Abstract
Considerable functional heterogeneity within human natural killer (NK) cells has been revealed through the characterization of distinct NK-cell subsets. Accordingly, a small subset of CD56+NKp44+NK cells, termed NK-22 cells, was recently described within secondary lymphoid tissue (SLT) as IL-22− when resting, with a minor fraction of this population becoming IL-22+ when activated. Here we discover that the vast majority of stage 3 immature NK (iNK) cells in SLT constitutively and selectively express IL-22, a TH17 cytokine important for mucosal immunity, whereas earlier and later stages of NK developmental intermediates do not express IL-22. These iNK cells have a surface phenotype of CD34−CD117+CD161+CD94−, largely lack expression of NKp44 and CD56, and do not produce IFN-γ or possess cytolytic activity. In summary, stage 3 iNK cells are highly enriched for IL-22 and IL-26 messenger RNA, and IL-22 protein production, but do not express IL-17A or IL-17F.
Introduction
It is established that T- and B-cell developmental intermediates reside in the thymus and bone marrow (BM), respectively. Recent evidence shows human natural killer (NK) developmental intermediates are within secondary lymphoid tissue (SLT).1,2 We characterized 4 stages of NK-cell development in SLT by lineage commitment, response to cytokines, and NK-cell effector function.2 CD34+CD117− stage 1 pro-NK cells and CD34+CD117+ stage 2 pre-NK cells retain multipotency with T-cell and dendritic cell (DC) potential, whereas CD34−CD117+CD94− stage 3 immature NK (iNK) cells and CD34−CD117+/−CD94+ stage 4 NK cells are committed to the NK-cell lineage. Stage 3 iNK cells lack certain NK cell–surface receptors, capacity for IFN-γ production, and cytolytic activity, all characteristic of mature NK cells including the more differentiated stage 4 cells
Cella et al recently identified a population of SLT NK cells, termed NK-22, that is characterized by a CD3−NKp44+CD56+CCR6+ phenotype.3 NK-22 cells express little or no IL-22 mRNA at rest, yet less than 15% become IL-22+ upon activation. IL-22 is a TH17 cytokine that engages a receptor expressed by epithelial tissues resulting in production of antimicrobial peptides that promote the local innate immune response.4,5 Here we show that the majority of stage 3 resting human iNK cells found in SLT selectively express abundant transcripts for IL-22 and IL-26, and produce IL-22 protein without prior stimulation.
Methods
Isolation of human NK precursors from SLT
All procedures were approved by The Ohio State University (OSU) Institutional Review Board. NK developmental intermediates were isolated from fresh normal tonsil and analyzed as described by Freud et al.2 All populations were sorted to more than 99% purity.
cDNA preparation
RNA was purified from less than 5 × 105 NK cells using the Absolutely RNA Nanoprep Kit (Stratagene, La Jolla, CA), or using RNeasy (QIAGEN, Valencia, CA) if more than 5 × 105. cDNA was synthesized using the Moloney murine leukemia virus (MMLV) reverse transcriptase kit (Invitrogen, Frederick, MD) and random hexamers.
Real-time PCR
TaqMan primer/probe sets for IL-22, IL-26, IL-17A, and IL-17F were purchased from Applied Biosystems (Foster City, CA). Real-time polymerase chain reaction (PCR) was performed on an ABI Prism 7900HT, analyzed by the ΔΔCt method, and normalized to an 18S internal control.
Flow cytometric analyses
Total CD3−CD19−CD34− tonsillar mononuclear cells were isolated as previously described.2 All mAbs were purchased from BD Biosciences (San Jose, CA), except NKp44 APC, BDCA-2 APC, CD161 APC (Miltenyi Biotec, Auburn, CA); CD94 FITC (clone 131412; R&D Systems, Minneapolis, MN); and CD56 APC and NKp44 PE (Beckman Coulter, Hialeah, FL). Intracellular staining was performed immediately after CD34 depletion, and without a protein transport inhibitor, using the BD Cytofix/Cytoperm Plus Fixation/Permeabilization Kit and anti–IL-22-PE mAB (clone 142928; R&D Systems). Nonspecific staining was detected through the use of an appropriately labeled isotype control mAB in all analyses. Cytometric and data analyses were performed as previously described.1
Statistical analysis
Data were analyzed using a Student 2-tailed t test. A P value less than .05 was considered significant.
