Abstract
The knowledge concerning fetal hepatic stellate cells (HSCs) is scarce, and their cell lineage and functions are largely unknown. The current study isolated fetal liver mesenchymal cells from a mouse expressing β-galactosidase under the control of Msx2 promoter by fluorescence-activated cell sorting (FACS) and surveyed marker genes by microarray analysis. Based on the location and immunostaining with conventional and newly disclosed markers, we have identified three distinct populations of fetal liver mesenchymal cells expressing both desmin and p75 neurotrophin receptor (p75NTR): HSCs in the liver parenchyma; perivascular mesenchymal cells expressing α-smooth muscle actin (α-SMA); and submesothelial cells associated with the basal lamina beneath mesothelial cells and expressing activated leukocyte cell adhesion molecule (ALCAM) and platelet-derived growth factor receptor α. A transitional cell type from the submesothelial cell phenotype to fetal HSCs was also identified near the liver surface. Mesothelial cells expressed podoplanin and ALCAM. Ki-67 staining showed that proliferative activity of the submesothelial cells is higher than that of mesothelial cells and transitional cells. Using anti-ALCAM antibodies, submesothelial and mesothelial cells were isolated by FACS. The ALCAM+ cells expressed hepatocyte growth factor and pleiotrophin. In culture, the ALCAM+ cells rapidly acquired myofibroblastic morphology and α-SMA expression. The ALCAM+ cells formed intracellular lipid droplets when embedded in collagen gel and treated with retinol, suggesting the potential for ALCAM+ cells to differentiate to HSCs. Finally, we demonstrated that fetal HSCs, submesothelial cells, and perivascular mesenchymal cells are all derived from mesoderm by using MesP1-Cre and ROSA26 reporter mice.
Conclusion
Fetal HSCs, submesothelial cells, and perivascular mesenchymal cells are mesodermal in origin, and ALCAM+ submesothelial cells may be a precursor for HSCs in developing liver.
Hepatic stellate cells (HSCs) are located in the space of Disse between hepatocytes and sinusoidal endothelial cells (SECs) in the adult liver and play a pivotal role in liver physiology.1 In normal adult liver, quiescent HSCs are characterized by the expression of desmin, storage of vitamin A, and extensive dendrite-like processes along the sinusoid. On liver injury, HSCs express α-smooth muscle actin (α-SMA), lose vitamin A, and acquire a myofibroblastic phenotype. Adult liver HSCs express not only mesenchymal cell markers, but also neural cell markers, including nestin, glial fibrillary acidic protein (GFAP), and p75 neurotrophin receptor (p75NTR).2 Based on this HSC phenotype with expression of both mesenchymal and neural cell lineage markers, the neural crest was suggested to be the origin of HSCs. However, a cell lineage analysis using Wnt1-Cre and ROSA26 reporter (R26R) mice failed to support this hypothesis.3
Pluripotent mesenchymal stem cells give rise to different cell types, including osteoblasts, chondrocytes, smooth muscle cells, adipocytes, and neural cells, and HSCs express markers of those cell types.2 Our studies revealed that adipogenic regulation is essential for HSC quiescence, and its loss underlies transdifferentiation to myofibroblastic cells,4,5 much like transdifferentiation between pre-adipocytic fibroblasts and adipocytes.6 Thus it is plausible that HSCs originate from the pluripotent mesenchyme derived from mesoderm. Such a notion was proposed by a morphological study more than a decade ago, which indicated that as hepatoblasts invade into the adjacent septum transversum mesenchyme around embryonic day (E) 9.5, mesenchymal cells in the septum transversum seem to be trapped between growing heaptoblasts and endothelial cells and eventually become HSCs.7 However, a definitive answer has not been attained as to whether the septum transversum mesenchyme gives rise to HSCs because of the lack of appropriate markers to trace this lineage.
Little is known about characteristics, differentiation capacity, and function of HSCs during liver development. In fetal liver, several transcription factors, Hlx, Foxf1, and Lhx2, are expressed in HSCs.8–10 Analysis of Lhx2-null mice revealed that this transcription factor inhibits activation of HSCs and is important for liver morphogenesis.10 Although vitamin A–storing HSCs in adult liver can be purified by density gradient centrifugation based on their buoyancy or by fluorescence-activated cell sorting (FACS) using an ultraviolet laser,1,11 these isolation techniques are not applicable to fetal HSCs because of the absence of vitamin A storage.12 Furthermore, the lack of specific markers has hampered further studies on cell lineage and functions of fetal HSCs.
As an initial approach to investigate fetal HSCs, we attempted to isolate fetal liver mesenchymal cells using the transgenic mouse carrying a LacZ gene under the control of Msx2 (msh-like 2) promoter, which is sufficient for correct expression in developing embryos.13 During the course of expression analysis in this mouse, we found that liver mesenchymal cells including fetal HSCs express the lacZ gene. Isolation of the lacZ+ cells and subsequent complementary DNA (cDNA) microarray analysis have revealed novel markers that distinguish different populations of liver mesenchymal cells during liver development. One such unique mesenchymal cell population we have identified is termed “submesothelial cell,” which exists just beneath the mesothelial surface of the developing liver. We also demonstrate that fetal HSCs, submesothelial cells and perivascular mesenchymal cells are derived from mesoderm during embryogenesis.
Materials and Methods
Mice
The 560-base-pair Δ4Msx2-hsplacZ transgenic mouse (Msx2-lacZ) and MesP1-Cre knock-in mouse were described previously.13,14 Male MesP1-Cre mice were crossed with female R26R mice.15 The use of animals for this study was approved by the Institutional Animal Care and Use Committee of the University of Southern California.
X-gal Staining and Fluorescence Immunohistochemistry
Whole embryos were fixed with 4% paraformaldehyde and stained with 1% X-gal solution at 37°C for 4 hours.13 For detection of lacZ activities in sections, frozen sections (7 µm) were incubated in X-gal solution as above and counter-stained with eosin Y (Sigma, St. Louis, MO).
For immunostaining, cryosections were permeabilized with phosphate-buffered saline (PBS) containing 0.1% Triton X-100. After washing with PBS, the sections were blocked with 0.2% bovine serum albumin and 5% serum that is from the same species as that for secondary antibody for 30 minutes. After blocking, the sections were incubated with diluted primary antibodies for 1 hour. The primary antibodies used in immunostaining are listed in Table 1. For double immunostaining, the sections were washed with PBS and incubated with primary antibodies for 1 hour. After washing with PBS, the sections were incubated with secondary antibodies conjugated with AlexaFluor 488, 555, or 568 (Invitrogen, Carlsbad, CA) for 30 minutes. The sections were mounted with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen) and fluorescence images were visualized under a microscope (Nikon Eclipse 90i; Nikon, Tokyo, Japan). For assessment of proliferative activity, we prepared cryosections from three different embryos and performed double immunostaining with Ki-67 and activated leukocyte cell adhesion molecule (ALCAM). After staining, a total of 55 photographs were taken, and the Ki-67+ cells were counted.
Table 1.
List of Antibodies for Immunohistochemistry
| Primary Antibodies | Maker (Catalog No.) | Dilution |
|---|---|---|
| FITC-labeled mouse anti-αSMA* | Sigma (F3777) | 100 |
| Goat anti-human desmin† | Santa Cruz (sc-7559) | 50 |
| Goat anti-mouse albumin | Bethyl (A90-134A) | 2,000 |
| Hamster anti-mouse podoplanin | eBioscience (14-5381) | 50 |
| Mouse anti-human WT1 | Cell Marque (6F-H2) | 50 |
| Rabbit anti-β-galactosidase | Cappel (55976) | 4,000 |
| Rabbit anti-cow cytokeratin | Dako (Z0622) | 100 |
| Rabbit anti-human desmin | Abcam (ab8592) | 200 |
| Rabbit anti-human PDGFRα | Santa Cruz (sc-338) | 200 |
| Rabbit anti-human Ki-67 | Zymed (18-0191Z) | 100 |
| Rabbit anti-mouse collagen type IV | Millipore (AB756P) | 200 |
| Rabbit anti-mouse p75NTR | Abcam (ab8874) | 1,000 |
| Rat anti-mouse ALCAM | eBioscience (14-1661) | 100 |
| Rat anti-mouse CD31 | BD Pharmingen (550274) | 100 |
| Rat anti-mouse CD45 | eBioscience (14-0451) | 200 |
| Rat anti-mouse E-cadherin | Zymed (13-1900) | 800 |
| Rat anti-mouse F4/80 | eBioscience (14-4801) | 500 |
| Rat anti-mouse Flk1 | eBioscience (14-5821) | 200 |
αSMA was detected with FITC without secondary antibodies.
