Abstract
Gap-junction channels connect the cytoplasm of adjacent cells, allowing the diffusion of ions and small metabolites. They are formed at the appositional plasma membranes by a family of related proteins named connexins. Mutations in connexins 26, 31, 30, 32, and 43 have been associated with nonsyndromic or syndromic deafness. The majority of these mutations are inherited in an autosomal recessive manner, but a few of them have been associated with dominantly inherited hearing loss. Mutations in the connexin26 gene (GJB2) are the most common cause of genetic deafness. This review summarizes the most relevant and recent information about different mutations in connexin genes found in human patients, with emphasis on GJB2. The possible effects of the mutations on channel expression and function are discussed, in addition to their possible physiologic consequences for inner ear physiology. Finally, we propose that connexin channels (gap junctions and hemichannels) may be targets for age-related hearing loss induced by oxidative damage. Antioxid. Redox Signal. 11, 309–322.
Introduction
Connexins (Cxs) are the protein subunits that constitute the gap-junction channel (GJCh), one of the most important pathways for intercellular communication that mediates the ionic and metabolic coupling of adjacent cells. Gap junctions are plasma-membrane structures formed by hundreds or thousands of GJCh units. Each GJCh is constituted by the docking of two independent hemichannels (or connexons) present in the appositional plasma membranes of the two contacting cells. Six connexin subunits oligomerize in an intracellular compartment (ER-Golgi) to constitute a hemichannel that is distributed to the plasma membrane by the secretory pathway, likely aided by microtubules tethered to the vesicles (82, 88). At sites of cell apposition, the hemichannel must dock with its complementary hemichannel to constitute a GJCh, allowing the formation of an aqueous pore that connects cytoplasms of adjacent cells. The channels are permeable to ions and small molecules, like second messengers (cAMP, cGMP, ATP, etc.) or diverse metabolites (sugars, amino acids, glutathione, etc.), with a size cutoff about 1 kDa (for comprehensive reviews, see refs. 82 and 102).
In addition to this traditional view, strong evidence suggests that undocked or unopposed hemichannels can open to allow communication between the cellular interior and the extracellular space under both physiologic and pathologic conditions (5, 83). Connexins are encoded by a family of homologous genes. A screening of the human genomic database identified 20 connexin genes (102). Connexins all have the same topology in the plasma membrane; with the amino and carboxyl termini, and one intracellular loop facing the cytoplasm, four transmembrane domains, and two extracellular loops. Although the homology between connexins is high, important differences between these proteins are found in the intracellular loop and the carboxyl terminus where many regulatory elements act, like kinases and cytoskeletal binding proteins. Additional diversity of GJCh is produced by the formation of heteromeric channels (in which a hemichannel is constituted from more than one connexin type) and/or heterotypic channels (produced by the docking of two hemichannels, each made by a different connexin). These connexin combinations may produce channels with particular functional and regulatory properties (6, 7, 66). The importance of GJCh for human physiology was pointed out by the findings of many genetic diseases associated with mutations in different connexin genes. Nine connexin genes have been implicated in diverse human hereditary disorders, like cataracts, Charcot-Marie-Tooth disease, oculodentodigital dysplasia, and inherited nonsyndromic or syndromic deafness. The latter condition is associated with a variety of mild to profound skin disorders (39). Of all connexin-associated diseases, deafness is the most important in terms of frequency in the human population. Although inherited deafness is genetically heterogeneous, mutations in the gene encoding Cx26 (GJB2) have been shown to account for a large proportion of cases in every population tested, whereas four other connexins, Cx30, Cx31, Cx32, and Cx43, have also been linked to either nonsyndromic or syndromic sensorineural hearing loss (48, 49, 75). The Cx43 mutation in deafness is controversial. Whereas the two mutations found in Cx43, L11F and V24A, are probably located in the Cx43 pseudo gene on chromosome 5, a recent report by Jian-Juo Yang et al. (107) describes a new mutation in the functional Cx43 gene in Taiwanese deaf patients. This review summarizes the most relevant and recent information about different mutations in connexin genes found in human patients, with emphasis on Cx26. The possible effects of the mutations on channel expression and function are discussed, in addition to their possible physiologic consequences for inner ear and skin physiology. Finally, we propose that connexin channels (gap junctions and hemichannels) may be targets for age-related hearing loss induced by oxidative damage.
Gap-Junction Networks in the Cochlea
The cochlea is the structure in the inner ear that contains the transduction machinery to sense the vibration transmitted from the middle ear after a sound stimulus. It is formed by three adjacent and paralleled tubular compartments: the scala media, the scala tympani, and the scala vestibule (Fig. 1). The principal cellular components of the cochlea are epithelial cells, fibrocytes, and receptor cells named hair cells, which are located in the wall of the tubular compartments. These compartments are filled with two types of solutions: (a) the perilymph, the ionic composition of which is identical to that of the extracellular solution, fills the scala tympani and scala vestibule; and (b) the endolymph, which possesses a high concentration of K+ (150 mM), fills the scala media (Fig. 1). Another important functional property of the cochlea is the high positive potential of the endolymph (approximately +80 mV), termed the endocochlear potential (Fig. 1). The endocochlear potential is probably a K+ equilibrium potential produced by the stria vascularis, a two-layered epithelium forming the wall of the scala media (Fig. 1) (101). The key elements are the potassium channels Kir 4.1 in the plasma membrane of intermediate cells and K+ transporters in the basal membrane of marginal cells of the stria vascularis (see review in ref. 101). The endocochlear potential is critical during the activation of hair cells. Hair cells in the basilar membrane of the scala media transform the mechanical stimuli produced by sounds into electrical signals transmitted to the brain. They are polarized cells, with the ciliated apical membrane exposed to the endolymph, and the cell body contacting the perilymph (or cortilymph). Sound causes vibration of the basilar membrane, inducing the deflection of hair cell cilia with subsequent opening of mechanosensitive nonselective cation channels (21) that allow endolymphatic K+ to enter into hair cells, resulting in their depolarization. The endocochlear potential directly contributes to the high sensitivity of hair cells to mechanical stimulation because it creates a large driving force for K+ influx of ~160 mV, the difference between the resting potential of hair cells (−80 mV) and the endolymph potential (+80 mV). After activation of hair cells, K+ is released to the perilymph (Fig. 1). It has then been proposed that K+ can circulate from the perilymph to the endolymph through the “cochlear lateral wall” (reviewed in ref. 41). This is supported by observations that inhibition of K+ flow from the perilymphatic space inhibits endocochlear potential, and perilymphatic perfusion of K+-free solution rapidly and prominently suppresses the endocochlear potential (41). This recirculation pathway may include different cellular components of the cochlear wall. K+ moves first through supporting and epithelial cells in the basilar membrane, to fibrocytes in the spiral ligament (lateral wall connective tissue), and eventually to epithelial cells of the stria vascularis, from which the K+ is released to the endolymph. It has been hypothesized that the K+ circulation may be favored by the formation of two independent syncytia in the cochlea lateral wall: an epithelial gap-junction network (supporting cells and epithelial cells on the basilar membrane), and a connective tissue gap-junction network (fibrocytes of spiral ligament and epithelial cells of stria vascularis (Fig. 1) (51). It is proposed that these two networks mediate the K+ circulation from perilymph to endolymph (41, 100). However, the loss of Cx30 in the mouse ear does result in significant loss of the endocochlear potential generated by the stria vascularis (17) without changes in the K+ concentration and volume of the endolymph. Mutations in Cx26 (V84L) that do not affect ionic conductance, but selectively affect IP3 permeability, have been genetically linked with deafness (4). Thus, the exact role of gap-junction channels in the K+ circulation, and in the generation of the endocochlear potential, has yet to be directly demonstrated.
FIG. 1.
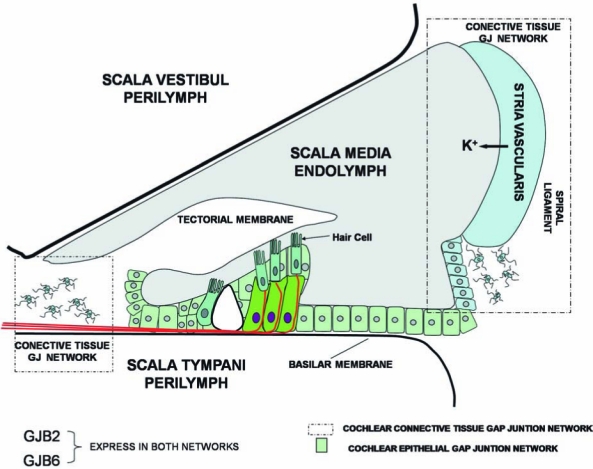
Diagram of the cochlea cellular systems showing the gap-junction networks. Deiter cells (green) and supporting and epithelial cells (light green) constitute the epithelial gap-junction network. Connective cells (fibrocytes; star morphology) and cells in the stria vascularis (light blue) constitute the connective tissue gap-junction network. Cells in both networks coexpress Cx26 (GJB2) and Cx30 (GJB6). It has been proposed that K+ recirculates back to the endolymph through these networks. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Connexin Expression in Inner Ear and Gap-Junction Function
For obvious reasons, most of the information with respect to connexin distribution in the inner ear has been obtained from animal models, mainly rodents, restricting our understanding of connexin physiology to the supposition that similar distributions would happen in humans. In rodents, several connexins (Cx26, Cx30, Cx31, Cx32, and Cx43) have been detected in the epithelia and connective tissue of the cochlea (15, 32, 36, 47, 55, 56, 60, 104, 112), supporting the formation of a gap-junction network in these tissues. In addition, gap-junction plaques (32–35, 50, 51) and extensive intercellular coupling (4, 35, 64, 84–86, 110, 111) were observed in both the cochlear connective tissue and the organ of Corti. Strong antibody co-labeling has been observed for Cx26 and Cx30 in these regions (1, 35, 55, 56, 68, 92), and immunogold studies have shown an equal distribution of these connexins within individual gap-junction plaques (33). Moreover, coimmunoprecipitation experiments under conditions that favor isolation of hemichannels suggested that Cx26 and Cx30 co-oligomerize in cochlear tissue (1, 33, 92). Together, these observations strongly support the existence of heteromeric Cx26/Cx30 gap-junction channels in the cochlea, although heterotypic forms could also exist. Although the GJChs are largely nonselective for ions, they do present selectivity to larger molecules like second-messengers nutrients and fluorescent tracers. The molecular permeability of gap junctions consisting of Cx26 and Cx30 was studied previously in vitro by using dyes of different charge and molecular weight, including Lucifer Yellow (LY) (charge, −2; molecular mass, 443 Da) and neurobiotin (NB) (charge, +1; molecular mass, 287 Da). In heterologous expression systems, gap junctions between cells expressing Cx26 alone are equally permeable to both LY and NB, whereas Cx30 gap junctions are far more permeable to NB than to LY. In addition, human Cx26 and Cx30, coexpressed in HeLa or human kidney cells, form functional heteromeric channels that are permeable to cationic and anionic tracers (92, 108). However, because homotypic Cx30 channels are more permeable to cationic tracers (108), it is possible that heteromeric Cx26/Cx30 channels may have different permeability properties than their respective homomeric counterparts, increasing the functional diversity of GJCh in the cochlea. In addition, cells expressing Cx26/Cx30 heteromeric channels showed faster Ca2+ intercellular signaling than did cells expressing homomeric Cx26 or Cx30 channels (92). These permeability differences may have critical functional consequences. Interestingly, in rat after the onset of hearing (P12–P13), LY transfer was not evident in any recordings from supporting cells immediately adjacent to hair cells (Deiters cells, inner border cells), suggesting a significant decrease in the numbers of Cx26 homomeric channels between these cells in hearing animals (45). The relative contributions of homotypic and heterotypic GJCh to the cochlea physiology remains to be resolved.
