Abstract
Synthetic linear peptide chimeras (LPCscys+) show promise as delivery platforms for malaria subunit vaccines. Maximal immune response to LPCscys+ in rodent malaria models depends upon formation of cross-linkages to generate homopolymers, presenting challenges for vaccine production. To replicate the immunogenicity of LPCscys+ using a recombinant approach, we designed a recombinant LPC (rLPC) based on Plasmodium yoelii circumsporozoite protein-specific sequences of 208 amino acids consisting of four LPC subunits in series. BALB/c or CAF1/J mice were immunized with synthetic or recombinant LPCs. Antibody concentrations, cytokine production and protection against challenge were compared. Recombinant peptide replicated the robust, high avidity antibody responses obtained with the synthetic linear peptide chimera. After in vitro stimulation spleen cells from mice immunized with rLPC or synthetic LPCcys+ produced gamma interferon and IL-4 suggesting the efficient priming of T cells. Immunization of mice with either recombinant or synthetic LPCcys+ provided comparable protection against experimental challenge with P. yoelii sporozoites. Recombinant LPCs reproduced the immunogenicity of synthetic LPCcys+ without requiring polymerization, improving prospects for use as malaria vaccines.
Keywords: MALARIA, VACCINE, SYNTHETIC PEPTIDE, RECOMBINANT CHIMERA
1. Introduction
Malaria is responsible for more than 300 million clinical infections and one million fatalities annually [1]. The search for an effective malaria vaccine has been elusive. The complexity of the parasite life cycle and the lack of surrogate markers of protection complicate the assessment of vaccine efficacy. Clinical trials and studies of natural immunity suggest that anti-parasite immunity induced by a single antigen may not be sufficient to induce long lasting protective immunity. An optimal malaria vaccine should include several antigens and generate immune responses against different parasite life stages [2]. Genomic information available for P. falciparum and the recently completed genome sequence of P. vivax provide resources that can be mined for novel vaccine candidates [3, 4]. The combination of protective antigens in a single polyvalent molecule would be a cost-effective strategy for vaccine development.
Synthetic peptides are versatile and affordable delivery systems for subunit vaccines (reviewed in [5, 6]). As the first step to develop chimeric multi-epitope vaccine candidates, we have reported proof of principle studies using synthetic peptides, that we have called Linear Peptide Chimeras (LPCcys+). These linear peptides combines the synthesis in tandem of a promiscuous CD4+ T-cell epitope followed by B-cell and CD8+ T cell epitopes, with the inclusion of flanking cysteine residues to promote spontaneous polymerization [7]. The first generation of LPCscys+, based on the P. yoelii or P. berghei circumsporozoite protein (CSP), included the immunodominant B cell epitope derived from the repeat sequence within the central domain that elicit neutralizing antibodies and a CD8+ T cell epitope that target the infected hepatocytes [8]. We have reported that the immunogenicity of these linear synthetic peptides is highly dependent on the inclusion of promiscuous malaria CD4+ T-cell epitopes and the formation of various peptide species through homo-polymerization [7, 8]. Polymerized LPCscys+ are immunogenic in such mouse models of malaria and demonstrate substantial protection against parasite challenge [8]. Homo-polymerization is a random process that generates a variety of peptide species with different molecular masses. This product heterogeneity is a demanding feature for LPCscys+ vaccine mass production. To optimize the delivery of polymeric subunit vaccines, we sought to replicate the immunogenicity of LPCscys+ by engineering a recombinant LPC molecule (rLPC) consisting of four LPC sequences in series. We determined the immunogenicity and protective activity of the P. yoelii-specific rLPC in mice and compared immune responses elicited by synthetic and recombinant LPC molecules. Results suggested that recombinant LPC replicates the protective effect of the polymeric synthetic peptide.
2. Materials and Methods
2.1. Parasites
P. yoelii 17X/MRA-267 parasites were obtained from the Malaria Research and Reference Reagent Resource Center (MR4/ATCC, Manassas, VA). The complete parasite life cycle was maintained using Anopheles stephensi and sporozoites isolated from salivary glands [8]. For in vitro sporozoite neutralization assays, P. yoelii 17XNL/MRA-886 sporozoites in A. stephensi were obtained from the New York University School of Medicine through MR4/ATCC. For irradiated sporozoites experiments, P. yoelii-infected mosquitoes taken 12 days after an infectious blood meal were irradiated with 10,000 rad of gamma radiation from a 137Cesium source (Gammacell 3001, MDS Nordion, Vancouver, Canada). To isolate sporozoites infected mosquitoes were initially washed in 70% ethanol, followed by medium M199 containing Fungizone (5%) and M199 containing penicillin and streptomycin (5%). Salivary glands were dissected and homogenized on ice. For irradiated sporozoite immunization, mice received three doses of 20,000 irradiated sporozoites one week apart.
