed F12 medium supplemented with 20% FBS. At 16 hr post-transfection, cells were washed twice with PBS, and then incubated in F12 medium with 15% FBS. All experimental treatments were done at 48 hr post-transfection with the following exception: After c-Raf and Ras plasmid transfection, cells were treated at 24 hr post-transfection since the maximum expression of these plasmids was achieved at this time. Respective siRNA for a non-target luciferase control and siRNA SMARTpool for Akt1, Erk1 and Erk2 were purchased from Dharmacon (Chicago, IL). Constitutively active (c/a) Mek1 plasmid (R4F) was a gift from Dr. Natalie G. Ahn, University of Colorado [9, 10]. The c/a Akt1 (MyrAkt1) plasmid was obtained from Dr. Philip Tschlis at Tufts University School of Medicine. The dominant negative (d/n) Ras, c/a Ras, d/n c-Raf, and c/a c-Raf plasmids (RasN17, RasL61, dnRaf-1, and Raf1-CAAX, respectively) were gifts from Dr. Kang-Yell Choi, YonSei University [11, 12]. The pmax vector from Amaxa was used for mock control transfection.
2.4 Immunoblotting
Total protein lysates were extracted and Western blotting was performed as we have previously described [13]. Antibodies used were as follows: Akt1 (Upstate, Lake Placid, NY), p-Akt(ser473), Total Akt, Total c-Raf, p-c-Raf (ser338), p-c-Raf (ser289/296/301), total Mek1/2, p-Mek1/2(ser217/221), p-Erk1/2(thr202/tyr204), total Erk1/2, p-p90 RSK(ser380), and HA-tag (Cell Signaling, Denvers, MA), pan-Ras (Pierce, Rockford, IL), Mek1, Mek2 (Santa Cruz Biotechnology, Santa Cruz, CA ), β-actin and α-tubulin (Sigma, St. Louis, MO).
2.5 Clonogenicity assay
HLFs, at 24 – 48 hr post-transfection with the indicated siRNA or plasmid, were incubated with 0 - 2 μM Na2CrO4 for 24 hr in the absence or presence of 10 μM SOV. For studies with chemical inhibitors, i.e., U0126, geldanamycin (GA) and GW5074, cells were pre-incubated with chemical inhibitors for 0.5 hr at 24 hr post-plating and then treated with Cr(VI) ± SOV for 24 hr. Cells were collected by trypsinization, washed and reseeded at 8 × 102/60 mm dish and colonies were stained as previously described [8].
2.6 Ras activity assay
The EZ-Detect Ras Activation kit (Pierce, Rockford, IL) was utilized to measure Ras activity according to the manufacturer’s instructions and as previously described [14]. A GST-fusion protein containing the Ras-binding domain of c-Raf was used to specifically pull down GTP-bound Ras. The active Ras was then detected by immunoblotting. Negative and positive controls were prepared with 500 μg of control protein lysates with the addition of GDP (1 mM) and GTPγS (0.1 mM), respectively.
2.7 Statistical analysis
To assess significant differences among experimental groups, a two-tailed, unpaired Student’s t test was performed when comparing two groups. ANOVA was used when more than two groups were compared with an untreated control group and Tukey’s multiple comparison was used as a post-hoc test (GraphPad Prism 4, San Diego, CA).
3. Results
3.1 PTP inhibition increases tyrosine phosphorylation of specific proteins that are upstream effectors of both Akt and Erk pathways
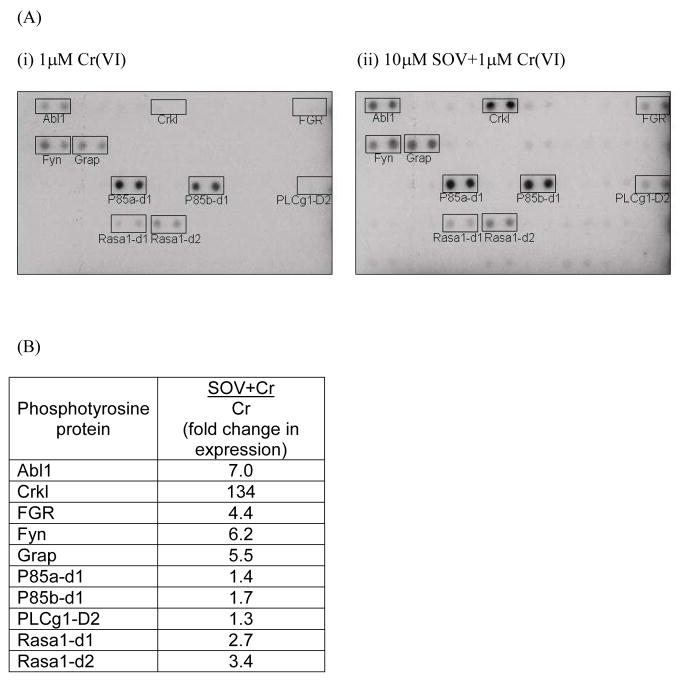
In order to explore the molecular mechanism(s) of enhanced survival in the presence of PTP inhibition after Cr(VI) exposure, we first examined possible alterations in protein tyrosine phosphorylation after Cr(VI) exposure in the presence or absence of PTP inhibition utilizing a phosphotyrosine array. Tyrosine phosphorylation of Abl1, Crkl, FGR, Fyn, Grap, and Rasa1 were increased by 3- to 134-fold upon co-treatment with Cr(VI) and the PTP inhibitor, as compared to Cr(VI) treatment alone (Fig. 1A and B). There was a moderate increase by 1.4–1.7 fold in levels of tyrosine phosphorylation of the respective p85a and b subunit, indicative of PI3K/Akt activation. Also, there was a weak increase of PLCg1 domain 2 upon SOV treatment following Cr(VI) insult. Given the facts that the tyrosine phosphorylation of several known upstream effectors (i.e., FGR and ABL) of both Akt and Erk pathways were increased by SOV from phosphotyrosine array data and protein expression pattern of p-Akt(ser473) were abrogated by co-treatment with Cr(VI) and the PTP inhibitor as compared to that of Cr(VI) alone (Lal et al., unpublished results), we postulated that the PI3K/Akt and/or Mek/Erk pathways may play a role in the enhanced clonogenic survival induced by PTP inhibition after Cr(VI) exposure.
Fig. 1. PTP inhibition increases tyrosine phosphorylation of specific proteins that are upstream effecters of both Akt and Erk pathways.
(A)Protein lysates were isolated from HLFs treated with 1 μM Na2CrO4 in the absence or presence of 10 μM SOV for 24 hr and incubated with TranSignal Phosphotyrosine Profiling Array. Representative blot for (i) 1 μM Cr(VI)- or (ii) 10 μM SOV + 1 μM Cr(VI)-treated sample is shown from one experiment. Full description for selected 10 proteins delineated by boxes: Abl1:v-abl Abelson murine leukemia viral oncogene homolog 1, Crkl: CrRK(v-crk avian sarcoma virus CT-10)-like protein, FGR: Gardner-Rasheed feline sarcoma viral (v-fgr) oncogene homolog, Fyn: FYN oncogene related to SRC, FGR, YES, Grap: Grb2-related adaptor protein, P85a/b: PI3K regulatory p85 subunit a and b for domain 1, PLCg1-D2: 1-phosphatidylinositol-4,5-bisphosphate phosphodiesterase gamma 1, SH2 domain 2, and Rasa1: Ras p21 GTPase activating protein domain 1 and 2. (B) Tabular presentation for 10 phosphotyrosine proteins altered by PTP inhibition. Value for fold change in expression in SOV+Cr/Cr column represents expression level after 10 μM SOV + 1 μM Cr co-treatment divided by expression level after 1 μM Cr treatment alone from averaged value of duplicate spots for each protein from one experiment.