In addition to the well-known function of GJCh in intercellular communication, hemichannels present in the non-appositional plasma membrane may open under several physiologic and pathologic conditions (19, 20) to increase plasma-membrane permeability. Functional hemichannels are present in the mature organ of Corti and allow uptake of large anionic molecules under certain conditions (113), as well as the release of ATP.
The importance of GJCh in the cochlea has been highlighted because of its disruption in several forms of nonsyndromic and syndromic deafness (100). Notably, abnormality of the genes GJB2 and GJB6, encoding Cx26 and Cx30, respectively, are the most frequent genetic causes of deafness (for a complete updated list of mutations, see http://davinci.crg.es/deafness/). However, mutations in other connexin genes, like GJB3 (encoding Cx31), have also been reported in certain deaf patients. Animal models with conditional knockout of the GJB2 gene in the ear (16), targeted deletion of GJB6 (93), or transgenic mice overexpressing a dominant-negative form of Cx26 (53), all have severe hearing loss, supporting the idea that Cx26 and Cx30 gap-junction proteins play important roles in the physiology of the cochlea and hearing.
Nonsyndromic Deafness
The most common form of genetic deafness, nonsyndromic hearing loss, has been predominantly associated with mutations in the GJB2 gene, encoding Cx26. To date, 90 mutations in the Cx26 gene have been associated with nonsyndromic deafness, which account for half of congenital cases of hearing impairments (a complete and updated list of mutations can be found in: http://davinci.crg.es/deafness/). Most of these mutations cause mild to severe hearing loss that is inherited recessively (Fig. 2 and Table 1). However, a few mutations cause dominant forms of inherited disease (Fig. 2 and Table 1). One such mutant, M34T, appeared to display dominant characteristics in some families (8), and recessive in others (22, 42). Analysis in the exogenous Xenopus oocyte expression system revealed that the dominant-negative effects of M34T on coexpressed wtCx26 was strongly dependent on the ratio of expression, so that promoter differences between the wt and mutant genes could account for familial variances (55).
FIG. 2.
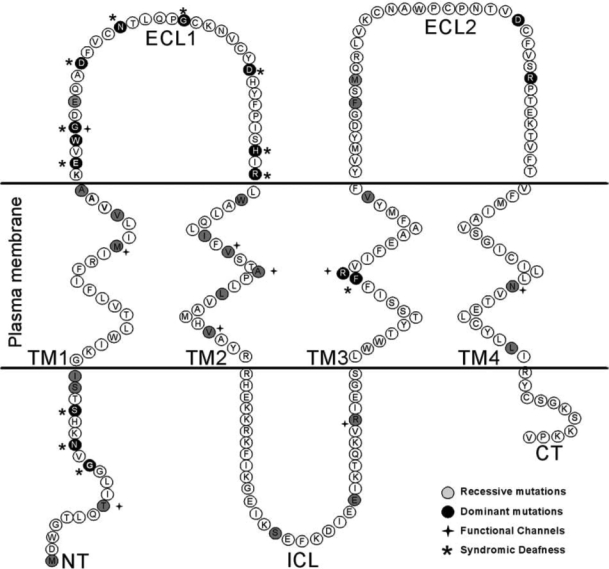
Schematic topology of Cx26 relative to plasma membrana, showing the distribution of deafness-related mutations of Cx26. The amino acid position mutated (missense point substitutions) are labeled.
Table 1.
Mutations in GJB2 That Cause Non-Syndromic Deafness
| Mutation | Type | Domain | Mechanism | Reference |
|---|---|---|---|---|
| M1V | R | NT | Truncated protein | 96 |
| T8M | R | NT | Makes functional GJCh with lower conductance. Voltage gating properties different from wt. | 71 |
| G12V | D | NT | Plasma membrane localization. GJCh not permeable to LY | 24 |
| S19T | R | NT | Some plasma membrane localization. GJCh not permeable to LY | 24 |
| 120T | R | NT | Unable to form functional GJCh. | 109 |
| M34T | D | TM1 | Plasma membrane localization. GJCh permeable to NB but not to LY. Reduced propagation of calcium wave. When expressed in oocyte; difficult to detect electrical coupling between paired oocytes. Possibly because it makes channels with small conductance (10-fold less than wild type). Dominant negative of wt Cx6 and Cx30. | 8, 24, 74, 90, 96 |
| V37I | R/D | TM1 | Did not induce the formation of homotypic junctional channels in paired Xenopus oocytes or KEK293 cells. Possible dominant negative of wt Cx26 and Cx30. | 13, 74, 109 |
| A40G | R | TM1 | Unable to form functional GJCh. | 109 |
| W44C | D | ECL1 | Plasma membrane localization. Unable to form functional channels in oocytes and mammal cells. Dominant negative of wt Cx26 and Cx30. | 12, 35, 65, 68, 81 |
| W44S | ||||
| E47K | R | ECL1 | Plasma membrane unable to form functional GJCh or hemichannels. | 91 |
| G59V | ECL1 | Unable to form functional GJCh in oocytes. Dominant negative of wt Cx30. | 74 | |
| W77R | R | TM2 | Protein is neither trafficked to membrane nor able oligomerize efficiently. Did not induce the formation of homotypic junctional channels in paired Xenopus oocytes | 65 |
| I82M | TM2 | Unable to form functional GJCh in oocytes. Dominant negative of wt Cx26 and Cx30 | 74 | |
| V84L | R | TM2 | Plasma membrane localization. Functional channels permeable to fluorescent tracers. Reduced permeability to IP3. Form intercellular channels in oocytes. Conductance similar to that of wtCx26 | 4, 13, 98, 109 |
| A87S | R | TM2 | Form functional channels. Reduced permeability to fluorescent tracers and IP3. | 109 |
| L90P | R | TM2 | Plasma membrane localization (low levels of junctional plaques). Very low incidence dye transfer. Did not induce the formation of homotypic junctional channels in paired Xenopus oocytes. Possible dominant negative of wt Cx30. | 13, 24, 74, 96 |
| V95M | R | TM2 | Form functional channels that are permeable to fluorescent tracers in transfected N2A cells | 98, 109 |
| Conductance similar to that of wt Cx26. Reduced permeability to IP3. | ||||
| S113R | R | ICL | Did not induce the formation of homotypic junctional channels in paired Xenopus oocytes | 13 |
| delE120 | R | ICL | Did not induce the formation of homotypic junctional channels in paired Xenopus oocytes | 13 |
| R127H | R | ICL | Plasma membrane localization. Reducing junctional permeability. Unable to form functional GJCh in Xenopus oocytes. Dominant negative of wt Cx30. | 24, 74, 96, 98 |
| R143W | R/D | TM3 | Form functional channels that are permeable to fluorescent tracers in transfected N2A cells. Conductance similar to that of wt Cx26. However, does not induce the formation functional GJCh in paired Xenopus oocytes. Dominant negative of wt Cx30. | 71, 74, 98 |
| V153I | TM3 | Unable to form functional GJCh in Xenopus oocytes. | 71 | |
| F161S | ECL2 | Plasma membrane localization. GJCh not permeable to NB | 96 | |
| M163V | R | ECL2 | Unable to form functional GJCh in Xenopus oocytes. | 13 |
| D179N | D | ECL2 | Unable to form functional GJCh in Xenopus oocytes. | 109 |
| R18RQ | D | ECL2 | Unable to form functional GJCh in Xenopus oocytes. | 109 |
| R184P | ECL2 | Protein is neither trafficked to membrane nor able to oligomerize efficiently. Unable to form functional GJCh | 13, 96 | |
| N206S | TM4 | Form functional GJCh in oocytes | 71 | |
| L214P | R | TM4/CT | Unable to form functional GJCh | 71 |
The list includes only mutations that have been study in some detail using exogenous expression systems. For a complete list of mutation see (http://davinci.crg.es/deafness). R: recessive mutation; D: dominant mutation; NT: Amino Terminal; TM1: Transmembrane Domain 1; ECL1: Extracellular loop 1; TM2: Transmembrane Domain 2; ICL: Intracellular loop; TM3: Transmembrane Domain 3; ECL2: Extracellular loop 2; TM4: Transmembrane Domain 4; CT: Carboxi Terminal. wt: wild type.
By far the most commonly found disease-associated mutations of Cx26 are deletions in two regions of GJB2 (35delG and 235delC (28, 52, 57, 99, 106). 35delG and 235delC are the most common Cx26 mutations found in caucasoid families and east Asian populations, respectively. These mutations result in a frameshift and premature termination of the protein. 35delG is caused by errors in replication of a string of Gs at this location, similar to the high-frequency mutation site in CFTR. Less-common point mutations of Cx26 associated with deafness have been characterized in recombinant expression systems (shown in Table 1). The majority of Cx26 mutations tested are loss-of-function mutations (Table 1), resulting from mistargeting or trafficking of the channels to the plasma membrane, or failure to form normal open channels (Fig. 3). Three loss-of-function mutations have been shown to allow targeting to the plasma membrane but do not support gap-junction plaque formation [G12V, W77R, and R184P (13, 24, 65, 96)]. Mutations S19T, W44C (W44S), E47K, L90P, R127H, and F161S are loss-of-function mutations that form gap-junction plaques in exogenous expression systems but fail to open intercellular channels (13, 24, 65, 68, 74, 91, 96, 98). Mutations V37I, A40G, G59V, I82M, S113R, delE120, V153I, M163V, D179N, R184Q, and L214P were shown to be nonfunctional in Xenopus oocytes, but difficulties with immunofluorescence in these cells did not allow assessment of whether these mutants reach the plasma membrane or are trapped in an intracellular compartment (13, 71, 74, 109).