2.2. Synthetic linear peptide chimeras
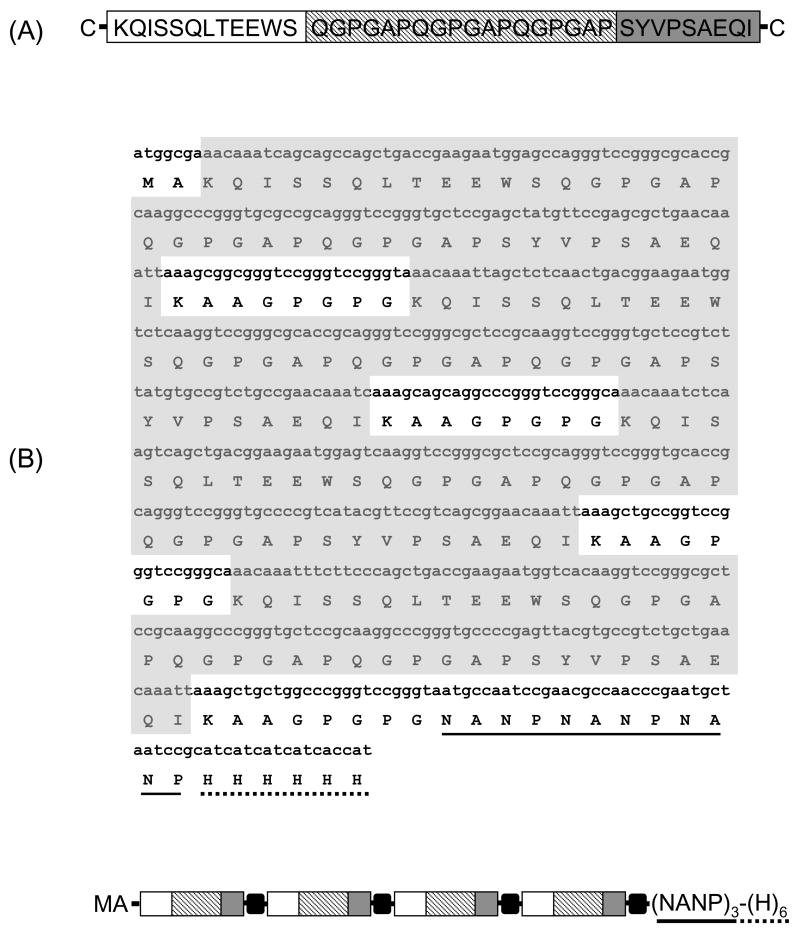
The topology of the P. yoelii-specific LPCcys+ used here (designated cys-T*-B-CTL-cys) has been previously described [8]. Briefly, the polymeric peptide sequence derived from the P. yoelii circumsporozoite protein (GenBank accession P06914), includes three linear epitopes synthesized in tandem (Fig. 1A): 1) An amino terminal promiscuos CD4+ T cell epitope KQISSQLTEEWS (K293-S304, numbers indicate relative amino acid position), that represents the orthologous sequences originally described in P. falciparum [9]; 2) (QGPGAP)3 that represent the minimal B cell epitope of the repeat region; and 3) the CD8+, H-2d restricted, T cell epitope SYVPSAEQI (S280-I288) [10]. Cysteine residues at amino and carboxyl terminal end of the sequence are involved in spontaneous polymerization. As a control for antigen specificity, a linear peptide that would not form polymers was synthesized without cysteine residues (LPCcys−). Peptides were synthesized at the Emory Microchemical facility by a multiple solid-phase technique using standard Fmoc protection chemistry [8].
Figure 1.
Topology and sequence of synthetic rlpc gene. (A) Schematic representation and sequence of synthetic LPCcys+. The sequence, derived from the P. yoelii circumsporozoite protein, includes a promiscuous CD4+ T cell epitope, T*-cell (□), an immunodominant B cell epitope from the CSP repeat domain, B-cell (striped bar) and a single CD8+ T cell epitope, CTL (■) arranged in tandem. (B) Amino acid and nucleotide sequences of the synthetic DNA encoding rlpc. A schematic representation of the chimeric protein is included for reference. The amino acid sequence is shown in the single letter code. Four subunits are arranged in tandem interspaced with KAAGPGPG spacers (black boxes in the diagram). The amino acid residues KAA are included downstream of the CD8+ T cell epitope. The carboxyl terminal P. falciparum tag sequence (NANP)3 (underlined) was included for biochemical characterization of the antigenic integrity and to provide optional affinity purification tag. The carboxyl terminal H6 tag sequence (dotted line) was used for protein purification.
2.3. Design of P. yoelii recombinant LPC
A 624 bp gene based on the P. yoelii-specific LPC amino acid sequence and optimized for expression in E. coli was synthesized by Geneart (Regensburg, Germany) (Fig. 1B). The synthetic gene include the following design features: 1) MA on the N-terminus provided the start signal and decreased degradation in E. coli (Ala); 2) Four P. yoelii-specific T*-B-CTL subunits with the sequence KQISSQLTEEWS(QGPGAP)3SYVPSAEQI; 3) KAA spacers downstream of the CD8+ T cell epitope to facilitate processing and presentation [11]; 4) GPGPG spacers between individual P. yoelii-specific T*-B-CTL subunits to enhance stability and antigen processing [12]; 5) (NANP)3 amino terminal tag sequence derived from P. falciparum circumsporozoite protein for biochemical characterization of antigenic integrity and to provide an alternative affinity purification tag; 6) carboxy terminal His-tag for protein purification; and 7) restriction sites for subcloning in an expression vector. The chimeric synthetic gene was cloned into a pET24d(+) vector and transformed to E. coli BL21(DE3) cells (Novagen, Madison, WI). Protein expression was induced with 1 mM IPTG for 3 hours. The recombinant chimeric protein was purified with a Ni-NTA affinity column according to the manufacturer’s instructions (Qiagen, Valencia, CA).
2.4. Protein characterization
Proteins and peptides were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Electrophoresis was performed under reducing conditions on 15% polyacrylamide for protein characterization or after alkylation with iodoacetamide (Sigma, Saint Louis, MO) under non-reducing conditions for peptide analysis on 16.5% Tris-Tricine ready gel system (Bio-Rad Laboratories, Hercules, CA) [8]. Following electrophoresis, proteins were transferred to PVDF (Polyvinylidene difluoride) membranes for Western blotting. Membranes were incubated with P. falciparum species-specific anti-circumsporozoite protein mAbs 2A10 [13], mouse anti-LPCcys+ or anti-rLPC polyclonal antibodies diluted 1:5000, or anti-His tag antibody (0.2 μg/ml). After washing, membranes were incubated with goat anti mouse IgG (H+L) conjugated to alkaline phosphatase (Promega, Madison, WI) and visualized using 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitroblue tetrazolium (NBT). For synthetic LPCcys+, membranes were incubated with goat anti-mouse IgG conjugated to horseradish peroxidase and visualized using a chemiluminescent substrate (Pierce Biotechnology, Rockford, IL).