To reduce background staining and detect desmin, we purified this antibody with Quick spin column (Sephadex G-25; Roche Applied Science, Indianapolis, IN) and the sections were partially digested with 4 µg/mL proteinase K (Invitrogen) for 5 minutes before blocking.
FACS Analysis of LacZ+ Liver Cells
E12.5 livers were digested in Dulbecco’s modified Eagle’s medium containing 0.2 U/mL Dispase I (Roche Applied Science) for 20 minutes at 37°C.16 For detection of lacZ activity in liver cells, we loaded fluorescein di-β-D-galactoside (FDG, Invitrogen) into the liver cells by hypotonic shock as previously described.17 Dead cells were detected using 5 µM propidium iodide. For FACS analysis, the cells were analyzed using FACS Vantage SE (Becton Dickinson, San Jose, CA), and the data were processed using CellQuest Pro software (Becton Dickinson).
Cell Depletion Using Antibodies and Magnetic Beads
The lacZ+ cells sorted by FACS were incubated with antibody-coated magnetic beads as previously reported.16 Briefly, 5 × 105 beads (Dynabeads Sheep antirat immunoglobulin G; Invitrogen) were coated with each of rat anti-mouse E-cadherin (ECCD-1; Takara Bio, Shiga, Japan), F4/80, Flk1, CD45, TER-119 (eBioscience, San Diego, CA), and CD31 (BD Biosciences) antibodies. After washing, each of the magnetic beads was combined and incubated with the lacZ+ cells for 30 minutes on ice. The antibody+ cells were depleted with a magnetic particle concentrator (Invitrogen).
Quantitative Polymerase Chain Reaction
Total RNAs were extracted with RNAqueous Micro (Ambion, Austin, TX). Complementary DNAs were synthesized from 30 ng total RNAs using SuperScript III and Oligo(dT)20 primers (Invitrogen). Quantitative polymerase chain reaction (qPCR) was performed with SYBR Green PCR master mix (Applied Biosystems, Foster City, CA) in M×3000P QPCR System (Stratagene, La Jolla, CA) as previously described.5 The primers are listed in Table 2.
Table 2.
List of Primers for qPCR
| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Albumin | tgctgctgattttgttgagg | agagttggggttgacacctg |
| Alcam | ctcgttgctggtgtcgtcta | aatccgctcctctcttaggc |
| β-actin | gacggccaggtcatcactat | tgatccacatctgctggaag |
| Cd31 | gaatgacacccaagcgtttt | ggcttccacactaggctcag |
| Cd45 | gggttgttctgtgccttgtt | ctggacggacacagttagca |
| Cd68 | ccaattcagggtggaagaaa | ttgcatttccacagcagaag |
| Col1a1 | caccaccctcaagagcctgagtc | gttcgggctgatgtaccagt |
| Col4a1 | tgtggatcggctattccttc | agcggggtgtgttagttacg |
| Desmin | caggacctgctcaatgtgaa | gtagcctcgctgacaacctc |
| E-cadherin | cctgccaatcctgatgaaat | tcagggaaggagctgaaaga |
| Flk1 | ccagatgacagccagacaga | agcagcacctctctcgtgat |
| Foxf1 | ggcctcctacatcaagcaac | taagatcctccgcctgttgt |
| Gapdh | cgtcccgtagacaaaatggt | gaatttgccgtgagtggagt |
| Gfap | cacgaacgagtccctagagc | ccttctgacacggatttggt |
| Hgf | ttcccagctggtctatggtc | tggtgctgactgcatttctc |
| Hlx | cttcagcgggacagttcttc | ctggaaccacaccttcacct |
| LacZ* | aatacctgttccgtcatagc | cttacgccaatgtcgttatc |
| Lhx2 | gagaaagcgcaagagtccag | tttcctgccgtaaaaggttg |
| Msx2 | aattccgaagacggagcac | gcagccattttcagcttttc |
| p75ntr | cagcagacccacacacagac | tctgtgggggctagaacatc |
| Pdgfrα | acagagactgagcgctgaca | ctcgatggtctcgtcctctc |
| Pleiotrophin | ttttcatcttggcagctgtg | ggcttggagatggtgacagt |
| Podoplanin | gtgaccccaggtacaggaga | atggctaacaagacgccaac |
| αSma | ctgagcgtggctattccttc | cttctgcatcctgtcagcaa |
| Wt1 | ggttttctcgctcagaccag | ggtgagtgggaggaatttca |
All primers were designed on different exons for each gene except lacZ gene.
Microarray Analysis
From each 60 ng total RNA prepared from E12.5 liver cells and lacZ+Ab− cells, we obtained 26 µg and 18 µg cDNAs using the Ovation RNA amplification system V2 (NuGen, San Carlos, CA). Then, 3.75 µg from each cDNA was fragmented and labeled with Biotin using FL-Ovation cDNA Biotin module V2 (NuGen). The labeled probes were hybridized with GeneChip Mouse Genome 430 2.0 arrays (Affymetrix, Santa Clara, CA) and signals were analyzed at the USC/CHLA Genome Core Laboratory.
Isolation and Culture of ALCAM+ Cells
E12.5 livers were digested with 0.05% trypsin-ethylene diamine tetraacetic acid for 5 minutes, and the blood cells were depleted using a lineage cell depletion kit (Miltenyi Biotec, Auburn, CA). After blocking with anti-CD16/32 antibodies (eBioscience), the blood cell lineage− cells were incubated with phycoerythrin-labeled rat anti-mouse ALCAM antibodies (eBioscience) for 30 minutes. For FACS analysis, the cells were analyzed using FACS Vantage SE (Becton Dickinson), and the antibody positive and negative fractions were sorted. After FACS, 1 × 104 cells were plated on 24-well plates in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and were subjected to immunostaining and qPCR. Collagen gel culture was performed as described previously.18 Briefly, ALCAMhigh cells were mixed with PureCol (Inamed, Fremont, CA) and loaded in 24-well plates (1 × 104 cells/ well). On day 4 in culture, the cells were treated with 5 µM retinol (Sigma) with 100 µM palmitic acid (Sigma) for 24 hours.19 For lipid staining, cells were incubated with 0.2% Oil Red O (Sigma) for 5 hours.5
Results
LacZ Expression in the Msx2 Transgenic Mouse Liver
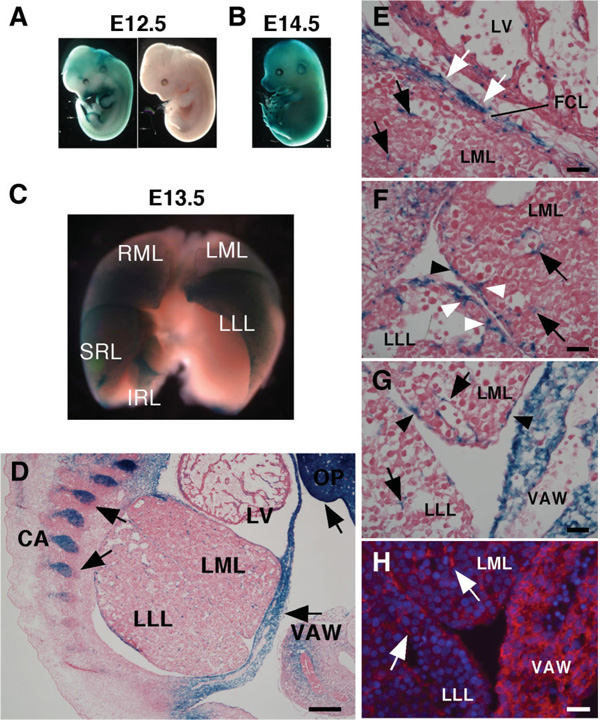
TheMsx2-lacZ embryos showed X-gal staining in the developing limbs and head of E12.5 to E14.5 whole embryos (Fig. 1A, B).13 The lacZ expression was also found in developing liver, which was not previously reported (Fig. 1C). The developing liver at E13.5 is macroscopically divided into the right and left median lobes (RML, LML), left lateral lobe (LLL), and superior and inferior right lobes (SRL, IRL), and intense X-gal staining was detected, particularly at the ventral surface of the RML, SRL, and LLL (Fig. 1C). The lacZ expression was found in mesenchymal cells in the parenchyma and around the blood vessels (Fig. 1D–G). The “flat cell layers” covering the median lobe right adjacent to heart were also lacZ+ (Fig. 1E). This tissue appears to be the transitional structure from septum transversum to diaphragm. Between liver lobes, some mesothelial cells and mesenchymal cells beneath the mesothelial cells also expressed lacZ (Fig. 1F). Because the staining patterns by lacZ immunostaining and X-gal staining were shown to be identical (Fig. 1G, H), subsequent analysis was performed by lacZ immunostaining.
Fig. 1.
