The CD4+CD25+FoxP3+ regulatory T (Treg) cells play an important role in regulating the immune response. These Treg cells are present in peripheral blood and lymphoid organs and have a high potential for immunotherapy in clinics. Adoptive Cell transfer therapy using CD4+CD25+ cells has been shown to prevent autoimmune diseases and has also induced transplant tolerance in mice. Treg cells low frequency in peripheral blood will necessitate its ex-vivo expansion to enable adaptive immunotherapy. Recently, it has been reported that rapamycin, an immunosuppressive agent, inhibits T cell proliferation while selectively increasing the number of Treg cells. Based on this additional mode of action, rapamycin can be used to expand Treg cells for ex-vivo cellular therapy in T-cell-mediated diseases and in transplantation. We have reported the ex-vivo expansion of baboon Treg cells, using irradiated pig PBMC and IL-2, and have demonstrated the suppression of autologus CD4+CD25neg T-cell proliferation in response to pig PBMCs. In the present study, we have expanded baboon CD4+ T cells in the presence or absence of rapamycin (0.1-10 nM) using irradiated pig PBMCs and IL-2 to enrich the regulatory T cells. CD4+CD25+FoxP3+ Treg cells were increased up to two times in the presence of rapamycin as compared without rapamycin in-vitro. However, a higher dose of rapamycin (≥10nM) considerably decreases the number of Treg cells. Furthermore, purified CD4+CD25+ Treg cells enriched from CD4+ cells in the presence of rapamycin were able to suppress the baboon anti-porcine xenogeneic immune responses in vitro up to 90% at a 1:1 ratio (T regulatory Cells: T effector cells) and suppression ability exists even at a 1:256 ratio whereas freshly isolated natural Treg cells suppress only 70% at 1:1 and lose their suppressive ability (>50%) at 1:16. Our results demonstrate that the addition of rapamycin to the culture enriches the Treg phenotype and induces functional regulatory T cells. This method may allow the production of large numbers of regulatory cells for the preclinical testing of Treg cell therapy in a non human primate model.
CD4+CD25+ Regulatory T (Treg) cells play an important role in regulating and/or suppressing immune responses by controlling Ag-driven T-cell proliferation. These cells are a low frequency subpopulation of CD4+ cells, representing 1-2% of total lymphocytes. Treg cells that are thymic derived are characterized as naturally occurring regulatory T cells and are believed to control autoreactive T cells, maintain homeostasis, and induce peripheral tolerance. Treg cells also express forkhead box protein 3 (FoxP3), a Forkhead family transcription factor that converts CD4+CD25neg cells into suppressive CD4+CD25hi T cells. As most T cells express CD25 after stimulation, FoxP3 is considered to be a specific marker of Treg cells. Experiments in small animal models suggest that the adoptive transfer of natural Treg cells can prevent autoimmune diseases. Edinger et al reported that CD4+CD25+ regulatory T cells prevent graft-versus-host disease (GVHD) without affecting graft versus tumor (GVT) activity after bone marrow transplantation (BMT) in mice(1). For clinical Treg immunotherapy, high numbers of cells are needed. Successful ex-vivo expansion of naturally occurring human and murine CD4+CD25+ T cells is achieved after TCR stimulation in the presence of T cell growth factors such as IL-2, IL-7 and IL-15(2). Ex-vivo expanded cells retain suppressive function in both in-vitro and in-vivo animal models and induce experimental transplantation tolerance(3). Furthermore, other studies have demonstrated that fresh and ex-vivo expanded CD4+CD25+ regulatory T cells can also prevent the development of GVHD and inhibit cardiac graft rejection in murine models(4, 5).
Rapamycin is an immunosuppressive agent which blocks the intracellular signaling in response to T cell growth factor, favors expansion of CD4+CD25+ T cells in-vitro and in-vivo(6). Recently, we have identified the phenotype and expanded ex-vivo baboon naturally occurring CD4+CD25+ Treg cells(7). The aim of the present study was to evaluate the effect and utility of rapamycin on Treg cell induction and expansion of unmanipulated baboon CD4+ T cells.
Materials and Methods
Isolation of cells
Peripheral blood samples and spleen of baboon were obtained from Southwest Tx. Mini-pig blood for irradiated pig PBMCs was obtained from the NIH ungulate facility (Poolesville, MD). Lymphocytes were isolated as described before(7). Isolated mononuclear cells from spleens and blood from the baboons were stained for flow cytometric analyses with CD4 FITC (L200) and CD25 PE (4E3). 7-AAD (BD Biosciences) staining was used to exclude non-viable cells. Human PBMCs were stained with CD4 FITC (RPA-T4), CD25 PE (4E3), and CD3 APC (UCHT1). Intracellular staining for FoxP3 in baboon cells was performed as described(7).
Purification and expansion of Treg cells
As described earlier(7), isolated mononuclear cells were first enriched for CD4+ T cells by MACS, and CD4+ CD25high and CD4+ CD25neg populations were further sorted by FACS aria after immunostaining. Purified natural CD4+ CD25high cells (5×104) were either stimulated with irradiated pig PBMC (1×105) or beads coated with anti CD3/CD28 (Dynal) and IL-2 (500IU/ml) for 4 weeks.