2.5. Immunization
Six to 8-week old female CAF1/J [(BALB/cJ × A/J) H-2a/d] and BALB/c mice (H-2d) (Jackson Laboratory, Bar Harbor, ME) were immunized subcutaneously at days 0, 20 and 40, in the base of the tail and in the inter-scapular area, using 50 μg of the synthetic LPCcys+ or 10 μg of the rLPC emulsified in Montanide ISA51 (Seppic, Fairfield, NJ). CAF1/J mice (H-2a/d) were selected based on the described high responder status of H-2a mice to the promiscuous CD4+ T* cell epitope and the H-2Kd restriction of the CD8+ T cell epitope [8]. To determine if differences in the frequency of cells with helper activity play a role in protective efficacy, we compared the immune responses elicited by recombinant or synthetic LPCs in CAF1/J and BALB/c mice. Control groups received a placebo immunization with PBS emulsified in Montanide ISA51. Twenty days after the third immunization the mice were challenged with 100 P. yoelii 17X sporozoites. Forty hours after challenge, the parasite load was measured by quantitative real time PCR [8].
2.6. Antibody assays and cellular immune response
The specificity of the antibodies elicited by immunization with rLPC or synthetic LPCcys+ was determined by ELISA using Immulon-2 plates (Dynatech Laboratories, Chantilly, VA) coated with 1 μg/ml LPCcys+, rLPC or synthetic peptides representing the T- or B-cell epitopes and tested 20 days after each immunization. IgG isotype profiles were also determined by ELISA using biotinylated rat anti-mouse mAbs IgG1 (A85-1), IgG2a (clone R19-15), IgG2b (R12-3) and IgG3 (R40–82) (BD PharMingen, Franklin Lakes, NJ) using sera samples obtained 20 days after the third immunization.
To test avidity of anti-LPC antibodies, sera were obtained 20 days after the third immunization, before experimental challenge. Avidity of total IgG antibody was assessed by thiocyanate elution-based ELISA [7], with varying concentration of sodium thiocyanate (0.0175 to 10 M). The concentration of thiocyanate required to dissociate 50% of bound antibody was estimated by linear regression.
In vitro sporozoite neutralization assays were performed using HepG2-A16 cells (American Type Culture Collection, HB 8065, Rockville, MD) seeded at 5×104 cells/well in 8-well chamber slides (Lab-tek, Naperville, IL) as described [14]. All neutralization assays were performed in duplicates by incubating 2×104 P. yoelii sporozoites with pre-immune or immune sera dilutions (1:5) for 40 min on ice. The sporozoites were then added to HepG2 cultures that incubator. The development of intracellular were maintained at 37°C in a humidified 5% CO2 hepatic-stage parasites was assayed after 40 hours incubation by real-time PCR as described [8].
The percent of T lymphocytes specific for LPC was determined by IFN-γ and IL-4 specific ELISPOT assays conducted ex vivo using pooled spleen cells obtained from four mice 20 days after priming. Assays were performed in nitrocellulose microplates (Millipore, Bedford, MA) coated with rat anti-mouse IFN-γ or rat anti-mouse IL-4 capture antibodies (BD Biosciences Pharmingen, San Diego, CA) following instructions from the manufacturer [8]. T cells were stimulated by the addition of rLPC, synthetic LPCcys+, or synthetic peptides representing T*-cell or CD8+ T cell epitopes at 10 μg/ml. Intracellular IL-2 producing cells were quantified by flow cytometry using Cytofix/cytoperm Plus Kit with GolgiStop (Pharmingen, San Jose, CA). Freshly isolated splenocytes from mice primed with rLPC or synthetic LPCcys+ were stimulated in vitro with 10 μg of rLPC, synthetic LPC cys+ or synthetic peptides representing the T*-cell or CD8+ T cell epitopes. After six hours, the cells were surface labeled for CD3/CD4 or CD3/CD8 followed by intracellular IL-2 staining. Data were acquired using CellQuest and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
2.7. Statistics
Antibody levels were log-transformed and compared using Student’s t-test. Differences in parasite loads and cytokine SFCs were assessed using multiple comparison tests. To evaluate the correlation between antibody titers, affinity assays and inhibition of parasite load, Spearman’s rank correlation coefficient was calculated. p-values of 0.05 or less were considered significant.
3. Results
3.1. rLPC expression and antigenicity
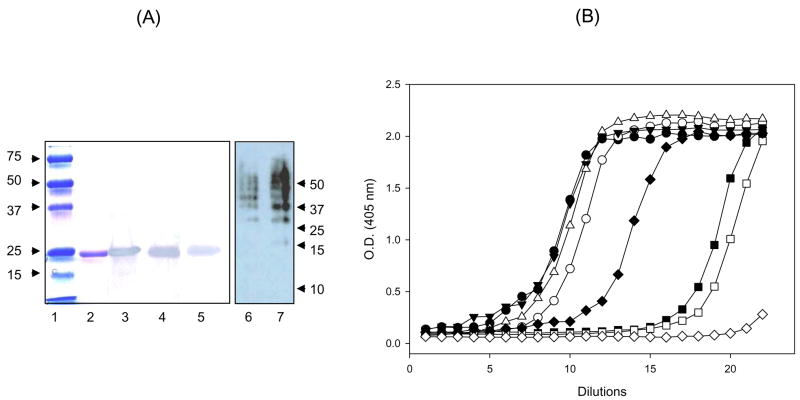
Protein electrophoresis showed a single band at 21 kDa relative mobility, the predicted size of recombinant LPC (Fig. 2A). Recombinant LPC was identified by Western blotting using anti-His tag antibody, 2A10 monoclonal antibodies that recognize the amino-terminal portion of the chimeric protein, and polyclonal anti-synthetic LPCcys+ or anti-rLPC antibodies (Fig. 2A). Anti-rLPC antibodies recognized several peptide species in the synthetic LPCcys+ that confirm the heterogeneity of the product (Fig. 2A). Fig. 2B compares antigenicity of purified recombinant versus synthetic LPCcys+, using polyclonal antibody produced by immunization with purified rLPC, synthetic LPCcys+ polymer or synthetic LPCcys− monomer. The antigenicity of recombinant LPC was higher than synthetic LPCcys+ following incubation with anti-rLPC (p =0.0011), anti-LPCcys+ (p =0.0033) or anti-LPCcys− (p =0.005) antibodies. As expected the 2A10 monoclonal antibody only recognized the rLPC (p <0.0001).