LacZ expression in Msx2 promoter driven-lacZ embryos. Whole mount X-gal staining in (A) E12.5 embryos, (B) E14.5 embryo, and (C) E13.5 liver. (A) LacZ expression in the limbs and head of the transgenic embryo (left), but not in the wild-type embryo (right). (B) Strong lacZ expression at the digits and olfactory placode. (C) An frontal view of liver X-gal staining. E13.5 liver is divided into the right and left median lobes (RML, LML), left lateral lobe (LLL), and superior and inferior right lobes (SRL, IRL). Intense X-gal staining is detected particularly at the ventral surface of the RML, SRL, and LLL. (D–H) Detection of lacZ expression on the E13.5 cryosections by X-gal staining (D–G) and immunohistochemistry of anti-β-galactosidase antibody (H). (D) Arrows indicate lacZ expression in the ventral abdominal wall (VAW), cartilage (CA), and olfactory placode (OP). LV; left ventricle. (E–G) Magnified views of cranial (E), dorsal (F), and ventral (G) regions of (D). Black arrows indicate lacZ expression in mesenchymal cells in the parenchyma and around the vein. LacZ expression is also found in the flat cell layers (FCL) covering the LML (white arrows), mesothelial cells (black arrowheads), and mesenchymal cells beneath the mesothelial cells (white arrowheads). (H) LacZ expression in the liver mesenchymal cells (white arrows) detected by β-galactosidase antibodies (red). Bar, 100 µm (D) and 10 µm (E–H).
LacZ Expression in Liver Mesenchymal Cells
We determined whether lacZ+ liver mesenchymal cells express desmin or α-SMA by immunostaining of E12.5 embryos. Desmin was expressed in the fetal HSCs, perivascular mesenchymal cells, and mesenchymal cells beneath the mesothelium (Fig. 2A). Almost all desmin+ cells expressed lacZ (Fig. 2A–D). The lacZ+ flat cell layers covering the median lobe weakly expressed desmin (Fig. 2D). Many of the perivascular mesenchymal cells expressed α-SMA (Fig. 2E, G) and coexpressed desmin and lacZ (Fig. 2F, H). Near the vessels, there were rare desmin+ α-SMA+ HSCs, which accounted for 3.7% of the desmin+ HSCs (Fig. 2E, F, arrows). The lacZ+ mesenchymal cells beneath the mesothelium was negative for α-SMA (Fig. 2I, J). Expression of Flk1 and CD31, markers for SECs, F4/80, a marker for Kupffer cells, and E-cadherin and albumin, markers for hepatoblasts, were not colocalized with lacZ+ staining in E12.5 (Fig. 2K–O) and E13.5 livers (data not shown). These data indicate that desmin+ cells including fetal HSCs, perivascular mesenchymal cells, and mesenchymal cells beneath the mesothelium, express Msx2 messenger RNA, and the lacZ expression is a suitable marker for these three populations of fetal liver mesenchymal cells in this model.
Fig. 2.
LacZ expression in mesenchymal cells of the developing liver. Double immunostaining was performed on cryosections prepared from E12.5 Msx2-lacZ embryos. (A,B) Detection of desmin (green) and lacZ (red) at the liver surface. HSCs (arrows), perivascular mesenchymal cells (double arrows), and mesenchymal cells beneath the mesothelium (arrowheads) coexpress desmin and lacZ. (C) A merged image of (A) and (B). (D) Double staining of desmin (green) and lacZ (red) in the cranial region of the liver. Desmin and lacZ coexpression in HSCs (arrows) and the flat cell layers (arrowheads). PC, pericardial cavity. (E–J) Double staining of α-SMA (green) and desmin or lacZ (red). (E, F) Many of the α-SMA+ perivascular mesenchymal cells coexpress desmin (arrowheads). Double arrows indicate α-SMA− cells in the desmin+ perivascular mesenchymal cells. Near the vessels, there are rare desmin+ α-SMA+ HSCs (arrows). Of 484 desmin+ HSCs, 3.7% are coexpressed with α-SMA. (G, H) α-SMA+ perivascular mesenchymal cells coexpress lacZ (arrowheads). Double arrows indicate α-SMA− cells in the lacZ+ perivascular mesenchymal cells. (I, J) Alpha-SMA−lacZ+ mesenchymal cells beneath the mesothelium (arrowheads). (K–O) Double staining of lacZ (red) and Flk1 (K, green), CD31 (L, green), F4/80 (M, green), E-cadherin (N, green), or albumin (O, green). No coexpression of lacZ (arrows) with these markers (arrowheads). (P) Negative control staining of isotype immunoglobulin G. Nuclei were counterstained with DAPI (blue). Bar, 25 µm.
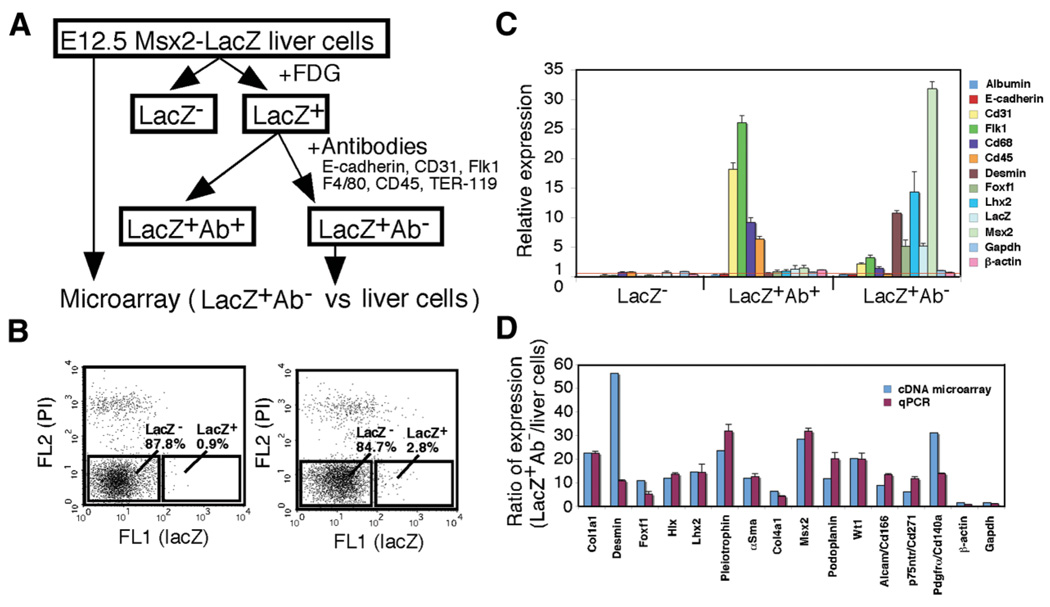
Isolation of LacZ+ Cells
To identify markers for liver mesenchymal cells, including fetal HSCs, we attempted to isolate lacZ+ cells from Msx2-lacZ fetal livers by FACS using FDG. Fig. 3A illustrates the procedure of fetal liver mesenchymal cell isolation. When liver cells prepared from E12.5 lacZ+ livers were incubated with FDG, 2% to 3% of the cells showed high FL1 caused by hydrolysis of FDG to fluorescein by β-galactosidase (right panel, Fig. 3B). However, the cells prepared from the LacZ− livers also showed approximately 1% FL1 cells, which appeared to be caused by endogenous β-galactosidase–like activity (left panel, Fig. 3B). LacZ+ cells sorted from E12.5 lacZ+ livers expressed desmin messenger RNA, although other cell markers, including albumin, CD31, and CD68, were also detected because of inevitable contamination (data not shown). Using antibodies that recognize cell surface markers of hepatoblasts (E-cadherin), SECs (CD31, Flk1), Kupffer cells (F4/80), and blood cells (CD45, TER-119) plus magnetic beads, we depleted these cells to further purify the lacZ+ cells (Fig. 3A). From 1.6 × 107 E12.5 liver cells treated with FDG, 8.9 × 104 (0.6%) lacZ+ and 6.9 × 106 (43.1%) lacZ− cells were obtained by FACS. Then, the LacZ+ cells that were pull-down with beads (LacZ+ Ab+) and other remaining cells (LacZ+ Ab−) were subjected to qPCR analysis for the cell type markers. The data were compared against those obtained from isolated liver cells without FACS and magnetic bead separation. As shown in Fig. 3C, the LacZ+ Ab− fraction was enriched by the cells expressing desmin, Foxf1, and Lhx2, markers for HSCs, whereas LacZ+ Ab+ cells expressed other liver cell markers. The LacZ+ Ab− cells expressed 5-fold and 32-fold higher lacZ and Msx2, respectively, validating the enrichment efficiency and confirming endogenous expression of Msx2 by this population.
Fig. 3.