Expansion of Treg cells in presence of Rapamycin
Enriched CD4+ T cells (105) were stimulated with (1:4) anti-CD3/CD28 Dynal beads (Invitrogen) and, or 1 × 105 irradiated pig PBMCs in the presence of human rIL-2 without and with different concentrations (0.1nM to 10nM) of rapamycin (Sigma Aldrich) for 4 weeks. Repeated re-stimulations were done with irradiated PBMCs of the same pig. The percentage and absolute number of CD4+CD25+FoxP3+ were analyzed by flow cytometry.
Suppression Assay
After 4 weeks, CD4+CD25high cells from the cultures were purified by FACS sorting and these cells (5×104) were co-cultured with baboon CD4+ CD25neg T cells and stimulated with 1×105 irradiated pig PBMC in triplicate in 96 well round bottom tissue culture plates (Costar) in a final volume of 200 μl complete RPMI (cRPMI: RPMI, 10% FCS, 50 μg/ml Gentamycin, 2 mM L-glutamine). The cells were cultured for 5 days in a humidified incubator with 5% CO2. Proliferation of CD4+CD25neg was assessed by the MTT-based Cell Titer 96® AQueous One Solution Cell Proliferation ((Promega) kit. MTS Tetrazolium compound is bioreduced by cells into a colored Formazan product that is soluble in tissue culture medium. The quantity of Formazan product was measured by the absorbance at 490 nm, which is directly proportional to the number of living cells in culture. Percentages of suppression of CD4+CD25neg T cells proliferation were measured by the following formula:
Results and Discussion
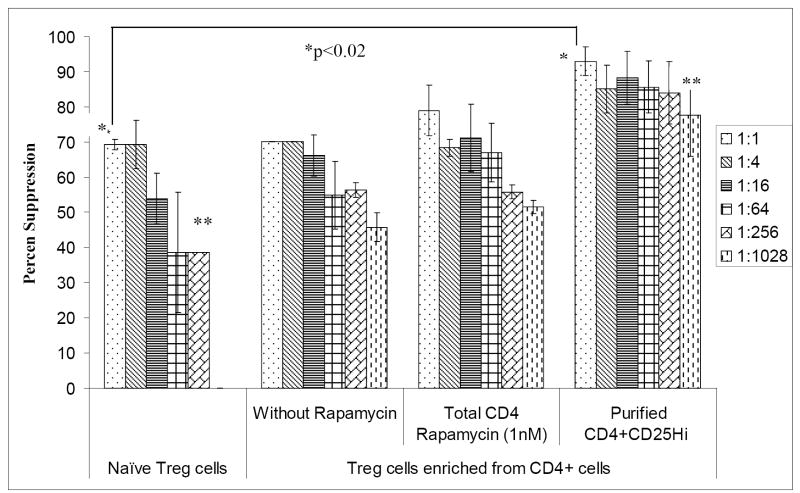
In our recent report, we have identified and characterized naturally occurring baboon regulatory T cells by flow cytometry in different lymphoid organs and compared with adult human blood7. We also successfully expanded Treg cells 200 -1000 fold in four weeks. We further demonstrated that these expanded Treg cells are functional and more potent suppressors of pig-to- baboon xeno MLR. Since a high number of Treg cells are required over a shorter period of time for effective immunotherapy, in this study, we decided to evaluate the effect of rapamycin on the induction and expansion of Treg cells. Immunosuppressive drugs, such as rapamycin, have shown to selectively expand CD4+CD25+FoxP3+ Treg cells and can possibly promote expansion of Treg cells from CD4+ T cells. Therefore, in this study, we analyzed the effect of rapamycin on the expansion of CD4+CD25+FoxP3+ directly from CD4+ T cells. First, the optimal concentration of rapamycin was determined for the ex-vivo expansion of CD4+CD25high Treg cells. Enriched CD4+ cells were stimulated with anti CD3/CD28 beads, and additional IL-2 was added with different concentrations of rapamycin (0.1-10nM). The percentages of CD4+CD25high cells analyzed by flow cytometry were found to increase in the presence of 1nM rapamycin together with CD3/CD28 beads and 500 IU/ml IL-2 after 7 and 14 days in culture. Consistent with other studies (8), we have also found that higher doses of rapamycin (≥10nM) considerably decrease the CD4+CD25high cells in culture whereas lower dose showed no difference in expansion rates for MACS-purified CD4+CD25+ cells. Furthermore, enriched CD4+ cells were stimulated with irradiated pig PBMCs, and additional IL-2 was added without and with 1 nM rapamycin. Though a much greater proliferation of CD4+ cells was seen in the absence of rapamycin, the numbers of CD4+CD25+ Treg cells were significantly increased only when rapamycin was added to the culture (Table). In vitro expanded CD4+ T cells were also stained with FoxP3 mAb. The percentages of CD4+CD25+FoxP3+ were 1.5x-2x times in presence of rapamycin (1nM) as compared to without rapamycin (20.6%±5.26 vs 11.2%±3.6)(Fig 1). Absolute numbers of CD4+CD25+FoxP3+ were also determined and were found to be increased in 4 weeks in presence of rapamycin (1nM). Next, we purified the CD4+CD25high from CD4+ cells expanded ex-vivo in the presence of rapamycin. Further, in order to analyze the suppressive potential of these cells, these CD4+CD25high were co-cultured with baboon CD4+CD25neg cells, irradiated pig PBMC, and rIL-2. As shown in Fig 2, purified CD4+CD25+ Treg cells enriched from CD4+ cells in the presence of rapamycin were able to suppress the baboon anti-porcine xenogeneic immune response in vitro up to 92.9%± 4.1 at a 1:1 ratio (T regulatory Cells: T effector cells) and suppression ability exists (84.1% ± 8.9) even at a 1:256 ratio whereas freshly isolated natural Treg cells suppress only 69.3 ± 1.4 at 1:1 and loose their suppressive ability (53.9% ±7.2) at a 1:16.