Figure 2.
Expression, purification and antigenic characterization of LPCs. A) Coomassie-stained reducing SDS page analysis (lane 1, MWM; lane 2, purified protein) and western blot analysis of rLPC expressed in E. coli BL21 (DE3). Western blot analysis of purified rLPC using anti-(NANP)3 tag mAb (lane 3), anti-synthetic LPCcys+ polyclonal antibodies (lane 4) and anti-rLPC polyclonal antibodies (lane 5). Western blot analysis of the synthetic LPCcys+ using anti-rLPC polyclonal antibodies is included for comparative purposes (lane 6, 0.25 μg; lane 7, 0.5 μg). B) Antigenicity of rLPC (1 μg/ml; closed symbols) or synthetic LPCcys+ (1 μg/ml; open symbols) determined by ELISA using the anti-(NANP)3 monoclonal antibody 2A10 or polyclonal antibodies. Data presented as geometric mean O.D. obtained at concentrations of mAb from 0.0009 ng/ml (dilution 1) to 1 μg/ml (dilution 22) or reciprocal serum dilution obtained from mice immunized with different antigens (from 1:80, dilution 1, to 1:41,943,040, dilution 22). rLPC or synthetic LPCcys+ were tested using the anti-(NANP)3 tag monoclonal antibody (◇◆) or pooled sera from mice immunized with rLPC (○●), LPCcys+ polymer (△▼;), LPCcys− monomer (□■).
3.2. Humoral and cellular immune response in mice immunized with rLPC or LPCcys+
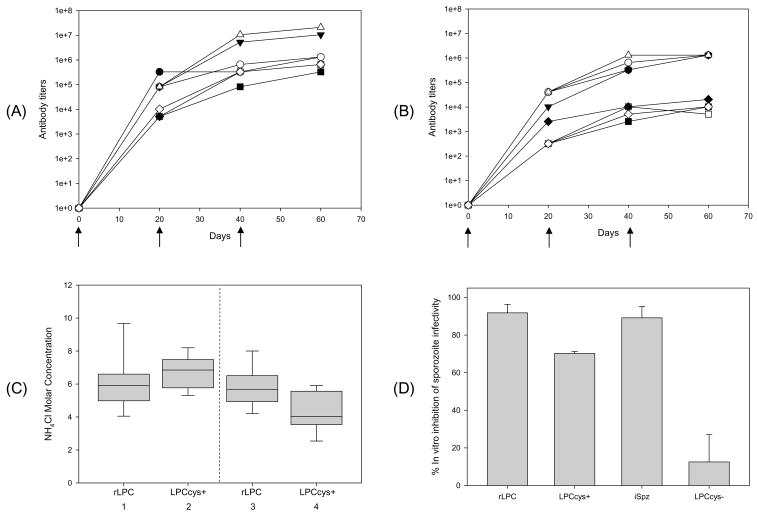
Kinetics of antibody responses to immunization of CAF1/J and BALB/c mice with recombinant or synthetic LPCcys+ followed similar trends whether antisera were tested against homologous or heterologous immunogen. Final antibody titers in mice of the same strain immunized with recombinant or synthetic LPCcys+ were not significantly different. However, anti-synthetic LPCcys+ antibody titers were 8-fold higher in CAF1/J mice than in BALB/c mice following immunization with rLPC (reciprocal final antibody titers 10,485,760 versus 1,310,720 respectively; p = 0.031) and 16-fold higher following immunization with synthetic LPCcys+ (reciprocal final antibody titers 20,971,520 versus 1,310,720; p = 0.05) (Fig. 3A–B). Using the P. yoelii-specific synthetic B-cell or T* epitopes as antigens, antibody titers were consistently higher in immunized CAF1/J versus BALB/c mice (PyB, p = 0.03; T*, p = 0.02) in animals immunized with synthetic LPCcys+ but not with rLPC. (Fig. 3A–B).
Figure 3.
Kinetics of antibody response, anti-LPC affinities and functional activity of antibodies elicited by immunization of mice with rLPC or synthetic LPCcys+. Antibody titers were measured 20 days after each immunization of CAF1/J (A) or BALB/c mice (B) with rLPC or synthetic LPCcys+ and expressed as reciprocal geometric mean antibody titers in pooled sera. The data are representative of one out of two independent experiments. Anti-sera from mice immunized with rLPC (closed symbols) or synthetic LPCcys+ (open symbols) were tested by ELISA with rLPC (●○), synthetic LPCcys+ (▼;△), synthetic peptide representing the B-cell epitope (□■) or synthetic peptide representing the T cell epitope (◇◆). (C) Antibody avidity (expressed as concentration of ammonium thiocyanate required to dissociate 50% of bound peptide) measured 20 days after the third immunization of CAF1/J (lanes 1 and 2) or BALB/c mice (lanes 3 and 4). Avidity of anti-rLPC antibody is shown in lanes 1 (CAF1/J mice) and 3 (BALB/c); anti-synthetic LPCcys+ in lanes 2 (CAF1/J) and 4 (BALB/c). The box-whisker plot represents median, 25th and 75th percentiles from six mice. (D) Sporozoite-neutralizing antibodies \in sera of BALB/c mice immunized with rLPC or synthetic LPCcys+. Viable P. yoelii sporozoites \were incubated for 40 minutes on ice with pools of immune sera, from BALB/c mice immunized with rLPC, LPCcys+, LPCcys− or irradiated sporozoites (iSpz), prior to the infection of HepG2 hepatoma cells. P. yoelii parasite loads were determined measuring 18S rRNA using RT-PCR after 40 hour incubation. The results are expressed as percentage of in vitro inhibition of sporozoite infectivity determined as follows: % Inhibition = [(1 − copy number obtained with immune sera/copy number obtained with pre-immune sera) × 100]
Consistent with differences in the magnitude of antibody response, total IgG anti-rLPC antibodies had higher avidity in CAF1/J mice than in BALB/c mice following immunization (NH4Cl molar concentration mean values 6.9 versus 5.9; p<0.0001) (Fig. 3C). The total IgG anti-synthetic LPCcys+ antibodies also had higher avidity in CAF1/J mice than in BALB/c mice following immunization (NH4Cl molar concentration mean values 7.8 versus 4.9; p<0.0001). The anti-rLPC antibody avidity in BALB/c mice immunized with recombinant LPC was higher than following immunization with irradiated sporozoites (concentration of thiocyanate required to dissociate 50% of total IgG was 5.81M for rLPC versus 4.85M for irradiated sporozoites immunized mice). This is in contrast with the 4.28M concentration required to dissociate 50% of anti-synthetic LPCcys+ in mice immunized with synthetic LPCcys+ versus 4.73M for irradiated sporozoites immunized mice.
