Abstract
Angiogenesis is essential for tumor growth, metastasis, arteriosclerosis as well as embryonic development and wound healing. Its process is dependent on cell proliferation, migration and capillary tube formation in endothelia cells (ECs). High levels of reactive oxygen species (ROS) such as superoxide and H2O2 are observed in various cancer cells. Accumulating evidence suggests that ROS function as signaling molecules to mediate various growth-related responses including angiogenesis. ROS-dependent angiogenesis can be regulated by endogenous antioxidant enzymes such as SOD and thioredoxin. Vascular endothelial growth factor (VEGF), one of the major angiogenesis factor, is induced in growing tumors and stimulates EC proliferation and migration primarily through the VEGF receptor type2 (VEGFR2, Flk1/KDR). Major source of ROS in ECs is a NADPH oxidase which consists of Nox1, Nox2, Nox4, Nox5, p22phox, p47phox and the small G protein Rac1. NADPH oxidase is activated by various growth factors including VEGF and angiopoietin-1 as well as hypoxia and ischemia, and ROS derived from this oxidase are involved in VEGFR2 autophosphorylation, and diverse redox signaling pathways leading to induction of transcription factors and genes involved in angiogenesis. Dietary antioxidants appear to be effective for treatment of tumor angiogenesis. The aim of this review is to provide an overview of the recent progress on role of ROS derived from NADPH oxidase and redox signaling events involved in angiogenesis. Understanding these mechanisms may provide insight into the NADPH oxidase and redox signaling components as potential therapeutic targets for tumor angiogenesis.
Introduction
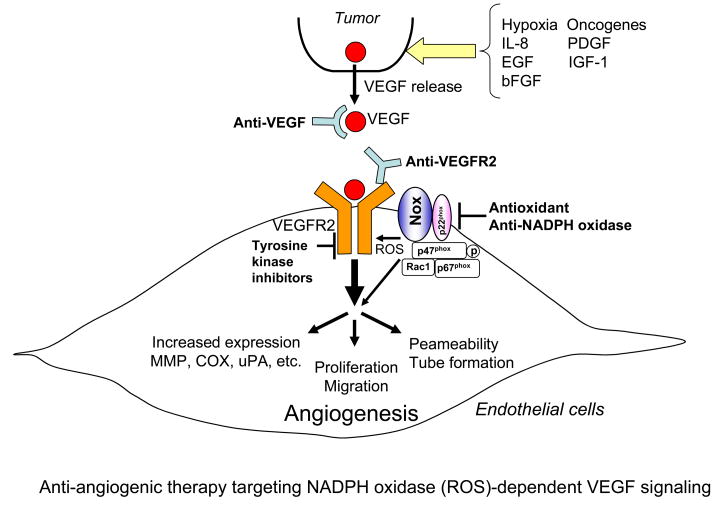
Angiogenesis, the process of new blood vessel formation from the pre-existing vessels, plays an important role in tumor growth, metastasis, embryonic development, wound healing, and arteriosclerosis 1. It is required for tumors to grow beyond a few millimeters in diameter, because successful growth and metastasis of tumors requires the establishment of an efficient blood supply 2. Progression to a growing tumor is characterized by induction of proangiogenic factors such as vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs), and VEGF receptors (VEGFR) in the growing endothelial cells (ECs)(Figure 1). VEGF is one of the most important angiogenesis growth factor 3 and stimulates permeability, proliferation, migration and tube formation of ECs primarily through the VEGF receptor type2 (VEGR2, KDR/Flk1) 4(Figure 1). VEGF plays a critical role in tumor angiogenesis not only through its effect on EC but also through mobilization of bone marrow-derived endothelial progenitor cells 5. Many stimuli including hypoxia, growth factors, cytokines and oxidative stress can increase VEGF expression in tumor cells, which is correlated with increased microvessel counts and poor prognosis in many human cancers (Figure 1). The conversion to the angiogenic phenotype in previously dormant tumors is known as the “angiogenic switch”. Thus, anti-angiogenic therapy is essential strategy for the treatment of tumor 2, 6, which has been focused on targeting VEGF and VEGFR2 using specific antibodies and tyrosine kinase inhibitors (Figure 1).
Figure 1. Anti-angiogenic therapy targeting NADPH oxidase (ROS)-dependent VEGF signaling.
Progression to a growing tumor is characterized by induction of proangiogenic factors such as vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs), and VEGF receptors (VEGFR) in the growing endothelial cells (ECs). VEGF is induced from tumor and stimulates permeability, proliferation, migration and tube formation of ECs primarily through the VEGFR type2 (VEGFR2). The conversion to the angiogenic phenotype in previously dormant tumors is known as the “angiogenic switch”. Thus, anti-angiogenic therapy is essential strategy for the treatment of tumor. In addition to VEGF and VEGFR2, ROS and NADPH oxidase are potential therapeutic targets for the treatment of tumor angiogenesis.
Reactive oxygen species (ROS) such as such as superoxide (O2•−) and hydrogen peroxide (H2O2) are found in a large number of tumors 7. ROS are conventionally thought as cytotoxic and mutagenic, and in high levels they induce cell death, apoptosis and senescence. In contrast, ROS at low levels function as signaling molecules to mediate cell growth, migration, differentiation and gene expression. Of note, ROS play an important role in angiogenesis 8, 9. ROS are produced in response to hypoxia, ischemia, angiogenic growth factors such as VEGF and angiopoietin-1, thereby stimulating EC proliferation and migration 10–12. ROS in tumor cells also contribute to angiogenesis and mitogenesis 13–15. Anti-tumor activity of conventional chemotherapeutic agents is enhanced by antioxidants treatment 16. Tumor cells may be inherently more resistant to oxidative stress than normal cells, or oxidative stress may provide a selective advantage in tumor growth. ROS-generating enzymes such as NADPH oxidase (Nox) has been shown to contribute to activation of redox signaling pathways leading to angiogenic responses in ECs as well as postnatal angiogenesis in vivo 11, 17–25. The aim of this review is to summarize the recent progress and information on ROS and NADPH oxidase as potential therapeutic targets for treatment of tumor angiogenesis (Figure 1).
1. Generation and metabolism of ROS
Oxygen is fundamental to cellular respiration and cells have evolved several enzyme systems that use this ubiquitous substrate as an acceptor of electron transfer. The reduction of oxygen by one electron increases the formation of O2−, which can be either dismutated to H2O2 spontaneously or in a reaction catalyzed by superoxide dismutase (SOD). Further reactions lead to the formation of hydroxyl radicals (•OH), especially in the presence of metal ions through the Fenton or Haber-Weiss reactions 26, 27. O2− reacts with nitric oxide (NO•) at a near diffusion-limited rate to form peroxynitrite (ONOO−), which is a potent oxidant. NO• is protective and thus loss of NO• bioavailability via these reactions contributes to various pathophysiologies. Mammalian peroxidases such as myeloperoxidase (MPO) are activated by H2O2 to form a highly reactive radical that can oxidize NO• to NO2− and react with NO2− to form NO2•. NO2• can, in turn, participate in nitrating events, such as the formation of nitrotyrosines. Under homeostatic conditions, antioxidant defences are critical to modulate the steady state balance.
2. Role of ROS in Angiogenesis
ROS such as such as O2•− and H2O2 are increased in a large number of cancer cells 7. ROS act as key mediators of the cellular signaling induced by the ligation of the cell surface receptors as well as by many classes of environmental agents 8, 28–32. Cell stimulation by such agents has been shown to increase the cellular ROS levels, which regulate various cellular functions. Exogenous ROS stimulate induction of VEGF by various cell types, and promote cell proliferation and migration 10, 12, 13, 33, cytoskeletal reorganization 34 and tubular morphogenesis 35 in ECs. Hypoxia/reoxygenation and adhesion of activated polymorphonuclear leukocytes to ECs causes ROS production, which results in capillary tube formation 36, 37. Angiogenesis growth factors such as VEGF and angiopoietin-1 (Ang1) induce EC migration and/or proliferation through an increase in ROS 11, 21, 23, 25. Leptin, a circulating adipocytokine, is also defined as angiogenesis factor 38 and upregulates VEGF mRNA and stimulates cell proliferation through an increase in ROS in ECs 39. Pigment epithelium-derived factor (PEDF), a natural inhibitor of angiogenesis 40, blocks leptin-induced ROS production in ECs 39, suggesting that it may function as an endogenous antioxidant factor. Thus, many of angiogenenic-related responses are mediated through ROS in ECs.
In vivo, ROS are also involved in tumor angiogenesis as well as various physiological and pathological angiogenesis. ROS play an important role in neovascularization during tumor growth 41, and thiol antioxidant, N-acetylcysteine (NAC) attenuates EC invasion and angiogenesis in a tumor model in vivo 42. PEDF which has antioxidant properties inhibits angiogenesis and melanoma growth 43. ROS are increased during the reperfusion of the ischemic retina, which upregulates VEGF mRNA 44. Short periods of ischemia/reperfusion or preconditioning induce an increase in ROS, thereby stimulating myocardial angiogenesis or collateral development 45–47. Moreover, ROS are involved in physiological repair processes such as ischemia-induced angiogenesis and wound healing in vivo 22, 48. The ROS inhibitors 49 or antioxidants such as pyrrolidine dithiocarbamate 50 inhibit neovascularization in the mouse model of angiogenesis. Recently, Kim et al. 51 demonstrated that Ang1-induced angiogenesis is enhanced in catalase−/− mice as compared to catalase+/+ mice, suggesting that Ang1-induced H2O2 plays an essential roles in angiogenesis in vivo. In pathophysiological state, strong correlation between ROS production, neovascularization and VEGF expression has been reported in eyes of diabetics 52–54 and balloon injured arteries 55. Anti-angiogenic therapy reduces plaque growth and intimal neovascularization in atherosclerosis 56. Antioxidants vitamins C and E reduce VEGF and VEGFR-2 expression in apolipoprotein-E deficient mice 57. These reports indicate that ROS play an important role in postnatal neovascularization in physiological and pathophysiological states.
