Abstract
Adult T-cell leukemia/lymphoma (ATL) is an aggressive and fatal disease. We have examined 32 patients with smoldering, chronic, lymphoma and acute leukemia using Affymetrix HG-U133A2.0 arrays. Using the BRB array program, we identified genes differentially expressed in leukemia cells compared with normal lymphocytes. Several unique genes were identified that were overexpressed in leukemic cells, including TNFSF11, RGS13, MAFb, CSPG2, C/EBP-α, and TCF4; 200 of the most highly overexpressed ATL genes were analyzed by the Pathway Studio, version 4.0 program. ATL leukemia cells were characterized by an increase in genes linked to “central” genes CDC2/cyclin B1, SYK/LYN, proliferating cell nuclear antigen, and BIRC5. Because of its potential therapeutic importance, we focused our studies on the regulation and function of BIRC5, whose expression was increased in 13 of 14 leukemia samples. TCF4 reporter assays and transfection of DN-TCF4 demonstrated that TCF4 regulates BIRC5 gene expression. Functionally, transfection of ATL cells with BIRC5 shRNA decreased BIRC5 expression and cell viability 80%. Clinical treatment of ATL patients with Zenapax or bortezomib decreased BIRC5 expression and cell viability. These experiments represent the first direct experimental evidence that BIRC5 plays an important role in ATL cell viability and provides important insight into ATL genesis and potential targeted therapies.
Introduction
Human T-lymphotropic virus type 1 (HTLV-1) is a complex delta-retrovirus that infects 15 to 20 million people worldwide.1 HTLV-1 is associated with 2 diseases: adult T-cell leukemia (ATL) and the neurodegenerative disease tropical spastic paraparesis/HTLV-1–associated myelopathy.2–4 HTLV-1 is endemic in areas of southern Japan, the Caribbean basin, intertropical Africa, the Middle East, South America, and Papua New Guinea.5 HTLV-1 is transmitted primarily through breastfeeding (mother-to-infant), sexual transmission, and blood transfusion
Although the majority of HTLV-1–infected people are asymptomatic carriers, approximately 2% to 5% will develop ATL.6 In most cases, the disease develops over a 40- to 70-year period. This suggests that, as with other human cancers, genetic and epigenetic changes must occur for the development of ATL.7 Because of the diverse clinical features of ATL, it has been subclassified as smoldering, chronic, lymphoma and acute.
Although several therapeutic approaches have been used, ATL continues to carry a very poor prognosis. Resulting in part from overexpression of the multidrug resistance gene and p53 inactivation,8,9 ATL is often resistant to chemotherapy with median survival time of 6.2, 10.2, and 24.3 months for the acute, lymphoma, and chronic subtypes, respectively.10 Cyclophosphamide, doxorubicin, viscristine, and prednisolone are usually the first-line therapy for ATL. Although several reports indicate that approximately 60% of patients exhibit a partial or complete remission,11–13 overall survival of patients on various chemotherapeutic regimens remains poor, with an estimated survival ranging from 5 to 13 months. Treatment of patients with antiretrovirals and interferon-α can result in a partial, but dramatic, clinical response, albeit short-lived.14
Targeting cell differentiation markers on malignant cells using monoclonal antibodies have been used as an alternative approach. Because of the high level of interleukin-2α (IL-2α) receptor (CD25) expression on ATL cells, Waldmann et al were the first to treat patients with anti-CD25 (anti-Tac) monoclonal antibodies and achieved a response in 3 patients lasting up to 8 months in one study and 2 complete remissions and 4 partial remissions in 19 patients in a follow-up study.15,16 Yttruim90 labeling of the antibody and development of a humanized version of anti-CD25 (Zenapax, daclizumab) showed a slight improvement in ATL treatment.17 Waldmann's group has used a humanized monoclonal anti-CD52 (Campath-1H, alemtuzumab) antibody, which showed efficacy in a nonobese diabetic/severe combined immunodeficiency animal model for ATL.18 To date, reports on 2 patients treated with Campath-1H suggest that the monoclonal antibody alone or in combination with pentostatin reduces viral load and restored normal cell counts.19,20 Further studies are necessary to assess the use of anti–Campath-1H for ATL therapy. The poor prognosis and frequent relapse of patients highlight the need for development of new approaches and novel therapeutic targets for the treatment of ATL.