3.2 Silencing of Akt1 and Erk1/2 has no effect on the PTP inhibitor-induced increase in clonogenic survival following Cr(VI) treatment
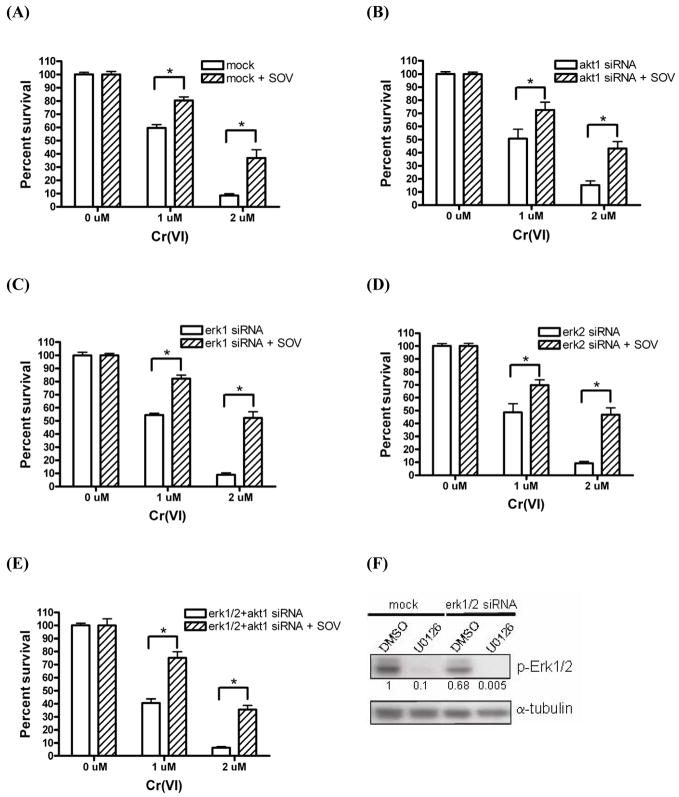
In order to explore the respective role of Akt and Erk in the enhanced clonogenic survival after Cr(VI) exposure and PTP inhibition in HLFs, we silenced Akt1 and Erk1/2 protein expression using akt1 and erk1/2 siRNA. We focused on akt1 since we found the relative mRNA expression of this isoform to be around 3 – 7-fold higher than that of akt2 and akt3, respectively, in HLFs by PCR (data not shown). Transient transfection of 0.12, 0.5, and 1.0 nmoles of akt1, erk1 and erk2 siRNA resulted in approximately 75%, 97%, and 92% knockdown of Akt1, Erk1 and Erk2 protein, respectively, at 72 hr post-transfection (Suppl. Fig. 1). Akt1 silencing effectively inhibited the expression of the pan-active form, i.e., p-Akt(ser473) by 80% on average, thereby confirming that akt1 is the predominant isoform transcript in HLFs. We also observed similar knockdown of total Akt protein expression by 70% after akt1 siRNA transfection. Transfection of non-target luciferase siRNA (mock) showed no effect on either Akt1 or Erk1/2 protein expression (Suppl. Fig. 1). Similarly, Erk1 protein expression was not affected by Erk2 silencing, and vice-versa, indicating the high specificity of erk1 and erk2 siRNA (Suppl. Fig. 1B). Furthermore, the respective silencing of Akt1, Erk1 and Erk2 after combined transfection with akt1, erk1 and erk2 siRNA was similar as that observed after transfection with the respective individual siRNA (Suppl. Fig. 1C).
As shown in Fig. 2A, Cr(VI) induced a significant dose-dependent decrease (p<0.001) in clonogenic survival in mock-transfected HLFs as we have previously seen in non-transfected HLFs [13]. SOV alone, at a concentration of 10 μM had no effect on clonogenic survival. However, PTP inhibition induced a significant increase (p<0.001) in clonogenic survival after Cr(VI) exposure as we have recently reported [8], which was, on average, 1.4-fold with 1 μM Cr(VI) and 4-fold with 2 μM Cr(VI) (Fig. 2A). As shown in Fig. 2B-E, neither individual nor simultaneous Akt1 and Erk1/2 silencing had any effect on the PTP inhibitor-induced increase in clonogenic survival after Cr(VI) exposure. In other words, neither Akt1 nor Erk1/Erk2 was required for the PTP inhibitor effect on clonogenic survival. In addition, transient silencing of the expression of these proteins also had no effect on HLF clonogenic survival in the absence or presence of Cr(VI) alone.
Fig. 2. Silencing of Akt1 and Erk1/2 has no effect on the PTP inhibitor-induced increase in clonogenic survival following Cr(VI) treatment.
HLFs were transfected with (A) mock, (B) akt1, (C) erk1, (D) erk2, or (E) erk1/2 + akt1 siRNA via nucleofection. HLFs at 48 hr post-transfection were incubated with 0, 1, and 2 μM Na2CrO4 for 24 hr in the absence or presence of 10 μM SOV, then seeded for colony formation. Data are expressed as percent clonogenic survival of Cr 0 μM control, and are the average ± SE from 2–5 independent experiments performed in triplicate. *: statistically significant difference between the samples with and without SOV at p<0.05. (F)p-Erk1/2 protein expression level after transfection with either mock or erk1/2 siRNA in the presence of DMSO or U0126. HLFs at 48 hr post-transfection with indicated siRNA were treated with 100 μM U0126 or its vehicle control DMSO for 24 hr, then protein lysates were extracted and analyzed for p-Erk1/2 and α-tubulin (loading control) by immunoblotting. Numbers under the p-Erk1/2 blot show p-Erk1/2 expression normalized by α-tubulin level.
Only phosphorylated/active forms of Akt1 and Erk1/2 transduce their upstream survival signal to downstream effectors in cells. Akt1 silencing effectively decreased the expression level of p-Akt(ser473) as shown in Suppl. Fig. 1A. However, combined Erk1 and Erk2 silencing was associated with the persistent expression of p-Erk1/2(thr202/tyr204), which remained at 68% of the control value at 72 hr post-transfection, given a 70–80% transfection efficiency in HLFs (Fig. 2F). These results suggested that residual p-Erk1/2 activity may play a role in maintaining enhanced clonogenic survival after Cr(VI) exposure and PTP inhibition despite complete silencing of total Erk1/2 protein expression. In order to investigate such a possibility, we additionally inhibited Erk1/2 phosphorylation with the Mek inhibitor U0126 in the presence of combined Erk1/2 silencing (Fig. 2F) and examined clonogenic potential. Mek inhibition by U0126 did not alter the PTP inhibitor-mediated increase in clonogenic survival after Cr(VI) exposure in HLFs (data not shown). In addition, neither PI3K inhibition with LY294002 nor Mek inhibition with U0126 in non-transfected HLFs altered the ability of the PTP inhibitor to enhance clonogenic survival following Cr(VI) insult (data not shown). Taken together, these data suggest the presence of a non-Akt/non-Erk-mediated alternative survival pathway(s) which governs enhanced clonogenic survival upon Cr(VI) insult in the presence of PTP inhibition.