3. Role of NADPH oxidase in Angiogenesis
ROS are generated from a number of sources including the mitochondrial electron transport system, xanthine oxidase, the cytochrome p450, the NADPH oxidase, uncoupled NO synthase (NOS) and MPO. The mitochondria and the Nox family of NADPH oxidase have emerged as major sources of ROS induction 31, 58, 59. Before the recent discovery of the NADPH oxidase family as a major source of ROS generation 18, the main intracellular ROS were known to be derived from mitochondria. Mitochondrial DNA mutations are responsible for aberrant ROS production, leading to development of neoplastic lesions and progression 60. It is unclear whether mitochondria-dependent ROS generation are directly associated with tumor angiogenesis.
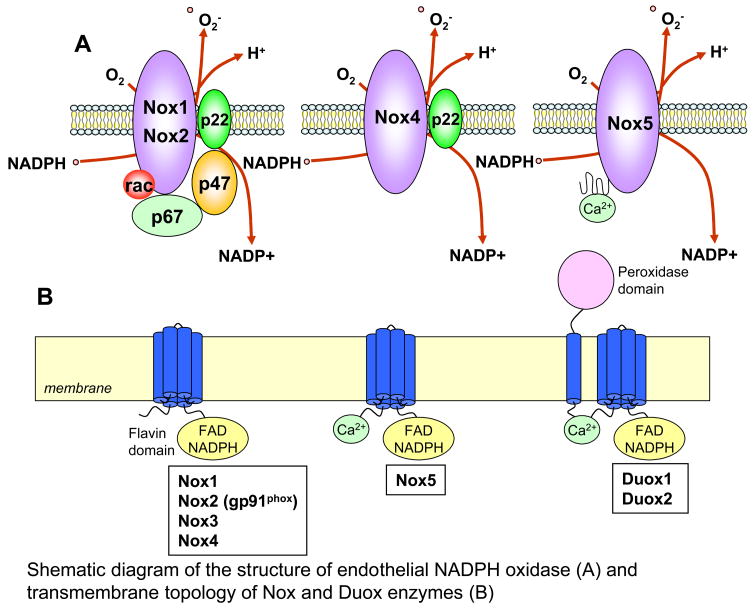
One of the major sources of ROS in ECs is NADPH oxidase, also known as Nox enzymes. The prototypical Nox is the phagocyte NADPH oxidase that is normally quiescent but generates a large amount of O2•− upon activation during phagocytosis via the one electron-reduction of oxygen by NADPH, which participate in host defense by killing invading microbes 59, 61. This phagocytic type of NADPH oxidase consists of membrane-bound subunits, gp91phox and p22phox which form the flavocytochrome b558 complex, together with the cyctosolic subunits p40phox, p47phox, and p67phox as well as small GTPase, Rac. Superoxide production is induced by assembly of the cytosolic and membrane-bound subunits, which is mediated through the phosphorylation of p47phox 62. The neutrophil NADPH oxidase releases large amounts of O2•− in bursts, whereas the non-phagocytic NADPH oxidase(s) continuously produce low levels of O2•− intracellularly in basal state, yet it can be further stimulated acutely by various agonists and growth factors 31, 63. Several human homologs of gp91phox (also termed as Nox2) have been identified in non-phagocytic cells. The first described homolog is Nox1 which was cloned from the colon epithelial cells and involved in mitogenic activity. 17. Subsequently, additional homologs including Nox3, Nox4, Nox5, and the Dual oxidases (Duox1, and Duox2) were cloned 59, 64–66 (Figure 2). Of note, each is encoded by different genes 59, 63. Recently, isoforms of p47phox and p67phox were discovered. These were termed NoxO1 (for Nox organizer 1) and NoxA1 (for Nox activator 1), which substitute for p47phox and p67phox respectively 67, 68. An important difference between p47phox and NoxO1 is that the latter lacks the p47phox domain that is regulated by phosphorylation; therefore NoxO1 may influence oxidase activity quite differently from p47phox.
Figure 2. A, Shematic diagram of the structure of NADPH oxidase in ECs.
gp91phox (Nox2) and its homologues (Nox1, Nox4 and Nox5) and cytosolic components p47phox, p67phox and small GTPase Rac1 have been identified in ECs. B, Transmembrane topology of Nox and Duox enzymes. The predicted transmembrane α-helices contain conserved histidine residues which comprise binding sites for haems. The carboxyl-terminal domain folds within the cytoplasm and binds to flavin adenine dinucleotide (FAD) and NADPH. The enzymes catalyze the transfer of electrons from NADPH to molecular oxygen, to form O2− across the membrane. The amino-terminal calcium-binding domain of Nox5 and Duox enzymes are also predicted to be on the cytosolic side of the membrane. Additional transmembrane α-helix of the Duox enzymes at the amino-terminus localize the peroxidase domain to the opposite side of the membrane, where it can use ROS generated by the catalytic core to generate more powerful oxidant species that then oxidize extracellular substrates.
Nox isozymes have been shown to increase in association with ROS production and tumorgenicity in various cancer cells. Nox1 is highly expressed in human colon cancers and prostate cancers 69. Overxpression of Nox1 in NIH3T3 fibroblasts induced malignant transformation, rendering them slightly tumorigenic in athymic mice 17. NIH 3T3 cells that stably express Nox1 exhibit cell growth, and coexpression of catalase along with Nox1 reverses the growth phenotype, suggesting that one of the signaling species generated by Nox1 is H2O2 18. Arbiser et al. 19 demonstrated that Nox1-induced H2O2 increases VEGF and VEGF receptor expression and MMP activity, markers of the angiogenic switch, thereby promoting vascularization and rapid expansion of the tumors. In colon epithelial cells, Nox1 oxidase activity requires NoxO1 and NoxA1 instead of p47phox and p67phox. In addition to Nox1, expression of Nox4 and Nox5 has been shown to be increased in melanoma cells 20 and prostate cancer cells 70, respectively. Of note, there is cross-talk between mitochondria and O2− generating NADPH oxidase in breast and ovarian tumors in that mitochondria controls Nox1 redox signaling and the loss of control of this signaling contributes to tumorigenesis 71.
Various Nox enzymes, Nox1, Nox2, Nox4 and Nox5 as well as cytosolic regulatory subunits p47phox, p67phox and Rac1 have been shown to be involved in ROS production in ECs (Figure 2), a major site of angiogenesis as well as in vivo model of angiogenesis, as listed below. To our knowledge, role of Nox3, Duox, NoxO1 and NoxA1 in ECs/cancer cells has not been demonstrated.
Nox1
Nox1 is upregulated by oscillatory shear stress, mediating ROS-dependent leukocyte adhesion to ECs 72. Nox1 stimulates branching morphogenesis in sinusoidal ECs 73. Given that leukocyte adhesion to ECs causes disruption of cell-cell junction which is required for initiating EC migration and proliferation, and that morphogenesis is a critical component of angiogenic process, it is likely that Nox1 may be involved in angiogenesis in ECs. Of note, Petry et al. 74 showed that Nox1 depletion has no effect on ROS levels and proliferation in basal EC.
Nox2
Nox2 is a critical component of ROS-generating NADPH oxidase activated by various stimulants and agonists in ECs 11, 75–78. VEGF and Ang1 stimulate Nox2-based NADPH oxidase in EC, which is involved in angiogenesis in ECs 11, 25. We have shown that Nox2 binds to actin and IQGAP1, an actin- and Rac1-binding scaffold protein, at the leading edge in migrating ECs 79. IQGAP1 seems to tether Nox2 to actin cytoskeleton at the leading edge to direct ROS production and EC migration, which may contribute to angiogenesis. Consistent with this, Petry et al. 74 reported that Nox2 is co-localized not only with endoplasmic reticulum (ER) marker calreticulin but also with F-actin at the plasma membrane. Li et al. 80 showed that IL-1b stimulation of MCF-7 epithelial cells causes Nox2 internalization into endosomes in a Rac1-dependent manner together with the IL receptor. H2O2 generation within the endosomes plays a critical role in subsequent activation of an IκB (inhibitory κB) kinase complex and NF-κB. Neovascularization in response to ischemia or VEGF is inhibited in Nox2−/− mice and in wild-type mice treated with antioxidant ebselen or NADPH oxidase inhibitor apocynin or gp91ds-tat 11, 22, 24. Furthermore, Nox 2 expression is increased in association with ROS production in mice ischemia hindlimb and retinopathy model 22, 24. Khatri et al. 81 have shown that vascular NADPH oxidase-derived ROS promotes VEGF expression and neovascularization of experimental atheroma in transgenic mice overexpressing p22phox, a binding partner of Nox.
Nox4
Nox4 is expressed more abundantly compared to other Nox proteins in ECs, and seems to be involved in basal O2•− production 82. Kuroda et al. 83 demonstrated that Nox4 preferentially localizes to the nucleus in human ECs, which is involved in basal- and PMA-stimulated NADPH oxidase activity in nuclear fraction as well as oxidative stress responsive gene expression. Thus it is temping to speculate that Nox4 might act as a sensor for nuclear redox 65, which may contribute to gene expression linked to cell growth, differentiation, senescence or apoptosis. Nox4 is also localized at endoplasmic reticulum where it interacts with p22phox, which contributes to basal ROS production and proliferation in ECs 74. Most recently, Datla et al. 84 reported that Nox4 siRNA inhibits VEGF-induced EC migration and proliferation, while overexpression of dominant negative Nox4 blocks PDGF receptor autophosphorylation. It should be noted that this dominant negative Nox4 also blocks Nox2 function. Role of endogenous Nox4 in VEGFR2 autophosphorylation and downstream signaling remains unclear. Vallet et al. 85 reported that Nox4 expression is upregulated in new capillaries in brain ischemia-induced angiogenesis of mice.