With the advent of global gene expression analysis, DNA microarray technology has allowed researchers to develop expression profiles that can identify and classify discrete subsets of disease, predict disease outcome, or predict disease response to therapy. Expression profiles of HTLV-1–infected cells and Tax-expressing cells grown in vitro have provided a list of HTLV-1/Tax-regulated genes.21–24 Many of these genes are involved in regulation of T-cell proliferation, such as IL-2, IL-2Rα (allowing for an autocrine loop), IL-15, IL-15R, granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor-α (TNF-α).1,2,25 As noted, however, the oncogenic features of HTLV-1–infected cells versus malignant cells from ATL patients have distinct phenotypes.1,26 This underscores the importance of examining the gene expression profiles from ATL patient samples.
We have examined the gene expression profile of uncultured lymphocytes from 32 ATL patients who included smoldering, chronic, lymphoma, acute leukemia, and drug-treated patients using Affymetrix arrays containing 14 500 genes. This study is the most comprehensive to date of ATL patients.27,28 Leukemia cells were characterized by a striking increase in genes linked to the cell cycle (CDC2, cyclin B), hypercalcemia (RANKL, PTHLH), tyrosine kinase signaling (SYK, LYN) pathways, and antiapoptosis (BIRC5) factors.
In view of the antiapoptotic function of BIRC5 (survivin) and its importance in other human cancers, we focused our initial analysis of the complex microarray data on this protein. Our rationale was that, if overexpression of BIRC5 in ATL cells could be linked to cell viability, there are several clinically targeted BIRC5 therapies that could be tested for the treatment of ATL. In addition to showing that BIRC5 gene expression is linked to TCF4 transcription activity, the importance of BIRC5 in ATL cell viability was demonstrated using shRNA. Introduction of BIRC5 shRNA into ATL cells specifically down-regulated BIRC5 expression and decreased ATL cell viability. Our studies also provide the first analysis of BIRC5 gene expression in uncultured ATL patient cells after clinical treatment. Acute ATL patients were treated with either Zenapax or bortezomib, and gene expression profiles were examined before and after treatment. As the level of circulating ATL cells decreased because of cell death, a decrease in BIRC5 gene expression was observed. Our findings support BIRC5 and TCF4 as biomarkers for ATL cells and BIRC5 as a rational clinical target in the treatment of ATL.
Methods
Cells and transfection
Diagnosis of ATL was confirmed by clinical features, immunophenotypes, anti–HTLV-1 antibody, viral load, and monoclonal proviral integration. Peripheral blood lymphocyte (PBL) samples were from National Cancer Institute Institutional Review Board–approved studies. Informed consent was obtained from all subjects, in accordance with the Declaration of Helsinki. Peripheral blood or leukocyte packs were obtained from ATL patients by leukopheresis and purified over Ficoll Paque (GE Healthcare, Little Chalfont, United Kingdom) to obtain peripheral blood mononuclear cells (PBMCs). Surface marker expression for CD4, CD25, and CD8 was determined by flow cytometric analysis of patient PBLs. PBMCs were obtained from 6 healthy volunteers. Purified CD4+ cells were obtained through positive and negative selection using CD4+ selection following the manufacturer's instructions (Dynal, Oslo, Norway). HTLV-1 cell lines MT2 and C8166 used in the bortezomib (PS-341) studies were cultured as described previously.29 Cells from ATL patients 15 and 46 were cultured ex vivo in RPMI medium containing 20% fetal bovine serum, 2 mM l-glutamine, and 50 U interleukin 2. Transfection of shRNA (Superarray Bioscience, Frederick, MD) or plasmid constructs (DN-TCF4, Top [pGL3-OT], and Fop [pGL3-OF] kindly provided by Bert Vogelstein, Ludwig Center for Cancer Genetics and Therapeutics, and The Howard Hughes Medical Institute at The Johns Hopkins Kimmel Cancer Center, Baltimore, MD) was performed using Amaxa Nucleofector as described by the manufacturer (Amaxa Biosystems, Gaithersburg, MD). Top and Fop constructs contain 3 copies of the TCF site wild-type (5′-AGATCAAAGG-3′) or mutant (5′-AGGCCAAAGG-3′), respectively, upstream of the c-fos promoter driving luciferase gene expression. Cell viability was measured using CellTiter-Glo (Promega, Madison, WI). Western blot analysis was performed on 80 μg whole-cell lysate with antibodies to BIRC5 (Abcam, Cambridge, MA) and actin (Sigma-Aldrich, St Louis, MO).