FIG. 3.
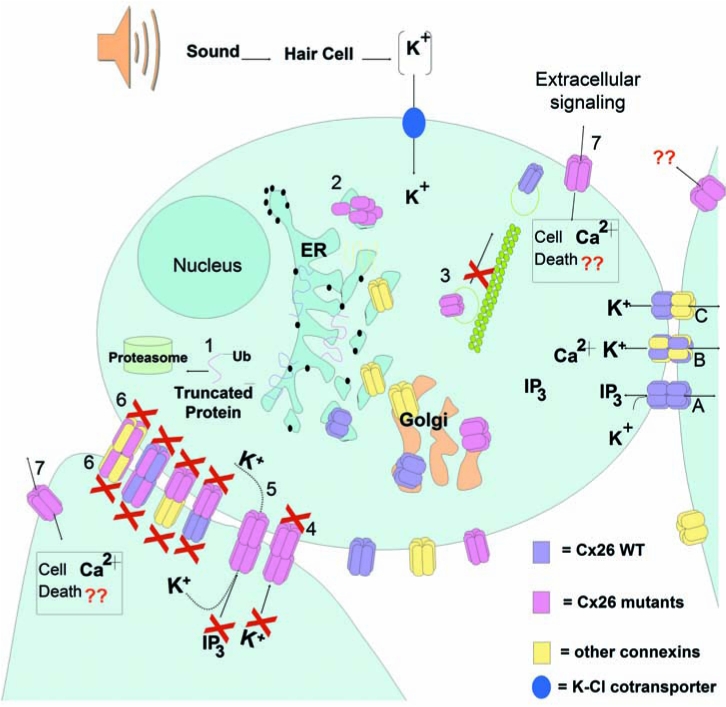
Pathogenic mechanism of deafness-associated Cx26 mutations. Wild-type connexins oligomerize in the ER/Golgi. Hemichannels traffic to plasma membrane through the secretory pathway by a cytoskeletal-dependent mechanism. Epithelial and supporting cells in the cochlea express both Cx26 and Cx30. (A) Cx26 homomeric GJCh are permeable to ions, like K+, and bigger molecules, like IP3. Cx30 homomeric GJCh have high permeability to K+ but lower permeability to IP3. (B) Heteromeric Cx26–Cx30 GJCh. (C) Heterotypic channels. Deafness-associated Cx26 mutations may produce 1. Truncated protein connexin subunits; 2. Oligomerization defects impeding the assembly of hemichannels; 3. Defective trafficking of the hemichannels, impeding targeting to the plasma membrana; 4. Nonfunctional channels; normal trafficking and assembly into the plasma membrane and gap-junction plaque formation, but the GJCh are closed or their pore structure severely affected, impeding the diffusion of ions and small metabolites; 5. Functional channels permeable to ions but with reduced permeability to bigger molecules like IP3, affecting propagation of calcium waves or other metabolites; 6. Mutant Cx26 that can act as dominant negative of co-expressed wild-type connexins. Mutant Cx26 can oligomerize with wild-type connexins, producing nonfunctional heteromeric channels. Heterotypic combination between mutant Cx26 hemichannel and wild-type hemichannels can also lead to nonfunctional channels; 7. Aberrant functionality of free hemichannels in the plasma membrane, allowing an increase in plasma-membrane permeability that may lead to cell death due to either loss of important intracellular metabolites (like ATP or NAD), or increase intracellular calcium concentration. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Several deafness-causing mutations (T8M, M34T, V84L, A87S, V95M, R143W, N206S) form functional channels with similar conductance to wtCx26 channels, but more subtle differences in gating or permeability (4, 8, 13, 71, 90, 96, 98, 109). Interpretation of these differences is complicated by the observation that they can vary between expression systems. For example, whereas Wang and collaborators (98) found that mutant R143W, expressed in N2A cells, forms gap-junction channels with junctional conductance similar to that of the wild type, Mese and colleagues (71) found the opposite when the mutant was expressed in oocytes. A similar situation was found for mutant V95M, which is apparently not functional when expressed in HeLa cells (4) but forms functional channels between HEK293 cells (109). These discrepancies suggest that important regulatory elements for gap-junction function may be missing in some cellular systems, supporting the need to use the most physiologically relevant system to study the pathogenic mechanism of deafness-associated Cx26 mutations.
The ability of some pathogenic mutations to produce functional channels that conduct ionic current similarly to the wild type, suggests that, in some cases of deafness, potassium recirculation in the inner ear may be normal. Therefore, abnormalities in the exchange of other metabolites through the cochlear gap-junction network may also produce deafness (Fig. 3). For example, mutations V84L, A87S, and V95M produce functional channels that present reduced permeability to the second-messenger IP3 compared with wtCx26 gap-junction channels (4, 109). In organotypic culture of mouse cochlea, injection of IP3 into one supporting cell elicited Ca2+ waves that propagate to the neighboring cells in seconds. The Ca2+ wave is not generated by Ca2+-induced Ca2+ release, but is dependent on the diffusion of IP3 through GJCh (4, 109).
The mechanism by which loss of IP3 intercellular transfer leads to deafness is a matter for speculation. One possibility is that it may indirectly affect K+ spatial buffering through cochlear supporting cells. For example, release of Ca2+ from intracellular stores may gate Ca2+-activated ion currents, especially Cl− currents, increasing anion efflux into the endolymph (Fig. 4). Such a movement of Cl− is likely to promote an equivalent efflux of K+ through membrane channels, preserving the electroneutrality of the cytoplasm and the endolymphatic fluid. This hypothetic mechanism might contribute to the homeostasis of K+, facilitating its re-absorption into the endolymph (54). In summary, loss of gap-junction permeability to IP3 may reduce Ca2+ signalling between supporting cells, thus affecting the KCl balance of cochlear fluids, leading to excitotoxic death of the hair cells. It is important to note that this is a hypothetic model that requires testing, and that the selective loss of gap-junction coupling could affect other metabolites important for cellular viability.
FIG. 4.
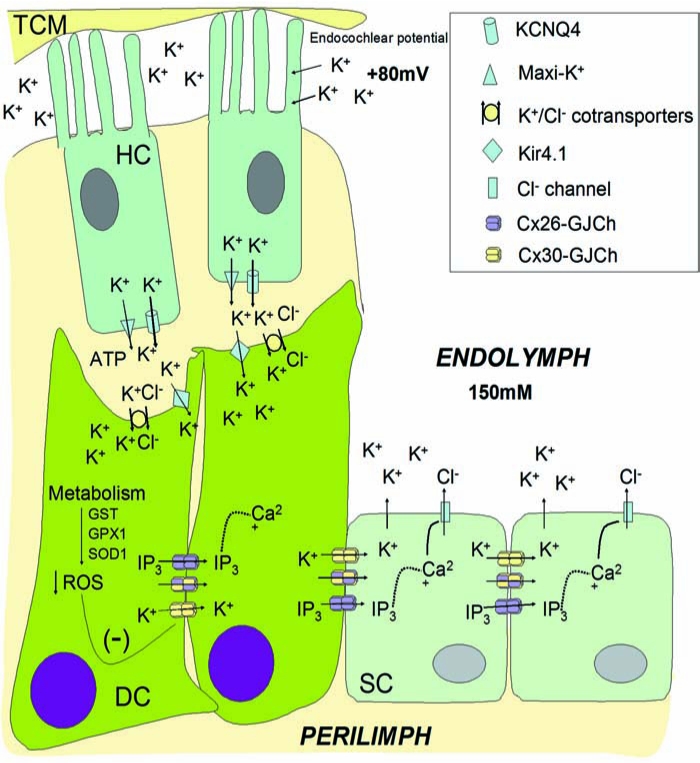
K+ recirculation in the cochlea may be affected by the level and properties of gap-junction intercellular communication. Activation of hair cells (HCs) by sound-induced movement of the tectorial membrane (TM) produces an increase in extracellular K+ at the basal surface by activation of KCNQ4 and maxi-K+ potassium channels in the basal membrane of HC. Thereafter, K+ is buffered by supporting and epithelial cells. K+ enters Deiter cells (DCs) through K+/Cl− cotransporters and K+ channels like Kir4.1. Then K+ is redistributed to supporting cells (SCs) and epithelial cell by means of GJCh connecting these cells (epithelial GJ network). In addition, some extracellular signals, like ATP (activating purinergic receptors), induce IP3 production and Ca2+ release from the ER compartment. Diffusion of IP3 through Cx26-GJCh allows calcium wave propagation, a signal for many cellular functions. It has been proposed that Ca2+ may activate Cl− channels of supporting cells, allowing the efflux of Cl− to the extracellular milieu that favors K+ circulation to the endolymph. High metabolism of cochlear cells favors generation of reactive oxygen species (ROS) that are under the control of antioxidant enzymatic systems (GTS, GTX1, SOD1). ROS are negative regulators of GJCh, reducing intercellular coupling in many systems. Aging-induced hearing loss may be associated with inhibition of GJCh by increasing ROS production and reduction in the function of antioxidant enzymatic systems in the cochlea. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Syndromic Deafness
Connexin-related deafness is sometimes associated with congenital skin disorders, such us Vohwinkel syndrome, keratitis–ichthyosis–deafness syndrome (KID) and palmoplantar keratodermas (Fig. 2 and Table 2). In these syndromes, hearing loss is associated with abnormal epidermal keratinization. As in the sensory epithelium in the inner ear, abundant gap junctions are found in the epidermis, with multiple and overlapping expression of several connexins (Cx43, Cx31, Cx26, Cx30) (10, 14, 29, 31, 63, 103). Thus, to cause skin disease, it has been hypothesized that the mutated Cx26 protein affects the normal gap-junction function of other connexins through a dominant-negative effect over wild-type coexpressed connexins. This hypothesis is supported by: (a) all syndromic mutations are inherited in a dominant way; and (b) in exogenous expression systems, some of these mutants have been shown to act as dominant-negative inhibitors of coexpressed wild-type connexins 26, 30, or 43 (Table 2).
Table 2.