Isolation of lacZ+ cells from E12.5 Msx2-lacZ livers and cDNA microarray analysis. (A) The procedure of lacZ+ liver mesenchymal cell isolation. After digestion of E12.5 livers, cells were incubated with FDG, and lacZ− and lacZ+ cells were sorted by FACS. The lacZ+ cells were further incubated with antibodies against E-cadherin, CD31, Flk1, F4/80, CD45, and TER-119. Then, populations positive (lacZ+ Ab+) and negative (lacZ+ Ab−) for those antibodies were obtained. The lacZ+ Ab− population and liver cells were subjected to cDNA microarray analysis. (B) FACS analysis of E12.5 liver cells incubated with FDG prepared from lacZ− (left) and lacZ+ embryonic livers (right). Dead cells were detected by propidium iodide (PI, FL2). In the lacZ+ liver cells, 2.8% of the cells show high FL1 caused by hydrolysis of FDG to fluorescein (right). (C) Quantitative PCR of the liver cells, lacZ−, lacZ+Ab+, and lacZ+Ab− populations separated from E12.5 livers. The results are expressed as relative expression compared with the liver cells (arbitrarily set at 1; red line). Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) and β-actin were used as internal controls. (D) Comparison of gene expression levels obtained by cDNA microarray and those by qPCR from the lacZ+Ab− and liver cells. Each value of qPCR is the mean ± standard deviation of triplicate measurements.
Microarray Analysis
Having isolated the lacZ+ Ab− cells, we surveyed their differentially expressed genes by comparing with total isolated liver cells using the cDNA microarray. Among 45,037 genes analyzed, 4793 showed 5-fold or higher expression in the lacZ+ Ab− cells than the total liver cells. Table 3 is a partial list of the upregulated genes that include those previously reported to be expressed by HSCs. Confirmation of the microarray results by qPCR analysis was performed for some of these genes (Fig. 3D).
Table 3.
Up-Regulated Genes in the LacZ+ Ab− Cells Against Liver Cells
| Entrez Gene ID | Gene Symbol | Gene Name | Liver Cells | LacZ+ Ab− | Ratio |
|---|---|---|---|---|---|
| 18008 | Nes | Nestin | 1.27 | 72.87 | 57.37 |
| 13346 | Des | Desmin | 1.55 | 87.30 | 56.31 |
| 12305 | Ddr1 | Discoidin domain receptor family, member 1 | 2.83 | 111.15 | 39.23 |
| 17390 | Mmp2 | Matrix metalloproteinase 2 | 22.20 | 856.11 | 38.57 |
| 18595 | Pdgfra | PDGF receptor α | 5.10 | 158.34 | 31.07 |
| 18214 | Ddr2 | Discoidin domain receptor family, member 2 | 21.17 | 653.20 | 30.85 |
| 17702 | Msx2 | Homeo box, msh-like 2 | 0.39 | 11.12 | 28.66 |
| 21808 | Tgfb2 | Tgfβ2 | 54.87 | 1491.54 | 27.18 |
| 21858 | Timp2 | Tissue inhibitor of metalloproteinase 2 | 4.30 | 105.64 | 24.57 |
| 14114 | Fbln1 | Fibulin 1 | 5.99 | 145.36 | 24.27 |
| 19242 | Ptn | Pleiotrophin | 19.60 | 460.45 | 23.50 |
| 12842 | Col1a1 | Procollagen, type I, α1 | 179.70 | 4040.91 | 22.49 |
| 22431 | Wt1 | Wilms tumor homolog | 49.41 | 996.44 | 20.17 |
| 26561 | Mmp23 | Matrix metalloproteinase 23 | 6.26 | 112.68 | 17.99 |
| 17967 | Ncam1 | Neural cell adhesion molecule 1 | 1.48 | 26.47 | 17.92 |
| 14115 | Fbln2 | Fibulin 2 | 8.68 | 154.84 | 17.83 |
| 17385 | Mmp11 | Matrix metalloproteinase 11 | 17.65 | 299.96 | 17.00 |
| 14463 | Gata4 | GATA binding protein 4 | 55.14 | 899.02 | 16.30 |
| 13003 | Vcan | Versican | 163.38 | 2636.66 | 16.14 |
| 14118 | Fbn1 | Fibrillin 1 | 61.74 | 996.28 | 16.14 |
| 16870 | Lhx2 | LIM homeobox protein 2 | 9.00 | 130.69 | 14.52 |
| 12843 | Col1a2 | Procollagen, type I, α2 | 321.27 | 4488.27 | 13.97 |
| 12825 | Col3a1 | Procollagen, type III, α1 | 245.58 | 3348.73 | 13.64 |
| 12111 | Bgn | Biglycan | 61.11 | 824.68 | 13.49 |
| 13179 | Dcn | Decorin | 120.98 | 1611.85 | 13.32 |
| 16776 | Lama5 | Laminin, alpha 5 | 11.58 | 151.15 | 13.05 |
| 17387 | Mmp14 | Matrix metalloproteinase 14 | 14.64 | 184.71 | 12.61 |
| 15284 | Hlx | H2.0-like homeobox | 31.23 | 370.67 | 11.87 |
| 11475 | Acta2 | Actin, α2, smooth muscle | 156.83 | 1852.08 | 11.81 |
| 14726 | Pdpn | Podoplanin | 65.01 | 761.54 | 11.71 |
| 17389 | Mmp16 | Matrix metalloproteinase 16 | 28.76 | 322.35 | 11.21 |
| 17967 | Ncam1 | Neural cell adhesion molecule 1 | 351.73 | 3817.77 | 10.85 |
| 15227 | Foxf1a | Forkhead box F1a | 137.22 | 1487.05 | 10.84 |
| 11658 | Alcam | Activated leukocyte cell adhesion molecule | 342.22 | 2990.88 | 8.74 |
| 14165 | Fgf10 | Fibroblast growth factor 10 | 1.36 | 10.70 | 7.89 |
| 16449 | Jag1 | Jagged 1 | 142.58 | 1079.85 | 7.57 |
| 12826 | Col4a1 | Procollagen, type IV, α1 | 58.57 | 367.77 | 6.28 |
| 18053 | Ngfr | Nerve growth factor receptor | 20.79 | 125.88 | 6.05 |
| 22352 | Vim | Vimentin | 619.19 | 3699.38 | 5.97 |
| 23849 | Klf6 | Kruppel-like factor 6 | 9.86 | 54.04 | 5.48 |
Identification of Submesothelial Cells Beneath the Liver Surface
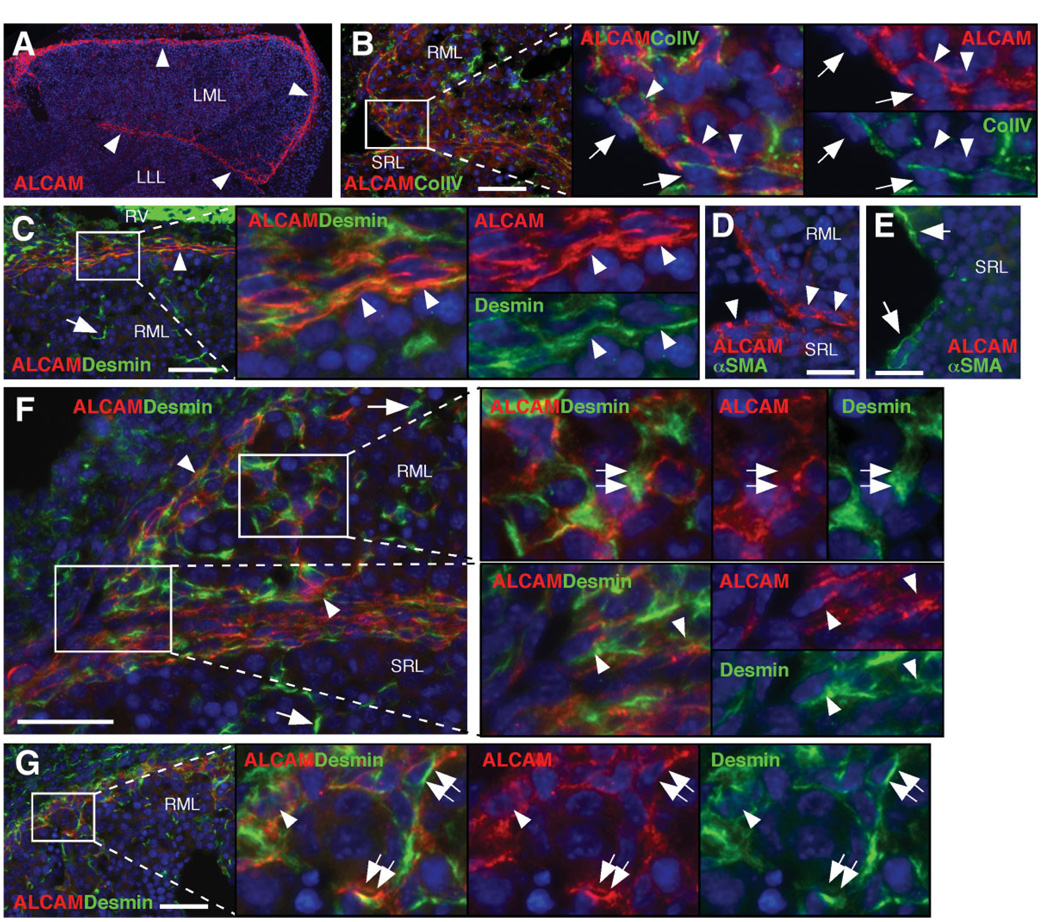
Of the up-regulated genes confirmed by qPCR (Fig. 3D), we selected CD antigens, including activated leukocyte cell adhesion molecule (ALCAM/CD166), p75NTR/CD271, and platelet-derived growth factor receptor α (PDGFRα/CD140a) and developmental markers including type IV collagen, podoplanin, and WT1 for immunohistochemical analysis in E12.5 livers. ALCAM staining was found along the liver surface (Fig. 4A). Type IV collagen, a marker for basal lamina, is deposited beneath the mesothelial cells at the liver surface (Fig. 4B). Along the dorsal liver surface, the mesenchymal cells were separated from the mesothelial cells by the type IV collagen (Fig. 4B). Immunostaining of ALCAM was found in the mesenchymal cells beneath the mesothelium (Fig. 4B, arrowheads). ALCAM expression was overlapped with type IV collagen and was also detected at the basolateral side of mesothelial cells (Fig. 4B). ALCAM was co-expressed with desmin in the flat cell layers on the surface of the median lobe (Fig. 4C, arrowheads). The ALCAM+ mesenchymal cells beneath the mesothelial cells coexpressed desmin (Fig. 4F, arrowheads), but not α-SMA (Fig. 4D). Conversely, fetal HSCs (Fig. 4C,F, arrows) and perivascular mesenchymal cells (Fig. 4E) were negative for ALCAM. From these staining patterns, we defined the desmin+ ALCAM+ cells associated with type IV collagen of the basal lamina as “submesothelial cells.” Near the liver surface, desmin+ ALCAM+ cells, not associated with the basal lamina, were also identified in the parenchyma a short distance (3–5 hepatoblast layers) away from the submesothelial cells (Fig. 4F, G, double arrows), suggestive of transitional cells migrating inward from the submesothelial cells.