To characterize the biological function of anti-sporozoite antibodies elicited by immunization with rLPC or synthetic LPCcys+, sporozoite-neutralization assays were conducted as described [14]. Preincubation of P. yoelii sporozoites with sera from mice immunized with rLPC or synthetic LPCcys+ prior to the hepatoma cell line infection reduce 91±4.5 or 70±1.2% respectively the infectivity compared to that of sporozoites incubated with sera from naïve mice (Fig. 3D). These levels of inhibition of sporozoite infectivity were significantly higher to that obtained when the sporozoites were incubated with sera from mice immunized with the peptide without flanking cysteine residues (LPCcys−) (p <0.001). Relevantly, levels of functional antibodies elicited by immunization with rLPC or synthetic LPCcys+ were equivalent to that obtained with sera derived from P. yoelii-irradiated sporozoite immunized mice (89±6%, Fig. 3D).
Analysis of IgG isotypes induced by immunization with recombinant or synthetic LPCcys+ in CAF1/J mice differed from those in BALB/c (Table 1). In CAF1/J mice the specific antibody concentration for each isotype induced by immunization could be ranked as IgG1=IgG2a=IgG2b=IgG3 for rLPC and IgG1=IgG2b=IgG3>IgG2a for synthetic LPCcys+. In BALB/c mice the specific antibody concentration for each isotype induced by immunization could be ranked as IgG2a >IgG1=IgG2b=IgG3 for rLPC and IgG1=IgG2a=IgG2b=IgG3 for synthetic LPCcys+. Comparison of LPCcys+-specific IgG1 and IgG2a isotypes titers did not show a clear Th1 or Th2 bias following immunization with recombinant or synthetic LPCcys+, suggesting that both Th1 and Th2 helper activity were induced (Table 1).
Table 1.
Comparison of the IgG isotypes induced by immunization with recombinant or synthetic LPC
| Strain | Haplotype | rLPCa | Synthetic LPCcys+a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IgG1 | IgG2a | IgG2b | IgG3 | IgG1 | IgG2a | IgG2b | IgG3 | ||
| CAF1/J | H-2a/d | 5,242,880 | 5,242,880 | 5,242,880 | 5,242,880 | 5,242,880 | 10,485,760 | 5,242,880 | 5,242,880 |
| BALB/c | H-2d/d | 655,360 | 1,310,720 | 655,360 | 655,360 | 655,360 | 655,360 | 655,360 | 655,360 |
Data are presented as median reciprocal final antibody titers in a pool of sera obtained from six mice 20 days after the third immunization.
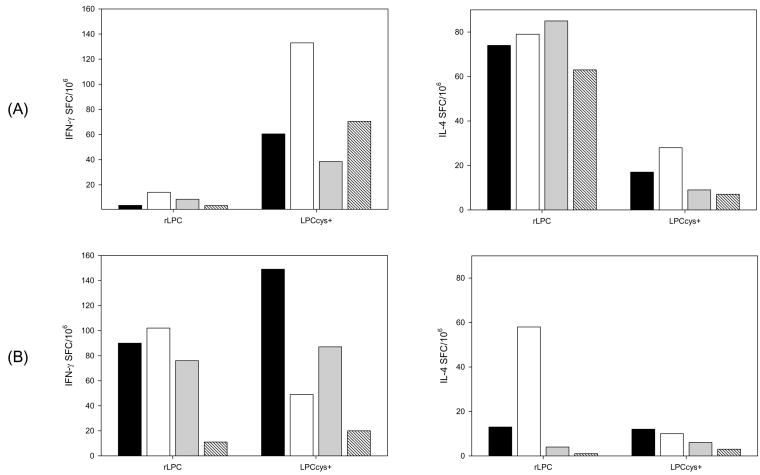
T-cell responses to immunization with LPCs were evaluated by quantifying IFN-γ or IL-4 secreting cells in pooled splenocytes. Fig. 4 compares T-cell responses in CAF1/J and BALB/c mice primed with recombinant or synthetic LPCcys+ following stimulation with peptides. IFN-γ and IL-4 secreting cells were confirmed in CAF1/J and BALB/c mice immunized with LPCs. The frequency of IFN-γ SFC was significantly lower following immunization with recombinant versus synthetic LPCcys+ in CAF1/J (p = 0.0078) but not in BALB/c mice. This is in contrast with the frequency of IL-4 secreting cells that was lower following immunization with synthetic LPCcys+ in CAF1/J (p < 0.0001) but not in BALB/c mice. Differences in the frequency of IFN-γ and IL-4 secreting cells between CAF1/J and BALB/c mice that received rLPC were also significant (p = 0.011 for IFN-γ; p = 0.0035 for IL-4) suggesting preferential Th1 recall response in BALB/c mice and preferential Th2 recall response in CAF1/J. Relevantly, we identified cellular recall responses to the individual T cell epitopes included in the recombinant or synthetic chimeras (Fig. 4).
Figure 4.