Dominant Mutations in GJB2 Gene That Cause Syndromic Deafness (Deafness Associated with Skin Disorders)
| Mutation | Domain | Skin disorder | Mechanism | Reference |
|---|---|---|---|---|
| G12R | NT | KID | Form Gap Junction plaques. Incapable of inducing intercellular coupling. Changes may interfere with conformation and flexibility of the NT and gating polarity. | 76, 79 |
| N14Y | NT | KID | Impaired gap junction communication. Change in local structural flexibility of the NT. | 2 |
| N14K | NT | Clouston-syndrome-like | Non determined | 97 |
| S17F | NT | KID | Form Gap Junction plaques. Incapable of inducing intercellular coupling. | 79 |
| DelE42 | ECL1 | Palmoplantar keratoderma | Failed to induce intercellular coupling. Dominant negative effect on co-expressed wt Cx26, Cx30 and Cx43. | 81 |
| G45E | ECL1 | KID Fatal | Form functional GJCh. Hemichannels have significantly greater whole cell currents than the while type Cx26 hemichannels. Expression may produce cell death. | 38, 91 |
| D50N/D50Y | ECL1 | KID and HID | Impairs trafficking (rescue partially by Cx30 and Cx26). Incapable of inducing intercellular coupling. | 29, 79 |
| N54K | ECL1 | BPS | Form gap junction plaques. Functionality not determined. | 78 |
| G59A | ECL1 | Palmoplantar keratoderma | Impairs trafficking. Affects the ability to form functional channels. Dominant-negative effect on co-expressed wt Cx26, Cx30 or Cx43. | 35, 68 |
| D66H | ECL1 | Vohwinkel syndrome | Interfering with assembly into connexon, docking with adjacent cells or gating properties of the gap junction. Dominant negative effect on co-expressed wt Cx26, Cx30 or Cx43. | 35, 68, 81, 95 |
| H73R | ECL1 | Palmoplantar keratoderma | Impairs trafficking to plasma membrane. Dominant dominant-negative effect on co-expressed wt Cx26. | 25 |
| R75W | ECL1 | Palmoplantar keratoderma | Incapable of inducing electrical conductance; does not affect protein trafficking and suppressed the activity of co-expressed wtCx26, Cx30 or Cx43. | 23, 27, 35, 68, 80, 81, 109 |
| F142L | TM3 | Psoriasiform mucocutaneous | Non determined | 11 |
The list includes only mutations that have been study in some detail using exogenous expression systems. For a complete list of mutation see (http://davinci.crg.es/deafness). KID: keratitis-ichthyosis-deafness syndrome; HID: Hystrix-like ichthyosis with deafness syndrome; BPS: Bart-Pumphrey syndrome; VS: Vohwinkel syndrome. NT: Amino Terminal; TM1: Transmembrane Domain 1; ECL1: Extracellular loop 1; TM2: Transmembrane Domain 2; ICL: Intracellular loop; TM3: Transmembrane Domain 3; ECL2: Extracellular loop 2; TM4: Transmembrane Domain 4; CT: Carboxi Term.
Vohwinkel syndrome is characterized by relatively mild sensorineural deafness, hyperkeratosis of the soles, palms, and knuckles, with constriction rings on the digits, sometimes leading to autoamputation. Affected individuals in all Vohwinkel syndrome families analyzed to date are carriers of the D66H mutation (62). In addition, mutation H73R causes Vohwinkel-like syndrome (25). Both mutations are located in extracellular loop 1 (ECL1; Fig. 2), a critical domain for assembly and function of GJCh. Consistently over several experimental systems, these mutations act as dominant-negative effectors of wild type Cx26 or Cx30 gap junction, but not hemichannels (35, 68, 95). Transgenic mice designed to express D66H specifically in the suprabasal epidermal keratinocytes [the cells where Cx26 is upregulated after minor skin trauma (61)] have skin abnormalities similar to those observed in true Vohwinkel syndrome patients (3). In addition, in these transgenic mice, Cx26 accumulates in the cytoplasm of suprabasal keratinocytes, like the observed staining for Cx26 in Vohwinkel patients' skin (3). Interestingly, abundant TUNEL staining in the affected epidermis indicates that either excessive apoptosis, or premature terminal differentiation, contribute to the disease phenotype. In addition, high levels of cell-proliferation markers were observed in the underlying basal epidermis (where the transgene is not expressed), indicating enhanced proliferation of adjacent tissue (3). The authors of this work hypothesized that the mutant protein in the suprabasal keratinocytes disrupts the epidermal gap-junction network, leading to premature terminal differentiation, with retention of cohesion between corneocytes in the stratum corneum. Premature keratinocyte death might produce compensatory basal cell proliferation, leading to massive thickening of the stratum corneum (3).
KID syndrome is a rare ectodermal dysplasia characterized by vascularizing keratitis, profound sensorineural hearing loss, and progressive erythrokeratoderma, a clinical triad that indicates a failure in development and differentiation of multiple stratifying epithelia. All Cx26 dominant mutations linked to KID syndromic deafness are located in the N terminal (NT) or in the ECL1 of the protein (Fig. 2). Previous studies suggest that the NT region of connexins is involved in the voltage gating of gap-junction channels and in the recognition between different connexin subunits during oligomerization. Cx26 mutations, G12R, N14Y, and S17F, produced gap-junction plaques, indicating that they do not affect trafficking and gap-junction formation (2, 79). However, these mutations either do not form open channels or significantly affect channel permeability. Previous studies suggest that charged amino acid residues in the amino terminus of connexins form part of the transjunctional voltage sensor of GJCh and play a fundamental role in ion selectivity (76, 77). Results from studies of the voltage dependence of NT mutants predict that residues 1–10 lie within the channel pore. The NT is proposed to contribute to the channel vestibule by folding back toward the pore mouth via the conserved G12 residue (76). In support of this interpretation, the three-dimensional structure of M34A shows that the channel vestibule is blocked by a physical structure that looks like a plug (73). In this work, it is proposed that the plug most likely corresponds to the NT, because the pore region is an ideal location to detect the transjunctional voltage field (73). More recently, these authors showed that partial deletion of the NT results in a marked decrease in this plug mass (72a).
The dynamic properties of the synthetic NT peptide containing the KID mutation N14Y, as revealed by two-dimensional nuclear magnetic resonance and circular dichroism, suggest that this mutation induces profound changes in the local structural flexibility of the NT (2). Therefore, a possibility is that some mutations in the NT domain may produce channels that open with low probability because the plug is stabilized within the pore vestibule. Other Cx26 mutations associated with KID syndrome are clustered in the first half of ECL1 (Fig. 2). Belonging to this group are mutations A40V, G45E, and D50N or D50Y, which have been studied in exogenous expression systems (29, 38, 79, 91). In two different functional expression systems [i.e., Xenopus oocytes and HEK (human embryonic kidney)-293 cells], G45E led to increased hemichannel activity and cell lysis (38, 91) (Fig. 3). Similar results were observed in oocytes expressing A40V (38). In either case, this severe phenotype was rescued by increasing extracellular Ca2+, which closes the hemichannels. These studies support the proposal that treatment strategies should include the development of pharmacologic agents that modulate extracellular Ca2+ concentration, or specifically block Cx26 hemichannels in the epidermis and cochlea.
The other KID mutation located in the ECL1, D50N, did not produce functional channels when expressed in NEB1 cells (an immortalized keratinocyte cell line) but displayed impaired trafficking to the plasma membrane (29). However, plasma-membrane localization of Cx26 is observed in the sweat gland of a KID patient heterozygous for the D50N mutation (29).
These differences between in vivo and in vitro studies suggest that connexin–connexin interactions may change mutant protein behavior with respect to localization and possibly functionality. In support of this idea, D50N is found at gap-junction plaques in cells co-transfected with fluorescently tagged D50N and wtCx26 or wtCx30 (29). FRET analysis demonstrates a proximity between mutant and wt subunits consistent with co-assembly into heteromeric channels that could explain the expression of the mutant at the cell surface.
Finally, the missense mutations G59A and R75W (or R75Q) cause autosomal-dominant, profound hearing loss that has been associated with a mild skin disorder, palmoplantar keratoderma. Expression of Cx26 R75W in transgenic mice causes deafness, which is associated with death of both supporting cells and hair cells (53). Cx26 mutants with different amino acid substitutions of residue R75, including R75W, are unable to form functional gap-junction channels between oocyte pairs (27). However, they do insert normally into the plasma membrane (27) and form functional hemichannels (27), albeit with slightly modified gating. This mutant has a dominant-negative effect on gap-junctional communication mediated by wtCx26 (23, 27, 35, 53, 68, 80), consistent with the dominant nature of deafness cause by the R75W mutation (80). The possible mechanism of gap-junctional communication dysfunction for mutant R75W could be a defective docking, in which interaction of apposing hemichannels does not result in channel opening. However, some form of stable interaction is likely to occur, as at the immunofluorescent and ultrastructural level, R75W gap-junction plaques have been observed in different expression systems (72, 94). Connexons purified from the Sf9 insect cells expressing R75W have very low stability in the detergent dodecyl maltoside compared with those formed by wtCx26, suggesting that R75 is important for intersubunit interactions (72). Because functional hemichannels can be detected in cells transfected with wtCx26, we can speculate that the supposed connexon instability has no biologic relevance, but these studies in exogenous systems are hard to quantitate. The other palmoplantar keratoderma mutation, G59A, is also a loss-of-function mutation (35, 68, 95). Some discrepancies with respect to the effects of this mutation on Cx26 trafficking have been reported, with both complete intracellular localization (35, 68), and effective assembly into gap-junction plaques (95) being reported. Co-expression experiments showed that the plasma-membrane localization of Cx26 G59A is rescued by wtCx26 or wtCx30, but that this mutant has a dominant-negative effect on wild-type connexins (35, 68, 95). The mechanism by which this mutant affects gap-junction function is unknown.
Other Connexin Mutations Involved in Deafness
Mutations in other connexins have been detected in deaf patients. Deletion in GJB6 (Cx30), “del(GJB6-D13S1830),” is the second most frequent mutation causing prelingual hearing impairment in Spain and is also common in other European countries and in Israel (26). Dominant mutations in the Cx30 gene have been identified, T5M (40) and 63delG (9). Normal trafficking and gap-junction plaques were observed in HeLa cells or keratinocyte cells transfected with TM5/EGFP, but dye-coupling experiments fail to showed functionality (18), suggesting that T5M is a loss-of-function mutation. Interestingly, other Cx30 mutations associated with skin disease (G11R, V37E, A88V) showed impaired trafficking of the protein to the plasma membrane (18).