RNA and probe synthesis
Total RNA was isolated from lymphocytes using RNA-Bee reagent following the manufacturer's instructions (Tel-Test, Friendswood, TX) followed by purification using RNeasy Mini-columns (QIAGEN, Valencia, CA). cRNA probes were prepared using the Affymetrix protocol and hybridized to HG-U133A2.0 GeneChip oligonucleotide arrays (Affymetrix, Santa Clara, CA). Analysis was performed with GeneChip Operating Software (MAS5.0). After identifying genes increased or decreased by at least 2-fold for all ATL samples compared with CD4+ controls, the gene list was analyzed using Pathway Studio, version 4.0 software (Ariadne Genomics, Rockville, MD). Semiquantitative reverse transcription polymerase chain reaction (RT-PCR) was performed to validate RNA expression. One microgram RNA was reverse transcribed to cDNA as described by the manufacturer (Ambion, Austin, TX). Real-time qPCR was then performed on a Stratagene Mx3000P system using primers specific for BIRC5: 5′-GAGGAGACAGAATAGAGTGATAG-3′; 5′-GAGCTGCTGCCTCCAAAGAA-3′ and GAPDH: 5′-GCCAGTGGACTCCACGAC-3′; 5′-CAACTACATGGTTTACATGTTC-3′ (Stratagene, La Jolla, CA). PCR was performed at multiple cycles to maintain the amplification in a linear range.
MTT assay
The methyl-thiazol-tetrazolium (MTT) assay was performed following the manufacturer's instructions (Chemicon International, Temecula, CA). Briefly, cells were plated in duplicate in a 24-well plate in RPMI media containing 0, 3, 5, 10, or 20 nM bortezomib or 2% Triton X-100 as a positive control. After 24 or 48 hours, the cells were suspended and 0.1 mL was transferred in triplicate to a microtiter plate for MTT analysis. Measurements were taken on an enzyme-linked immunosorbent assay plate reader at a wavelength of 570 nm and a reference wavelength of 630 nm.
Apoptosis cDNA array
Cells were treated with 0 nM or 10 nM bortezomib for 48 hours. Total RNA was harvested and the protocol for cDNA GEArray was followed as outlined by the manufacturer (Superarray Bioscience). Expression was quantified using FluorChem (Imgen Technologies, Alexandria, VA).
All microarray data have been deposited with Gene Expression Omnibus under accession number GSE14317.30
Results
Clinical characteristics of ATL patients used in this study
Thirty-two HTLV-1–associated ATL patients with smoldering, chronic, lymphoma and acute leukemia were analyzed (Table 1). Also included were 2 HTLV-1–seropositive patients (ATL9 and ATL17) with no evidence of disease, 5 patients (ATL2, ATL3, ATL10, ATL11, and ATL21) treated with Zenapax, and 1 lymphoma patient (ATL8) with transformation to acute leukemia treated with bortezomib. Diagnosis of ATL was made on the basis of clinical features, hematologic characteristics, flow cytometric analysis (CD3 dim, CD25 bright, CD7 negative), serum antibodies against HTLV-1 antigens, elevated levels of soluble IL-2Rα, and detection of the HTLV-1 proviral genome in leukemia cells by Southern blot hybridization or PCR analysis.
Table 1.