IFN-γ or IL-4 secreting cells in CAF1/J (A) or BALB/c mice (B) twenty days after priming with rLPC or synthetic LPCcys+. Results from duplicate wells are expressed as mean IFN-γ spot forming cells (SFC) or mean IL-4 spot forming cells (SFC) per 106 splenocytes obtained from four mice, after subtractions of the background values obtained with medium alone. The data are representative of one out of two independent experiments. Splenocytes were stimulated ex vivo with rLPC (black bars), synthetic LPCcys+ (white bars), a synthetic peptide representing the CD8+ T cell epitope (gray) or a synthetic peptide representing the promiscuous CD4+ T* epitope (stripped bars). Frequencies of IFN-γ or IL-4 SFC in mice immunized with adjuvant alone ranged from 3 to 11.
To characterize the phenotype of the antigen-responding cells, IL-2 CD4+ secreting cells were quantified following in vitro stimulation of splenocytes with the corresponding immunogen. LPC-specific IL-2 secreting T cells induced by immunization with recombinant or synthetic LPCcys+ were CD4+ in BALB/c mice (frequency of 0.37% for mice immunized with rLPC and 0.72% for mice immunized with synthetic LPCcys+). This is in contrast with the low frequency of IL-2-CD4+ secreting cells derived from placebo-immunized mice used as a control (0.001% for mice immunized with rLPC and 0.015% for mice immunized with synthetic LPCcys+). We were unable to identify IL-2 secreting cells in CAF1/J mice at this time point suggesting qualitative differences in the cellular responses.
3.3. Protection against P. yoelii challenge
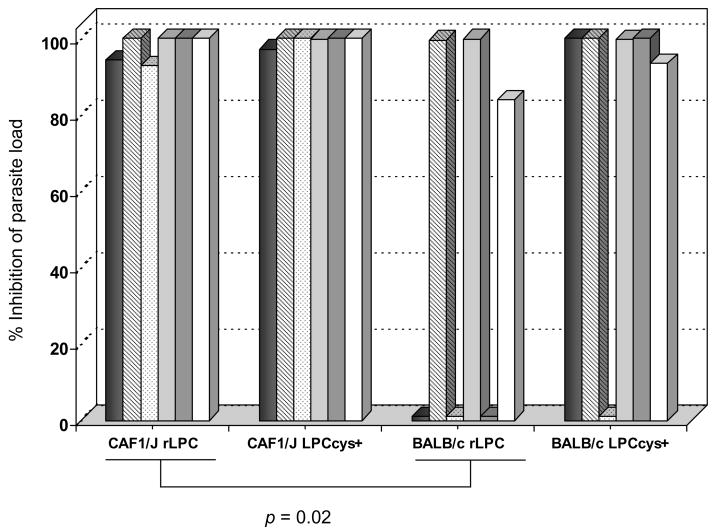
Immunization of CAF1/J and BALB/c mice with recombinant or synthetic LPCcys+ resulted in significantly lower P. yoelii parasite loads following challenge compared with control mice that received adjuvant alone (p < 0.0001). Differences in protective immunity were observed between CAF1/J and BALB/c mice immunized with recombinant LPC (p < 0.02) but not in mice immunized with synthetic LPCcys+ (Fig. 5). Interestingly, higher number of BALB/c mice immunized with the recombinant LPC exhibited poor reduction in parasite loads in comparison with BALB/c mice that received the synthetic LPCcys+. However, these differences were not statistically significant (Fig. 5). There was no correlation between reduction in parasite loads and IgG antibody titers or antibody avidity.
Figure 5.
Comparative evaluation of protective efficacy against malaria in mice immunized with rLPC or synthetic LPCcys+ after experimental challenge with P. yoelii sporozoites (n=6). Bars represent the percentage of inhibition of parasite load in the liver of individual mice and determined as follows: % Inhibition = [(1 − copy number obtained in the liver of immune mice/copy number mean value obtained in placebo immunized mice) × 100]. Results are shown for one out of two independent experiments that showed similar results.
4. Discussion
To overcome the need for polymerization of linear peptide chimeras for protective immunity, we designed a recombinant LPC protein that induced comparable antibody and cellular immune responses to the polymeric peptide construct. Cellular and humoral immune responses were elicited to individual epitopes included in the chimeric protein. Recombinant LPC reproduced the protective effect on parasite load in the liver observed with synthetic peptide polymers. These results suggest that stoichiometry rather than the presence of several peptide species in polymers of LPCcys+ are responsible for the immunological features of this unique vaccine construct.
To avoid the use of carrier molecules, different approaches have been used to improve the immunogenicity of multiepitope synthetic peptides (reviewed in [5, 6]). Such totally synthetic vaccines are aimed at increasing the valency of individual epitopes or enhancing the geometric complexity of the antigen. The use of peptides containing CTL epitopes and synthesized with extended flanking sequences have revealed that the length of the peptide is also critical for efficient priming of professional APC and to induce sustained immunity [15]. Multiepitope platforms can be designed using ligation chemistry [16, 17], branched peptide synthesis with oligolysine cores [18, 19] or orthogonally protected lysine residues [20] or using homo [21] or hetero polymerization [22]. These methodologies in general require complex biosynthesis procedures, are associated with low yields, or have been linked to deleterious effects on the immune response [23–25]. Two chimeric proteins that include a string of several B and T cell epitopes derived from pre-erythrocytic, erythrocytic and gametocytic proteins and a single non-malarial universal CD4+ T cell epitope have been previously reported [26–28]. The first generation of such protein chimeras faced problems associated with protein stability and low protein yield [28]. The inclusion of GPGPG spacers in the second generation seemed to improve both protein stability and antibody specificity [28]. We have included the same spacers in recombinant LPC resulting in a very stable recombinant product giving a final yield of 49.33 mg/liter. We are investigating more complex synthetic structures that include several subunits derived from human malaria parasites. This approach would facilitate the incorporation of multiple chimeras in a single recombinant construct with the resulting reduction of manufacturing and formulation costs.