Mutations in the gene for human Cx31 (GJB3) are associated with disorders of the skin and auditory system. Mutations in Cx31 gene (GJB3) produce dominant or recessive inherited deafness (58, 105). Recessive mutations in Cx31 (141delI, I141V) (58) are located in the third transmembrane domain, whereas dominant mutations (R180X, E183K) (105) are clustered in the ECL2. The pathogenic mechanism of these mutants is unknown. However, mutation 66delD, associated with a dominant syndrome of hearing loss and peripheral neuropathy, has defective trafficking to the plasma membrane, and the functionality assessed with dye transfer is impaired compared with wtCx31 (30).
Gap-Junction Channel and Environmental and Age-Related Hearing Loss
Although age-related hearing loss is polygenic and multifactorial in etiology, a consensus indicates that the cochlea is the main affected auditory organ (59). The low-frequency pattern of hearing loss is interpreted as possibly representing a disorder of the stria vascularis, whereas the high-frequency loss is probably due to changes in hair cell function (59). The effects of aging on connexin expression and gap-junction function in the cochlea have yet to be investigated. However, in other systems, aging induces changes in connexin expression. For example, in the aging heart, Cx43 is drastically reduced in the sinoatrial node, contributing to decreases in the conduction velocity observed in older hearts (46). Similar studies should be done in the cochlea to determine the effect of aging on Cx26 or Cx30 expression and its potential phenotypic consequences.
Noise is the most-studied environmental factor causing hearing loss, with long-term noise exposure leading to loss of the outer hair cells and ultimately loss of the inner hair cells (59). In rats after acoustic trauma induced by noise exposure (54.2 dB), Cx26 expression was upregulated in the cochlea lateral wall (43), suggesting that noise may regulate connexin expression. The cochlea is also a very metabolically active tissue that produces significant levels of reactive oxygen species (ROS), which increase to detrimental levels with reduced production or function of the endogenous enzymes that protect the cell from ROS damage. Loss of antioxidant defense or increase in ROS production appears to be consistently associated with the aging process in many tissues and has also been associated with changes in gap-junction expression or function (37, 67, 87). Loss of antioxidant enzymes has also been associated with noise-induced damage in the ear (44). Two classes of antioxidant enzymes are active in the cochlea: enzymes involved in glutathione (GSH) metabolism (glutathione S-transferase, GST; glutathione peroxidase, GPX1; and glutathione reductase, GSR) and enzymes involved in the breakdown of superoxide anions and hydrogen peroxide (e.g., catalase, CAT; and Cu/Zn superoxide dismutase, SOD1) (44, 59, 69, 70). Studies of knockout models of Gpx1 and Sod1 have shown that deletion in these two antioxidant genes can lead to both age-related and noise-induced hearing loss (59, 70). Oxidative stress affects connexin expression and GJCh function in many systems. For example, in astrocytes, reoxygenation after hypoxia disrupts gap-junctional communications (67). In addition, treatments with antioxidant agents modify connexin expression. Cx26 was induced in a transgenic mouse line that expresses the gene CrtB, encoding phytoene synthase, which could produce the potent antioxidant phytoene (a type of carotenoid) endogenously (87). This was consistent with previous findings that carotenoids enhance gap-junctional communications by inducing the expression of connexins genes and resistance to oxidative stress. Taurine in rat hepatocytes prevents the reduction in Cx32 induced by oxidative stress (37), thus protecting against H2O2-induced reduction in gap-junctional communication in the liver. Melatonin, the potent free radical scavenger hormone, protects astrocyte gap junctions from oxidative damage induced by reoxygenation (67). Thus, in addition to its role in familial deafness, Cx26 may be affected by oxidative stress in the cochlea, contributing to age-related hearing loss or damage cause by sound (Fig. 4). The latter hypothesis should be tested experimentally. However, we can speculate that treatment with antioxidants (like carotens, taurine, or melatonin) may protect gap-junction function from oxidative damage induced by age or sound, contributing to protection from hearing loss.
Conclusions
In summary, Cx26 mutations produce deafness and skin disease through multiple pathogenic mechanisms. In terms of disease, deafness-associated Cx26 mutants may be broadly classified into two categories: (a) mutations that produce nonsyndromic deafness, and (b) mutations that produce syndromic deafness, in which deafness is associated with skin disorders. Nonsyndromic mutations are very diverse and located in most Cx26 protein domains. They are generally recessive mutations, but a few dominant mutations cluster mainly in the third transmembrane domain and second extracellular loop [important protein domains for constitution of the channel, as defined by mutagenic mapping of the pore lining (89) (Fig. 2)]. All Cx26 syndromic mutations are dominant and are located in the NT domain or in the first extracellular loop (critical domains for voltage sensing, channel gating, and hemichannel permeability) (Fig. 2). In terms of GJCh formation and function, deafness-associated Cx26 mutations could be classified into four types: (a) mutations that affect hemichannel trafficking to the plasma membrane or GJCh assembly; (b) mutations that produce gap junctions, but the channels are non-functional; (c) mutations that produce functional GJCh that have aberrant gating or permeability properties, like reduced IP3 permeability; and (d) mutations that produce functional hemichannels at the plasma membrane that may open under physiologic conditions, affecting ionic balance or the homeostasis of vital metabolites that diminish cellular viability (Fig. 3).
Most functional mutations are located in the transmembrane domains, especially clustering in the second trans-membrane domain, which has also been implicated in lining the pore (89) (Fig. 2). However, nonfunctional mutations are located in any part of the protein, suggesting that the structure of Cx26 gap-junction channels is very sensitive to minor changes in the amino acid sequence, independent of the protein domain where they are present. Deafness associated with Cx26 mutations can be inherited in recessive or dominant forms, depending on whether the mutant connexin can act as dominant-negative subunits for the function of coexpressed wild-type connexins, like Cxs 26, 30, or 43. The mechanism by which some dominant mutations are syndromic and others are nonsyndromic is a matter for speculation. However, it is reasonable to think that constitution of aberrant heteromeric channels is behind the mechanism. This serves to emphasize the fact that, although exogenous expression of connexins in model systems has focused on homomeric connexon channels, in situ, heteromeric channels likely exist or even predominate. Each unique heteromeric connexin assembly may have its own unique properties and physiologic role.
Although it is clear that gap-junction intercellular communication is vital for cochlea and skin physiology, its exact function is unknown. The hypothesis that gap junctions participate in K+ recirculation in the cochlea is both logical and consistent with the physiology of the ear, but remains to be directly tested experimentally. Gap junctions may also be important for other reasons. The permeability properties of some pathogenic Cx26 mutations indicates that circulation of metabolites, like IP3, is critical for cochlea-supporting cell function or survival and that K+ flow cannot be the only function of Cx26 (Fig. 4). Finally, connexin genes are potential candidates for susceptibility to age-related hearing loss or noise-induced hearing damage.
Acknowledgments
This work was supported by Anillo de Ciencia y Tecnología, ACT-46 for ADM and NIH-NCI (CA48049) and NIH-NIGMS (GM55437) for BJN.
Abbreviations
CT, carboxyl-terminus domain; Cx, connexin protein; ECL1, ECL2, extracellular loop 1 and 2, respectively; GJB2 and GJB6, Cx26 and Cx30 genes, respectively; GJ, gap junction; GJCh, gap-junction channels; GPX1 glutathione peroxidase 1; GST, glutathione S-transferase; ICL, intracellular loop; KID, keratitis–ichthyosis–deafness syndrome; NT, protein amino-terminus domain; SOD, superoxide dismutase; TM, transmembrane domain; wtCx, wild-type connexin.
References
- 1. Ahmad S. Chen S. Sun J. Lin X. Connexins 26 and 30 are co-assembled to form gap junctions in the cochlea of mice. Biochem Biophys Res Commun. 2003;307:362–368. doi: 10.1016/s0006-291x(03)01166-5. [DOI] [PubMed] [Google Scholar]
- 2. Arita K. Akiyama M. Aizawa T. Umetsu Y. Segawa I. Goto M. Sawamura D. Demura M. Kawano K. Shimizu H. A novel N14Y mutation in connexin26 in keratitis-ichthyosis-deafness syndrome: analyses of altered gap junctional communication and molecular structure of N terminus of mutated Connexin26. Am J Pathol. 2006;169:416–423. doi: 10.2353/ajpath.2006.051242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bakirtzis G. Choudhry R. Aasen T. Shore L. Brown K. Bryson S. Forrow S. Tetley L. Finbow M. Greenhalgh D. Hodgins M. Targeted epidermal expression of mutant connexin 26(D66H) mimics true Vohwinkel syndrome and provides a model for the pathogenesis of dominant connexin disorders. Hum Mol Genet. 2003;12:1737–1744. doi: 10.1093/hmg/ddg183. [DOI] [PubMed] [Google Scholar]