3.3 Geldanamycin abrogates the PTP inhibitor-induced increase in clonogenic survival following Cr(VI) treatment
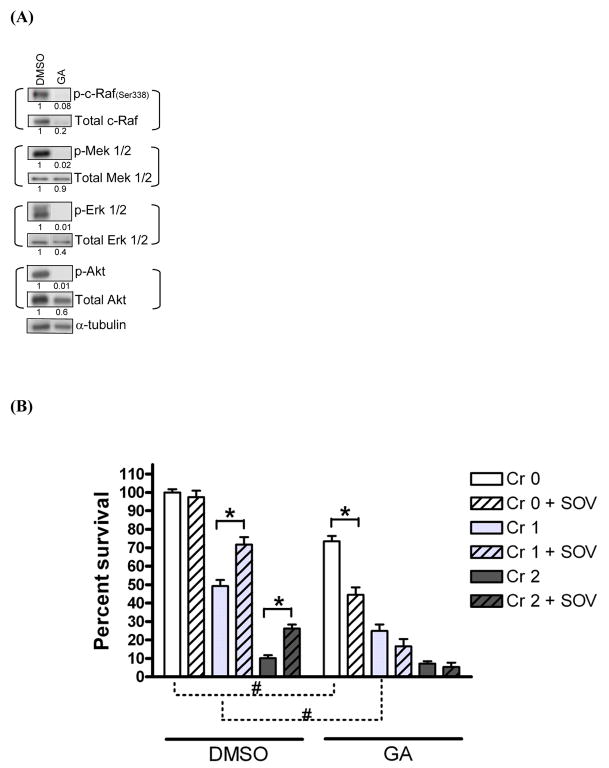
Geldanamycin (GA) is an inhibitor of HSP90 that regulates many client proteins downstream of the pathways that appear to be activated by SOV, as assessed by phosphotyrosine array (Fig 1). Indeed, GA has been used as a non-specific Raf inhibitor [15]. First, we examined the ability of GA to inhibit the total expression/activity of c-Raf, Mek, Erk, and Akt by immunoblotting in HLFs (Fig. 3A). As reported previously [16], the c-Raf activity, as measured by p-c-Raf (ser338) protein expression, was completely inhibited by 1 μM GA, while the expression of total c-Raf was inhibited by 80%. As expected, the activity of Mek1/2 and Erk1/2, as measured by the expression of their phosphorylated forms, p-Mek1/2(ser217/221) and p-Erk1/2(thr202/tyr204), respectively, was completely abolished by GA. Neither total expression of Mek1/2 nor Erk1/2 was significantly altered by GA treatment. Finally, p-Akt (ser473) expression was completely inhibited by GA while total Akt expression was inhibited by 40%.
Fig. 3. Geldanamycin abrogates the PTP inhibitor-induced increase in clonogenic survival following Cr(VI) treatment.
(A) HLFs at 24 hr post-seeding were treated with either 1 μM geldanamycin (GA) or DMSO for 24 hr and total lysates were collected and analyzed for expression of indicated proteins by immunoblotting. Numbers under the blot show individual protein expression normalized by α-tubulin level. (B) HLFs at 24 hr post-seeding were pretreated with either 1 μM GA or DMSO for 30 min and treated with 1 and 2 μM Cr(VI) with or without 10 μM SOV for 24 hr, then seeded for colony formation. Data are expressed as percent clonogenic survival of Cr 0μM control, and are the average ± SE from 4 experiments performed in triplicate. *: statistically significant difference between the samples with and without SOV at p<0.05. #: statistically significant difference for two compared groups indicated by dotted line at p<0.05.
These results prompted us to examine whether inhibition of Mek and c-Raf activity as well as Akt and Erk activity in the presence of GA could alter clonogenic survival in HLFs before and after co-treatment with Cr(VI) and SOV. At a concentration of 1 μM, GA alone induced a 25% decrease in clonogenic survival, which was further augmented in the presence of SOV (Fig. 3B). The Cr(VI)-induced dose-dependent decrease in clonogenic survival was also observed in GA-treated HLFs, but was more pronounced after 1 μM exposure. Importantly, GA completely abrogated the PTP inhibitor-mediated enhanced clonogenic survival following Cr(VI) exposure (Fig. 3B). Taken together, these data suggest that c-Raf activity alone or in combination with Mek activity may be necessary for the PTP inhibitor effect on clonogenic survival in the presence of Cr(VI) insult in HLFs.
3.4 c-Raf activity drives enhanced clonogenic survival after Cr(VI) exposure and PTP inhibition
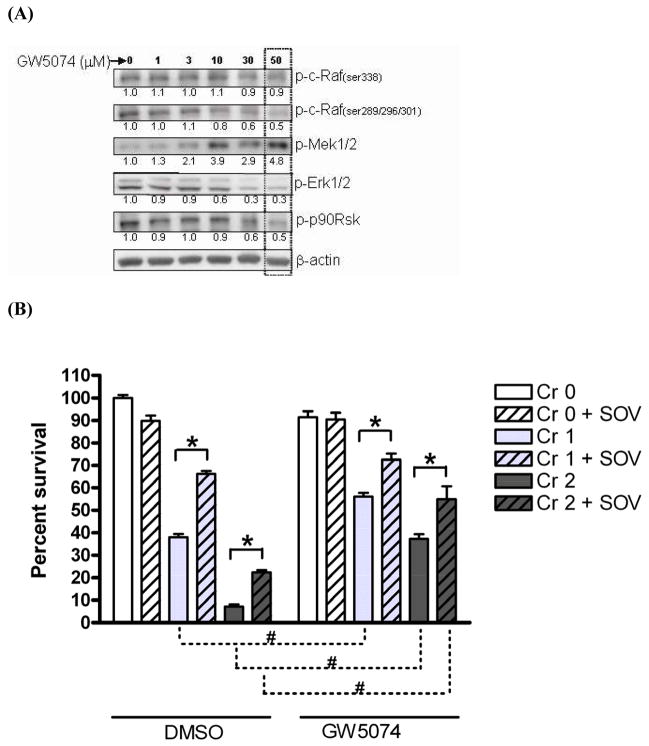
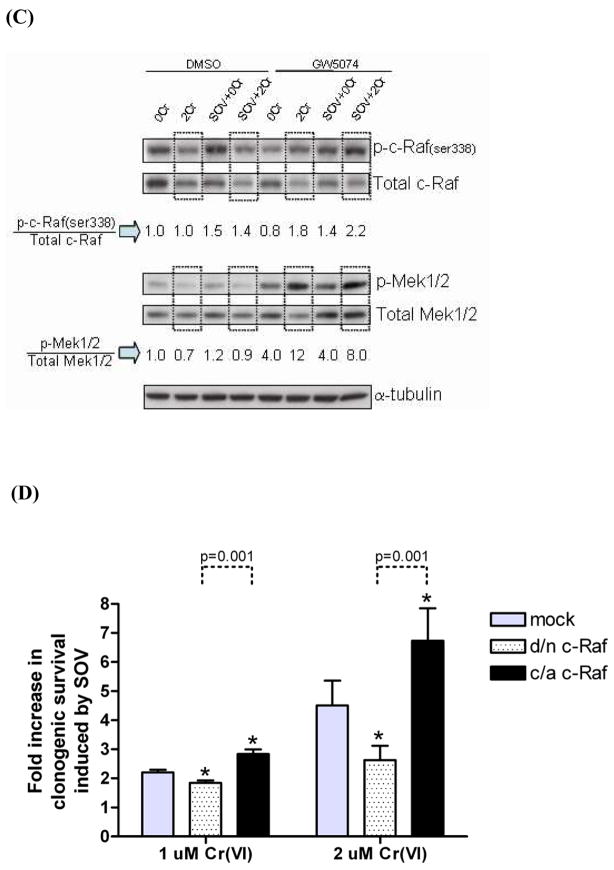
In order to determine the direct role of c-Raf activity in enhanced clonogenic survival after Cr(VI) exposure and PTP inhibition, we employed a combined pharmacologic and genetic approach. We used GW5074, a potent and selective inhibitor, which has been reported to inhibit the Raf/Mek/Erk kinase cascade by blocking the kinase activity of c-Raf [17]. As expected, protein expression of p-Erk1/2(thr202/tyr204) and p-p90Rsk(ser380), two downstream mediators of the Raf signaling cascade, were decreased to 30% and 50% of their respective control level by 50 μM GW5074 (Fig. 4A). This decrease was dose-dependent up to 50 μM, and higher concentrations were cytotoxic. Unexpectedly and in contrast, we observed an apparent hyperactivation of Mek1/2 as shown by the approximate 5-fold increase of p-Mek1/2 (ser217/221) protein expression after 50 μM GW5074 treatment, which was also dose-dependent, and maximum at 50 μM. In addition, no change was observed in the activating phosphorylation of c-Raf [p-c-Raf (ser338)] while a gradual decrease in the inhibitory phosphorylation of c-Raf [p-c-Raf (ser289/296/301)] was observed after treatment with 1–50 μM GW5074 (Fig. 4A). Protein expression of p-Akt (ser473) was not altered by GW5074 in HLFs (data not shown).