Nox5
NOX5 contains four EF-hand domains in its N terminus, is FAD and NADPH dependent as other Nox1-Nox4 but p22phox independent 86 and is activated by calcium 87. NOX5 is expressed in lymphoid cells and the testis 87 as well as in prostate cancer cells 70. Most recently, BelAiba et al. 88 reported that Nox5β, Nox5δ as well as a short variant lacking calcium-binding domains, Nox5S are expressed in human microvascular ECs. They also showed that Nox5 is mainly localized at ER as other Nox enzymes, and that overexpression of Nox5 stimulates ROS production, proliferation and formation of capillary-like structures whereas depletion of Nox5 by siRNA prevents these responses by thrombin in ECs. Whether Nox5 is involved in VEGF or Ang-1-induced ROS production and angiogenesis remains unknown. Of note, overexpression of p22phox interacts with Nox5 but it is not essential for Nox5-mediated ROS production. Nox5 is present in most mammals but is absent in rodents where its function may have been taken over by another Nox or Duox. These suggest that Nox5 variants may play a role in controlling ROS-dependent process involved in angiogenesis.
Rac1
Rac1 is involved in VEGF- and Ang-1-induced increase in ROS production which is involved in EC migration, a critical angiogenesis process 11, 25. Overexpession of active form of Rac1 induces loss of cell-cell adhesion 89 and cytoskeletal reorganization 90 through increase of H2O2, which are required for EC migration. Rac1 activity is highest at the leading edge in wound-induced migrating cells 91 and endogenous H2O2 accumulates at the membrane ruffles in actively migrating ECs 79, 92. Thus, Rac1 seems to play an important role in angiogenesis in ECs. Of note, HMG-CoA reductase inhibitor, statins which inhibit Rac1 activity 93 can block angiogenesis in vivo 94.
p47phox
In ECs, p47phox phosphorylation is involved in oxidase activation in response to angiotensin II, TNFα, VEGF and oscillatory shear stress, and these agonist-induced O2− production is inhibited in ECs isolated from p47phox−/− mice 95–97. Protein kinase C isoforms are the major kinases responsible for p47phox phosphorylation although other kinases such as Akt, p38 MAP kinase and PAK (p21-activated kinase) also play a role depending on the stimulus 59, 98, 99. Gu et al. 100 reported that p47phox localizes to the cytoskeletal elements and plays a role in TNFα-induced c-terminal Jun kinase activation in ECV304 cells. After agonist stimulation or during directed cell migration, p47phox translocates from the perinucleus to the membrane ruffles through binding to the WAVE1 and to the leading edge of focal complexes through binding to the adaptor TRAF4 and Hic-5, a focal contact scaffold, in ECs 101–103. Thus, p47phox is a functional component of NADPH oxidase associated with actin cytoskeleton in ECs, implicating its role in angiogenesis. Recently, Chen et al. 104 reported that Ang1-stimulated ROS, Akt and ERK phosphorylation, cell migration and capillary growth from aortic ring are inhibited in heart microvascular ECs from p47phox−/− mice. Thus, p47phox is a critical component of NADPH oxidase involved in angiogenesis in EC and isolated vessels. Definitive role of p47phox in postnatal angiogenesis including tumor angiogenesis in vivo will require further investigation using p47phox−/− mice.
4. Role of SOD in Angiogenesis
The reduction of oxygen by one electron increases the formation of O2−, which can be either dismutated to H2O2 spontaneously or in a reaction catalyzed by SOD. Overexpression of extracellular SOD (ecSOD) inhibits tumor vascularization in mice 105, suggesting that antioxidant ecSOD treatment is useful therapeutic strategy for angiogenesis-dependent diseases. Using ecSOD deficient mice, we recently demonstrated that endogenous ecSOD is required for reparative neovascularization in response to ischemic injury by protecting ischemic tissues and bone marrow from overproduction of O2− 48. This study suggests that optimal low level of ROS is necessary but overproduction of ROS is inhibitory for postnatal angiogenesis in vivo. Furthermore, cytosolic Cu/ZnSOD overexpressing transgenic mice show enhancement of FGF-induced angiogenesis and tumor development 106, and gene transfer of Cu/ZnSOD in NIH3T3 fibroblasts enhances VEGF synthesis through an increase in H2O2 107. Connor et al. 108 have reported that MnSOD promotes mitochondrial H2O2 production, thereby stimulating EC sprouting and neovascularization in the CAM assay. VEGF-induced ROS produced via activation of Rac1 upregulate MnSOD expression in ECs 109, which could represent a feed-forward mechanism by which ROS-triggered H2O2 plays an important role in angiogenesis. Thus, SOD may serve as a H2O2-generating, pro-angiogenic enzyme, rather than anti-angiogenic, antioxidant enzyme, in some settings 107.
5. Role of Thioredoxin in Angiogenesis
Thioredoxins (Trx) are a family of small redox active proteins that undergo reversible oxidation/reduction and play an important role to maintain the redox state of cells. Trx serves as a cofactor in many Trx-catalyzed reductions in a manner similar to glutathione in thioltransferase reactions to control the reduced intracellular redox environment, cellular growth, and defense against oxidative stress in mammalian cells including cancer cells 110. Trx-1 is involved in many of the hallmarks of cancer including increased proliferation, resistance to cell death and increased angiogenesis 111. Trx-1 is a validated cancer drug target associated with aggressive tumor growth, resistance to standard therapy and decreased patient survival.
6. Role of ROS as Signaling Molecules in Angiogenesis
ROS can act as a signaling molecule for activation of diverse signaling pathways by oxidation of reactive cysteine on the specific target molecules including kinases, phosphatases 28–30, redox sensitive transcripition factors 112, cell cycle regulators 113.
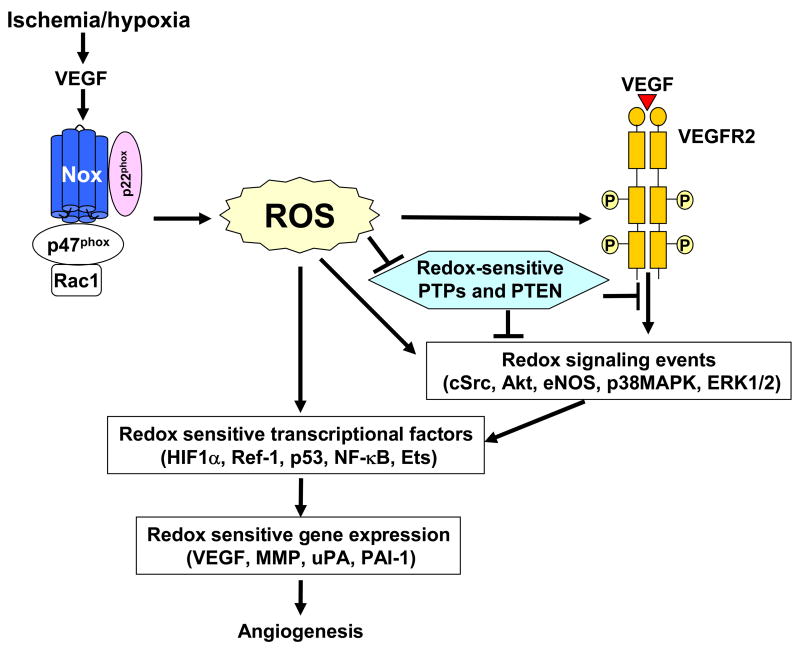
NADPH oxidase is activated by numerous stimuli including VEGF, EGF, cytokines, shear stress, hypoxia and G-protein coupled receptor agonists including Ang II in ECs 31. VEGF binds to two tyrosine kinase receptors, VEGF receptor-1 (VEGFR1, Flt-1) and VEGFR2 in ECs. The mitogenic and chemotactic effects of VEGF in ECs are mediated mainly through VEGFR2 4 which is activated through autophosphorylation of tyrosine residues in the cytoplasmic kinase domain. This event is followed by activation of downstream signaling pathways such as mitogen-activated protein kinases, Akt and eNOS, which are essential for EC migration and proliferation 4. VEGF stimulation increases ROS production via activation of Rac1-dependent NADPH oxidase in ECs 11, 21, 23, 109, 114, 115. We and others have shown that ROS are involved in VEGF-induced VEGFR2 autophosphorylation in ECs 11, 23, 114 (Figure 3). Ang-1 also stimulates ROS production through activation of Rac1-dependent, Nox2-based NADPH oxidase through Tie-2 receptor (Tie-2 R), which is required for EC chemotaxis 25. ROS are also important for VEGF-induced cSrc activation, phosphorylation of VE-cadherin and Akt, thereby stimulating angiogenesis in ECs 21, 89, 115. Evidence suggests that VEGFR2-mediated signaling is temporally and spatially controlled and that NADPH oxidases are localized within discrete subcellular compartments, which is required for localizing ROS production and activation of specific redox signaling events 32, 116.
Figure 3. Role of ROS derived from NADPH oxidase in VEGF signaling linked to induction of transcription factors and genes involved in angiogenesis.
Ischemia/hypoxia stimulates induction of VEGF which stimulates NADPH oxidase to produce ROS, thereby inducing oxidative inactivation of protein tyrosine phosphatases (PTPs) and PTEN to promote VEGFR2 autophosphorylation and downstream redox signaling events or directly activating redox signaling kinases. These events are converged and integrated to induce various redox sensitive transcriptional factors and gene expression, which are involved in angiogenesis.
The signaling properties of ROS are due, in part, to reversible oxidative inactivation of redox-sensitive target proteins including protein tyrosine phosphatases (PTPs) 28–30. SHP-2, one of the SH2-containing PTP, can be inactivated by ROS in PDGF-treated Rat-1 cells, which is associated with autophophorylation of PDGFR leading to MAPK activation 117. Several PTPs including SHP-1, SHP-2 and low molecular weight PTP (HCPTPA) inducibly associate with VEGFR2 after VEGF stimulation 118–120. HCPTPA overexpression inhibits VEGF-induced VEGFR2 autophosphorylation but its endogenous role has not been demonstrated 120. SHP-1 mediates TNF-induced inhibitory effect on VEGFR2 phosphorylation without affecting VEGF-induced responses in ECs 121. SHP-2 negatively regulates VEGFR2 signaling in ECs which are cultured only on type I collagen 122. High cell density-enhanced PTP1 (DEP-1)/CD148 attenuates phosphorylation of VEGFR2 in contact-inhibited confluent ECs 123. A small molecule inhibitor of PTP1B enhances VEGF-induced VEGFR2 autophosphorylation, migration and proliferation of EC as well as neovascularization in a mouse matrigel model 124. Ang-1 stimulates association of SHP-2 to the phosphorylated Tie-2 R in ECs 125, which in turn inhibits PI3 kinase-dependent signaling pathways involved in EC migration.