- 4. Beltramello M. Piazza V. Bukauskas FF. Pozzan T. Mammano F. Impaired permeability to Ins(1,4,5)P3 in a mutant connexin underlies recessive hereditary deafness. Nat Cell Biol. 2005;7:63–69. doi: 10.1038/ncb1205. [DOI] [PubMed] [Google Scholar]
- 5. Bennett MV. Contreras JE. Bukauskas FF. Saez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bevans CG. Kordel M. Rhee SK. Harris AL. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem. 1998;273:2808–2816. doi: 10.1074/jbc.273.5.2808. [DOI] [PubMed] [Google Scholar]
- 7. Beyer EC. Gemel J. Martinez A. Berthoud VM. Valiunas V. Moreno AP. Brink PR. Heteromeric mixing of connexins: compatibility of partners and functional consequences. Cell Commun Adhes. 2001;8:199–204. doi: 10.3109/15419060109080723. [DOI] [PubMed] [Google Scholar]
- 8. Bicego M. Beltramello M. Melchionda S. Carella M. Piazza V. Zelante L. Bukauskas FF. Arslan E. Cama E. Pantano S. Bruzzone R. D'Andrea P. Mammano F. Pathogenetic role of the deafness-related M34T mutation of Cx26. Hum Mol Genet. 2006;15:2569–2587. doi: 10.1093/hmg/ddl184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Birkenhager R. Zimmer AJ. Maier W. Schipper J. [Pseudodominants of two recessive connexin mutations in nonsyndromic sensorineural hearing loss?] Laryngorhinootologie. 2006;85:191–196. doi: 10.1055/s-2005-870302. [DOI] [PubMed] [Google Scholar]
- 10. Brissette JL. Kumar NM. Gilula NB. Hall JE. Dotto GP. Switch in gap junction protein expression is associated with selective changes in junctional permeability during keratinocyte differentiation. Proc Natl Acad Sci U S A. 1994;91:6453–6457. doi: 10.1073/pnas.91.14.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown CW. Levy ML. Flaitz CM. Reid BS. Manolidis S. Hebert AA. Bender MM. Heilstedt HA. Plunkett KS. Fang P. Roa BB. Chung P. Tang HY. Richard G. Alford RL. A novel GJB2 (connexin 26) mutation, F142L, in a patient with unusual mucocutaneous findings and deafness. J Invest Dermatol. 2003;121:1221–1223. doi: 10.1046/j.1523-1747.2003.12550_4.x. [DOI] [PubMed] [Google Scholar]
- 12. Bruzzone R. Gomes D. Denoyelle E. Duval N. Perea J. Veronesi V. Weil D. Petit C. Gabellec MM. D'Andrea P. White TW. Functional analysis of a dominant mutation of human connexin26 associated with nonsyndromic deafness. Cell Commun Adhes. 2001;8:425–431. doi: 10.3109/15419060109080765. [DOI] [PubMed] [Google Scholar]
- 13. Bruzzone R. Veronesi V. Gomes D. Bicego M. Duval N. Marlin S. Petit C. D'Andrea P. White TW. Loss-of-function and residual channel activity of connexin26 mutations associated with non-syndromic deafness. FEBS Lett. 2003;533:79–88. doi: 10.1016/s0014-5793(02)03755-9. [DOI] [PubMed] [Google Scholar]
- 14. Butterweck A. Elfgang C. Willecke K. Traub O. Differential expression of the gap junction proteins connexin45, −43, −40, −31, and −26 in mouse skin. Eur J Cell Biol. 1994;65:152–163. [PubMed] [Google Scholar]
- 15. Cohen-Salmon M. Maxeiner S. Kruger O. Theis M. Willecke K. Petit C. Expression of the connexin43- and connexin45-encoding genes in the developing and mature mouse inner ear. Cell Tissue Res. 2004;316:15–22. doi: 10.1007/s00441-004-0861-2. [DOI] [PubMed] [Google Scholar]
- 16. Cohen-Salmon M. Ott T. Michel V. Hardelin JP. Perfettini I. Eybalin M. Wu T. Marcus DC. Wangemann P. Willecke K. Petit C. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr Biol. 2002;12:1106–1111. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen-Salmon M. Regnault B. Cayet N. Caille D. Demuth K. Hardelin JP. Janel N. Meda P. Petit C. Connexin30 deficiency causes intrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proc Natl Acad Sci U S A. 2007;104:6229–6234. doi: 10.1073/pnas.0605108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Common JE. Becker D. Di WL. Leigh IM. O'Toole EA. Kelsell DP. Functional studies of human skin disease- and deafness-associated connexin 30 mutations. Biochem Biophys Res Commun. 2002;298:651–656. doi: 10.1016/s0006-291x(02)02517-2. [DOI] [PubMed] [Google Scholar]
- 19. Contreras JE. Saez JC. Bukauskas FF. Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci U S A. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Contreras JE. Sanchez HA. Eugenin EA. Speidel D. Theis M. Willecke K. Bukauskas FF. Bennett MV. Saez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci U S A. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corey DP. Garcia-Anoveros J. Holt JR. Kwan KY. Lin SY. Vollrath MA. Amalfitano A. Cheung EL. Derfler BH. Duggan A. Geleoc GS. Gray PA. Hoffman MP. Rehm HL. Tamasauskas D. Zhang DS. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- 22. Cucci RA. Prasad S. Kelley PM. Green GE. Storm K. Willocx S. Cohn ES. Van Camp G. Smith RJ. The M34T allele variant of connexin 26. Genet Test. 2000;4:335–344. doi: 10.1089/109065700750065063. [DOI] [PubMed] [Google Scholar]
- 23. Chen Y. Deng Y. Bao X. Reuss L. Altenberg GA. Mechanism of the defect in gap-junctional communication by expression of a connexin 26 mutant associated with dominant deafness. FASEB J. 2005;19:1516–1518. doi: 10.1096/fj.04-3491fje. [DOI] [PubMed] [Google Scholar]
- 24. D'Andrea P. Veronesi V. Bicego M. Melchionda S. Zelante L. Di Iorio E. Bruzzone R. Gasparini P. Hearing loss: frequency and functional studies of the most common connexin26 alleles. Biochem Biophys Res Commun. 2002;296:685–691. doi: 10.1016/s0006-291x(02)00891-4. [DOI] [PubMed] [Google Scholar]
- 25. de Zwart-Storm EA. Hamm H. Stoevesandt J. Steijlen PM. Martin PE. van Geel M. van Steensel MA. A novel missense mutation in GJB2 disturbs gap junction protein transport and causes focal palmoplantar keratoderma with deafness. J Med Genet. 2008;45:161–166. doi: 10.1136/jmg.2007.052332. [DOI] [PubMed] [Google Scholar]
- 26. Del Castillo I. Moreno-Pelayo MA. Del Castillo FJ. Brownstein Z. Marlin S. Adina Q. Cockburn DJ. Pandya A. Siemering KR. Chamberlin GP. Ballana E. Wuyts W. Maciel-Guerra AT. Alvarez A. Villamar M. Shohat M. Abeliovich D. Dahl HH. Estivill X. Gasparini P. Hutchin T. Nance WE. Sartorato EL. Smith RJ. Van Camp G. Avraham KB. Petit C. Moreno F. Prevalence and evolutionary origins of the del(GJB6-D13S1830) mutation in the DFNB1 locus in hearing-impaired subjects: a multicenter study. Am J Hum Genet. 2003;73:1452–1458. doi: 10.1086/380205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deng Y. Chen Y. Reuss L. Altenberg GA. Mutations of connexin 26 at position 75 and dominant deafness: essential role of arginine for the generation of functional gap-junctional channels. Hear Res. 2006;220:87–94. doi: 10.1016/j.heares.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 28. Denoyelle F. Marlin S. Weil D. Moatti L. Chauvin P. Garabedian EN. Petit C. Clinical features of the prevalent form of childhood deafness, DFNB1, due to a connexin-26 gene defect: implications for genetic counselling. Lancet. 1999;353:1298–1303. doi: 10.1016/S0140-6736(98)11071-1. [DOI] [PubMed] [Google Scholar]
- 29. Di WL. Gu Y. Common JE. Aasen T. O'Toole EA. Kelsell DP. Zicha D. Connexin interaction patterns in keratinocytes revealed morphologically and by FRET analysis. J Cell Sci. 2005;118:1505–1514. doi: 10.1242/jcs.01733. [DOI] [PubMed] [Google Scholar]
- 30. Di WL. Monypenny J. Common JE. Kennedy CT. Holland KA. Leigh IM. Rugg EL. Zicha D. Kelsell DP. Defective trafficking and cell death is characteristic of skin disease-associated connexin 31 mutations. Hum Mol Genet. 2002;11:2005–2014. doi: 10.1093/hmg/11.17.2005. [DOI] [PubMed] [Google Scholar]
- 31. Di WL. Rugg EL. Leigh IM. Kelsell DP. Multiple epidermal connexins are expressed in different keratinocyte subpopulations including connexin 31. J Invest Dermatol. 2001;117:958–964. doi: 10.1046/j.0022-202x.2001.01468.x. [DOI] [PubMed] [Google Scholar]
- 32. Forge A. Becker D. Casalotti S. Edwards J. Evans WH. Lench N. Souter M. Gap junctions and connexin expression in the inner ear. Novartis Found Symp. 1999;219:134–150. doi: 10.1002/9780470515587.ch9. discussion 151–136. [DOI] [PubMed] [Google Scholar]
- 33. Forge A. Becker D. Casalotti S. Edwards J. Marziano N. Nevill G. Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessment of connexin composition in mammals. J Comp Neurol. 2003;467:207–231. doi: 10.1002/cne.10916. [DOI] [PubMed] [Google Scholar]
- 34. Forge A. Becker D. Casalotti S. Edwards J. Marziano N. Nickel R. Connexins and gap junctions in the inner ear. Audiol Neurootol. 2002;7:141–145. doi: 10.1159/000058299. [DOI] [PubMed] [Google Scholar]
- 35. Forge A. Marziano NK. Casalotti SO. Becker DL. Jagger D. The inner ear contains heteromeric channels composed of cx26 and cx30 and deafness-related mutations in cx26 have a dominant negative effect on cx30. Cell Commun Adhes. 2003;10:341–346. doi: 10.1080/cac.10.4-6.341.346. [DOI] [PubMed] [Google Scholar]
- 36. Frenz CM. Van De Water TR. Immunolocalization of connexin 26 in the developing mouse cochlea. Brain Res Brain Res Rev. 2000;32:172–180. doi: 10.1016/s0165-0173(99)00078-8. [DOI] [PubMed] [Google Scholar]
- 37. Fukuda T. Ikejima K. Hirose M. Takei Y. Watanabe S. Sato N. Taurine preserves gap junctional intercellular communication in rat hepatocytes under oxidative stress. J Gastroenterol. 2000;35:361–368. doi: 10.1007/s005350050361. [DOI] [PubMed] [Google Scholar]
- 38. Gerido DA. DeRosa AM. Richard G. White TW. Aberrant hemichannel properties of Cx26 mutations causing skin disease and deafness. Am J Physiol Cell Physiol. 2007;293:C337–C345. doi: 10.1152/ajpcell.00626.2006. [DOI] [PubMed] [Google Scholar]