Fig. 4. Mek activity is associated with decrease in Cr(VI)-induced clonogenic lethality and c-Raf activity is associated with increase in clonogenic survival after Cr(VI) insult and PTP inhibition.
(A)HLFs at 24 hr post-seeding were treated with a c-Raf inhibitor GW5074 at 1 – 50 μM in DMSO solvent for 24 hr, then protein lysates were extracted and analyzed for indicated proteins by immunoblotting. A box over blots at 50 μM GW5074 indicates the concentration that most effectively inhibited 2 downstream effectors, p-Erk1/2 and p-p90Rsk. Numbers under the blot show individual protein expression normalized by β-actin level. (B) HLFs at 24 hr post-seeding were incubated with 0, 1, and 2 μM Na2CrO4 for 24 hr in the absence or in presence of 10 μM SOV and GW5074, and then seeded for colony formation. GW5074 treatment was 30 min prior to SOV dosing at a concentration of 50 μM. Data are expressed as percent clonogenic survival of Cr 0 μM control, and are the average ± SE from 3 experiments performed in triplicate. *: statistically significant difference between the samples with and without SOV at p<0.05, #: statistically significant difference for two compared groups indicated by dotted line at p<0.05. (C) HLFs at 24 hr post-seeding were pretreated with either 50 μM GW5074 or DMSO for 30 min, and then treated with 2 μM Na2CrO4 for 24 hr in the absence or presence of 10 μM SOV. Protein lysates were analyzed for expression of indicated proteins by immunoblotting. The box indicates c-Raf and Mek1/2 protein expression for which HLFs were treated with 2 μM Cr(VI) without or with SOV. Numbers under a set of blots show the fold expression of phospho-protein/total protein for c-Raf and Mek after normalization by α-tubulin level. (D) HLFs at 24 hr post-transfection with 2 μg of mock, d/n c-Raf, or c/a c-Raf plasmid were assayed for clonogenicity as described in Fig. 4B. Data are expressed as fold increase in clonogenic survival induced by SOV following 1 μM or 2 μM Cr(VI) treatment. Data are the average ± SE of 3 experiments performed in triplicate. *: statistically significant difference from mock control of respective Cr(VI) group at p<0.05. Statistically significant difference between d/n c-Raf and c/a c-Raf is presented by dotted line with p-value.
Next, we studied the effect of GW5074 on clonogenic survival following Cr(VI) exposure with and without SOV co-treatment. A concentration of 50 μM GW5074 was selected since this dose showed the maximum change in relevant phosphoprotein expression with minimum cytotoxicity (Fig. 4A,B). As previously observed, Cr(VI) treatment induced a dose-dependent decrease (p<0.001) in clonogenic survival, while PTP inhibition significantly decreased (p<0.001) Cr(VI)-mediated clonogenic death in HLFs treated with the vehicle (DMSO). GW5074 treatment alone had no effect on clonogenic survival (Fig. 4B). Whereas pre-incubation of HLFs with GW5074 did not prevent the Cr(VI)-induced dose-dependent decrease in clonogenic survival, the presence of GW5074 significantly diminished clonogenic lethality induced by 1 μM and 2 μM Cr(VI), by approximately 2- and 5-fold, respectively (Fig. 4B). Moreover, the SOV-induced increase in clonogenic survival after Cr(VI) exposure was not altered in GW5074-treated cells (Fig. 4B).
Next, we attempted to identify a potential correlation between the enhanced clonogenic survival and c-Raf and Mek phospho-protein expression after GW5074 treatment from parallel analyses of clonogenicity and immunoblotting. As shown in Fig. 4C, the expression level of p-c-Raf (ser338) was increased by around 1.5-fold by SOV in the vehicle control both in the absence and presence of 2 μM Cr(VI) exposure. Notably, this activating phosphorylation of c-Raf was increased around 2-fold after GW5074 treatment in the presence of 2 μM Cr(VI) alone or in conjunction with that of SOV, which is concordant with the enhanced survival shown in Fig. 4B. The expression level of p-Mek1/2 was not altered by Cr(VI) or SOV treatment either alone or combined, in DMSO-treated control cells. In the presence of 50 μM GW5074 treatment alone, the expression level of p-Mek1/2 was increased by 4-fold on average, and was markedly increased to 12- and 8-fold by 2 μM Cr(VI) treatment alone, and in the presence of the PTP inhibitor, respectively.
Although p-Erk1/2 levels were decreased by GW5074 treatment (Fig. 4A), neither Cr(VI), SOV, nor the combination of Cr(VI) and SOV had any further effect on Erk1/2 phosphorylation (data not shown). Furthermore, there was no correlative change in protein expression level of pan-Ras (data not shown), total c-Raf, total Mek1/2 (Fig. 4C) and total Erk1/2 (data not shown) with clonogenic potential under any of these aforementioned conditions.
Taken together, these data suggest that active c-Raf, potentially through downstream Mek1/2 hyperactivation, might be the critical governor of Cr(VI)-mediated clonogenic lethality and that p-c-Raf(ser338) and p-Mek1/2 activity may be associated with the PTP inhibitor-induced increase in clonogenic survival in HLFs.
In order to further examine the role of c-Raf activity in clonogenic survival after the respective Cr(VI), SOV and combined treatment, we employed a genetic approach, and decreased and increased c-Raf activity by d/n c-Raf and c/a c-Raf plasmid transfection, respectively. As shown in Figure 4D, d/n c-Raf transfection decreased SOV-mediated clonogenic survival to 1.8-fold as compared to 2.2-fold induction by SOV in mock-transfected cells while c/a c-Raf transfection further increased SOV-mediated clonogenic survival by 2.9-fold after 1 μM Cr(VI) treatment. This respective attenuation and augmentation of the PTP inhibitor effect on clonogenic survival after transfection with d/n c-Raf and c/a c-Raf was also observed in the presence of 2 μM Cr(VI) treatment. Neither d/n c-Raf nor c/a c-Raf expression alone altered Cr(VI)-mediated clonogenic lethality.
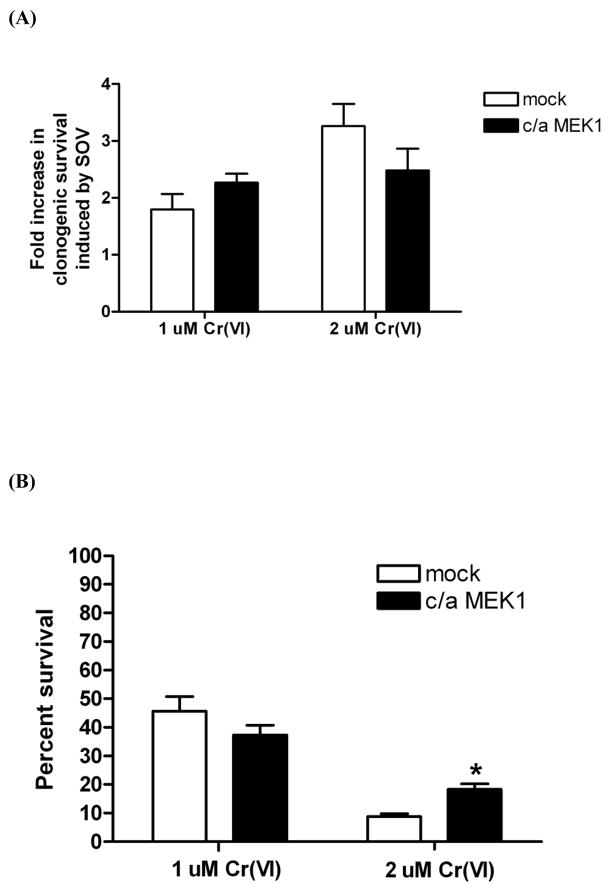
3.5 Increase in activating phosphorylation of Mek decreases Cr(VI)-induced clonogenic lethality, but has no role in the PTP inhibitor effect
The ability of GW5074 to elevate p-Mek1/2 levels and protect HLFs from Cr(VI)-mediated clonogenic death prompted us to investigate the direct role of the activating phosphorylation of Mek (ser217/ser221) in the Cr(VI)-induced clonogenic lethality utilizing a c/a Mek1 mutant in which ser217 and ser221 are substituted to glutamic acid and aspartic acid, respectively. Simultaneous phosphorylation on these 2 amino acids represents the best indirect index for Mek activity. HA-tagged c/a Mek1 plasmid was transiently transfected into HLFs to express activated Mek1 and its effect on clonogenic survival after Cr(VI) treatment in the presence or absence of the PTP inhibitor was examined (Fig. 5). Figure 5A shows that the SOV-induced increase in clonogenic survival after 1 or 2 μM Cr(VI) treatment is not altered by overexpression of activated Mek1. Furthermore, c/a Mek1 overexpression was associated with a statistically significant decrease (p<0.01) in 2 μM Cr(VI)-mediated clonogenic lethality suggesting that Mek1 activity alone is sufficient to decrease Cr(VI)-mediated clonogenic death (Fig. 5B and Fig. 4B). Taken together, activated Mek1 appeared to decrease Cr(VI)-mediated clonogenic lethality, but did not alter the PTP inhibitor’s effect.