Tumor suppressor PTEN dephosphorylates phosphatidylinositol 3,4,5-triphosphate, a product of the PI3 kinase (PI3K) reaction, and is critically involved in a wide variety of human tumors 126. ROS regulate EGF-induced increase in VEGF and HIF-1α expression through activation of PI3K/Akt/p706K pathway which is involved in tumorigenesis and angiogensis in ovarian cencer cells 127. Although underlying mechanisms remain unclear, it is tempting to speculate that ROS-dependent oxidative inactivation of PTEN 128 may be involved in this response. Low molecular weight PTPs are oxidized and inactivated by ROS during cell adhesion to matrix in fibrobrast 129. ROS derived from Rac1-induced NADPH oxidase inactivate PTP-PEST at focal contacts, thereby promoting membrane ruffling and endothelial migration 103. Thus, it is likely that PTPs and PTEN are reversibly oxidized and inactivated by angiogenesis growth factor-induced ROS, thereby promoting RTK-mediated redox signaling linked to angiogenesis (Figure 3).
7. Redox-sensitive Transcription Factors and Genes involved in Angiogenesis
Transcription factors and genes involved in angiogenesis are regulated by ROS. These redox-sensitive transcription factors and genes include hypoxia inducible factor-1 (HIF-1), Ref-1, p53, NF-κB and the ETS transcription factor, Ets-1 as well as VEGF, MMP, cyclooxygenase-2 (COX-2), urokinase plasminogen activator (uPA) and plasminogen activator inhibitor-1 (PAI-1) (Figure 3).
HIF-1
In response to tumor hypoxia, many angiogenesis-related genes including VEGF and erythropoietin are upregulated by HIF-1, which is a heterodimeric transcription factor composed of HIF-1α and HIF-1β subunits 130–132. HIF-1 activates the transcription of many genes involved in multiple aspects of tumor growth including angiogenesis, cell survival, and invasion. High levels of HIF-1 expression are observed in many human cancers, and are correlated with tumorigenesis 133. ROS derived from NADPH oxidase are also involved in induction of HIF-1α under normoxia and hypoxia in vascular cells 134–136. Görlach et al. 137 reported that overexpression of Rac1 increases HIF-1 expression through ROS. Thus, Rac1/Nox/ROS pathways play an important role for upregulation of HIF-1 and VEGF expression in response to VEGF and hypoxia. A cytochrome b-type NADPH oxidoreductase or mitochondria also produce ROS under hypoxia. As mentioned, ROS are involved in upregulation of HIF-1α and VEGF protein expression through activation of PI3K/Akt/p706K pathway or MEK/ERK pathway in cancer cells 138–142 Of interest, JunD, a member of the AP-1 family of transcription factors, promotes degradation of HIF-1α and reduces expression of VEGF by reducing Ras-mediated production of ROS, thereby inhibiting tumor angiogenesis 143. These reports suggest that ROS play an important role for stabilizing HIF-1α protein expression, which may contribute to VEGF expression and tumor angiogenesis.
Ref-1
The redox state of cysteines is in part under the control of the redox factor 1 (Ref-1), a nuclear protein whose reducing activity also increases DNA binding activity of other transcription factors such as Fos, Jun, NF-κB 144, and p53 145
p53
p53 is an important intracellular mediator of the stress response including ROS and is now also recognized as a modifier of the angiogenic response 146. p53 interacts with the HIF system but also has direct effects on angiogenesis regulators or interfere with translation mechanisms of angiogenesis factors and mediators such as VEGF 147 and FGF 148.
NF-κB
The redox-regulated transcription factor NF-κB is involved in NADPH oxidases-dependent tumor cell proliferation via regulating numerous genes involved in apoptosis, cell proliferation, metastasis and angiogenesis 149. H2O2 induces NF-κB-dependent IL-8 expression in ECs, which contributes to the angiogenic phenotype 35. NF-κB is also constitutively expressed in numerous malignancies 149, and is predominantly activated in adenoma and adenocarcinoma cells which express abundant Nox1, suggesting that Nox1 stimulates NF-κB-dependent pro-inflammatory pathways in colon tumors 150.
Ets
Ets-1 is a transcriptional mediator of vascular inflammation and remodeling 151. H2O2 stimulates induction of the transcription factor Ets-1, which are involved in EC proliferation and tube formation 13 as well as expression of chemokine monocyte chemoattractant protein-1, the adhesion molecule vascular cell adhesion molecule-1, and the PAI-1 151. Most recently, Ni et al. 152 demonstrated that Ets-1 is a critical transcriptional regulator of angiotensin II-mediated ROS generation and the induction of NADPH oxidase subunit p47phox. Thus, positive feed-forward mechanism may exist whereby ROS induce Ets-1 expression and activated Ets-1 increases expression of ROS-generating enzyme.
MMP
MMP is another ROS-regulated molecule involved in the progression of tumor-induced angiogenesis 19. The presence of MMPs is essential for endothelial cell ingression into the tumor tissue because they degrade the extracellular matrix of the tumor cells and free the way for migrating ECs 153. ROS can activate MMP-9 154 and VEGF stimulates MMP-1 and 2 expression in ECs 155. Wartenberg and his colleagues demonstrated that confrontation cultured ECs differentiated from embryonic stem cells invade tumor tissue, which results in tumor vascularization and growth with an increase in ROS 156. This data strongly suggests that tumor-induced angiogenesis requires the presence of ROS for EC invasion. In parallel with this phenomenon, up-regulation of MMPs was observed, which was abolished in the presence of free radical scavengers 157. Thus, ROS-dependent MMP expression is a prerequisite for vascular growth within the tumor tissue with subsequent tumor expansion (Figure 3).
COX-2
COX-2 is a key enzyme in the synthesis of prostaglandins and thromboxans and is highly upregulated in tumor cells and angiogenic ECs during tumor progressioin, and plays an important role in tumor angiogenesis. Involvement of NADPH oxidase-derived ROS in COX2 induction has been demonstrated 158–163.
Urokinase plasminogen activator (uPA)
uPA, its receptor (uPAR) and PAI-1-mediated signaling are involved in tumour cell invasion, survival, angiogenesis and metastasis 164–167. These genes are upregulated by ROS 168, 169. Silensing uPA promoter with siRNA inhibits tumor cell invasion and angiogenesis in vitro as well as prostate tumor growth and lung metastasis in a bioluminescence tumor/metastasis model 170.
8. Prevention of angiogenesis by dietary antioxidants
Because ROS play an important role in tumor angiogenesis, treatment with dietary antioxidants such as food phytochemicals which have antioxidant capacity seems to be a promising anti-angiogenic strategy. Especially dietary polyphenols are involved in protection against not only cardiovascular risk factors such as atherosclerosis but also cancer angiogenesis by inhibiting oxidative stress. Recent studies have indicated that both red wine and green tea polyphenols prevent effectively the thrombin-induced activation of MMP-2 in vascular smooth muscle cells 171. VEGF expression and its release are prevented by red wine polyphenols 172. In addition, green tea polyphenols and epigallocatechin-3-gallate reduce VEGF expression in several types of cancer cells by inhibiting epidermal growth factor receptor (EGFR)-related signaling pathways 173, 174 Furthermore, natural polyphenols can inhibit migration and proliferation of vascular cells 171. Consistent with these observations, either red wine polyphenols, green tea polyphenols or epigallocatechin-3-gallate inhibits angiogenesis in ECs 175 and in animal model of angiogenesis 172, 176–178. Thus, dietary antioxidants appear to be effective for treatment of tumor angiogenesis. Understanding the molecular mechanisms of their action should provide basis to design more effective anti-angiogenic drugs targeted to various cancers.