- 39. Gerido DA. White TW. Connexin disorders of the ear, skin, and lens. Biochim Biophys Acta. 2004;1662:159–170. doi: 10.1016/j.bbamem.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 40. Grifa A. Wagner CA. D'Ambrosio L. Melchionda S. Bernardi F. Lopez-Bigas N. Rabionet R. Arbones M. Monica MD. Estivill X. Zelante L. Lang F. Gasparini P. Mutations in GJB6 cause nonsyndromic autosomal dominant deafness at DFNA3 locus. Nat Genet. 1999;23:16–18. doi: 10.1038/12612. [DOI] [PubMed] [Google Scholar]
- 41. Hibino H. Kurachi Y. Molecular and physiological bases of the K+ circulation in the mammalian inner ear. Physiology (Bethesda) 2006;21:336–345. doi: 10.1152/physiol.00023.2006. [DOI] [PubMed] [Google Scholar]
- 42. Houseman MJ. Ellis LA. Pagnamenta A. Di WL. Rickard S. Osborn AH. Dahl HH. Taylor GR. Bitner-Glindzicz M. Reardon W. Mueller RF. Kelsell DP. Genetic analysis of the connexin-26 M34T variant: identification of genotype M34T/M34T segregating with mild-moderate non-syndromic sensorineural hearing loss. J Med Genet. 2001;38:20–25. doi: 10.1136/jmg.38.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hsu WC. Wang JD. Hsu CJ. Lee SY. Yeh TH. Expression of connexin 26 in the lateral wall of the rat cochlea after acoustic trauma. Acta Otolaryngol. 2004;124:459–463. doi: 10.1080/00016480310000584. [DOI] [PubMed] [Google Scholar]
- 44. Jacono AA. Hu B. Kopke RD. Henderson D. Van De Water TR. Steinman HM. Changes in cochlear antioxidant enzyme activity after sound conditioning and noise exposure in the chinchilla. Hear Res. 1998;117:31–38. doi: 10.1016/s0378-5955(97)00214-1. [DOI] [PubMed] [Google Scholar]
- 45. Jagger DJ. Forge A. Compartmentalized and signal-selective gap junctional coupling in the hearing cochlea. J Neurosci. 2006;26:1260–1268. doi: 10.1523/JNEUROSCI.4278-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jones SA. Lancaster MK. Boyett MR. Ageing-related changes of connexins and conduction within the sinoatrial node. J Physiol. 2004;560:429–437. doi: 10.1113/jphysiol.2004.072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kammen-Jolly K. Ichiki H. Scholtz AW. Gsenger M. Kreczy A. Schrott-Fischer A. Connexin 26 in human fetal development of the inner ear. Hear Res. 2001;160:15–21. doi: 10.1016/s0378-5955(01)00310-0. [DOI] [PubMed] [Google Scholar]
- 48. Kelsell DP. Di WL. Houseman MJ. Connexin mutations in skin disease and hearing loss. Am J Hum Genet. 2001;68:559–568. doi: 10.1086/318803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kelsell DP. Dunlop J. Stevens HP. Lench NJ. Liang JN. Parry G. Mueller RF. Leigh IM. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature. 1997;387:80–83. doi: 10.1038/387080a0. [DOI] [PubMed] [Google Scholar]
- 50. Kikuchi T. Kimura RS. Paul DL. Adams JC. Gap junctions in the rat cochlea: immunohistochemical and ultra-structural analysis. Anat Embryol (Berl) 1995;191:101–118. doi: 10.1007/BF00186783. [DOI] [PubMed] [Google Scholar]
- 51. Kikuchi T. Kimura RS. Paul DL. Takasaka T. Adams JC. Gap junction systems in the mammalian cochlea. Brain Res Brain Res Rev. 2000;32:163–166. doi: 10.1016/s0165-0173(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 52. Kudo T. Ikeda K. Kure S. Matsubara Y. Oshima T. Watanabe K. Kawase T. Narisawa K. Takasaka T. Novel mutations in the connexin 26 gene (GJB2) responsible for childhood deafness in the Japanese population. Am J Med Genet. 2000;90:141–145. doi: 10.1002/(sici)1096-8628(20000117)90:2<141::aid-ajmg10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 53. Kudo T. Kure S. Ikeda K. Xia AP. Katori Y. Suzuki M. Kojima K. Ichinohe A. Suzuki Y. Aoki Y. Kobayashi T. Matsubara Y. Transgenic expression of a dominant-negative connexin26 causes degeneration of the organ of Corti and non-syndromic deafness. Hum Mol Genet. 2003;12:995–1004. doi: 10.1093/hmg/ddg116. [DOI] [PubMed] [Google Scholar]
- 54. Lagostena L. Ashmore JF. Kachar B. Mammano F. Purinergic control of intercellular communication between Hensen's cells of the guinea-pig cochlea. J Physiol. 2001;531:693–706. doi: 10.1111/j.1469-7793.2001.0693h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lautermann J. Frank HG. Jahnke K. Traub O. Winterhager E. Developmental expression patterns of connexin26 and −30 in the rat cochlea. Dev Genet. 1999;25:306–311. doi: 10.1002/(SICI)1520-6408(1999)25:4<306::AID-DVG4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 56. Lautermann J. ten Cate WJ. Altenhoff P. Grummer R. Traub O. Frank H. Jahnke K. Winterhager E. Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res. 1998;294:415–420. doi: 10.1007/s004410051192. [DOI] [PubMed] [Google Scholar]
- 57. Liu XZ. Xia XJ. Ke XM. Ouyang XM. Du LL. Liu YH. Angeli S. Telischi FF. Nance WE. Balkany T. Xu LR. The prevalence of connexin 26 (GJB2) mutations in the Chinese population. Hum Genet. 2002;111:394–397. doi: 10.1007/s00439-002-0811-6. [DOI] [PubMed] [Google Scholar]
- 58. Liu XZ. Xia XJ. Xu LR. Pandya A. Liang CY. Blanton SH. Brown SD. Steel KP. Nance WE. Mutations in connexin31 underlie recessive as well as dominant non-syndromic hearing loss. Hum Mol Genet. 2000;9:63–67. doi: 10.1093/hmg/9.1.63. [DOI] [PubMed] [Google Scholar]
- 59. Liu XZ. Yan D. Ageing and hearing loss. J Pathol. 2007;211:188–197. doi: 10.1002/path.2102. [DOI] [PubMed] [Google Scholar]
- 60. Lopez-Bigas N. Arbones ML. Estivill X. Simonneau L. Expression profiles of the connexin genes, Gjb1 and Gjb3, in the developing mouse cochlea. Gene Expr Patterns. 2002;2:113–117. doi: 10.1016/s0925-4773(02)00299-x. [DOI] [PubMed] [Google Scholar]
- 61. Lucke T. Choudhry R. Thom R. Selmer IS. Burden AD. Hodgins MB. Upregulation of connexin 26 is a feature of keratinocyte differentiation in hyperproliferative epidermis, vaginal epithelium, and buccal epithelium. J Invest Dermatol. 1999;112:354–361. doi: 10.1046/j.1523-1747.1999.00512.x. [DOI] [PubMed] [Google Scholar]
- 62. Maestrini E. Korge BP. Ocana-Sierra J. Calzolari E. Cambiaghi S. Scudder PM. Hovnanian A. Monaco AP. Munro CS. A missense mutation in connexin26, D66H, causes mutilating keratoderma with sensorineural deafness (Vohwinkel's syndrome) in three unrelated families. Hum Mol Genet. 1999;8:1237–1243. doi: 10.1093/hmg/8.7.1237. [DOI] [PubMed] [Google Scholar]
- 63. Maher AC. Thomas T. Riley JL. Veitch G. Shao Q. Laird DW. Rat epidermal keratinocytes as an organotypic model for examining the role of Cx43 and Cx26 in skin differentiation. Cell Commun Adhes. 2005;12:219–230. doi: 10.1080/15419060500511818. [DOI] [PubMed] [Google Scholar]
- 64. Mammano F. Goodfellow SJ. Fountain E. Electrophysiological properties of Hensen's cells investigated in situ. Neuroreport. 1996;7:537–542. doi: 10.1097/00001756-199601310-00039. [DOI] [PubMed] [Google Scholar]
- 65. Martin PE. Coleman SL. Casalotti SO. Forge A. Evans WH. Properties of connexin26 gap junctional proteins derived from mutations associated with non-syndromal hereditary deafness. Hum Mol Genet. 1999;8:2369–2376. doi: 10.1093/hmg/8.13.2369. [DOI] [PubMed] [Google Scholar]
- 66. Martinez AD. Hayrapetyan V. Moreno AP. Beyer EC. Connexin43 and connexin45 form heteromeric gap junction channels in which individual components determine permeability and regulation. Circ Res. 2002;90:1100–1107. doi: 10.1161/01.res.0000019580.64013.31. [DOI] [PubMed] [Google Scholar]
- 67. Martinez AD. Saez JC. Regulation of astrocyte gap junctions by hypoxia-reoxygenation. Brain Res Brain Res Rev. 2000;32:250–258. doi: 10.1016/s0165-0173(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 68. Marziano NK. Casalotti SO. Portelli AE. Becker DL. Forge A. Mutations in the gene for connexin 26 (GJB2) that cause hearing loss have a dominant negative effect on connexin 30. Hum Mol Genet. 2003;12:805–812. doi: 10.1093/hmg/ddg076. [DOI] [PubMed] [Google Scholar]
- 69. McFadden SL. Ding D. Reaume AG. Flood DG. Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- 70. McFadden SL. Ohlemiller KK. Ding D. Shero M. Salvi RJ. The influence of superoxide dismutase and glutathione peroxidase deficiencies on noise-induced hearing loss in mice. Noise Health. 2001;3:49–64. [PubMed] [Google Scholar]
- 71. Mese G. Londin E. Mui R. Brink PR. White TW. Altered gating properties of functional Cx26 mutants associated with recessive non-syndromic hearing loss. Hum Genet. 2004;115:191–199. doi: 10.1007/s00439-004-1142-6. [DOI] [PubMed] [Google Scholar]
- 72. Oshima A. Doi T. Mitsuoka K. Maeda S. Fujiyoshi Y. Roles of Met-34, Cys-64, and Arg-75 in the assembly of human connexin 26: implication for key amino acid residues for channel formation and function. J Biol Chem. 2003;278:1807–1816. doi: 10.1074/jbc.M207713200. [DOI] [PubMed] [Google Scholar]
- 72a. Oshima A, Tani K, Hiroaki Y, Fujiyoshi Y, and Sosinsky GE. Projection structure of a N-terminal deletion mutant of connexin 26 channel with decreased central pore density. Cell Commun Adhes. 2008;15:85–93. doi: 10.1080/15419060802013588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Oshima A. Tani K. Hiroaki Y. Fujiyoshi Y. Sosinsky GE. Three-dimensional structure of a human connexin26 gap junction channel reveals a plug in the vestibule. Proc Natl Acad Sci U S A. 2007;104:10034–10039. doi: 10.1073/pnas.0703704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Palmada M. Schmalisch K. Bohmer C. Schug N. Pfister M. Lang F. Blin N. Loss of function mutations of the GJB2 gene detected in patients with DFNB1-associated hearing impairment. Neurobiol Dis. 2006;22:112–118. doi: 10.1016/j.nbd.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 75. Petit C. Levilliers J. Hardelin JP. Molecular genetics of hearing loss. Annu Rev Genet. 2001;35:589–646. doi: 10.1146/annurev.genet.35.102401.091224. [DOI] [PubMed] [Google Scholar]