Results and discussion
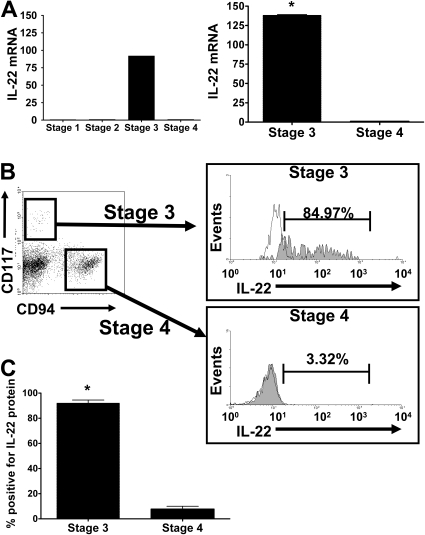
We quantified transcripts of IL-17A and IL-17F, IL-22, and IL-26 in purified human NK developmental intermediates from SLT. IL-17 mRNA was not detected in any of the 4 stages (not shown). IL-22 mRNA was detected only in resting stage 3 iNK cells in amounts that were more than 92-fold greater than trace quantities found in stages 1, 2, and 4 NK cells. In subsequent donors, IL-22 mRNA expression was 138-fold plus or minus 1.6-fold higher in stage 3 iNK cells compared with stage 4 (P = .001, n = 7; Figure 1A).
Figure 1.
IL-22 mRNA and protein expression during NK development. (A) (Left) Quantitative (Q) RT-PCR analysis of IL-22 expression was performed on fluorescence-activated cell sorting (FACS)–sorted NK stages 1 through 4 from human tonsil after pooling mRNA of each purified stage from 6 to 7 donors to achieve sufficient quantities for cDNA synthesis. Relative quantification was performed using the ΔΔCt method, and gene expression levels were normalized to 18S mRNA. Y-axis indicates fold increase over level of IL-22 mRNA quantified in stage 4 NK cells, arbitrarily normalized to 1. IL-22 was virtually absent from stages 1 and 2, so (right) subsequent RT-PCR measurements were performed using stage 3 iNK and stage 4 NK cells using cDNA from 7 individual donors. The average fold change in IL-22 mRNA present in stage 3 iNK cells compared with stage 4 NK cells is approximately 138. Error bars represent standard error of the mean from n = 7 donors. *P = .001. (B) IL-22 intracellular protein expression during NK development. Total CD3−CD19−CD34− tonsillar mononuclear cells were stained for surface expression of lineage markers, CD117 and CD94, followed by assessment for intracellular expression of IL-22 protein. Lin−CD117+CD94− identify stage 3 iNK cells that are then stained for intracellular expression of IL-22 (shaded) as shown in this representative donor, compared with isotype control (clear). Lin−CD117−CD94+ identify stage 4 NK cells that are then stained for intracellular expression of IL-22 (shaded) as shown in this representative donor, compared with isotype control (clear). (C) The average proportion of IL-22+ stage 3 iNK cells versus stage 4 NK cells in all donors examined (n = 6). Error bars represent standard error of the mean. *P = .001.
We measured IL-17A, IL-17F, and IL-26 mRNA in stage 3 and stage 4 NK cells from individual donor SLT. Again, no IL-17A or IL-17F was detected in more than 10 donors (not shown). IL-26 mRNA was expressed in resting stage 3 iNK cells from 5 of 7 donors, and absent or barely detectable in stage 4 NK cells from all 7 donors. When present in both stages, IL-26 transcript was 34.8-fold plus or minus 1.6-fold higher in stage 3 iNK cells compared with stage 4 cells (P = .002, n = 5; data not shown).
We detected IL-22 protein in stage 3 resting iNK cells using intracellular staining with an anti–IL-22 antibody. The majority of CD117+CD94− stage 3 iNK cells, a population previously described in our laboratory as NKp46−2B4+,2 coexpresses intracellular IL-22, whereas staining is very low in the CD117+/−CD94+ stage 4 population (Figure 1B). Indeed, 88.0% plus or minus 5.3% of stage 3 iNK cells were IL-22+ compared with 6.7% plus or minus 3.5% of stage 4 NK cells (Figure 1C; P < .001, n = 6;). We used an enzyme-linked immunosorbent assay (ELISA) to confirm that resting and IL-15–stimulated stage 3 iNK cells, but not stage 4 NK cells, secrete IL-22 protein (not shown). Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) documents the localization of these cells to the lamina propria and parafollicular region of the tonsil.
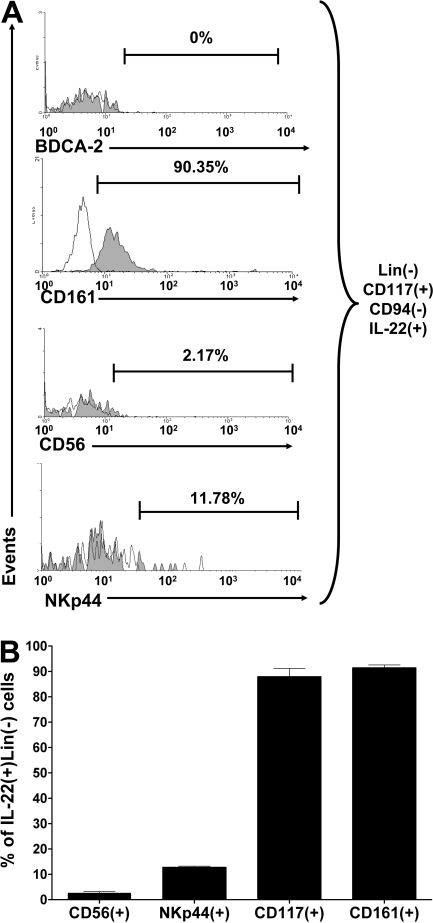
We found that most CD117+CD94−IL-22+ cells express CD161, but do not express BDCA-2, ruling out inclusion of NK dendritic cells6 (Figure 2A). Less than 3% of stage 3 iNK cells express CD56 and less than 15% of stage 3 iNK cells express NKp44, 2 markers associated with the inducible NK-22 phenotype as previously described.3 The average proportion of IL-22+ cells expressing CD56, NKp44, CD117, or CD161 from 4 donors is summarized in Figure 2B. Finally, in quantitative data not shown, we ascertained that stage 2 and stage 3 NK-cell intermediates selectively expressed RORC mRNA, the gene required for lymph node formation and TH17 differentiation.7,8
Figure 2.