9. Conclusion
Accumulating evidence suggest that ROS derived from NADPH oxidase play an important role in physiological and pathological angiogenesis. ROS function as signaling molecules to mediate various angiogenic-related responses such as cell proliferation, migration and angiogenic gene expression in ECs and cancer cells. However, significant work remains to be performed; 1) to define the role of each Nox and its regulatory subunits in tumor angiogenesis; 2) to determine the activation mechanisms of NADPH oxidase by various angiogenesis factors; and 3) to identify molecular targets of oxidase-derived ROS in signaling pathways involved in angiogenic switch in various cancer cells. The development of specific inhibitors of NADPH oxidases and redox signaling components (kinase, phosphatase, transcription factors and genes) as well as understanding the mechanism by which dietary antioxidants inhibit angiogenesis could provide useful therapeutic strategies for treatment of various angiogenesis-dependent pathophysiologies such as cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971 Nov 18;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001 Dec 11;2001(112):RE21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- 5.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001 Nov;7(11):1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000 Sep 14;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 7.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumour cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 8.Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res. 2006 Jul 15;71(2):226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem. 2004 Sep;264(1–2):85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 10.Stone JR, Collins T. The role of hydrogen peroxide in endothelial proliferative responses. Endothelium. 2002;9(4):231–238. doi: 10.1080/10623320214733. [DOI] [PubMed] [Google Scholar]
- 11.Ushio-Fukai M, Tang Y, Fukai T, Dikalov S, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91phox-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 12.Luczak K, Balcerczyk A, Soszynski M, Bartosz G. Low concentration of oxidant and nitric oxide donors stimulate proliferation of human endothelial cells in vitro. Cell Biol Int. 2004;28(6):483–486. doi: 10.1016/j.cellbi.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Yasuda M, Ohzeki Y, Shimizu S, Naito S, Ohtsuru A, Yamamoto T, Kuroiwa Y. Stimulation of in vitro angiogenesis by hydrogen peroxide and the relation with ETS-1 in endothelial cells. Life Sci. 1999;64:249–258. doi: 10.1016/s0024-3205(98)00560-8. [DOI] [PubMed] [Google Scholar]
- 14.Yeldandi AV, Rao MS, Reddy JK. Hydrogen peroxide generation in peroxisome proliferator-induced oncogenesis. Mutation research. 2000 Mar 17;448(2):159–177. doi: 10.1016/s0027-5107(99)00234-1. [DOI] [PubMed] [Google Scholar]
- 15.Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 16.Chinery R, Brockman JA, Peeler MO, Shyr Y, Beauchamp RD, Coffey RJ. Antioxidants enhance the cytotoxicity of chemotherapeutic agents in colorectal cancer: a p53-independent induction of p21WAF1/CIP1 via C/EBPbeta. Nat Med. 1997 Nov;3(11):1233–1241. doi: 10.1038/nm1197-1233. [DOI] [PubMed] [Google Scholar]
- 17.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401(6748):79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 18.Arnold RS, Shi J, Murad E, Whalen AM, Sun CQ, Polavarapu R, Parthasarathy S, Petros JA, Lambeth JD. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci U S A. 2001 May 8;98(10):5550–5555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci U S A. 2002 Jan 22;99(2):715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Govindarajan B, Sligh JE, Vincent BJ, Li M, Canter JA, Nickoloff BJ, Rodenburg RJ, Smeitink JA, Oberley L, Zhang Y, Slingerland J, Arnold RS, Lambeth JD, Cohen C, Hilenski L, Griendling K, Martinez-Diez M, Cuezva JM, Arbiser JL. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007 Mar;117(3):719–729. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaoka-Tojo M, Ushio-Fukai M, Hilenski L, Dikalov SI, Chen YE, Tojo T, Fukai T, Fujimoto M, Patrushev NA, Wang N, Kontos CD, Bloom GS, Alexander RW. IQGAP1, a novel vascular endothelial growth factor receptor binding protein, is involved in reactive oxygen species-dependent endothelial migration and proliferation. Circ Res. 2004 Aug 6;95(3):276–283. doi: 10.1161/01.RES.0000136522.58649.60. [DOI] [PubMed] [Google Scholar]
- 22.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev NA, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda S, Ushio-Fukai M, Zuo L, Tojo T, Dikalov S, Patrushev NA, Alexander RW. Novel role of ARF6 in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2005 Mar 4;96(4):467–475. doi: 10.1161/01.RES.0000158286.51045.16. [DOI] [PubMed] [Google Scholar]
- 24.Al-Shabrawey M, Bartoli M, El-Remessy AB, Platt DH, Matragoon S, Behzadian MA, Caldwell RW, Caldwell RB. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Pathol. 2005 Aug;167(2):599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harfouche R, Malak NA, Brandes RP, Karsan A, Irani K, Hussain SN. Roles of reactive oxygen species in angiopoietin-1/tie-2 receptor signaling. Faseb J. 2005 Oct;19(12):1728–1730. doi: 10.1096/fj.04-3621fje. [DOI] [PubMed] [Google Scholar]
- 26.Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 27.Deby C, Goutier R. New perspectives on the biochemistry of superoxide anion and the efficiency of superoxide dismutases. Biochem Pharmacol. 1990 Feb 1;39(3):399–405. doi: 10.1016/0006-2952(90)90043-k. [DOI] [PubMed] [Google Scholar]
- 28.Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000 Oct 10;2000(53):PE1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 29.Chiarugi P, Cirri P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem Sci. 2003 Sep;28(9):509–514. doi: 10.1016/S0968-0004(03)00174-9. [DOI] [PubMed] [Google Scholar]
- 30.Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273(25):15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 31.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86(5):494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 32.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006 Aug 21;2006(349):re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 33.Chua CC, Hamdy RC, Chua BH. Upregulation of vascular endothelial growth factor by H2O2 in rat heart endothelial cells. Free Radic Biol Med. 1998;25(8):891–897. doi: 10.1016/s0891-5849(98)00115-4. [DOI] [PubMed] [Google Scholar]
- 34.Vepa S, Scribner WM, Parinandi NL, English D, Garcia JG, Natarajan V. Hydrogen peroxide stimulates tyrosine phosphorylation of focal adhesion kinase in vascular endothelial cells. Am J Physiol. 1999;277:L150–L158. doi: 10.1152/ajplung.1999.277.1.L150. [DOI] [PubMed] [Google Scholar]
- 35.Shono T, Ono M, Izumi H, Jimi SI, Matsushima K, Okamoto T, Kohno K, Kuwano M. Involvement of the transcription factor NF-kappaB in tubular morphogenesis of human microvascular endothelial cells by oxidative stress. Mol Cell Biol. 1996;16(8):4231–4239. doi: 10.1128/mcb.16.8.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lelkes PI, Hahn KL, Sukovich DA, Karmiol S, Schmidt DH. On the possible role of reactive oxygen species in angiogenesis. Adv Exp Med Biol. 1998;454:295–310. doi: 10.1007/978-1-4615-4863-8_35. [DOI] [PubMed] [Google Scholar]
- 37.Yasuda M, Shimizu S, Tokuyama S, Watanabe T, Kiuchi Y, Yamamoto T. A novel effect of polymorphonuclear leukocytes in the facilitation of angiogenesis [In Process Citation] Life Sci. 2000;66(21):2113–2121. doi: 10.1016/s0024-3205(00)00537-3. [DOI] [PubMed] [Google Scholar]
- 38.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007 Sep;117(9):2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamagishi S, Amano S, Inagaki Y, Okamoto T, Takeuchi M, Inoue H. Pigment epithelium-derived factor inhibits leptin-induced angiogenesis by suppressing vascular endothelial growth factor gene expression through anti-oxidative properties. Microvasc Res. 2003 May;65(3):186–190. doi: 10.1016/s0026-2862(03)00005-0. [DOI] [PubMed] [Google Scholar]
- 40.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999 Jul 9;285(5425):245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 41.Monte M, Davel LE, Sacerdote de Lustig E. Hydrogen peroxide is involved in lymphocyte activation mechanisms to induce angiogenesis. Eur J Cancer. 1997;33:676–682. doi: 10.1016/s0959-8049(96)00506-0. [DOI] [PubMed] [Google Scholar]
- 42.Cai T, Fassina G, Morini M, Aluigi MG, Masiello L, Fontanini G, D’Agostini F, De Flora S, Noonan DM, Albini A. N-acetylcysteine inhibits endothelial cell invasion and angiogenesis. Lab Invest. 1999;79(9):1151–1159. [PubMed] [Google Scholar]
- 43.Abe R, Shimizu T, Yamagishi S, Shibaki A, Amano S, Inagaki Y, Watanabe H, Sugawara H, Nakamura H, Takeuchi M, Imaizumi T, Shimizu H. Overexpression of pigment epithelium-derived factor decreases angiogenesis and inhibits the growth of human malignant melanoma cells in vivo. Am J Pathol. 2004 Apr;164(4):1225–1232. doi: 10.1016/s0002-9440(10)63210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuroki M, Voest EE, Amano S, Beerepoot LV, Takashima S, Tolentino M, Kim RY, Rohan RM, Colby KA, Yeo KT, Adamis AP. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J Clin Invest. 1996;98(7):1667–1675. doi: 10.1172/JCI118962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lakshminarayanan V, Lewallen M, Frangogiannis NG, Evans AJ, Wedin KE, Michael LH, Entman ML. Reactive oxygen intermediates induce monocyte chemotactic protein-1 in vascular endothelium after brief ischemia. Am J Pathol. 2001 Oct;159(4):1301–1311. doi: 10.1016/S0002-9440(10)62517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu W, Weihrauch D, Tanaka K, Tessmer JP, Pagel PS, Kersten JR, Chilian WM, Warltier DC. Reactive oxygen species are critical mediators of coronary collateral development in a canine model. Am J Physiol Heart Circ Physiol. 2003 Oct;285(4):H1582–1589. doi: 10.1152/ajpheart.00318.2003. [DOI] [PubMed] [Google Scholar]