Clinical information on ATL patients
| Case | Race | Sex/age, y | Subtype at diagnosis | Subtype at sampling | WBC, cells/μL | CD4+CD25+, cells/μL | CD4+7G7+, cells/μL | TCRγ PCR | LDH, U/L | sIL2R, U/mL | Ca, mol/L | Therapy at sampling |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATL1 | Afro-Caribbean | M/48 | Leukemia | Leukemia | 40 500 | 31 646 | 31 290 | Pos | 897 | 44 196 | 2.76 | RIT |
| ATL2 | Hispanic | M/41 | Leukemia | Leukemia | 37 300 | 29 780 | 33 200 | Pos | 202 | 6741 | 2.15 | Zenapax |
| ATL2a | 25 300 | 24 | 12 472 | ND | 199 | 7266 | 2.18 | |||||
| ATL3 | Afro-Caribbean | M/53 | Leukemia | Leukemia | 39 700 | 30 211 | 29 782 | Pos | 288 | ND | 2.23 | Zenapax |
| ATL3a | 40 000 | 272 | 17 390* | ND | 277 | ND | 2.39 | |||||
| ATL4 | African American | F/49 | Smoldering | Smoldering | 19 000 | 331 | 330 | Pos | 1180 | WNL | 2.27 | None |
| ATL5 | Afro-Caribbean | F/50 | Lymphoma | Lymphoma | 2740 | 293 | 302 | Pos | 634 | 2695 | 3.22 | RIT |
| ATL5a | Leukemia | 49 300 | 40 640 | 40 270 | Pos | 340 | 1627 | 2.47 | CHOP | |||
| ATL6 | Afro-Caribbean | F/48 | Lymphoma | Lymphoma | 3070 | 303 | 268 | Pos | 390 | 10 856 | 2.76 | CHOP |
| ATL7 | Afro-Caribbean | F/49 | Lymphoma | Lymphoma | 3440 | 90 | 101 | Pos | 388 | 6329 | 2.50 | RIT |
| ATL8 | Afro-Caribbean | M/35 | Lymphoma | Lymphoma | 4450 | 897 | 835 | Pos | 128 | WNL | 2.33 | None |
| ATL8a | Leukemia | 67 500 | 39 393 | ND | ND | 1694 | ND | 2.81 | Steroids | |||
| ATL8b | Leukemia | 66 900 | ND | ND | ND | 1734 | ND | 2.24 | Steroids/Velcade | |||
| ATL8c | Leukemia | 27 700 | ND | ND | ND | 865 | ND | 2.06 | Steroids/Velcade | |||
| ATL9 | White | M/19 | Leukemia | In remission | 5680 | 1 | 44 | ND | 117 | ND | 2.74† | Zenapax |
| ATL10 | White | M/19 | Smoldering | Smoldering | 8650 | 793 | 701 | ND | 154 | ND | 2.32 | None |
| ATL11 | Afro-Caribbean | M/49 | Chronic | Chronic | 7310 | 4622 | 4551 | Pos | 149 | 2 500 | 2.38 | None |
| ATL12 | Afro-Caribbean | M/41 | Lymphoma | Leukemia | 10 500 | 4958 | 4858 | Pos | 280 | 11 773 | 2.34 | CVP |
| ATL13 | Afro-Caribbean | F/45 | Chronic | Chronic | 5700 | 230 | 159 | Pos | 216 | 1039 | 2.47 | CHOP |
| ATL14 | Afro-Caribbean | M/34 | Leukemia | Leukemia | 90 600 | 84 715 | 84 976 | Pos | 1528 | 51 846 | 3.45 | None |
| ATL15 | African American | F/57 | Leukemia | Leukemia | 62 100 | 46 548 | 46 980 | Pos | 633 | 28 802 | 2.34 | None |
| ATL16 | Afro-Caribbean | M/51 | Lymphoma | Lymphoma | 6230 | 111 | ND | Pos | 450 | ND | 2.11 | None |
| ATL17 | African American | M/78 | Leukemia | In remission | 3770 | 422 | 321 | Neg | 195 | WNL | 2.38 | None |
| ATL18 | Afro-Caribbean | M/52 | Lymphoma | Leukemia | 31 500 | 3722 | 3838 | Pos | 1073 | ND | 3.06 | CHOP |
| ATL19 | Afro-Caribbean | F/34 | Leukemia | Leukemia | 105 000 | 96 269‡ | ND | Pos | 3920 | 30 500 | 2.72 | None |
| ATL20 | Asian | F/62 | Leukemia | Leukemia | 62 700 | 49 056 | 47 652 | Pos | 693 | 23 580 | 2.21 | None |
| ATL21 | Afro-Caribbean | F/79 | Smoldering | Smoldering | 3720 | 158 | 139 | Ind | 234 | WNL | 2.33 | Radiation |
| ATL22 | Afro-Caribbean | F/41 | HTLV1 carrier | HTLV1 carrier | 4040 | 402 | 302 | Neg | 196 | 1455 | 2.27 | None |
| ATL24 | Afro-Caribbean | F/36 | Lymphoma | Lymphoma | 6680 | 1486 | 1486 | Pos | 376 | WNL | 2.53 | CHOP |
| ATL25 | Afro-Caribbean | F/77 | Smoldering | Lymphoma | 4630 | 9 | 277 | ND | 594 | 1500 | 2.52 | Zenapax |
| ATL26 | Afro-Caribbean | F/53 | Lymphoma | Lymphoma | 7540 | 1343 | 1299 | Pos | 622 | 99 000 | 2.91 | None |
| ATL29 | Afro-Caribbean | F/49 | Lymphoma | Lymphoma | 4580 | 678 | ND | Pos | 955 | 29 800 | 2.90 | CHOP |
| ATL30 | Afro-Caribbean | M/33 | Lymphoma | Lymphoma | 10 200 | 772 | 748 | Pos | 6030 | 4880 | 3.88 | CHOP |