Fig. 5. Mek1 activity is not responsible for the PTP inhibitor-induced increase in clonogenic survival and Mek1 activity decreases Cr(VI)-induced clonogenic lethality.
HLFs at 48 hr post-transfection with 2 μg of mock or c/a Mek1 plasmid were assayed for clonogenicity as described in Fig. 4B. (A) Data are expressed as fold increase in clonogenic survival induced by SOV following 1 μM or 2 μM Cr(VI) treatment. Data are the average ± SE of 2 experiments performed in triplicate. (B) Data are expressed as percent clonogenic survival of Cr 0 μM control, and are the average ± SE from 2 experiments performed in triplicate. *: statistically significant difference from mock control of 2 μM Cr(VI) group at p<0.05.
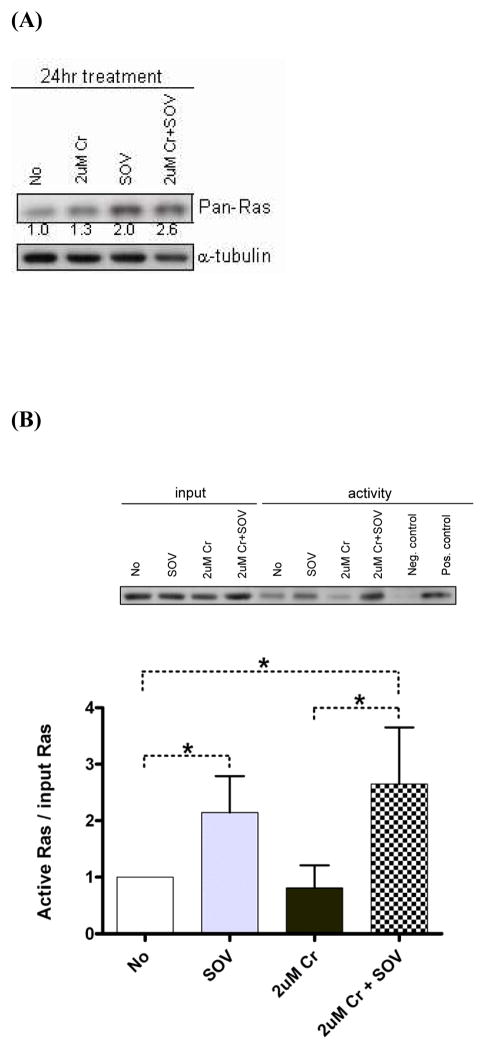
3.6 Ras activity also drives enhanced clonogenic survival after Cr(VI) exposure and PTP inhibition
We examined the role of Ras in clonogenic survival since we observed increased tyrosine phosphorylation of specific proteins that are upstream effecters of this pathway (Fig. 1A-B), and since Ras is one of the direct upstream regulators of c-Raf. We first determined whether total expression of Ras was altered by 24 hr Cr(VI) or SOV treatment either alone or combined in HLFs. Figure 6A shows that SOV alone increased pan-Ras expression by 2-fold, which was modestly augmented to 2.6-fold by co-treatment with Cr(VI). Due to the ability of active Ras to transduce its signal to downstream effectors, we performed a Ras activity assay in HLFs after treatment with SOV and Cr(VI) alone or in combination for 1 hr. A GST-fusion protein containing the Ras-binding domain of c-Raf was used to pull down GTP-bound/active Ras. As shown in Figure 6B, SOV alone increased Ras activity by 2.1-fold on average. While Cr(VI) alone had no effect, in the presence of SOV, Ras activity was increased to 2.8-fold of control, which was significantly higher than that observed in the presence of Cr(VI) alone.
Fig. 6. Ras activity is also responsible for the PTP inhibitor-induced increase in clonogenic survival.
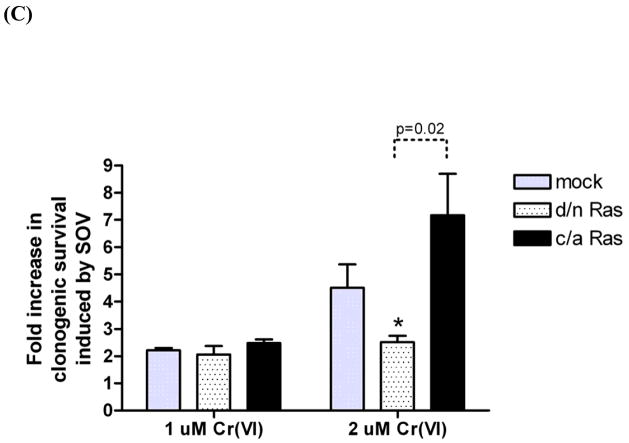
(A)HLFs at 24 hr post-seeding were treated with 2 μM Cr(VI) or 10 μM SOV alone or in combination for 24 hr, and pan-Ras protein expression was analyzed by immunoblotting. Numbers under the blot show pan-Ras protein expression normalized by α-tubulin level. (B) Level ofactive Ras in HLFs treated with 10 μM SOV or 2 μM Cr(VI) alone or in combination for 1 hr was measured by pull down of Ras-GTP bound to the Ras-binding domain of c-Raf followed by immunoblotting with pan-Ras antibody (shown as a insert). Negative and positive control was prepared with GDP and GTPγS, respectively. A bar plot shows the relative level of active Ras expressed as level of active Ras normalized by the Ras input from 3 experiments.*: statistically significant difference among compared groups indicated by dotted line at p<0.05. (C) HLFs at 24 hr post-transfection with 2 μg of indicated plasmid were assayed for clonogenicity as described in Fig. 2. Data are expressed as fold increase in clonogenic survival induced by SOV following 1 μM or 2 μM Cr(VI) treatment. Data are the average ± SE of 3 experiments performed in triplicate. *: statistically significant difference from mock control at p<0.05. Statistical significance is shown with p-value for compared groups indicated by dotted line.
Then, the direct role of Ras in clonogenic potential was assessed by transfection with either d/n Ras or c/a Ras plasmids in HLFs following Cr(VI) exposure with or without SOV co-treatment. As we observed for d/n c-Raf transfection in HLFs, d/n Ras transfection decreased SOV-mediated clonogenic survival to 2.5-fold as compared to 4.5-fold induction in mock-transfected cells after 2 μM Cr(VI) treatment while c/a Ras transfection augmented SOV-mediated clonogenic survival by 7.2-fold (Fig. 6C). Transfection of either d/n Ras or c/a Ras had no further effect on SOV-mediated clonogenic survival after 1 μM Cr(VI) treatment. Neither d/n Ras nor c/a Ras expression altered Cr(VI)-mediated clonogenic lethality in HLFs.