Table.
Absolute number of in vitro-expanded baboon CD4+, CD4+CD25- and CD4+CD25+ T cells in the presence or absence of rapamycin. Purified CD4+ (0.6×106) cells were cultured in presence of irradiated pig PBMCs and 500 IU/ml IL-2, with and without rapamycin, for 4-weeks. Viable cell counts and Flow cytometric analysis were performed at weekly intervals. The data shown is mean ± SD of experiments performed with cells from 4 different animals.
| After 3 weeks | After 4 weeks | |||||
|---|---|---|---|---|---|---|
| CD4+ (Mean± SD)* | CD4+CD25- (Mean± SD) * | CD4+ CD25+ (Mean± SD) * | CD4+ (Mean± SD)* | CD4+CD25- (Mean± SD)* | CD4+ CD25+ (Mean± SD)* | |
| Without Rapamycin | 63.7 ± 26.2 | 59.6 ± 23.0 | 4.2±3.2 | 147.0 ± 51.5 | 138.8 ± 48.2 | 8.8 ± 1.5 |
| With Rapamycin | 36.5 ± 9.4 | 28.5 ± 11.1 | 8.4±1.9 | 54.5 ± 12.3 | 42.8 ± 11.1 | 14.1 ± 2.2 |
in millions
Figure 1.
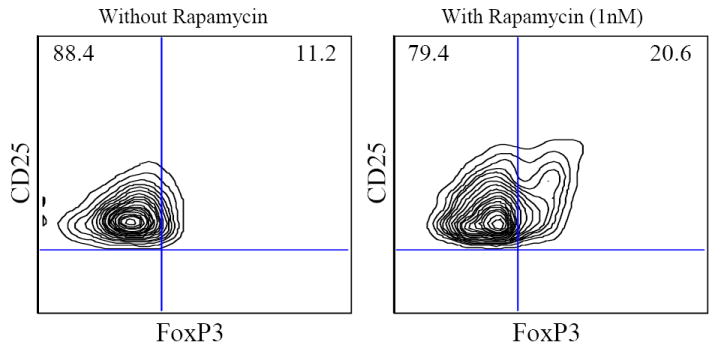
Flow cytometric analysis of FoxP3 staining on CD4+CD25+ T cells with and without rapamycin after 4 week expansion of CD4+ T cells in culture.
Fig: 2.
Percent suppression of the proliferation of baboon CD4+CD25neg cells. Naïve CD4+CD25high Treg cells, ex-vivo expanded CD4+ T (5×104) expanded without and with rapamycin and purified CD4+CD25high obtained from ex-vivo expanded CD4+ T in presence of Rapamycin were co-cultured with autologus CD4+CD25neg effector (Teff) cells (5×104) and stimulated with irradiated pig PBMCs. Percent suppression of CD4+CD25neg cell proliferation was assessed by using the above mentioned formula (p value *p<0.02, **P<0.03).
High numbers of Treg will be required for effective Treg immunotherapy and to facilitate tolerance. Ex vivo Treg expansion can provide the solution to obtain these high numbers. In this study, our results demonstrate that rapamycin selectively expands CD4+CD25highFoxP3+ cells from CD4+ cells and thereby are able to suppress the anti xenogenic response. These CD4+CD25hi cells cultured ex vivo in the presence of rapamycin may be used to test adoptive transfer therapy of Treg cells for tolerance induction and prevention of cellular rejection in the pig to baboon xenotransplantation model.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 2.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193(11):1303. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood. 2007;109(2):827. doi: 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- 4.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99(10):3493. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105(12):4743. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 7.Porter CM, Horvath-Arcidiacono JA, Singh AK, Horvath KA, Bloom ET, Mohiuddin MM. Characterization and expansion of baboon CD4+CD25+ Treg cells for potential use in a non-human primate xenotransplantation model. Xenotransplantation. 2007;14(4):298. doi: 10.1111/j.1399-3089.2007.00416.x. [DOI] [PubMed] [Google Scholar]
- 8.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178(1):320. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]