Fig. 4.
Identification of submesothelial cells in the developing livers. Immunostaining was performed on E12.5 Msx2-lacZ livers. (A) ALCAM immunostaining (red). ALCAM is expressed along the liver surface (arrowheads). (B) ALCAM (red) and collagen type IV (ColIV, green) staining in the dorsal region. Arrowheads indicate ALCAM+ submesothelial cells. Arrows indicate mesothelial cells. (C) ALCAM (red) and desmin (green) staining in the cranial region. Arrowheads indicate desmin+ ALCAM+ flat cells. An arrow indicates desmin+ ALCAM− HSCs. RV, right ventricle. (D, E) ALCAM (red) and α-SMA (green) staining in the dorsal region (D) and around the blood vessel (E). Submesothelial cells express ALCAM (arrowheads), but not α-SMA. Perivascular mesenchymal cells express α-SMA (arrows), but not ALCAM. (F, G) ALCAM (red) and desmin (green) staining in the dorsal region. Arrows and arrowheads indicate desmin+ ALCAM− HSCs and desmin+ ALCAM+ submesothelial cells, respectively. Double arrows indicate desmin+ ALCAM+ transitional cells away from the liver surface. Nuclei were counterstained with DAPI (blue). Bar, 20 µm.
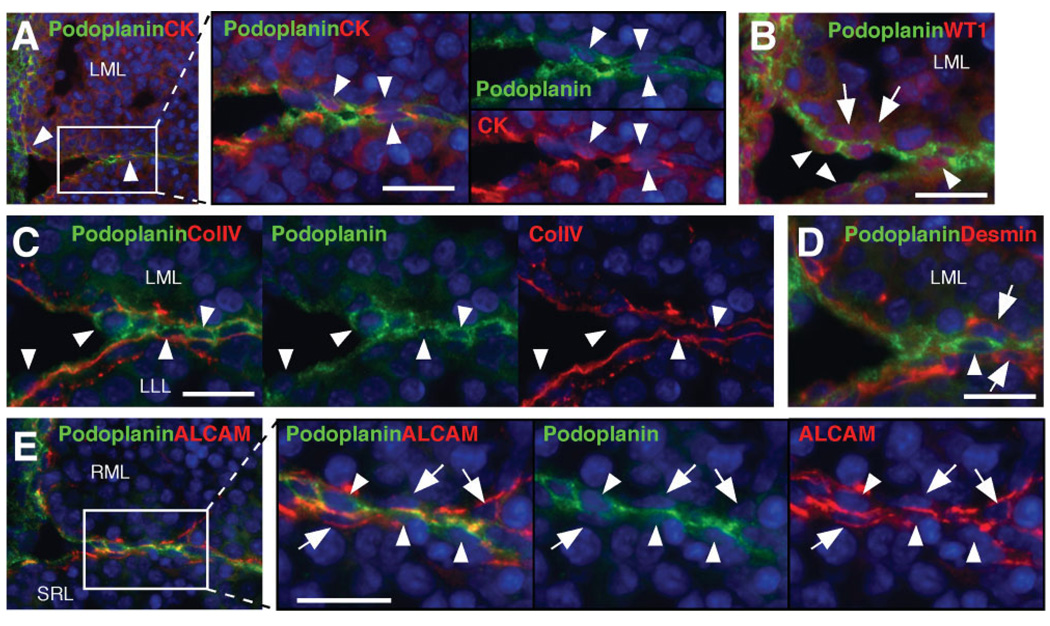
Podoplanin Is a Marker for Liver Mesothelial Cells
Podoplanin expression was exclusively found in the mesothelial cells positive for pan-cytokeratin or WT1, but not in the submesothelial cells expressing WT1 (Fig. 5A, B). Podoplanin+ mesothelial cells were located right above the basal lamina expressing type IV collagen (Fig. 5C) below which desmin+ submesothelial cells existed (Fig. 5D). ALCAM immunostaining was evident in the basal lamina side of both podoplanin+ mesothelial cells and podoplanin− submesothelial cells (Fig. 5E).
Fig. 5.
Identification of mesothelial cells in the developing livers. Double immunostaining was performed on E12.5 Msx2-lacZ livers. (A–D) Detection of podoplanin (green) and pan-cytokeratin (CK, red), WT1 (red), collagen type IV (ColIV, red), or desmin (red) in the dorsal region. Arrowheads indicate podoplanin+ mesothelial cells. Arrows indicate submesothelial cells. (E) Podoplanin (green) and ALCAM (red) staining in the dorsal region. Arrowheads and arrows indicate podoplanin+ mesothelial cells and ALCAM+ submesothelial cells, respectively. Nuclei were counterstained with DAPI (blue). Bar, 10 µm.
Definitions of HSCs, Submesothelial Cells, Mesothelial Cells, and Perivascular Mesenchymal Cells in the Developing Mouse Liver
p75NTR was expressed in desmin+ HSCs, ALCAM+ submesothelial cells, and α-SMA+ perivascular mesenchymal cells (Fig. 6A–C). PDGFRα expression was detected in the ALCAM+ submesothelial cells (Fig. 6D). From these expression patterns and location, we classified three populations of mesenchymal cells in developing liver: HSCs in the liver parenchyma (desmin+ p75NTR+ αSMA+/−), submesothelial cells beneath the mesothelium (desmin+ p75NTR+ ALCAM+ PDGFRα+), and perivascular mesenchymal cells in the vicinity of the vessels (desmin+ p75NTR+ α-SMA+) (Fig. 6E). The mesothelial cells were identifiable with podoplanin. Immunohistochemistry analysis suggests that cells showing the desmin+ ALCAM+ submesothelial cell phenotype, but not associated with the basal lamina near the liver surface, are transitional cells between the submesothelial cells and HSCs.
Fig. 6.