| ATL31 | Afro-Caribbean | M/45 | Leukemia | Leukemia | 271 000 | 249 510 | ND | Pos | 2633 | 52 935 | 2.72 | None |
| ATL32 | Afro-Caribbean | M/54 | Leukemia | Leukemia | 34 400 | 26 595 | 26 075 | Pos | 1437 | 18 000 | 4.09 | None |
| ATL33 | Afro-Caribbean | F/55 | Lymphoma | Lymphoma | 5990 | 1004 | 914 | Pos | 722 | 4470 | 2.85 | None |
| ATL34 | Asian | F/58 | Leukemia | Leukemia | 29 900 | 27 159 | 26 394 | Pos | 379 | 5530 | 2.31 | CHOP |
| ATL35 | White | F/39 | Lymphoma | Lymphoma | 6110 | 103 | 73 | ND | 965 | 10 630 | 3.70 | Steroids |
The majority of cases of patients described in the table are Afro-Caribbean. Three patients were white: 1 Romanian female and identical twins infected by a shared blood transfusion in the neonatal period. Subtype at diagnosis is the ATL disease subtype at initial diagnosis. Disease subtype changed over time in some individuals. Serum LDH, sIL2R, and corrected calcium values are the peak values measured within 30 days of sample collection. WBC, calcium, and LDH values outside the normal range are in bold (normal ranges: WBC, 3300-9600 cells/μL; LDH, 113-226 U/L; Ca, 2.05-2.50 mmol/L).
WBC indicates white blood cell count; TCRγ PCR, polymerase chain reaction for T-cell receptor gamma gene rearrangement; LDH, lactate dehydrogenase; sIL2R, serum soluble interleukin-2 receptor; Ca, calcium; M, male; F, female; ND, not done; WNL, within normal limits; Pos, positive; Neg, negative; Ind, indeterminate; RIT, radioimmunotherapy; CHOP; cyclophosphamide, doxorubicin, vincristine, and prednisone; CVP, cyclophosphamide, vincristine, and prednisone; and Zenapax, humanized monoclonal antibody to the IL-2Rα, also known as daclizumab.
CD4+/7G7+ counts on February 25, 2002, 6 weeks after Zenapax treatment. All other numbers in line and the Affymetrix sample were February 27, 2003.
This patient has coexistent sarcoidosis.
This value is CD4+-only cells.
Dendrogram analysis of ATL samples shows tight linkage of acute ATL patients
Because of dramatic changes in gene expression patterns of ATL cells after in vitro stimulation and tissue culture,31 we chose to analyze gene expression patterns in uncultured and unstimulated cells. Immediately after apheresis or collection of the blood sample, PBLs were isolated and RNA extracted. After the generation of cRNA, samples were hybridized to Affymetrix HG-U133A2.0 arrays and expression patterns analyzed using the BRB-Array Tools program (Biometric Research Branch, National Cancer Institute). The gene expression pattern of control and ATL samples, analyzed by unsupervised dendrogram analysis, revealed distinct clustering of leukemia, lymphoma, and control samples (Figure 1). The tightest clustering among the ATL patients was seen in the leukemia group (Figure 1 solid green), which branched from the control group (Figure 1 red), indicating that gene expression patterns clearly distinguish ATL patient lymphocytes from normal control lymphocytes. Lymphoma samples (Figure 1 brown) demonstrated the greatest degree of heterogeneity with patients ATL5, 6, 7, and 8 forming one expression cluster and ATL25, 28, 29, 30, and 33 forming a second distinct cluster. Strikingly, 3 other lymphoma patient samples (ATL5a, ATL8a, and ATL12), which had undergone transformation to acute leukemia, clustered with the leukemia samples. Using the BRB-Array Tools program, a class prediction was performed on the smoldering/chronic/carrier, lymphoma, leukemia, and control groups. By 1-nearest neighbor, 3-nearest neighbor, or newest centroid analysis, the clinical presentation of the patient samples can be predicted with 80% confidence based on the expression pattern of 9684 genes that pass the filter (P = .001).