Taken together, our data suggest that the activity of Ras also drives clonogenic survival after Cr(VI) exposure potentially though activation of its direct downstream target, c-Raf, playing a substantive role in the effect observed with the PTP inhibitor.
4. Discussion
In the present study, we demonstrate that the individual activity of two upstream regulators of Mek, i.e., Ras and c-Raf, is associated with enhanced clonogenic survival after PTP inhibition and Cr(VI) exposure. Interestingly, these pro-survival effects of Ras/MAPK pathway members were Mek/Erk-independent in normal human lung fibroblasts. Moreover, overexpression/activation of Mek protected human lung fibroblasts from Cr(VI)-induced clonogenic lethality.
Depending on the extent of the genotoxic insult, an arrested cell could either regain its replicative potential by repairing the damaged DNA faithfully or be removed from the dividing population. The fate of cells after exposure to a genotoxin can be further modulated by the presence of inappropriate growth signals such as perturbation of intracellular tyrosine phosphorylation levels. We have shown the involvement of upstream phospho-tyrosine regulation of survival pathway(s) after Cr(VI) treatment with PTP inhibition through phospho-tyrosine profiling array. Four of these proteins (Abl1, Crkl, Fyn and Grap) have been documented to play a role in cell survival and proliferation as adaptor kinases for receptor tyrosine kinases by regulating Ras/MAPK and/or PI3K/Akt pathways [18–21]. In addition, it has been suggested that FGR (member of Src family tyrosine kinases) may be involved in altering the Ras/MAPK and PI3K/Akt cascades and modifying apoptotic control in prostate cancers [22]. Consistent with our observations, the PTP inhibitor, SOV, has been shown to activate the PI3K/Akt and/or MAPK/Erk signaling pathway during and after ischemia in vivo and in vitro [23–26]. As early as 1 hr after treatment with SOV in HLFs, there was a ~4-fold increase in tyrosine phosphorylation of PTEN which was consistent with an increase in in vitro Akt kinse activity by co-treatment with the PTP inhibitor and Cr(VI) (Lal et al., unpublished results). In the present study, we explored roles of two specific survival pathways, PI3K/Akt and Erk MAPK, in clonogenic survival after Cr(VI) insult with or without PTP inhibition.
We have studied Cr(VI) as a model genotoxin in order to elucidate survival signaling pathways in the early stages of carcinogenesis. The Cr(VI) concentration used in the current studies, 1 – 2 μM, was shown to result in growth arrest and clonogenic lethality, whereas the maintenance of protein tyrosine phosphorylation by PTP inhibition during Cr(VI) exposure abrogated these two biological end points [8]. Akt1 was found to be required for the bypass of Cr(VI)-mediated G1/S checkpoint arrest (Lal et al., unpublished results), which was accompanied by an increase in short-term cell survival, as measured by cell proliferation assay up to 72 hr post-transfection (Suppl. Fig. 2A) and as previously reported [27]. Nevertheless, transient c/a Akt1 expression had no effect on Cr(VI)-mediated clonogenic death (Suppl. Fig. 2B). This suggests two possibilities to explain our findings on the unique role of Akt1 in short-term and long-term cell survival after Cr(VI) insult in the presence of either exogenously overexpressed Akt1 protein or PTP inhibition. First, it is possible that transient Akt1 activity is sufficient to release cell cycle arrest and growth arrest induced by Cr(VI) and sustained Akt1 activity may be required for surviving cells to maintain their replicative potential for longer periods after Cr(VI) exposure. Second, survival pathway(s) other than Akt1 may be involved in the modulation of Cr(VI)-mediated clonogenic death in HLFs. Our present data support this latter hypothesis.
The roles of the Erk MAPK pathway in cell survival and growth have been extensively studied alone or with other mitogenic pathways in immortalized or cancer cells. Inhibition of either PI3K/Akt or Erk MAPK signaling pathways suppressed growth of breast cancer cell lines, but Erk MAPK signaling was critical for cell survival [28]. Coutant et al (2002) reported that the antiapoptotic function of EGF in primary cultures of rat hepatocytes was dependent on the Erk MAPK pathway whereas the inhibition of the PI3K cascade had no effect on hepatocyte survival [29]. In contrast, McCubrey et al (2006) reported that Raf/Mek/Erk is associated with proliferation and the prevention of apoptosis while Akt is associated with the long-term clonogenicity in hematopoietic cells [15]. Based on published reports it is feasible that the contribution of specific survival pathways to determine long-term survival/death upon genotoxic stress is cell type-specific and cell stage-specific. A persistent activation of Erk MAPK in rat hepatoma cells following exposure to 0.3 – 3.0 μM Cr(VI) up to 16 hrs has been suggested as a mechanism of Cr-induced carcinogenesis [30]. High levels of Cr(VI) (>10μM) have been shown to activate MAPKs (i.e., Erk, p38) [31] while lower concentrations (<10μM) were more selective in activating JNK in immortalized lung epithelial cells [32]. Alternatively, we have previously shown that 6 μM Cr(VI) induced a burst of Erk activity in HLFs, ranging from 0.5–3 hr after exposure, which returned to basal levels by 24 hr. Neither sensitization to, nor inhibition of, Cr(VI)-induced clonogenic lethality was observed after Erk inhibition by 25–100 μM PD98059 indicating a lack of Erk involvement in Cr(VI)-mediated clonogenic death [13]. In addition, our present data show that both Erk silencing with siRNA and abrogation of Erk activity by additional U0126 treatment in Erk-silenced cells had no effect on Cr(VI)-induced clonogenic lethality.
Our present study is the first report that activated Mek, in the absence of Erk activity plays a role in the protection of normal human cells from genotoxin-induced clonogenic death. Indeed, we have shown that hyperphosphorylation of Mek after GW5074 treatment as well as Mek1 overexpression dramatically decreased Cr(VI)-induced clonogenic lethality in HLFs. These observations suggest the presence of a novel, Erk-independent signaling pathway, potentially involving a kinase substrate downstream of Mek that is able to transduce its signal to regulate cell growth/proliferation. Alternatively, Mek activation alone may be sufficient to regulate cell growth upon genotoxin exposure. It is possible that Mek translocates to the nucleus and regulates cell growth or interacts with cytosolic effectors that regulate cell survival/growth in HLFs. Indeed, Mek translocation to the nucleus has been reported and its nuclear localization was promoted by G2-M progression [33]. A potential role of Mek translocation in enhanced clonogenic survival after genotoxin exposure is currently under investigation in our laboratory.
In sharp contrast, in the absence of genotoxin exposure, either exogenously expressed or chemically induced Mek activity had no effect on HLF clonogenic potential. In other words, whereas induced Mek activity during Cr(VI) exposure was cytoprotective, it did not increase the basal level of clonogenic potential when the cells were not challenged by Cr(VI). This interesting phenomenon was not observed for Ras and c-Raf activity. This unique role of Mek activity during genotoxin stress may have resulted from the presence of a threshold for activity or activating phosphorylation level above which enhanced clonogenic survival can be achieved in HLFs. In support of this hypothesis, a very recent study reported that a precise threshold level of Myc is required for tumor maintenance, whereupon there is a switch in gene expression program from a state of proliferation to a state of proliferative arrest and apoptosis [34].