- 47.Maulik N. Reactive oxygen species drives myocardial angiogenesis? Antioxid Redox Signal. 2006 Nov–Dec;8(11–12):2161–2168. doi: 10.1089/ars.2006.8.2161. [DOI] [PubMed] [Google Scholar]
- 48.Kim HW, Lin A, Guldberg RE, Ushio-Fukai M, Fukai T. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circ Res. 2007 Aug 17;101(4):409–419. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 49.Polytarchou C, Papadimitriou E. Antioxidants inhibit angiogenesis in vivo through down-regulation of nitric oxide synthase expression and activity. Free Radic Res. 2004 May;38(5):501–508. doi: 10.1080/10715760410001684621. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida A, Yoshida S, Ishibashi T, Kuwano M, Inomata H. Suppression of retinal neovascularization by the NF-kappaB inhibitor pyrrolidine dithiocarbamate in mice. Invest Ophthalmol Vis Sci. 1999;40(7):1624–1629. [PubMed] [Google Scholar]
- 51.Kim YM, Kim KE, Koh GY, Ho YS, Lee KJ. Hydrogen peroxide produced by angiopoietin-1 mediates angiogenesis. Cancer Res. 2006 Jun 15;66(12):6167–6174. doi: 10.1158/0008-5472.CAN-05-3640. [DOI] [PubMed] [Google Scholar]
- 52.Ellis EA, Guberski DL, Somogyi-Mann M, Grant MB. Increased H2O2, vascular endothelial growth factor and receptors in the retina of the BBZ/Wor diabetic rat. Free Radic Biol Med. 2000;28(1):91–101. doi: 10.1016/s0891-5849(99)00216-6. [DOI] [PubMed] [Google Scholar]
- 53.Ellis EA, Grant MB, Murray FT, Wachowski MB, Guberski DL, Kubilis PS, Lutty GA. Increased NADH oxidase activity in the retina of the BBZ/Wor diabetic rat. Free Radic Biol Med. 1998;24(1):111–120. doi: 10.1016/s0891-5849(97)00202-5. [DOI] [PubMed] [Google Scholar]
- 54.Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Liou GI, Caldwell RW. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005 Jun;6(4):511–524. doi: 10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- 55.Ruef J, Hu ZY, Yin LY, Wu Y, Hanson SR, Kelly AB, Harker LA, Rao GN, Runge MS, Patterson C. Induction of vascular endothelial growth factor in balloon-injured baboon arteries. Circ Res. 1997;81:24–33. doi: 10.1161/01.res.81.1.24. [DOI] [PubMed] [Google Scholar]
- 56.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99:1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 57.Nespereira B, Perez-Ilzarbe M, Fernandez P, Fuentes AM, Paramo JA, Rodriguez JA. Vitamins C and E downregulate vascular VEGF and VEGFR-2 expression in apolipoprotein-E-deficient mice. Atherosclerosis. 2003 Nov;171(1):67–73. doi: 10.1016/j.atherosclerosis.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 58.Fleury C, Mignotte B, Vayssiere JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002 Feb–Mar;84(2–3):131–141. doi: 10.1016/s0300-9084(02)01369-x. [DOI] [PubMed] [Google Scholar]
- 59.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004 Mar;4(3):181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 60.Czarnecka AM, Golik P, Bartnik E. Mitochondrial DNA mutations in human neoplasia. Journal of applied genetics. 2006;47(1):67–78. doi: 10.1007/BF03194602. [DOI] [PubMed] [Google Scholar]
- 61.Babior BM. The respiratory burst oxidase. Curr Opin Hematol. 1995 Jan;2(1):55–60. doi: 10.1097/00062752-199502010-00008. [DOI] [PubMed] [Google Scholar]
- 62.Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore! . Trends Biochem Sci Sep. 2003;28(9):502–508. doi: 10.1016/S0968-0004(03)00194-4. [DOI] [PubMed] [Google Scholar]
- 63.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003 Aug;285(2):R277–297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 64.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001 May 16;269(1–2):131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 65.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A. 2000;97(14):8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res. 2006 Jul 15;71(2):289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 67.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003 Feb 7;278(6):3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 68.Geiszt M, Lekstrom K, Brenner S, Hewitt SM, Dana R, Malech HL, Leto TL. NAD(P)H oxidase 1, a product of differentiated colon epithelial cells, can partially replace glycoprotein 91phox in the regulated production of superoxide by phagocytes. J Immunol. 2003 Jul 1;171(1):299–306. doi: 10.4049/jimmunol.171.1.299. [DOI] [PubMed] [Google Scholar]
- 69.Lim SD, Sun C, Lambeth JD, Marshall F, Amin M, Chung L, Petros JA, Arnold RS. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate. 2005 Feb 1;62(2):200–207. doi: 10.1002/pros.20137. [DOI] [PubMed] [Google Scholar]
- 70.Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B, Arnold RS, Whorton AR, Sturrock AB, Huecksteadt TP, Quinn MT, Krenitsky K, Ardie KG, Lambeth JD, Hoidal JR. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003 Aug;285(2):C353–369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 71.Desouki MM, Kulawiec M, Bansal S, Das GM, Singh KK. Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer biology & therapy. 2005 Dec;4(12):1367–1373. doi: 10.4161/cbt.4.12.2233. [DOI] [PubMed] [Google Scholar]
- 72.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004 Oct 15;95(8):773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 73.Kobayashi S, Nojima Y, Shibuya M, Maru Y. Nox1 regulates apoptosis and potentially stimulates branching morphogenesis in sinusoidal endothelial cells. Exp Cell Res. 2004 Nov 1;300(2):455–462. doi: 10.1016/j.yexcr.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 74.Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal. 2006 Sep–Oct;8(9–10):1473–1484. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- 75.Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87(1):26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- 76.Li J-M, Shah AM. Intracellular Localization and Preassembly of the NADPH Oxidase Complex in Cultured Endothelial Cells. J Biol Chem. 2002;277:19952–19960. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- 77.Frey RS, Rahman A, Kefer JC, Minshall RD, Malik AB. PKCzeta regulates TNF-alpha-induced activation of NADPH oxidase in endothelial cells. Circ Res. 2002 May 17;90(9):1012–1019. doi: 10.1161/01.res.0000017631.28815.8e. [DOI] [PubMed] [Google Scholar]
- 78.Furst R, Brueckl C, Kuebler WM, Zahler S, Krotz F, Gorlach A, Vollmar AM, Kiemer AK. Atrial natriuretic peptide induces mitogen-activated protein kinase phosphatase-1 in human endothelial cells via Rac1 and NAD(P)H oxidase/Nox2-activation. Circ Res. 2005 Jan 7;96(1):43–53. doi: 10.1161/01.RES.0000151983.01148.06. [DOI] [PubMed] [Google Scholar]
- 79.Ikeda S, Yamaoka-Tojo M, Hilenski L, Patrushev NA, Anwar GM, Quinn MT, Ushio-Fukai M. IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler Thromb Vasc Biol. 2005 Nov;25(11):2295–2300. doi: 10.1161/01.ATV.0000187472.55437.af. [DOI] [PubMed] [Google Scholar]
- 80.Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B, Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006 Jan;26(1):140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SI, Harrison DG, Sung HJ, Rong Y, Galis ZS. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation. 2004 Feb 3;109(4):520–525. doi: 10.1161/01.CIR.0000109698.70638.2B. [DOI] [PubMed] [Google Scholar]
- 82.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004 Jan 20;109(2):227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 83.Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells. 2005 Dec;10(12):1139–1151. doi: 10.1111/j.1365-2443.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- 84.Datla SR, Peshavariya H, Dusting GJ, Jiang F. Important Role of Nox4 Type NADPH Oxidase in Angiogenic Responses in Human Microvascular Endothelial Cells In Vitro. Arterioscler Thromb Vasc Biol. 2007 Aug 23; doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- 85.Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132(2):233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 86.Kawahara T, Ritsick D, Cheng G, Lambeth JD. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J Biol Chem. 2005 Sep 9;280(36):31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 87.Banfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnar GZ, Krause KH, Cox JA. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J Biol Chem. 2004 Apr 30;279(18):18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 88.BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, Gorlach A. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med. 2007 Feb 15;42(4):446–459. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 89.van Wetering S, van Buul JD, Quik S, Mul FP, Anthony EC, ten Klooster JP, Collard JG, Hordijk PL. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J Cell Sci. 2002 May 1;115(Pt 9):1837–1846. doi: 10.1242/jcs.115.9.1837. [DOI] [PubMed] [Google Scholar]
- 90.Moldovan L, Irani K, Moldovan NI, Finkel T, Goldschmidt-Clermont PJ. The actin cytoskeleton reorganization induced by Rac1 requires the production of superoxide. Antioxid Redox Signal. 1999;1(1):29–43. doi: 10.1089/ars.1999.1.1-29. [DOI] [PubMed] [Google Scholar]
- 91.Kraynov VS, Chamberlain C, Bokoch GM, Schwartz MA, Slabaugh S, Hahn KM. Localized Rac activation dynamics visualized in living cells. Science. 2000 Oct 13;290(5490):333–337. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- 92.Moldovan L, Moldovan NI, Sohn RH, Parikh SA, Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res. 2000;86(5):549–557. doi: 10.1161/01.res.86.5.549. [DOI] [PubMed] [Google Scholar]
- 93.Laufs U, Kilter H, Konkol C, Wassmann S, Bohm M, Nickenig G. Impact of HMG CoA reductase inhibition on small GTPases in the heart. Cardiovasc Res. 2002 Mar;53(4):911–920. doi: 10.1016/s0008-6363(01)00540-5. [DOI] [PubMed] [Google Scholar]
- 94.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002 Feb 12;105(6):739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 95.Li JM, Mullen AM, Yun S, Wientjes F, Brouns GY, Thrasher AJ, Shah AM. Essential role of the NADPH oxidase subunit p47(phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alpha. Circ Res. 2002;90(2):143–150. doi: 10.1161/hh0202.103615. [DOI] [PubMed] [Google Scholar]