Figure 1.
Unsupervised hierarchical clustering of ATL and CD4+ samples was completed using a 1-correlation metric with centroid linkage. Control, CD4+ samples (orange), and leukemia (green) samples formed distinct groups. Whereas lymphoma samples (brown) formed a distinct group, smoldering (blue), chronic (purple), and carrier (white) samples were more diverse.
Gene expression patterns observed in acute leukemia versus CD4+ lymphocytes
The tight clustering and homogeneity of the cell populations for leukemia samples prompted us to focus our initial analysis on expression patterns of leukemia versus CD4+ lymphocyte controls. Of the 14 500 genes assayed, 1232 known genes were identified that were elevated an average of 4-fold or greater (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Quantitative RT-PCR studies on selected genes confirmed expression changes indicated by Affymetrix analysis (Figure S1). To note a few of the more prominent genes that have been previously reported to be up-regulated in ATL or Tax-expressing cells, we observed high levels of expression of IL2Rα, proliferating cell nuclear antigen, CD3, and major histocompatibility complex class II genes (Table S2).
Our analysis also identified several unique or singly referenced genes that were overexpressed in the leukemia cells. For example, TNFSF11 (RANKL) is increased in the leukemia cells. Although there is variation in the absolute levels of TNFSF11 expression in individual ATL samples, 13 of 14 leukemia patients showed significantly elevated expression over controls (data not shown). This observation is of particular interest because one report suggests that RANKL mediates the hypercalcemic effect of MIP-1α in ATL.32 In agreement with deregulation of genes involved in hypercalcemia, a significant increase in PTHLH expression, a gene known to play a role in hypercalcemia was observed in 11 of 16 leukemia samples.
We also note that RGS13 was overexpressed approximately 75-fold in the leukemia cells (Table S1). Analysis of individual patients indicated that RGS13 was significantly overexpressed in 14 of 14 leukemia samples tested (Figure S1). This gene is of interest because it regulates signaling of chemokine receptors and significantly impacts lymphocyte migration and function.33 Because RGS proteins enhance deactivation of G-protein α subunits, they reduce activation of downstream effectors, probably rendering the leukemia cells unresponsive to chemokine signals.
Oncogene MAFb was also significantly overexpressed in the ATL leukemia cells. Although overexpression of this cellular oncogene has been reported in peripheral T-cell lymphoma–not otherwise specified and large B-cell lymphoma, the gene has not been previously related to ATL. MAFb expression was also elevated in ATL lymphoma patient cells that had undergone transformation to leukemia.
Chondroitin sulfate proteoglycan (CSPG2) expression was elevated in leukemia cells. CSPG2 has been reported to be elevated in adenomas and carcinomas and may play an important role in cancer cell migration and proliferation.34–37 Interestingly, CSPG2 is positively regulated by p53, which has been shown to be inhibited in ATL cells, and by β-catenin, which is increased in ATL cells38–40 (data not shown). It will be of interest to determine what is regulating CSPG2 promoter function in the ATL cells.
Our studies further suggest that transcription factors C/EBP-α and TCF4 were overexpressed in ATL leukemia cells. C/EBP-α is known to regulate the balance between cell proliferation and differentiation during early hematopoietic development and myeloid differentiation. In AML, C/EBP-α is down-regulated or mutated and has been reported to be a myeloid cell tumor suppressor. In contrast, C/EBP-α is activated in precursor B-cell acute lymphoblastic leukemia by rearrangement with the immunoglobulin enhancer. The observed overexpression in ATL cells suggests that C/EBP-α may have oncogenic as well as tumor suppressor activities.
Transcription factor TCF4 was overexpressed on average greater than 10-fold in leukemia cells. Interaction of TCF4 with β-catenin, which is also overexpressed in ATL cells, results in the import of the heterodimer to the nucleus of the cell.41 Among the promoters potentially regulated by β-catenin/TCF4, we observed that the survival gene BIRC5/survivin was significantly overexpressed in ATL cells..