Characterization of submesothelial cells. Double immunostaining was performed on E12.5 Msx2-lacZ livers. (A–C) Detection of p75NTR and desmin, ALCAM, or α-SMA. Arrows indicate p75NTR expression in desmin+ HSCs (A), ALCAM+ submesothelial cells (B), and α-SMA+ perivascular mesenchymal cells (C). Mesothelial cells are negative for p75NTR (arrowhead). (D) Expression of PDGFRα (green) and ALCAM (red) in the dorsal region. Arrows indicate PDGFRα+ ALCAM+ submesothelial cells. Nuclei were counterstained with DAPI (blue). (E) Summary of immunostaining in E12.5 livers. (F–I) Proliferative activities of submesothelial cells, mesothelial cells, and transitional cells in the E12.5 livers. (F) A representative merged image of Ki-67 (red) and ALCAM (green) staining. (G) Single DAPI image of (F). Mesothelial cells are identifiable at the liver surface (white circles). Of 534 mesothelial cells, 30.0% are Ki-67+ (I). (H) ALCAM and DAPI image of (F). Of 1,506 ALCAM+ submesothelial cells (red circles), 41.8% are Ki-67+ (I). Transitional cells are identified by ALCAM expression and irregular nuclei apart from the liver surface (white squares). Of 767 submesothelial cells, 22.8% are Ki-67+ (I). Bar, 10 µm (A–D), 20 µm (F).
Proliferative Activity of Submesothelial Cells
We determined the proliferative activities of submesothelial cells, mesothelial cells, and transitional cells by double immunostaining of Ki-67 and ALCAM (Fig. 6F). Mesothelial cells were identifiable at the liver surface (Fig. 6G, I, white circles), and 30.0% were Ki-67+. Conversely, 41.8% of the ALCAM+ submesothelial cells were Ki-67+ (Fig. 6H, I, red circles). Transitional cells were identified by ALCAM expression and irregular nuclei apart from the liver surface (Fig. 6H, I, white squares), and 22.8% were Ki-67+. These staining data indicate that submesothelial cell are actively dividing in the fetal livers as compared with mesothelial cells and transitional cells.
Differentiation Potential of ALCAM + Cells In Vitro
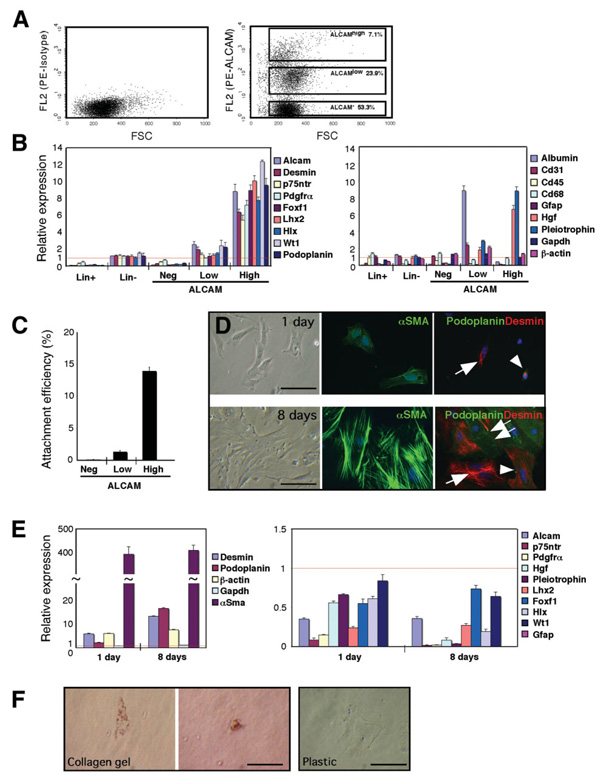
To test differentiation potential of submesothelial and mesothelial cells, we isolated the ALCAM+ cells by FACS for culture. After depletion of lineage+ blood cells, the lineage− population was incubated with anti-ALCAM antibodies. FACS analysis showed three distinct populations: ALCAMhigh, ALCAMlow, and ALCAM− in E12.5 liver cells (Fig. 7A). Quantitative PCR revealed that the ALCAMlow population highly expressed albumin (Fig. 7B), implying that hepatoblasts weakly express ALCAM at the level undetectable by immunohistochemistry (Fig. 4A) but detectable by qPCR. Conversely, the ALCAMhigh population expressed the genes related to submesothelial cells and mesothelial cells, but not albumin, indicating successful enrichment of these two cell types (Fig. 7B). The ALCAMhigh cells highly expressed hepatocyte growth factor (Hgf) and pleiotrophin, but did not express Gfap.
Fig. 7.
Isolation and culture of ALCAM+ submesothelial and mesothelial cells. (A) After digestion of the E12.5 liver, blood cell lineage+ population (Lin+) was depleted. The lineage− population (Lin−) were incubated with phycoerythrin-labeled anti-ALCAM (right) or isotype control (left) and analyzed by FACS. The ALCAMhigh, ALCAMlow, and ALCAM− populations were sorted. (B) Quantitative PCR of each population of E12.5 liver. The results are expressed as relative expression compared with the liver cells (arbitrarily set at 1; red lines). (C) Attachment efficiency of ALCAMhigh, ALCAMlow, and ALCAM− populations. After FACS, these three populations were plated on 24-well plates (1 × 104 cells/well). One day after culture, 20 photographs were randomly taken from four independent wells of each population, and the attached cell numbers were estimated. (D) Culture of the ALCAMhigh population at 1 and 8 days on plastic dish. The cells were stained with α-SMA (green) or desmin (red) and podoplanin (green). Arrowheads indicate cells coexpressing desmin and podoplanin. Arrows and double arrows indicate desmin+ and podoplanin+ cells, respectively. (E) Quantitative PCR of cultured ALCAMhigh population. The results are expressed as relative expression compared with the ALCAMhigh population before culture (arbitrarily set at 1; red lines). Increased expression was found in β-actin, but not Gapdh, at days 1 and 8. No Gfap expression was detected in both days 1 and 8 cultured cells. Each value of qPCR is the mean ± standard deviation of triplicate measurements. (F) Oil red O staining of day 5 ALCAMhigh cells in collagen gel or on plastic dish. Bar, 50 µm.
One day after plating, 13.9% of the ALCAMhigh cells attached to plastic well (Fig. 7C) and showed a fibroblastic morphology expressing α-SMA, desmin, or both desmin and podoplanin (Fig. 7D). Alpha-Sma messenger RNA showed a 393-fold increase after 1 day culture as compared with the preculture level (Fig. 7E). After 8 days in culture, all cells showed myofibroblastic morphology expressing α-SMA (Fig. 7D). Many of the cells were stained for desmin (86%), and 49% of these cells expressed podoplanin (Fig. 7D). Ten percent of the cells were podoplanin+ desmin−. Quantitative PCR showed that high expression levels of α-Sma, desmin, podoplanin, and β-actin were maintained in 8-day culture (Fig. 7E). In contrast, p75ntr, Pdgfrα, Hgf, pleiotrophin, Lhx2, and Hlx were down-regulated in the 8-day cells (Fig. 7E). No Gfap expression was found in the cultured cells. These results suggest that ALCAMhigh submesothelial cells and mesothelial cells rapidly acquire a myofibroblastic phenotype in vitro. Conversely, only 1.3% of the ALCAMlow population attached to plastic well (Fig. 7C), and these attaching cells showed a fibroblastic morphology expressing α-SMA on day 8 (data not shown). The ALCAM− population did not attach to the well (Fig. 7C).
Next, we tested whether the ALCAMhigh population has the ability to store lipids in vitro as HSCs do. The ALCAMhigh cells were cultured on a plastic well or in collagen gel for 4 days, and then incubated with retinol and palmitic acid for 24 hours. Oil Red O staining showed that 45% of the cells showed lipid storage in collagen gel (Fig. 7F), but no staining was found in the cells cultured on plastic dish despite the same treatment (Fig. 7F). These results suggest that the ALCAMhigh population may acquire a phenotype characteristic of HSCs in three-dimensional collagen culture.
Cell Lineage Analysis of Liver Mesenchymal Cells
Finally, we determined whether the three populations of liver mesenchymal cells described previously are derived from a mesoderm lineage during development by using MesP1-Cre mouse. MesP1 is known to be transiently expressed in the nascent mesoderm during gastrulation, and MesP1-expressing cells contribute to the cranial–cardiac mesoderm in the lateral plate mesoderm.14 We crossed MesP1-Cre and R26R mice and traced a mesodermal lineage during liver development (Fig. 8A). X-gal staining was found in the liver surface and mesenchymal cells (Fig. 8B). Immunofluorescence staining showed that desmin+ HSCs, α-SMA+ perivascular mesenchymal cells, and ALCAM+ submesothelial cells expressed lacZ (Fig. 8C–G). Some podoplanin+ liver (visceral) mesothelial cells coexpressed lacZ (Fig. 8H). Conversely, parietal mesothelial cells at the body wall were negative for lacZ (Fig. 8H). Vascular endothelial cells expressed lacZ (Fig. 8F, I). However, lacZ staining was rarely found in Flk1+ SEC (Fig. 8I). LacZ staining was also rare in CD45+ (Fig. 8J) or TER-119+ blood cells (data not shown). No lacZ expression was detected in hepatoblasts (Fig. 8K) or Kupffer cells (Fig. 8L). These staining patterns demonstrate that HSCs, submesothelial cells, and perivascular mesenchymal cells are derived from the MesP1-expressing mesoderm during development.