LPCscys+ were designed for proof of principle studies to test the relevance of the inclusion of homologous promiscuous CD4+ T cell epitopes in malaria vaccine peptides with a topology favoring spontaneous polymerization. Homo-polymerization is a random process that generates a variety of peptide species with different molecular mass. Although, we have tested several batches of the LPCcys+ used here with consistent results, the heterogeneity of the product is demanding for scaling up production. We have reproduced the high immunogenicity of a P. yoelii LPCcys+ by using a chimeric protein designed to increase the size and the valency of the peptide subunit. The resulting tetravalent rLPC was topologically feasible and easy to express in E. coli. We included GPGPG spacers to reduce potential steric hindrance between individual modules as described for other synthetic constructs [29]. Antigenicity and immunogenicity studies using rLPC and polymers of synthetic LPCcys+ indicated that the antibody specificity is equivalent. We also confirmed that immune responses were elicited to individual components of the string of epitopes. This feature opens the possibility of designing complex synthetic genes encoding structured antigens or several subunits of linear epitopes each one incorporating homologous promiscuous CD4+ T cell epitopes. Relevantly, the homogeneity of the rLPC, a single ~21 kDa band, in comparison with several peptide species present in polymers of synthetic LPCcys+ make the recombinant product more attractive for further development as a vaccine platform. From a logistical standpoint, the expression of single product ensures minimum batch to batch variability.
Compared to immunization with LPCcys+, immunization with recombinant LPC results in enhanced production of specific high avidity antibodies. Responses to immunization with LPCs differed in CAF1/J and BALB/c mice. Antibody production and IgG avidity was higher in CAF1/J mice and IgG isotype patterns differed in hybrid and inbred mice following immunization. It appeared that immunization with recombinant and synthetic LPCcys+ elicited both Th1 and Th2 subsets of T-helper cells. These results may also indicate differences in P. yoelii-specific T* epitope presentation associated with the H-2a haplotype. Plasmodium promiscuous CD4+ T cell epitopes delivered using polymeric peptides induce a mixed Th1/Th2 cellular immune response in H-2a, H-2b, H-2d and H-2q mice [8]. For these chimeric peptides, H-2a and H-2b appear to be high responder strains. Considering that the P. yoelii-specific CD8+ T cell epitope included in LPC constructs tested here is H-2d restricted, this might explain differences between hybrid CAF1/J mice (H-2a/d) and BALB/c mice (H-2d/d).
To our knowledge this is the first demonstration of protection against malaria challenge using a multivalent subunit recombinant vaccine synthesized as a single molecule. Epitopes included in a recombinant chimeric protein induced immune responses in mice comparable to immunization with synthetic LPCcys+. The synthetic gene that encodes recombinant LPC can be easily inserted in different viral vectors to implement a prime-boost vaccine strategy. Synergic effects have been recently demonstrated by using a combination of a vaccine regimes with a recombinant viral fowlpox, modified virus Ankara (MVA) and a protein construct expressing the P. berghei CSP [30]. Our results suggest that intra-molecular antigenic competition is a surmountable obstacle to develop recombinant vaccine constructs. The concept of using recombinant chimeric proteins can be optimized to design a vaccine targeting human malaria parasites.
Acknowledgments
This research was supported by the US National Institutes of Health, NIAID grants R01-AI052371 and R01-AI064766 and the Yerkes National Primate Research Center Base Grant No RR00165 awarded by the National Center for Research Resources of the National Institutes of Health. We thank Brendan L. Flannery for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuehn BM. “Roadmap” Aids Malaria Vaccine Efforts. JAMA. 2007;298:849–851. doi: 10.1001/jama.298.8.849. [DOI] [PubMed] [Google Scholar]
- 3.Mu J, Awadalla P, Duan J, McGee KM, Keebler J, Seydel K, McVean GAT, Su X-z. Genome-wide variation and identification of vaccine targets in the Plasmodium falciparum genome. Nat Genet. 2007;39:126–130. doi: 10.1038/ng1924. [DOI] [PubMed] [Google Scholar]
- 4.Kooij TWA, Janse CJ, Waters AP. Plasmodium post-genomics: better the bug you know? Nat Rev Micro. 2006;4:344–357. doi: 10.1038/nrmicro1392. [DOI] [PubMed] [Google Scholar]
- 5.Purcell AW, Zeng W, Mifsud NA, Ely LK, Macdonald WA, Jackson DC. Dissecting the role of peptides in the immune response: theory, practice and the application to vaccine design. J Pept Sci. 2003;9:255–281. doi: 10.1002/psc.456. [DOI] [PubMed] [Google Scholar]
- 6.Bijker MS, Melief CJ, Offringa R, van der Burg SH. Design and development of synthetic peptide vaccines: past, present and future. Expert Rev Vaccines. 2007;6:591–603. doi: 10.1586/14760584.6.4.591. [DOI] [PubMed] [Google Scholar]
- 7.Caro-Aguilar I, Rodriguez A, Calvo-Calle JM, Guzman F, De la Vega P, Patarroyo ME, Galinski MR, Moreno A. Plasmodium vivax promiscuous T-helper epitopes defined and evaluated as linear peptide chimera immunogens. Infect Immun. 2002;70:3479–3492. doi: 10.1128/IAI.70.7.3479-3492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caro-Aguilar I, Lapp S, Pohl J, Galinski MR, Moreno A. Chimeric epitopes delivered by polymeric synthetic linear peptides induce protective immunity to malaria. Microbes Infect. 2005;7:1324–1337. doi: 10.1016/j.micinf.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Moreno A, Clavijo P, Edelman R, Davis J, Sztein M, Herrington D, Nardin E. Cytotoxic CD4+ T cells from a sporozoite-immunized volunteer recognize the Plasmodium falciparum CS protein. Int Immunol. 1991;3:997–1003. doi: 10.1093/intimm/3.10.997. [DOI] [PubMed] [Google Scholar]