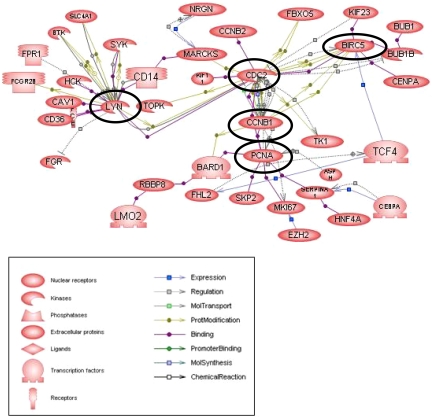
Analysis of increased ATL genes by Pathway Studio
In addition to the classic approach of subdividing genes into functional groups to assess involvement in ATL pathogenesis (Table S2), we analyzed 200 of the most highly overexpressed ATL genes by the Pathway Studio, version 4.0 program, which uses curated and RESNET databases to identify interactions among genes to build pathways, gene regulation networks, and protein interaction maps. Analysis of ATL overexpressed genes generated the network presented in Figure 2. Interestingly, genes, such as CDC2, CCNB1, BIRC5, and LYN, are “central” in linking activity of peripheral genes in the biologic networks. Not surprisingly, these genes are known to play prominent roles in proliferation, cell-cycle regulation, and antiapoptosis. For example, expression of CDC2 is required for entry into S-phase and mitosis. Cyclin B1 (CCNB1) couples with CDC2 to control cell-cycle progression at the G2/M (mitosis) transition. Tyrosine kinases SYK and LYN may have significance in IL-2R signaling in ATL cells. Although the cytoplasmic regions of the IL-2Rβ and γ chains lack an intrinsic protein tyrosine kinase, these chains recruit various non–receptor-type tyrosine kinases, including SYK.39,42 SYK was up-regulated 2-fold or greater in 12 of 14 and LYN in 10 of 14 leukemia patient samples.
Figure 2.
Schematic of molecular interactions using Pathway Studio, version 4.0, for genes increased in leukemia samples more than 4-fold above CD4+. The shape of a given protein is indicative of its functional class as shown in the legend. Also included in the legend is the definition of the lines connecting 2 genes. The “central” genes are circled in black.
Of significant interest to us was the appearance of BIRC5 as one of the “central” genes. BIRC5 has been reported to be an important determinant for progression in ATL,40 counteracting a default induction of apoptosis in G2/M by acting as an inhibitor of caspase-3 and caspase-7. The results shown in Figure 3A demonstrate that BIRC5 was overexpressed 3.5-fold or greater in 12 of 14 leukemia patient samples compared with control CD4+ cells. In contrast, the level of actin expression did not vary significantly among samples (Figure 3A bottom panel). These results were confirmed by quantitative RT-PCR (Figure S1).
Figure 3.
BIRCS expression in ATL patient samples. (A) The relative level of BIRC5 (top panel) and actin (bottom panel) expression obtained from the U133A2.0 arrays was graphed for the indicated ATL or control (CD4+) samples. (B) BIRC5 protein expression was analyzed in ATL or CD4+ whole-cell extracts by Western blot with antibodies to BIRC5 (top panel) or actin (bottom panel).
Western blot analysis for BIRC5 protein was performed on whole cell extracts from uncultured ATL patient cells or from CD4+ selected cells (Figure 3B). Consistent with overexpression at the RNA level, BIRC5 protein was detected in all ATL samples. In contrast, no BIRC5 protein was detected in control CD4+ lymphocytes.
TCF4 regulates BIRC5 gene expression
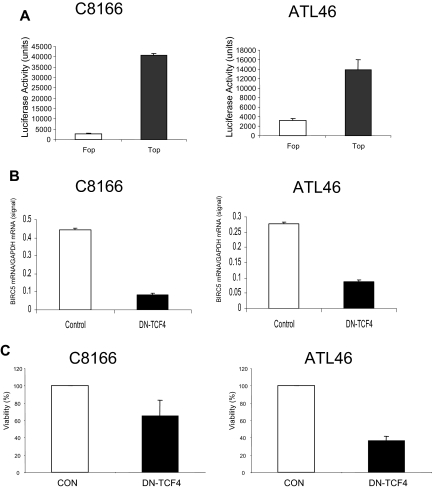
As noted in “Gene expression patterns observed in acute leukemia versus CD4+ lymphocytes,” we observed a significant increase in the level of transcription factor TCF4 in ATL cells. In the following studies, we analyzed TCF4 transcription activity and its relationship to BIRC5 gene expression in HTLV-1–transformed and ATL cells. To analyze TCF4 transcription activity, HTLV-1–transformed C8166 or ATL patient cells were transfected with a TCF4 reporter plasmid. Significant TCF4 activity was detected in both C8166 and ATL46 cells with the TCF4 promoter plasmid, Top-Flash (Figure 4A). In contrast, background transcription activity was detected after transfection of the Fop-Flash reporter plasmid carrying a mutation in the TCF4 promoter response element. It is important to note that ATL46 leukemia cells were used in this analysis because they could be obtained from fresh clinical apheresis samples, bypassing the need to analyze activities from frozen cells. The RT-PCR and TCF4 transcription assays presented in Figure 4 confirm elevated levels of BIRC5 and TCF4.