- 96.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2− from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003 Nov 21;278(47):47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 97.Li JM, Shah AM. Mechanism of endothelial cell NADPH oxidase activation by angiotensin II. Role of the p47phox subunit. J Biol Chem. 2003 Apr 4;278(14):12094–12100. doi: 10.1074/jbc.M209793200. [DOI] [PubMed] [Google Scholar]
- 98.Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004 Nov;287(5):R1014–1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 99.Frey RS, Gao X, Javaid K, Siddiqui SS, Rahman A, Malik AB. Phosphatidylinositol 3-kinase gamma signaling through protein kinase Czeta induces NADPH oxidase-mediated oxidant generation and NF-kappaB activation in endothelial cells. J Biol Chem. 2006 Jun 9;281(23):16128–16138. doi: 10.1074/jbc.M508810200. [DOI] [PubMed] [Google Scholar]
- 100.Gu Y, Xu YC, Wu RF, Souza RF, Nwariaku FE, Terada LS. TNFalpha activates c-Jun amino terminal kinase through p47(phox) Exp Cell Res. 2002 Jan 1;272(1):62–74. doi: 10.1006/excr.2001.5404. [DOI] [PubMed] [Google Scholar]
- 101.Wu RF, Gu Y, Xu YC, Nwariaku FE, Terada LS. Vascular endothelial growth factor causes translocation of p47phox to membrane ruffles through WAVE1. J Biol Chem. 2003 Sep 19;278(38):36830–36840. doi: 10.1074/jbc.M302251200. [DOI] [PubMed] [Google Scholar]
- 102.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005 Mar–Apr;7(3–4):308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 103.Wu RF, Xu YC, Ma Z, Nwariaku FE, Sarosi GA, Jr, Terada LS. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol. 2005 Dec 5;171(5):893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen JX, Zeng H, Lawrence ML, Blackwell TS, Meyrick B. Angiopoietin-1-induced angiogenesis is modulated by endothelial NADPH oxidase. Am J Physiol Heart Circ Physiol. 2006 Oct;291(4):H1563–1572. doi: 10.1152/ajpheart.01081.2005. [DOI] [PubMed] [Google Scholar]
- 105.Wheeler MD, Smutney OM, Samulski RJ. Secretion of extracellular superoxide dismutase from muscle transduced with recombinant adenovirus inhibits the growth of B16 melanomas in mice. Mol Cancer Res. 2003 Oct;1(12):871–881. [PubMed] [Google Scholar]
- 106.Marikovsky M, Nevo N, Vadai E, Harris-Cerruti C. Cu/Zn superoxide dismutase plays a role in angiogenesis. Int J Cancer. 2002 Jan 1;97(1):34–41. doi: 10.1002/ijc.1565. [DOI] [PubMed] [Google Scholar]
- 107.Grzenkowicz-Wydra J, Cisowski J, Nakonieczna J, Zarebski A, Udilova N, Nohl H, Jozkowicz A, Podhajska A, Dulak J. Gene transfer of CuZn superoxide dismutase enhances the synthesis of vascular endothelial growth factor. Mol Cell Biochem. 2004 Sep;264(1–2):169–181. doi: 10.1023/b:mcbi.0000044386.45054.70. [DOI] [PubMed] [Google Scholar]
- 108.Connor KM, Subbaram S, Regan KJ, Nelson KK, Mazurkiewicz JE, Bartholomew PJ, Aplin AE, Tai YT, Aguirre-Ghiso J, Flores SC, Melendez JA. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem. 2005 Apr 29;280(17):16916–16924. doi: 10.1074/jbc.M410690200. [DOI] [PubMed] [Google Scholar]
- 109.Abid MR, Tsai JC, Spokes KC, Deshpande SS, Irani K, Aird WC. Vascular endothelial growth factor induces manganese-superoxide dismutase expression in endothelial cells by a Rac1-regulated NADPH oxidase-dependent mechanism. Faseb J. 2001;15(13):2548–2550. doi: 10.1096/fj.01-0338fje. [DOI] [PubMed] [Google Scholar]
- 110.Arner ES, Holmgren A. The thioredoxin system in cancer. Seminars in cancer biology. 2006 Dec;16(6):420–426. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 111.Powis G, Kirkpatrick DL. Thioredoxin signaling as a target for cancer therapy. Current opinion in pharmacology. 2007 Aug;7(4):392–397. doi: 10.1016/j.coph.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 112.Storz G, Polla BS. Transcriptional regulators of oxidative stress-inducible genes in prokaryotes and eukaryotes. Exs. 1996;77:239–254. doi: 10.1007/978-3-0348-9088-5_16. [DOI] [PubMed] [Google Scholar]
- 113.Rudolph J. Redox regulation of the Cdc25 phosphatases. Antioxid Redox Signal. 2005 May–Jun;7(5–6):761–767. doi: 10.1089/ars.2005.7.761. [DOI] [PubMed] [Google Scholar]
- 114.Colavitti R, Pani G, Bedogni B, Anzevino R, Borrello S, Waltenberger J, Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J Biol Chem. 2002;277(5):3101–3108. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- 115.Lin MT, Yen ML, Lin CY, Kuo ML. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol. 2003 Nov;64(5):1029–1036. doi: 10.1124/mol.64.5.1029. [DOI] [PubMed] [Google Scholar]
- 116.Terada LS. Specificity in reactive oxidant signaling: think globally, act locally. J Cell Biol. 2006 Aug 28;174(5):615–623. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002 Feb;9(2):387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 118.Kroll J, Waltenberger J. The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem. 1997;272(51):32521–32527. doi: 10.1074/jbc.272.51.32521. [DOI] [PubMed] [Google Scholar]
- 119.Guo DQ, Wu LW, Dunbar JD, Ozes ON, Mayo LD, Kessler KM, Gustin JA, Baerwald MR, Jaffe EA, Warren RS, Donner DB. Tumor necrosis factor employs a protein-tyrosine phosphatase to inhibit activation of KDR and vascular endothelial cell growth factor-induced endothelial cell proliferation. J Biol Chem. 2000;275(15):11216–11221. doi: 10.1074/jbc.275.15.11216. [DOI] [PubMed] [Google Scholar]
- 120.Huang L, Sankar S, Lin C, Kontos CD, Schroff AD, Cha EH, Feng SM, Li SF, Yu Z, Van Etten RL, Blanar MA, Peters KG. HCPTPA, a protein tyrosine phosphatase that regulates vascular endothelial growth factor receptor-mediated signal transduction and biological activity. J Biol Chem. 1999;274(53):38183–38188. doi: 10.1074/jbc.274.53.38183. [DOI] [PubMed] [Google Scholar]
- 121.Sugano M, Tsuchida K, Maeda T, Makino N. SiRNA targeting SHP-1 accelerates angiogenesis in a rat model of hindlimb ischemia. Atherosclerosis. 2006 May 23; doi: 10.1016/j.atherosclerosis.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 122.Mitola S, Brenchio B, Piccinini M, Tertoolen L, Zammataro L, Breier G, Rinaudo MT, den Hertog J, Arese M, Bussolino F. Type I collagen limits VEGFR-2 signaling by a SHP2 protein-tyrosine phosphatase-dependent mechanism 1. Circ Res. 2006 Jan 6;98(1):45–54. doi: 10.1161/01.RES.0000199355.32422.7b. [DOI] [PubMed] [Google Scholar]
- 123.Grazia Lampugnani M, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, Dejana E. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol. 2003 May 26;161(4):793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Soeda S, Shimada T, Koyanagi S, Yokomatsu T, Murano T, Shibuya S, Shimeno H. An attempt to promote neo-vascularization by employing a newly synthesized inhibitor of protein tyrosine phosphatase. FEBS Lett. 2002 Jul 31;524(1–3):54–58. doi: 10.1016/s0014-5793(02)03002-8. [DOI] [PubMed] [Google Scholar]
- 125.Huang L, Turck CW, Rao P, Peters KG. GRB2 and SH-PTP2: potentially important endothelial signaling molecules downstream of the TEK/TIE2 receptor tyrosine kinase. Oncogene. 1995 Nov 16;11(10):2097–2103. [PubMed] [Google Scholar]
- 126.Leslie NR, Downes CP. PTEN function: how normal cells control it and tumour cells lose it. Biochem J. 2004 Aug 15;382(Pt 1):1–11. doi: 10.1042/BJ20040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu LZ, Hu XW, Xia C, He J, Zhou Q, Shi X, Fang J, Jiang BH. Reactive oxygen species regulate epidermal growth factor-induced vascular endothelial growth factor and hypoxia-inducible factor-1alpha expression through activation of AKT and P70S6K1 in human ovarian cancer cells. Free Radic Biol Med. 2006 Nov 15;41(10):1521–1533. doi: 10.1016/j.freeradbiomed.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 128.Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A. 2004 Nov 23;101(47):16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T, Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol. 2003 Jun 9;161(5):933–944. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996 Sep;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997 Aug 1;272(31):19253–19260. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- 132.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007 Jun;26(2):281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 134.BelAiba RS, Djordjevic T, Bonello S, Flugel D, Hess J, Kietzmann T, Gorlach A. Redox-sensitive regulation of the HIF pathway under non-hypoxic conditions in pulmonary artery smooth muscle cells. Biol Chem. 2004 Mar–Apr;385(3–4):249–257. doi: 10.1515/BC.2004.019. [DOI] [PubMed] [Google Scholar]
- 135.Kietzmann T, Gorlach A. Reactive oxygen species in the control of hypoxia-inducible factor-mediated gene expression. Semin Cell Dev Biol. 2005 Aug–Oct;16(4–5):474–486. doi: 10.1016/j.semcdb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 136.Dery MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol. 2005 Mar;37(3):535–540. doi: 10.1016/j.biocel.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 137.Gorlach A, Berchner-Pfannschmidt U, Wotzlaw C, Cool RH, Fandrey J, Acker H, Jungermann K, Kietzmann T. Reactive oxygen species modulate HIF-1 mediated PAI-1 expression: involvement of the GTPase Rac1. Thromb Haemost. 2003 May;89(5):926–935. [PubMed] [Google Scholar]
- 138.Skinner HD, Zheng JZ, Fang J, Agani F, Jiang BH. Vascular endothelial growth factor transcriptional activation is mediated by hypoxia-inducible factor 1alpha, HDM2, and p70S6K1 in response to phosphatidylinositol 3-kinase/AKT signaling. J Biol Chem. 2004 Oct 29;279(44):45643–45651. doi: 10.1074/jbc.M404097200. [DOI] [PubMed] [Google Scholar]
- 139.Gao N, Shen L, Zhang Z, Leonard SS, He H, Zhang XG, Shi X, Jiang BH. Arsenite induces HIF-1alpha and VEGF through PI3K, Akt and reactive oxygen species in DU145 human prostate carcinoma cells. Mol Cell Biochem. 2004 Jan;255(1–2):33–45. doi: 10.1023/b:mcbi.0000007259.65742.16. [DOI] [PubMed] [Google Scholar]
- 140.Shi YH, Wang YX, Bingle L, Gong LH, Heng WJ, Li Y, Fang WG. In vitro study of HIF-1 activation and VEGF release by bFGF in the T47D breast cancer cell line under normoxic conditions: involvement of PI-3K/Akt and MEK1/ERK pathways. The Journal of pathology. 2005 Mar;205(4):530–536. doi: 10.1002/path.1734. [DOI] [PubMed] [Google Scholar]