- 10.Romero P, Maryanski JL, Cordey AS, Corradin G, Nussenzweig RS, Zavala F. Isolation and characterization of protective cytolytic T cells in a rodent malaria model system. Immunol Lett. 1990;25:27–31. doi: 10.1016/0165-2478(90)90086-6. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann CC, Yao Q, Ho CK, Buckwold SL. Flanking residues alter antigenicity and immunogenicity of multi-unit CTL epitopes. J Immunol. 1996;157:3242–3249. [PubMed] [Google Scholar]
- 12.Livingston BD, Newman M, Crimi C, McKinney D, Chesnut R, Sette A. Optimization of epitope processing enhances immunogenicity of multiepitope DNA vaccines. Vaccine. 2001;19:4652–4660. doi: 10.1016/s0264-410x(01)00233-x. [DOI] [PubMed] [Google Scholar]
- 13.Nardin EH, Nussenzweig V, Nussenzweig RS, Collins WE, Harinasuta KT, Tapchaisri P, Chomcharn Y. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J Exp Med. 1982;156:20–30. doi: 10.1084/jem.156.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar KA, Oliveira GA, Edelman R, Nardin E, Nussenzweig V. Quantitative Plasmodium sporozoite neutralization assay (TSNA) J Immunol Methods. 2004;292:157–164. doi: 10.1016/j.jim.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Zwaveling S, Mota SCF, Nouta J, Johnson M, Lipford GB, Offringa R, van der Burg SH, Melief CJM. Established Human Papillomavirus Type 16-Expressing Tumors Are Effectively Eradicated Following Vaccination with Long Peptides. J Immunol. 2002;169:350–358. doi: 10.4049/jimmunol.169.1.350. [DOI] [PubMed] [Google Scholar]
- 16.Rose K, Zeng W, Brown LE, Jackson DC. A synthetic peptide-based polyoxime vaccine construct of high purity and activity. Mol Immunol. 1995;32:1031–1037. doi: 10.1016/0161-5890(95)00090-9. [DOI] [PubMed] [Google Scholar]
- 17.Tam JP, Yu Q, Miao Z. Orthogonal ligation strategies for peptide and protein. Biopolymers. 1999;51:311–332. doi: 10.1002/(SICI)1097-0282(1999)51:5<311::AID-BIP2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Tam JP. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu YA, Clavijo P, Galantino M, Shen ZY, Liu W, Tam JP. Chemically unambiguous peptide immunogen: preparation, orientation and antigenicity of purified peptide conjugated to the multiple antigen peptide system. Mol Immunol. 1991;28:623–630. doi: 10.1016/0161-5890(91)90131-3. [DOI] [PubMed] [Google Scholar]
- 20.Fitzmaurice CJ, Brown LE, Kronin V, Jackson DC. The geometry of synthetic peptide-based immunogens affects the efficiency of T cell stimulation by professional antigen-presenting cells. Int Immunol. 2000;12:527–535. doi: 10.1093/intimm/12.4.527. [DOI] [PubMed] [Google Scholar]
- 21.Patarroyo ME, Amador R, Clavijo P, Moreno A, Guzman F, Romero P, Tascon R, Franco A, Murillo LA, Ponton G. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature. 1988;332:158–161. doi: 10.1038/332158a0. [DOI] [PubMed] [Google Scholar]
- 22.Jackson DC, O’Brien-Simpson N, Ede NJ, Brown LE. Free radical induced polymerization of synthetic peptides into polymeric immunogens. Vaccine. 1997;15:1697–1705. doi: 10.1016/s0264-410x(97)00085-6. [DOI] [PubMed] [Google Scholar]
- 23.Barington T, Skettrup M, Juul L, Heilmann C. Non-epitope-specific suppression of the antibody response to Haemophilus influenzae type b conjugate vaccines by preimmunization with vaccine components. Infect Immun. 1993;61:432–438. doi: 10.1128/iai.61.2.432-438.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Yedidia T, Arnon R. Effect of pre-existing carrier immunity on the efficacy of synthetic influenza vaccine. Immunol Lett. 1998;64:9–15. doi: 10.1016/s0165-2478(98)00073-x. [DOI] [PubMed] [Google Scholar]
- 25.Joshi MB, Gam AA, Boykins RA, Kumar S, Sacci J, Hoffman SL, Nakhasi H, Kenney RT. Immunogenicity of well-characterized synthetic Plasmodium falciparum Multiple Antigen Peptide conjugates. Infect Immun. 2001;69:4884–4890. doi: 10.1128/IAI.69.8.4884-4890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi YP, Hasnain SE, Sacci JB, Holloway BP, Fujioka H, Kumar N, Wohlhueter R, Hoffman SL, Collins WE, Lal AA. Immunogenicity and in vitro protective efficacy of a recombinant multistage Plasmodium falciparum candidate vaccine. Proc Natl Acad Sci USA. 1999;96:1615–1620. doi: 10.1073/pnas.96.4.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi YP, Das P, Holloway B, Udhayakumar V, Tongren JE, Candal F, Biswas S, Ahmad R, Hasnain SE, Lal AA. Development, expression, and murine testing of a multistage Plasmodium falciparum malaria vaccine candidate. Vaccine. 2000;18:2902–2914. doi: 10.1016/s0264-410x(00)00045-1. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Z, Todd CW, Wohlhueter RM, Price A, Xiao L, Schnake P, Bonner PC, Martin AM, Goldman IF, De La Vega P, Udhayakumar V, Lal AA. Development, characterization and immunogenicity of a multi-stage, multi-valent Plasmodium falciparum vaccine antigen (FALVAC-1A) expressed in Escherichia coli. Hum Vaccin. 2006;2:14–23. doi: 10.4161/hv.2.1.2437. [DOI] [PubMed] [Google Scholar]
- 29.Livingston B, Crimi C, Newman M, Higashimoto Y, Appella E, Sidney J, Sette A. A rational strategy to design multiepitope immunogens based on multiple Th lymphocyte epitopes. J Immunol. 2002;168:5499–5506. doi: 10.4049/jimmunol.168.11.5499. [DOI] [PubMed] [Google Scholar]
- 30.Hutchings CL, Birkett AJ, Moore AC, Hill AVS. Combination of Protein and Viral Vaccines Induces Potent Cellular and Humoral Immune Responses and Enhanced Protection from Murine Malaria Challenge. Infect Immun. 2007;75:5819–5826. doi: 10.1128/IAI.00828-07. [DOI] [PMC free article] [PubMed] [Google Scholar]