Surface phenotype of IL-22+ stage 3 iNK cells. Total CD3−CD19−CD34− resting tonsillar mononuclear cells were stained for surface expression of lineage markers, CD117, CD94, followed by intracellular expression of IL-22, and events were gated on total Lin−CD117+CD94−IL-22+ stage 3 iNK cells. (A) Representative histograms show expression for each indicated surface marker (shaded) in a donor, compared with isotype control (clear). (B) Graphic summary of the mean proportion of IL-22+ stage 3 iNK cells expressing various surface markers from all (n = 4) donors is summarized. Error bars represent standard error of the mean.
Here we show that the vast majority of stage 3 iNK cells in SLT display robust constitutive IL-22 expression ex vivo. These findings are distinct from the earlier identification of NKp44+CD56+ cells in SLT that express little or no IL-22 at baseline, yet less than 10% of these cells express IL-22, IL-26, and leukemia inhibitory factor (LIF) upon in vitro culture with various cytokines or activated monocytes.3 The surface phenotype of NK-22 cells in our study includes a small percentage of CD56+NKp44+ cells, but these markers are neither necessary nor sufficient to unambiguously identify resting IL-22+ cells within human SLT. Rather, the surface phenotype of stage 3 iNK cells, CD34−CD117+CD161+CD94−, specifically identifies the NK-22 subset in SLT.
The finding that IL-22 transcript is absent from stages 1, 2, and 4 during NK development raises 2 possibilities: (1) IL-22 expression must be lost prior to differentiation to stage 4; or (2) the NK-22 subset represents a separate, terminally differentiated NK-cell population. In support of the latter, less than 10% of stage 3 iNK cells differentiate to the IFN-γ–producing stage 4 NK cell,2 which may reflect iNK-cell heterogeneity.
The abundant intracellular IL-22+ staining in stage 3 iNK cells isolated from human tonsil is consistent with in vivo exposure to a cellular milieu within SLT that is permissive for IL-22 production. Factors previously implicated in TH17 production and IL-22 synthesis by T cells include RORC and inflammatory cytokines such as IL-6, IL-23, and IL-1β, as well as Toll-like receptor (TLR) activation on DCs and monocyte/macrophages. Whether this selective population of iNK cells that constitutively produces IL-22 in SLT has a role in NK development, lymph node development, or mucosal immunity is unknown. It will be insightful to determine the contribution of TH17 polarizing cytokines and TLR activated cells to the differentiation of IL-22+ stage 3 iNK cells, as well as the effects of iNK cell IL-22 on surrounding cells within SLT.
Supplementary Material
Acknowledgments
We acknowledge the Tissue Procurement Shared Resource of The OSU Comprehensive Cancer Center, the Nucleic Acid Shared Resource, and the Flow Cytometry Shared Resource for provision of fresh human tonsil. Immunohistochemistry reagents were kindly provided by Ventan Medical Systems (Tucson, AZ) with thanks to Dr Christopher Roberts and Kathleen Sergott.
Note added in proof.
In an elegant study published online at the time of our submission, Cupedo et al9 reported IL-22 mRNA expression in human stage 3 iNK cells isolated from postnatal tonsil.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.H. performed the majority of experiments and contributed to the written paper; B.B. contributed to conceptual design, oversaw experimental design, and contributed to the written paper; S.M., E.B., H.M., and G.N. performed some of the experiments; X.Z. contributed to the statistical analysis; A.G.F. contributed to the ideas put forth in the paper and assisted with experiments; J.Y. contributed to experimental design, experimental work, and writing and editing of the paper; and M.A.C. contributed to conceptual idea for the paper, experimental design, and writing and editing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael A. Caligiuri, 521B James Cancer Hospital, 300 W 10th Ave, Columbus, OH 43210; e-mail: michael.caligiuri@osumc.edu.
References
- 1.Freud AG, Becknell B, Roychowdhury S, et al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Freud AG, Yokohama A, Becknell B, et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203:1033–1043. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cella M, Fuchs A, Vermi W, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Dzionek A, Sohma Y, Nagafune J, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Z, Unutmaz D, Zou YR, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 8.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Cupedo T, Crellin NK, Papazian N, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC(+) CD127(+) natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.