The expression level of total Mek1/2 protein was not altered after treatment with GA or GW5074 which is consistent with the idea that activating phosphorylation/activity of Mek is critical to the decrease in Cr(VI)-mediated clonogenic death in HLFs. Again this emphasizes the importance of level and duration of kinase activity in the Ras/MAPK axis during Cr(VI) insult and in the determination of cell fate (i.e., clonogenic survival and death). Duration of Akt and Mek activity as measured by the expression of their phosphorylated forms was monitored after transfection with c/a Mek1 or c/a Akt1 (Suppl. Fig. 3). A sustained expression level of HA tag and total Mek1 protein was observed up to 5 days post-transfection while HA tag and p-Akt(ser473) was expressed by 3 days post-transfection, suggesting that a sustained level of Mek activity during Cr(VI) exposure and recovery may contribute to an increase in long-term survival of Cr(VI)-challenged cells and that transient level of Akt activity may be responsible for short-term cell survival including cell cycle checkpoint override.
The Ras/Raf/Mek/Erk signaling cascade plays a critical role in the transmission of signals from the outside of the cell through Erk translocation to the nucleus to regulate gene expression and cell survival. Generally this signaling module is serially activated by extracellular stimuli and plays its roles in cell proliferation and survival in a context-dependent manner. Also the individual components of this cascade, i.e., Ras, c-Raf, Mek1, Mek2, or Erk1/2 have been shown to be sufficient to stimulate the cell growth accompanied by cellular transformation [35–43] [44]. In agreement with these reports, constitutively expressed Ras or c-Raf individual activity was sufficient to enhance the PTP inhibitor’s effect on clonogenic survival. In addition, neither Mek nor Erk was associated with the PTP inhibitor effect. Notably, the HSP90 chaperone protein was also shown to play a role in the PTP inhibitor effect on Cr(VI)-induced clonogenic death. Although GA, an HSP90 inhibitor and non-specific Raf inhibitor, disrupts numerous signaling pathways implicated in cancers (see review [45]), we focused on the PI3K/Akt and Ras/Raf/Mek/Erk pathways in the present study since tyrosine phosphorylation of several known upstream effectors of those pathways were increased by the PTP inhibitor, SOV. The effect of GA on Cr(VI)-induced clonogenic lethality was pronounced as it not only abrogated the SOV effect, but also augmented the Cr(VI) effect (Fig. 3B). In contrast, the extent of the decrease in the SOV-mediated effect on Cr(VI)-induced clonogenic lethality either by d/n c-Raf or d/n Ras was about 50% effective (Fig. 4D, Fig. 6C). These findings suggest that other client proteins of HSP90 may also be responsible for the PTP inhibitor effect. Based on our current data and published reports [45–47], ERBB2, BCR-ABL, B-Raf, and Fyn among > 100 known HSP90 client proteins are potential candidates to help us to fully understand the PTP inhibitor-mediated decrease in Cr(VI)-mediated clonogenic lethality, and consequent enhanced mutagenesis [8].
Nucleofection is a promising electroporation-based transfection method to perform gain- and loss-of-function studies in normal cells. Also, pharmacological inhibitors are very useful tools to block a particular target in a signaling cascade and determine its biological role in cells if there is high-specificity for target molecule. Transient transfection with siRNA or expression plasmids in HLFs was carried out successfully in our hands to study the respective and combined roles of Ras, c-Raf, Mek1, Erk1/2, and Akt1 in Cr(VI)-mediated clonogenic lethality with or without PTP inhibition. In contrast, a Raf-1 inhibitor, GW5074, resulted in an unexpected response in one of its target kinase effectors, Mek, in HLFs. GW5074 has been reported to be a potent and selective inhibitor for c-Raf kinase activity, thus accompanied by down-regulation of MAPK activity as measured by a cell-based assay of inhibition of EGF-stimulated Erk activation [17]. In agreement with this report we observed down-regulation of Erk and p90Rsk activity by 50 μM GW5074 treatment for 24 hrs in HLFs. Nevertheless, the direct downstream effector of c-Raf, Mek1/2, was not inhibited by GW5074, but rather activated by GW5074, as demonstrated by an increase in its activating phosphorylation. More recently and consistent with our present data, GW5074 treatment of neurons caused c-Raf activation and stimulated the Raf/Mek/Erk pathway [48]. These contradictory findings surrounding the use of the Raf inhibitor GW5074 emphasize that the blockade of one particular component in a signaling cascade by a small molecule chemical inhibitor could differentially affect its downstream or upstream targets due to the structural characteristics of this type of inhibitor as a general ATP competitor. Therefore, special caution is needed to thoroughly examine a chemical inhibitor’s functionality in an experimental system.
5. Conclusion
Our current study is the first to delineate the roles for specific components of the Ras/Raf/Mek/Erk pathway in determination of clonogenic survival/death following an acute exposure to low concentrations of Cr(VI) in normal human lung cells. Current studies highlight a novel pro-survival mechanism which is Mek/Erk-independent and Ras/c-Raf-dependent, which underlies the observed increased clonogenic survival in the face of genotoxic stress in the presence of PTP inhibition. We postulate that enhanced survival after genotoxin exposure may predispose normal cells to be more susceptible to malignant transformation and oncogenesis. Our findings provide insight into genotoxin-induced early carcinogenesis and highlight potential survival signaling pathway interactions relevant to molecularly targeted therapeutics for cancer prevention and treatment.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the support and encouragement of Dr. Steven Patierno. We thank Drs. Bernard Bouscarel and Travis O’Brien for helpful guidance and insightful comments. We thank Ashley Larrimore and Dr. Laura Beaver for technical help and Gina Chun, Madhu Lal, and Kristen Wright for helpful suggestions. The authors thank Dr. Philip Tschlis at Tufts University School of Medicine for c/a Akt1 plasmid, Dr. Natalie G. Ahn at University of Colorado for Mek plasmids, Dr. Bong-Hyun Ahn at Pusan National University for empty plasmids, and Dr. Kang-Yell Choi at YonSei University for c-Raf and Ras plasmids. Antibodies for Mek1 and Mek2 were gifts from Dr. Katherine Kennedy at GWU. This work was supported by National Institutes of Health Grant CA 107972 to SC.