Fig. 8.
Mesoderm lineage analysis using MesP1-Cre and R26R mice. (A) A schema of the strategy for mesoderm lineage analysis using MesP1-Cre and R26R mice. Once MesP1 is expressed in the nascent mesoderm during gastrulation, the Cre recombinase excises the stop sequence between loxP sites (yellow triangles) in the R26R locus, and then, lacZ gene is constitutively expressed in the R26R locus of the cells derived from the nascent mesoderm. (B) X-gal staining of an E13.5 MesP1-Cre/R26R embryo. Blue staining is detected in the mesenchymal cells at the liver surface and in the liver parenchyma. (C–L) Double immunostaining of lacZ (red) and liver cell markers (green) including desmin (C–E), α-SMA (F), ALCAM (G), podoplanin (H), Flk1 (I), CD45 (J), albumin (K), and F4/80 (L). The left panels show merged images. The right two panels show magnified images of red and green fluorescence from the white rectangles in the left panel. (C–E) LacZ expression in desmin+ HSCs (C,D, arrows), submesothelial cells (D, arrowheads), and perivascular mesenchymal cells (E, arrows). Dotted lines demarcate the boundary between LLL and LML. (F) Arrows and arrowheads indicate LacZ+ α-SMA+ perivascular mesenchymal cells and LacZ+α-SMA− endothelial cells, respectively. (G) LacZ+ ALCAM+ submesothelial cells between liver lobes (arrowheads). (H) LacZ+ podoplanin+ mesothelial cells (arrowheads) in the ventral region. Double arrows indicate lacZ− podoplanin+ mesothelial cells. No lacZ expression in the podoplanin+ parietal mesothelial cells (arrows) in the ventral abdominal wall (VAW). (I) LacZ+Flk1+ endothelial cells in the blood vessel (arrowheads). An arrow indicates few Flk1+ SECs expressing lacZ weakly. (J) Arrows indicate rare lacZ+CD45+ cells. (K,L) No coexpression of lacZ and albumin or F4/80. Nuclei were counterstained with DAPI (blue). Bar, 50 µm (B) and 10 µm (C–L).
Discussion
The knowledge concerning fetal HSCs is limited, including their origin, cell lineage, and functions. To help address these questions, we isolated fetal liver mesenchymal cells and identified new markers. The current study has led to two novel findings: identification of submesothelial cells and mesodermal origin of liver mesenchymal cells in fetal liver.
We identified ALCAM, p75NTR, PDGFRα, and type IV collagen as novel markers for fetal liver mesenchymal cells. Based on the immunostaining with antibodies against these and other known markers and location, we defined at least three different populations of liver mesenchymal cells: HSCs (desmin+ p75NTR+ αSMA+/−), submesothelial cells (desmin+ p75NTR+ ALCAM+-PDGFRα+), and perivascular mesenchymal cells (desmin+ p75NTR+ αSMA+) (Fig. 6E). Near the large vessels, there are minor desmin+ α-SMA+ HSCs. The morphology and marker expression of HSCs and submesothelial cells are similar, but submesothelial cells are distinguishable based on the expression of ALCAM and PDGFRα and association with the basal lamina type IV collagen. A previous morphological study identified capsular fibroblasts beneath the mesothelial cells in the adult rat liver.20 Similar to these capsular fibroblasts, ALCAM+ submesothelial cells in fetal liver are associated with the basal lamina. Thus, submesothelial cells identified in the current study are likely to be the counterpart of the capsular fibroblasts in adult rat livers. Recently, submesothelial cells were also recognized in human developing livers by neural cell adhesion molecule (NCAM) immunostaining.21 In addition, it has recently been reported that liver fibroblasts isolated from human liver express ALCAM and support the survival of cultured hepatocytes.22 Although we did not test NCAM immunostaining or FACS because of the lack of optimal antibodies, we identified NCAM as a candidate marker for lacZ+ Ab− cells by microarray analysis. We also found that ALCAMhigh cells isolated from developing livers highly express Hgf and pleiotrophin, the two important mitogens for hepatocytes.23 Therefore, our findings are consistent with the proposal that submesothelial cells support hepatoblast proliferation in developing liver.
We identified podoplanin as a marker for mesothelial cells, which are recognized as epithelial cells derived from mesenchymal cells. Liver mesothelial cells are distinguishable from the submesothelial cells by their spatial relationship to basal lamina and expression of podoplanin. ALCAM expression was detected between submesothelial cells and mesothelial cells at the adjoining side of basal lamina. ALCAM is a member of the immunoglobulin superfamily and mediates homophilic and heterophilic (ALCAM/CD6) interactions. In metastatic melanoma, ALCAM regulates membrane type 1-matrix metalloproteinase expression and subsequently activates matrix metalloproteinase-2.24 Although it is speculative at this time, ALCAM may mediate migration of mesothelial cells and submesothelial cells via matrix metalloproteinase-2.
In chick embryos, mesothelial cells were shown to contribute to both HSCs and SECs.25 Ijpenberg et al.26 referred mesothelial cells and submesothelial cells as coelomic epithelium in mice, and assumed that the coelomic epithelium delaminates and gives rise to HSCs and SECs as proposed for chicken. In agreement with their notion, we frequently observed the transitional cells showing the submesothelial cell phenotype, but not associated with the basal lamina, appear to be migrating into the liver parenchyma. Ki-67 staining revealed that proliferative activity of submesothelial cells is higher than that of mesothelial cells or transitional cells. These results imply that submesothelial cells are precursor cells for the transitional cells. To further test this notion, we isolated ALCAM+ cells and examined their differentiation in culture. All of the ALCAMhigh cells cultured on a plastic dish expressed α-SMA, and half of the cells expressed both desmin and podoplanin, suggesting that both submesothelial cells and mesothelial cells acquire a myofibroblastic phenotype in vitro. Furthermore, we found that the ALCAMhigh population cultured in three-dimensional collagen gel stored lipid droplets on retinol treatment, indicating that ALCAMhigh cells acquire an HSC phenotype under this culture condition. Although these results suggest ALCAMhigh mesothelial or submesothelial cells as precursor cells for HSCs, further cell lineage studies are obviously necessary to test this proposal.
Suzuki et al. recently identified p75NTR as a marker for HSCs and perivascular mesenchymal cells in developing mouse livers, and p75NTR+ cells isolated from E11.5 livers were shown to induce α-SMA and Gfap in culture.27 In our study, p75NTR is also expressed by ALCAMhigh submesothelial cells, and ALCAMhigh cells cultured on plastic express α-SMA, but not Gfap. This difference in Gfap expression suggests that our ALCAMhigh cells are distinct from Suzuki’s cells and do not contain HSCs or perivascular mesenchymal cells that may express Gfap. In fact, we believe the p75NTR+ cells that Suzuki et al. isolated contained the three mesenchymal populations identified by the current study. Interestingly, cultured ALCAMhigh cells down-regulate “adult activated HSC markers,” including p75ntr, Pdgfrα, Hgf, and pleiotrophin, suggesting the unique feature of ALCAMhigh cells. The reason for this behavior is unknown but may be related to the undifferentiated state of ALCAMhigh cells.
Using MesP1-Cre and R26R mice, we presented the evidence that the three distinct populations of fetal liver mesenchymal cells shown in this study are derived from lateral plate mesoderm. Interestingly, body wall/parietal mesothelial cells were not derived from the same lineage, but some liver/visceral mesothelial cells were lacZ+ in MesP1Cre/R26R embryos. LacZ expression was also found in the endothelial cells in the large vessels, but rarely in Flk1+ SECs and CD45+ blood cells, suggesting different mesodermal lineages for SECs and vascular endothelial cells.
The 560-base-pair promoter fragment of the Msx2-lacZ mouse contains bone morphogenic protein–responsive and Tcf/Lef1–binding elements.13 These pathways might regulate heterogeneous expression of the lacZ in the liver lobes shown in Fig. 1C. Msx2 is known to control cell proliferation and differentiation. However, the role of Msx2 in liver mesenchymal cells is unclear. Msx2-null mice show no major defects in the liver development (unpublished data). It is known that Msx1 compensates the loss of Msx2 during mouse embryogenesis. Analysis of double mutant mouse for Msx2 and Msx1 will be necessary to clarify the role of Msx1/2 in liver mesenchymal cells.