- 141.Gao N, Ding M, Zheng JZ, Zhang Z, Leonard SS, Liu KJ, Shi X, Jiang BH. Vanadate-induced expression of hypoxia-inducible factor 1 alpha and vascular endothelial growth factor through phosphatidylinositol 3-kinase/Akt pathway and reactive oxygen species. J Biol Chem. 2002 Aug 30;277(35):31963–31971. doi: 10.1074/jbc.M200082200. [DOI] [PubMed] [Google Scholar]
- 142.Trisciuoglio D, Iervolino A, Zupi G, Del Bufalo D. Involvement of PI3K and MAPK signaling in bcl-2-induced vascular endothelial growth factor expression in melanoma cells. Mol Biol Cell. 2005 Sep;16(9):4153–4162. doi: 10.1091/mbc.E04-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, Pouyssegur J, Yaniv M, Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004 Sep 17;118(6):781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 144.Xanthoudakis S, Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. Embo J. 1992 Feb;11(2):653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jayaraman L, Murthy KG, Zhu C, Curran T, Xanthoudakis S, Prives C. Identification of redox/repair protein Ref-1 as a potent activator of p53. Genes Dev. 1997 Mar 1;11(5):558–570. doi: 10.1101/gad.11.5.558. [DOI] [PubMed] [Google Scholar]
- 146.North S, Moenner M, Bikfalvi A. Recent developments in the regulation of the angiogenic switch by cellular stress factors in tumors. Cancer Lett. 2005 Jan 31;218(1):1–14. doi: 10.1016/j.canlet.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 147.Zhang L, Yu D, Hu M, Xiong S, Lang A, Ellis LM, Pollock RE. Wild-type p53 suppresses angiogenesis in human leiomyosarcoma and synovial sarcoma by transcriptional suppression of vascular endothelial growth factor expression. Cancer Res. 2000 Jul 1;60(13):3655–3661. [PubMed] [Google Scholar]
- 148.Galy B, Creancier L, Zanibellato C, Prats AC, Prats H. Tumour suppressor p53 inhibits human fibroblast growth factor 2 expression by a post-transcriptional mechanism. Oncogene. 2001 Mar 29;20(14):1669–1677. doi: 10.1038/sj.onc.1204271. [DOI] [PubMed] [Google Scholar]
- 149.Brar SS, Kennedy TP, Quinn M, Hoidal JR. Redox signaling of NF-kappaB by membrane NAD(P)H oxidases in normal and malignant cells. Protoplasma. 2003 May;221(1–2):117–127. doi: 10.1007/s00709-002-0059-y. [DOI] [PubMed] [Google Scholar]
- 150.Fukuyama M, Rokutan K, Sano T, Miyake H, Shimada M, Tashiro S. Overexpression of a novel superoxide-producing enzyme, NADPH oxidase 1, in adenoma and well differentiated adenocarcinoma of the human colon. Cancer Lett. 2005 Apr 18;221(1):97–104. doi: 10.1016/j.canlet.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 151.Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, Oettgen P. Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest. 2005 Sep;115(9):2508–2516. doi: 10.1172/JCI24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ni W, Zhan Y, He H, Maynard E, Balschi JA, Oettgen P. Ets-1 Is a Critical Transcriptional Regulator of Reactive Oxygen Species and p47phox Gene Expression in Response to Angiotensin II. Circ Res. 2007 Sep 13; doi: 10.1161/CIRCRESAHA.107.152439. [DOI] [PubMed] [Google Scholar]
- 153.Yoon SO, Park SJ, Yun CH, Chung AS. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. Journal of biochemistry and molecular biology. 2003 Jan 31;36(1):128–137. doi: 10.5483/bmbrep.2003.36.1.128. [DOI] [PubMed] [Google Scholar]
- 154.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wary KK, Thakker GD, Humtsoe JO, Yang J. Analysis of VEGF-responsive genes involved in the activation of endothelial cells. Mol Cancer. 2003 Jul 9;2:25. doi: 10.1186/1476-4598-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wartenberg M, Donmez F, Ling FC, Acker H, Hescheler J, Sauer H. Tumor-induced angiogenesis studied in confrontation cultures of multicellular tumor spheroids and embryoid bodies grown from pluripotent embryonic stem cells. Faseb J. 2001 Apr;15(6):995–1005. doi: 10.1096/fj.00-0350com. [DOI] [PubMed] [Google Scholar]
- 157.Wartenberg M, Budde P, De Marees M, Grunheck F, Tsang SY, Huang Y, Chen ZY, Hescheler J, Sauer H. Inhibition of tumor-induced angiogenesis and matrix-metalloproteinase expression in confrontation cultures of embryoid bodies and tumor spheroids by plant ingredients used in traditional chinese medicine. Lab Invest. 2003 Jan;83(1):87–98. doi: 10.1097/01.lab.0000049348.51663.2f. [DOI] [PubMed] [Google Scholar]
- 158.Feng L, Xia Y, Garcia GE, Hwang D, Wilson CB. Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-alpha, and lipopolysaccharide. J Clin Invest. 1995 Apr;95(4):1669–1675. doi: 10.1172/JCI117842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jaimes EA, Tian RX, Pearse D, Raij L. Up-regulation of glomerular COX-2 by angiotensin II: role of reactive oxygen species. Kidney international. 2005 Nov;68(5):2143–2153. doi: 10.1111/j.1523-1755.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 160.Massaro M, Habib A, Lubrano L, Del Turco S, Lazzerini G, Bourcier T, Weksler BB, De Caterina R. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc Natl Acad Sci U S A. 2006 Oct 10;103(41):15184–15189. doi: 10.1073/pnas.0510086103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Saha D, Sekhar KR, Cao C, Morrow JD, Choy H, Freeman ML. The antiangiogenic agent SU5416 down-regulates phorbol ester-mediated induction of cyclooxygenase 2 expression by inhibiting nicotinamide adenine dinucleotide phosphate oxidase activity. Cancer Res. 2003 Oct 15;63(20):6920–6927. [PubMed] [Google Scholar]
- 162.Barbieri SS, Cavalca V, Eligini S, Brambilla M, Caiani A, Tremoli E, Colli S. Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free Radic Biol Med. 2004 Jul 15;37(2):156–165. doi: 10.1016/j.freeradbiomed.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 163.Cosentino F, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, Kouroedov A, Delli Gatti C, Joch H, Volpe M, Luscher TF. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation. 2003 Feb 25;107(7):1017–1023. doi: 10.1161/01.cir.0000051367.92927.07. [DOI] [PubMed] [Google Scholar]
- 164.Pulukuri SM, Gondi CS, Lakka SS, Jutla A, Estes N, Gujrati M, Rao JS. RNA interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival, and tumorigenicity in vivo. J Biol Chem. 2005 Oct 28;280(43):36529–36540. doi: 10.1074/jbc.M503111200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 165.Gondi CS, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. Intraperitoneal injection of a hairpin RNA-expressing plasmid targeting urokinase-type plasminogen activator (uPA) receptor and uPA retards angiogenesis and inhibits intracranial tumor growth in nude mice. Clin Cancer Res. 2007 Jul 15;13(14):4051–4060. doi: 10.1158/1078-0432.CCR-06-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kunigal S, Lakka SS, Gondi CS, Estes N, Rao JS. RNAi-mediated downregulation of urokinase plasminogen activator receptor and matrix metalloprotease-9 in human breast cancer cells results in decreased tumor invasion, angiogenesis and growth. Int J Cancer. 2007 Jul 26; doi: 10.1002/ijc.22962. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 167.Pulukuri SM, Rao JS. Small interfering RNA directed reversal of urokinase plasminogen activator demethylation inhibits prostate tumor growth and metastasis. Cancer Res. 2007 Jul 15;67(14):6637–6646. doi: 10.1158/0008-5472.CAN-07-0751. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 168.Kim MH, Cho HS, Jung M, Hong MH, Lee SK, Shin BA, Ahn BW, Jung YD. Extracellular signal-regulated kinase and AP-1 pathways are involved in reactive oxygen species-induced urokinase plasminogen activator receptor expression in human gastric cancer cells. Int J Oncol. 2005 Jun;26(6):1669–1674. [PubMed] [Google Scholar]
- 169.Swiatkowska M, Szemraj J, Al-Nedawi KN, Pawlowska Z. Reactive oxygen species upregulate expression of PAI-1 in endothelial cells. Cellular & molecular biology letters. 2002;7(4):1065–1071. [PubMed] [Google Scholar]
- 170.Pulukuri SM, Estes N, Patel J, Rao JS. Demethylation-linked activation of urokinase plasminogen activator is involved in progression of prostate cancer. Cancer Res. 2007 Feb 1;67(3):930–939. doi: 10.1158/0008-5472.CAN-06-2892. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 171.Stoclet JC, Chataigneau T, Ndiaye M, Oak MH, El Bedoui J, Chataigneau M, Schini-Kerth VB. Vascular protection by dietary polyphenols. Eur J Pharmacol. 2004 Oct 1;500(1–3):299–313. doi: 10.1016/j.ejphar.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 172.Oak MH, El Bedoui J, Schini-Kerth VB. Antiangiogenic properties of natural polyphenols from red wine and green tea. J Nutr Biochem. 2005 Jan;16(1):1–8. doi: 10.1016/j.jnutbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 173.Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. Journal of experimental therapeutics & oncology. 2002 Nov–Dec;2(6):350–359. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 174.Sartippour MR, Shao ZM, Heber D, Beatty P, Zhang L, Liu C, Ellis L, Liu W, Go VL, Brooks MN. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. The Journal of nutrition. 2002 Aug;132(8):2307–2311. doi: 10.1093/jn/132.8.2307. [DOI] [PubMed] [Google Scholar]
- 175.Tang FY, Meydani M. Green tea catechins and vitamin E inhibit angiogenesis of human microvascular endothelial cells through suppression of IL-8 production. Nutr Cancer. 2001;41(1–2):119–125. doi: 10.1080/01635581.2001.9680622. [DOI] [PubMed] [Google Scholar]
- 176.Cao Y, Cao R. Angiogenesis inhibited by drinking tea [letter] Nature. 1999;398(6726):381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- 177.Dona M, Dell’Aica I, Calabrese F, Benelli R, Morini M, Albini A, Garbisa S. Neutrophil restraint by green tea: inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J Immunol. 2003 Apr 15;170(8):4335–4341. doi: 10.4049/jimmunol.170.8.4335. [DOI] [PubMed] [Google Scholar]
- 178.Brakenhielm E, Cao R, Cao Y. Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. Faseb J. 2001 Aug;15(10):1798–1800. doi: 10.1096/fj.01-0028fje. [DOI] [PubMed] [Google Scholar]