Abbreviations
- PTP
protein tyrosine phosphatase
- FGR
Gardner-Rasheed feline sarcoma viral (v-fgr) oncogene homolog
- ABL
v-abl Abelson murine leukemia viral oncogene homolog
- HLFs
human lung fibroblasts
- MEK
mitogen extracellular kinase
- ERK
extracellular signal-related kinase
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- d/n
dominant-negative
- c/a
constitutively active
- HSP90
90-kDa heat shock protein
- EGF
epidermal growth factor
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dy GK, Adjei AA. J Clin Oncol. 2002;20(13):3016–3028. doi: 10.1200/JCO.2002.02.112. [DOI] [PubMed] [Google Scholar]
- 2.Hahn WC, Weinberg RA. Nat Rev Cancer. 2002;2(5):331–341. doi: 10.1038/nrc795. [DOI] [PubMed] [Google Scholar]
- 3.Ostman A, Hellberg C, Bohmer FD. Nat Rev Cancer. 2006;6(4):307–320. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- 4.Tonks NK. Nat Rev Mol Cell Biol. 2006;7(11):833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 5.Chang F, Steelman LS, Shelton JG, Lee JT, Navolanic PM, Blalock WL, Franklin R, McCubrey JA. Int J Oncol. 2003;22(3):469–480. [PubMed] [Google Scholar]
- 6.Lewis TS, Shapiro PS, Ahn NG. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 7.Yoon S, Seger R. Growth Factors. 2006;24(1):21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 8.Bae D, Camilli TC, Chun G, Lal M, Wright K, O’Brien TJ, Patierno SR, Ceryak S. Mutat Res. 2008 doi: 10.1016/j.mrfmmm.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, Vande Woude GF, Ahn NG. Science. 1994;265(5174):966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 10.Whalen AM, Galasinski SC, Shapiro PS, Nahreini TS, Ahn NG. Mol Cell Biol. 1997;17(4):1947–1958. doi: 10.1128/mcb.17.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon SH, Yoon JY, Park YN, Jeong WJ, Kim S, Jho EH, Surh YJ, Choi KY. 2007;282(19):14482–14492. doi: 10.1074/jbc.M611129200. [DOI] [PubMed] [Google Scholar]
- 12.Yun MS, Kim SE, Jeon SH, Lee JS, Choi KY. 2005;118(Pt 2):313–322. doi: 10.1242/jcs.01601. [DOI] [PubMed] [Google Scholar]
- 13.Ceryak S, Zingariello C, O’Brien T, Patierno SR. 2004;255:139–149. doi: 10.1023/b:mcbi.0000007270.82431.3e. [DOI] [PubMed] [Google Scholar]
- 14.Clark JA, Black AR, Leontieva OV, Frey MR, Pysz MA, Kunneva L, Woloszynska-Read A, Roy D, Black JD. J Biol Chem. 2004;279(10):9233–9247. doi: 10.1074/jbc.M312268200. [DOI] [PubMed] [Google Scholar]
- 15.McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA, D’Assoro AB, Salisbury JL, Mazzarino MC, Stivala F, Libra M. Adv Enzyme Regul. 2006;46:249–279. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Schulte TW, Blagosklonny MV, Ingui C, Neckers L. J Biol Chem. 1995;270(41):24585–24588. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- 17.Lackey K, Cory M, Davis R, Frye SV, Harris PA, Hunter RN, Jung DK, McDonald OB, McNutt RW, Peel MR, Rutkowske RD, Veal JM, Wood ER. Bioorg Med Chem Lett. 2000;10(3):223–226. doi: 10.1016/s0960-894x(99)00668-x. [DOI] [PubMed] [Google Scholar]
- 18.Feng GS, Ouyang YB, Hu DP, Shi ZQ, Gentz R, Ni J. J Biol Chem. 1996;271(21):12129–12132. doi: 10.1074/jbc.271.21.12129. [DOI] [PubMed] [Google Scholar]
- 19.Pendergast AM. Adv Cancer Res. 2002;85:51–100. doi: 10.1016/s0065-230x(02)85003-5. [DOI] [PubMed] [Google Scholar]
- 20.Sattler M, Salgia R. Leukemia. 1998;12(5):637–644. doi: 10.1038/sj.leu.2401010. [DOI] [PubMed] [Google Scholar]
- 21.Tang X, Feng Y, Ye K. Cell Death Differ. 2007;14(2):368–377. doi: 10.1038/sj.cdd.4402011. [DOI] [PubMed] [Google Scholar]
- 22.Edwards J, Krishna NS, Witton CJ, Bartlett JM. Clin Cancer Res. 2003;9(14):5271–5281. [PubMed] [Google Scholar]
- 23.Feng Y, Bhatt AJ, Fratkin JD, Rhodes PG. Brain Res Bull. 2008;76(1–2):102–108. doi: 10.1016/j.brainresbull.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Gao N, Ding M, Zheng JZ, Zhang Z, Leonard SS, Liu KJ, Shi X, Jiang BH. J Biol Chem. 2002;277(35):31963–31971. doi: 10.1074/jbc.M200082200. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto J, Morioka M, Hasegawa Y, Kawano T, Yoshinaga Y, Maeda T, Yano S, Kai Y, Fukunaga K, Kuratsu J. J Pharmacol Exp Ther. 2006;318(3):982–991. doi: 10.1124/jpet.106.104562. [DOI] [PubMed] [Google Scholar]
- 26.Wu DN, Pei DS, Wang Q, Zhang GY. Neurosci Lett. 2006;404(1–2):98–102. doi: 10.1016/j.neulet.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Lee SR, Park JH, Park EK, Chung CH, Kang SS, Bang OS. J Cell Physiol. 2005;205(2):270–277. doi: 10.1002/jcp.20395. [DOI] [PubMed] [Google Scholar]
- 28.Ripple MO, Kalmadi S, Eastman A. Breast Cancer Res Treat. 2005;93(2):177–188. doi: 10.1007/s10549-005-4794-6. [DOI] [PubMed] [Google Scholar]
- 29.Coutant A, Rescan C, Gilot D, Loyer P, Guguen-Guillouzo C, Baffet G. Hepatology. 2002;36(5):1079–1088. doi: 10.1053/jhep.2002.36160. [DOI] [PubMed] [Google Scholar]
- 30.Kim G, Yurkow EJ. Cancer Res. 1996;56(9):2045–2051. [PubMed] [Google Scholar]
- 31.Wang S, Shi X. Carcinogenesis. 2001;22(5):757–762. doi: 10.1093/carcin/22.5.757. [DOI] [PubMed] [Google Scholar]
- 32.O’Hara KA, Klei LR, Barchowsky A. Toxicol Appl Pharmacol. 2003;190(3):214–223. doi: 10.1016/s0041-008x(03)00188-1. [DOI] [PubMed] [Google Scholar]
- 33.Tolwinski NS, Shapiro PS, Goueli S, Ahn NG. J Biol Chem. 1999;274(10):6168–6174. doi: 10.1074/jbc.274.10.6168. [DOI] [PubMed] [Google Scholar]
- 34.Shachaf CM, Gentles AJ, Elchuri S, Sahoo D, Soen Y, Sharpe O, Perez OD, Chang M, Mitchel D, Robinson WH, Dill D, Nolan GP, Plevritis SK, Felsher DW. Cancer Res. 2008;68(13):5132–5142. doi: 10.1158/0008-5472.CAN-07-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bond J, Jones C, Haughton M, DeMicco C, Kipling D, Wynford-Thomas D. Exp Cell Res. 2004;292(1):151–156. doi: 10.1016/j.yexcr.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 36.Bos JL. EMBO J. 1998;17(23):6776–6782. doi: 10.1093/emboj/17.23.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. Proc Natl Acad Sci USA. 1995;92(17):7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fedorov YV, Rosenthal RS, Olwin BB. J Cell Biol. 2001;152(6):1301–1305. doi: 10.1083/jcb.152.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frost JA, Geppert TD, Cobb MH, Feramisco JR. Proc Natl Acad Sci USA. 1994;91(9):3844–3848. doi: 10.1073/pnas.91.9.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerkhoff E, Rapp UR. Mol Cell Biol. 1997;17(5):2576–2586. doi: 10.1128/mcb.17.5.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pages G, Lenormand P, L’Allemain G, Chambard JC, Meloche S, Pouyssegur J Proc Natl Acad Sci USA. 1993;90(18):8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seger R, Seger D, Reszka AA, Munar ES, Eldar-Finkelman H, Dobrowolska G, Jensen AM, Campbell JS, Fischer EH, Krebs EG. J Biol Chem. 1994;269(41):25699–25709. [PubMed] [Google Scholar]
- 43.Skinner J, Bounacer A, Bond JA, Haughton MF, DeMicco C, Wynford-Thomas D. Oncogene. 2004;23(35):5994–5999. doi: 10.1038/sj.onc.1207798. [DOI] [PubMed] [Google Scholar]
- 44.Kerkhoff E, Rapp UR. Cancer Res. 1998;58(8):1636–1640. [PubMed] [Google Scholar]
- 45.Powers MV, Workman P. Endocr Relat Cancer. 2006;13(Suppl 1):S125–S135. doi: 10.1677/erc.1.01324. [DOI] [PubMed] [Google Scholar]
- 46.Shimamura T, Li D, Ji H, Haringsma HJ, Liniker E, Borgman CL, Lowell AM, Minami Y, McNamara K, Perera SA, Zaghlul S, Thomas RK, Greulich H, Kobayashi S, Chirieac LR, Padera RF, Kubo S, Takahashi M, Tenen DG, Meyerson M, Wong KK, Shapiro GI. Cancer Res. 2008;68(14):5827–5838. doi: 10.1158/0008-5472.CAN-07-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yun BG, Matts RL. Cell Signal. 2005;17(12):1477–1485. doi: 10.1016/j.cellsig.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Chin PC, Liu L, Morrison BE, Siddiq A, Ratan RR, Bottiglieri T, D’Mello SR. J Neurochem. 2004;90(3):595–608. doi: 10.1111/j.1471-4159.2004.02530.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.