In summary, this study has identified three populations of fetal liver mesenchymal cells that can all be traced to the MesP1-expressing mesoderm lineage. Our results suggest that ALCAM+ submesothelial cells are the precursor for HSCs in developing liver. Further characterization of submesothelial cells may contribute to new insights into mesenchyme–epithelial interactions in both liver development and regeneration.
Acknowledgement
Supported by the grants R24-AA12885 (Non-Parenchymal Liver Cell Core) and P50-AA11999 (Southern California Research Center for ALPD and Cirrhosis) from the National Institute on Alcohol Abuse and Alcoholism, and Medical Research Service of the Department of Veterans Affairs.
We thank Jiaohong Wang, Melissa Yang, and Yusuke Watanabe for helpful suggestions.
Abbreviations
- α-SMA
alpha-smooth muscle actin
- ALCAM
activated leukocyte cell adhesion molecule
- cDNA
complementary DNA
- DAPI
4′,6-diamidino-2-phenylindole
- E
embryonic day
- FACS
fluorescence-activated cell sorting
- FDG
fluorescein di-β-D-galactoside
- GFAP
glial fibrillary acidic protein
- HGF
hepatocyte growth factor
- HSC
hepatic stellate cell
- IRL
inferior right lobe
- LLL
left lateral lobe
- LML
left median lobe
- Msx2
msh-like 2
- NCAM
neural cell adhesion molecule
- p75NTR
p75 neurotrophin receptor
- PBS
phosphate-buffered saline
- PDGFRα
platelet derived growth factor receptor α
- qPCR
quantitative polymerase chain reaction
- RML
right median lobe
- SEC
sinusoidal endothelial cells
- SRL
superior right lobe
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21:311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- 3.Cassiman D, Barlow A, Vander Borght S, Libbrecht L, Pachnis V. Hepatic stellate cells do not derive from the neural crest. J Hepatol. 2006;44:1098–1104. doi: 10.1016/j.jhep.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Hazra S, Xiong S, Wang J, Rippe RA, Krishna V, Chatterjee K, et al. Peroxisome proliferator-activated receptor γ induces a phenotypic switch from activated to quiescent hepatic stellate cells. J Biol Chem. 2004;279:11392–11401. doi: 10.1074/jbc.M310284200. [DOI] [PubMed] [Google Scholar]
- 5.She H, Xiong S, Hazra S, Tsukamoto H. Adipogenic transcriptional regulation of hepatic stellate cells. J Biol Chem. 2005;280:4959–4967. doi: 10.1074/jbc.M410078200. [DOI] [PubMed] [Google Scholar]
- 6.MacDougald OA, Lane MD. Transcriptional regulation of gene expression during adipocyte differentiation. Annu Rev Biochem. 1995;64:345–373. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- 7.Enzan H, Himeno H, Hiroi M, Kiyoku H, Saibara T, Onishi S. Development of hepatic sinusoidal structure with special reference to the Ito cells. Microsc Res Tech. 1997;39:336–349. doi: 10.1002/(SICI)1097-0029(19971115)39:4<336::AID-JEMT4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Hentsch B, Lyons I, Li R, Hartley L, Lints TJ, Adams JM, et al. Hlx homeo box gene is essential for an inductive tissue interaction that drives expansion of embryonic liver and gut. Genes Dev. 1996;10:70–79. doi: 10.1101/gad.10.1.70. [DOI] [PubMed] [Google Scholar]
- 9.Kalinichenko VV, Bhattacharyya D, Zhou Y, Gusarova GA, Kim W, Shin B, et al. Foxf1+/− mice exhibit defective stellate cell activation and abnormal liver regeneration following CCl4 injury. Hepatology. 2003;37:107–117. doi: 10.1053/jhep.2003.50005. [DOI] [PubMed] [Google Scholar]
- 10.Wandzioch E, Kolterud Å, Jacobsson M, Friedman SL, Carlsson L. Lhx2−/− mice develop liver fibrosis. Proc Natl Acad Sci U S A. 2004;101:16549–16554. doi: 10.1073/pnas.0404678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogawa T, Tateno C, Asahina K, Fujii H, Kawada N, Obara M, et al. Identification of vitamin A-free cells in a stellate cell-enriched fraction of normal rat liver as myofibroblasts. Histochem Cell Biol. 2007;127:161–174. doi: 10.1007/s00418-006-0237-7. [DOI] [PubMed] [Google Scholar]
- 12.Kubota H, Yao HL, Reid LM. Identification and characterization of vitamin A-storing cells in fetal liver: implications for functional importance of hepatic stellate cells in liver development and hematopoiesis. Stem Cells. 2007;25:2339–2349. doi: 10.1634/stemcells.2006-0316. [DOI] [PubMed] [Google Scholar]
- 13.Brugger SM, Merrill AE, Torres-Vazquez J, Wu N, Ting MC, Cho JY, et al. A phylogenetically conserved cis-regulatory module in the Msx2 promoter is sufficient for BMP-dependent transcription in murine and Drosophila embryos. Development. 2004;131:5153–5165. doi: 10.1242/dev.01390. [DOI] [PubMed] [Google Scholar]
- 14.Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- 15.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 16.Nitou M, Sugiyama Y, Ishikawa K, Shiojiri N. Purification of fetal mouse hepatoblasts by magnetic beads coated with monoclonal anti-E-cadherin antibodies and their in vitro culture. Exp Cell Res. 2002;279:330–343. doi: 10.1006/excr.2002.5615. [DOI] [PubMed] [Google Scholar]
- 17.Berg T, Rountree CB, Lee L, Estrada J, Sala FG, Choe A, et al. Fibroblast growth factor 10 is critical for liver growth during embryogenesis and controls hepatoblast survival via β-catenin activation. Hepatology. 2007;46:1187–1197. doi: 10.1002/hep.21814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han YP, Zhou L, Wang J, Xiong S, Garner WL, French SW, et al. Essential role of matrix metalloproteinases in interleukin-1-induced myofibroblastic activation of hepatic stellate cell in collagen. J Biol Chem. 2004;279:4820–4828. doi: 10.1074/jbc.M310999200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogel S, Piantedosi R, Frank J, Lalazar A, Rockey DC, Friedman SL, et al. An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro. J Lipid Res. 2000;41:882–893. [PubMed] [Google Scholar]
- 20.Bhunchet E, Wake K. Role of mesenchymal cell populations in porcine serum-induced rat liver fibrosis. Hepatology. 1992;16:1452–1473. doi: 10.1002/hep.1840160623. [DOI] [PubMed] [Google Scholar]
- 21.Loo CKC, Wu XJ. Origin of stellate cells from submesothelial cells in a developing human liver. Liver Int. 2008;28:1437–1445. doi: 10.1111/j.1478-3231.2008.01788.x. [DOI] [PubMed] [Google Scholar]
- 22.Jodon de Villeroché V, Brouty-Boyé D. Establishment and characterization of atypical fibroblasts from human adult liver contributing to hepatocyte cord-like arrangement. Cell Biol Int. 2008;32:605–614. doi: 10.1016/j.cellbi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Asahina K, Sato H, Yamasaki C, Kataoka M, Shiokawa M, Katayama S, et al. Pleiotrophin/heparin-binding growth-associated molecule as a mitogen of rat hepatocytes and its role in regeneration and development of liver. Am J Pathol. 2002;160:2191–2205. doi: 10.1016/S0002-9440(10)61167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lunter PC, van Kilsdonk JW, van Beek H, Cornelissen IM, Bergers M, Willems PH, et al. Activated leukocyte cell adhesion molecule (ALCAM/CD166/MEMD), a novel actor in invasive growth, controls matrix metalloproteinase activity. Cancer Res. 2005;65:8801–8808. doi: 10.1158/0008-5472.CAN-05-0378. [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Pomares JM, Carmona R, González-Iriarte M, Macías D, Guadix JA, Muñoz-Chápuli R. Contribution of mesothelium-derived cells to liver sinusoids in avian embryos. Dev Dyn. 2004;229:465–474. doi: 10.1002/dvdy.10455. [DOI] [PubMed] [Google Scholar]
- 26.Ijpenberg A, Pérez-Pomares JM, Guadix JA, Carmona R, Portillo-Sánchez V, Macías D, et al. Wt1 and retinoic acid signaling are essential for stellate cell development and liver morphogenesis. Dev Biol. 2007;312:157–170. doi: 10.1016/j.ydbio.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki K, Tanaka M, Watanabe N, Saito S, Nonaka H, Miyajima A. p75 neurotrophin receptor is a marker for precursors of stellate cells and portal fibroblasts in mouse fetal liver. Gastroenterology. 2008;135:270–281. doi: 10.1053/j.gastro.2008.03.075. [DOI] [PubMed] [Google Scholar]