- 76. Purnick PE. Benjamin DC. Verselis VK. Bargiello TA. Dowd TL. Structure of the amino terminus of a gap junction protein. Arch Biochem Biophys. 2000;381:181–190. doi: 10.1006/abbi.2000.1989. [DOI] [PubMed] [Google Scholar]
- 77. Purnick PE. Oh S. Abrams CK. Verselis VK. Bargiello TA. Reversal of the gating polarity of gap junctions by negative charge substitutions in the N-terminus of connexin 32. Biophys J. 2000;79:2403–2415. doi: 10.1016/S0006-3495(00)76485-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Richard G. Brown N. Ishida-Yamamoto A. Krol A. Expanding the phenotypic spectrum of Cx26 disorders: Bart-Pumphrey syndrome is caused by a novel missense mutation in GJB2. J Invest Dermatol. 2004;123:856–863. doi: 10.1111/j.0022-202X.2004.23470.x. [DOI] [PubMed] [Google Scholar]
- 79. Richard G. Rouan F. Willoughby CE. Brown N. Chung P. Ryynanen M. Jabs EW. Bale SJ. DiGiovanna JJ. Uitto J. Russell L. Missense mutations in GJB2 encoding connexin26 cause the ectodermal dysplasia keratitis-ichthyosisdeafness syndrome. Am J Hum Genet. 2002;70:1341–1348. doi: 10.1086/339986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Richard G. White TW. Smith LE. Bailey RA. Compton JG. Paul DL. Bale SJ. Functional defects of Cx26 resulting from a heterozygous missense mutation in a family with dominant deaf-mutism and palmoplantar keratoderma. Hum Genet. 1998;103:393–399. doi: 10.1007/s004390050839. [DOI] [PubMed] [Google Scholar]
- 81. Rouan F. White TW. Brown N. Taylor AM. Lucke TW. Paul DL. Munro CS. Uitto J. Hodgins MB. Richard G. trans-dominant inhibition of connexin-43 by mutant connexin26: implications for dominant connexin disorders affecting epidermal differentiation. J Cell Sci. 2001;114:2105–2113. doi: 10.1242/jcs.114.11.2105. [DOI] [PubMed] [Google Scholar]
- 82. Saez JC. Berthoud VM. Branes MC. Martinez AD. Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 83. Saez JC. Retamal MA. Basilio D. Bukauskas FF. Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta. 2005;1711:215–224. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Santos-Sacchi J. The effects of cytoplasmic acidification upon electrical coupling in the organ of Corti. Hear Res. 1985;19:207–215. doi: 10.1016/0378-5955(85)90140-6. [DOI] [PubMed] [Google Scholar]
- 85. Santos-Sacchi J. Dye coupling in the organ of Corti. Cell Tissue Res. 1986;245:525–529. doi: 10.1007/BF00218553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Santos-Sacchi J. Electrical coupling differs in the in vitro and in vivo organ of Corti. Hear Res. 1987;25:227–232. doi: 10.1016/0378-5955(87)90094-3. [DOI] [PubMed] [Google Scholar]
- 87. Satomi Y. Misawa N. Maoka T. Nishino H. Production of phytoene, a carotenoid, and induction of connexin 26 in transgenic mice carrying the phytoene synthase gene crtB. Biochem Biophys Res Commun. 2004;320:398–401. doi: 10.1016/j.bbrc.2004.05.179. [DOI] [PubMed] [Google Scholar]
- 88. Shaw RM. Fay AJ. Puthenveedu MA. von Zastrow M. Jan YN. Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Skerrett IM. Aronowitz J. Shin JH. Cymes G. Kasperek E. Cao FL. Nicholson BJ. Identification of amino acid residues lining the pore of a gap junction channel. J Cell Biol. 2002;159:349–360. doi: 10.1083/jcb.200207060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Skerrett IM. Di WL. Kasperek EM. Kelsell DP. Nicholson BJ. Aberrant gating, but a normal expression pattern, underlies the recessive phenotype of the deafness mutant Connexin26M34T. FASEB J. 2004;18:860–862. doi: 10.1096/fj.03-0763fje. [DOI] [PubMed] [Google Scholar]
- 91. Stong BC. Chang Q. Ahmad S. Lin X. A novel mechanism for connexin 26 mutation linked deafness: cell death caused by leaky gap junction hemichannels. Laryngoscope. 2006;116:2205–2210. doi: 10.1097/01.mlg.0000241944.77192.d2. [DOI] [PubMed] [Google Scholar]
- 92. Sun J. Ahmad S. Chen S. Tang W. Zhang Y. Chen P. Lin X. Cochlear gap junctions coassembled from Cx26 and 30 show faster intercellular Ca2+ signaling than homomeric counterparts. Am J Physiol Cell Physiol. 2005;288:C613–C623. doi: 10.1152/ajpcell.00341.2004. [DOI] [PubMed] [Google Scholar]
- 93. Teubner B. Michel V. Pesch J. Lautermann J. Cohen-Salmon M. Sohl G. Jahnke K. Winterhager E. Herberhold C. Hardelin JP. Petit C. Willecke K. Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum Mol Genet. 2003;12:13–21. doi: 10.1093/hmg/ddg001. [DOI] [PubMed] [Google Scholar]
- 94. Thomas T. Aasen T. Hodgins M. Laird DW. Transport and function of cx26 mutants involved in skin and deafness disorders. Cell Commun Adhes. 2003;10:353–358. doi: 10.1080/cac.10.4-6.353.358. [DOI] [PubMed] [Google Scholar]
- 95. Thomas T. Telford D. Laird DW. Functional domain mapping and selective trans-dominant effects exhibited by Cx26 disease-causing mutations. J Biol Chem. 2004;279:19157–19168. doi: 10.1074/jbc.M314117200. [DOI] [PubMed] [Google Scholar]
- 96. Thonnissen E. Rabionet R. Arbones ML. Estivill X. Willecke K. Ott T. Human connexin26 (GJB2) deafness mutations affect the function of gap junction channels at different levels of protein expression. Hum Genet. 2002;111:190–197. doi: 10.1007/s00439-002-0750-2. [DOI] [PubMed] [Google Scholar]
- 97. van Steensel MA. Steijlen PM. Bladergroen RS. Hoefsloot EH. van Ravenswaaij-Arts CM. van Geel M. A phenotype resembling the Clouston syndrome with deafness is associated with a novel missense GJB2 mutation. J Invest Dermatol. 2004;123:291–293. doi: 10.1111/j.0022-202X.2004.23204.x. [DOI] [PubMed] [Google Scholar]
- 98. Wang HL. Chang WT. Li AH. Yeh TH. Wu CY. Chen MS. Huang PC. Functional analysis of connexin-26 mutants associated with hereditary recessive deafness. J Neurochem. 2003;84:735–742. doi: 10.1046/j.1471-4159.2003.01555.x. [DOI] [PubMed] [Google Scholar]
- 99. Wang YC. Kung CY. Su MC. Su CC. Hsu HM. Tsai CC. Lin CC. Li SY. Mutations of Cx26 gene (GJB2) for prelingual deafness in Taiwan. Eur J Hum Genet. 2002;10:495–498. doi: 10.1038/sj.ejhg.5200838. [DOI] [PubMed] [Google Scholar]
- 100. Wangemann P. K+ cycling and the endocochlear potential. Hear Res. 2002;165:1–9. doi: 10.1016/s0378-5955(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 101. Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol. 2006;576:11–21. doi: 10.1113/jphysiol.2006.112888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Willecke K. Eiberger J. Degen J. Eckardt D. Romualdi A. Guldenagel M. Deutsch U. Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 103. Wiszniewski L. Limat A. Saurat JH. Meda P. Salomon D. Differential expression of connexins during stratification of human keratinocytes. J Invest Dermatol. 2000;115:278–285. doi: 10.1046/j.1523-1747.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- 104. Xia AP. Ikeda K. Katori Y. Oshima T. Kikuchi T. Takasaka T. Expression of connexin 31 in the developing mouse cochlea. Neuroreport. 2000;11:2449–2453. doi: 10.1097/00001756-200008030-00022. [DOI] [PubMed] [Google Scholar]
- 105. Xia JH. Liu CY. Tang BS. Pan Q. Huang L. Dai HP. Zhang BR. Xie W. Hu DX. Zheng D. Shi XL. Wang DA. Xia K. Yu KP. Liao XD. Feng Y. Yang YF. Xiao JY. Xie DH. Huang JZ. Mutations in the gene encoding gap junction protein beta-3 associated with autosomal dominant hearing impairment. Nat Genet. 1998;20:370–373. doi: 10.1038/3845. [DOI] [PubMed] [Google Scholar]
- 106. Yan D. Park HJ. Ouyang XM. Pandya A. Doi K. Erdenetungalag R. Du LL. Matsushiro N. Nance WE. Griffith AJ. Liu XZ. Evidence of a founder effect for the 235delC mutation of GJB2 (connexin 26) in east Asians. Hum Genet. 2003;114:44–50. doi: 10.1007/s00439-003-1018-1. [DOI] [PubMed] [Google Scholar]
- 107. Yang JJ. Huang SH. Chou KH. Liao PJ. Su CC. Li SY. Identification of mutations in members of the connexin gene family as a cause of nonsyndromic deafness in Taiwan. Audiol Neurootol. 2007;12:198–208. doi: 10.1159/000099024. [DOI] [PubMed] [Google Scholar]
- 108. Yum SW. Zhang J. Valiunas V. Kanaporis G. Brink PR. White TW. Scherer SS. Human connexin26 and connexin30 form functional heteromeric and heterotypic channels. Am J Physiol Cell Physiol. 2007;293:C1032–C1048. doi: 10.1152/ajpcell.00011.2007. [DOI] [PubMed] [Google Scholar]
- 109. Zhang Y. Tang W. Ahmad S. Sipp JA. Chen P. Lin X. Gap junction-mediated intercellular biochemical coupling in cochlear supporting cells is required for normal cochlear functions. Proc Natl Acad Sci U S A. 2005;102:15201–15206. doi: 10.1073/pnas.0501859102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhao HB. Santos-Sacchi J. Effect of membrane tension on gap junctional conductance of supporting cells in Corti's organ. J Gen Physiol. 1998;112:447–455. doi: 10.1085/jgp.112.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhao HB. Santos-Sacchi J. Voltage gating of gap junctions in cochlear supporting cells: evidence for nonhomotypic channels. J Membr Biol. 2000;175:17–24. doi: 10.1007/s002320001051. [DOI] [PubMed] [Google Scholar]
- 112. Zhao HB. Yu N. Distinct and gradient distributions of connexin26 and connexin30 in the cochlear sensory epithelium of guinea pigs. J Comp Neurol. 2006;499:506–518. doi: 10.1002/cne.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhao HB. Yu N. Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci U S A. 2005;102:18724–18729. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]