Figure 4.
TCF4 regulates BIRC5 expression. (A) To determine whether TCF transcription activity was present in HTLV-1–infected cells, promoter plasmids containing 3 wild-type (pGL3-OT/Top; ■) or mutant (pGL3-OF/Fop; □) copies of the TCF4 binding site driving luciferase expression were transfected into C8166 or primary cells from an ATL patient (ATL46) using Human T-cell Nucleofection kit (Amaxa). The CMV–β-galactosidase control plasmid was cotransfected to adjust for transfection efficiency. Graphs represent luciferase activity from at least 2 independent experiments. (B) Control (□) plasmid or plasmid expressing a dominant-negative TCF4 protein (DN-TCF4; ■) were transiently transfected into C8166 or ATL46 cells as described in “Methods.” Forty-eight hours after transfection, cells were harvested, total RNA extracted, and RT-PCR performed for BIRC5 and GAPDH mRNA. The graph represents the BIRC5 mRNA levels per GAPDH message done in triplicate. (C) Transfection of C8166 and ATL46 cells was performed as described in “Methods.” Forty-eight hours after transfection, the viability of the cells was determined using trypan blue dye exclusion. The viability of the cells from the vector control (CON) transfection was set at 100%. The graph represents values from at least 3 experiments.
To analyze the link between TCF4 activity and BIRC5 gene expression, a plasmid encoding a dominant-negative TCF4 protein (DN-TCF4) was transfected into C8166 and ATL46 cells. Subsequently, RNA was extracted and RT-PCR analysis performed to analyze BIRC5 and control GAPDH gene expression. The results of this study demonstrate that the dominant-negative TCF4 protein inhibits BIRC5 gene expression (Figure 4B). To control for nonspecific effects of the transfection, BIRC5 gene expression was normalized to GAPDH mRNA expression, which did not vary significantly.
We also determined the effect of TCF4 and BIRC5 gene expression on cell viability. After transfection of C8166 or ATL46 cells with the control or DN-TCF4 plasmid, cells were incubated for 24 hours and cell viability determined by trypan blue staining 48 hours after transfection. The results presented in Figure 4C demonstrate that decreased viability was observed in both cell types after DN-TCF4 expression. An average decrease in cell viability of 35% in C8166 and 65% in ATL46 was observed. These results suggest that BIRC5 gene expression contributes to cell viability of HTLV-1 and ATL cells.
BIRC5 shRNA decreases viability of ATL leukemia cells
To more directly analyze the importance of BIRC5 in ATL cell viability, cultured lymphocytes from patient ATL15 were electroporated with a plasmid expressing a BIRC5 specific shRNA. Forty-eight hours after transfection, cells were harvested and analyzed for cell viability using the CellTiter-Glo assay (Promega) and BIRC5 RNA levels using RT-PCR. Transfection of cells with the shRNA caused a 7- to 8-fold decrease in BIRC5 RNA expression (Figure 5A). In contrast, there was no decrease in BIRC5 expression in the shRNA control-transfected cells. In the cell viability assay, we observed a dramatic decrease in viability in cells transfected with BIRC5 shRNA (Figure 5B). No decrease in cell viability was observed in cells transfected with the control plasmid. These studies provide the first direct experimental evidence that BIRC5 expression is linked to viability of ATL cells.
Figure 5.
BIRCS expression contributes to cell viability. BIRC5 RNA levels (A) and cell viability (B) for ATL15 PBLs electroporated with shRNA control or BIRC5 vectors (Superarray Bioscience). The control values were set to 1. For these studies, cells directly from the patient were cultured in media containing 20% fetal bovine serum, 2 mM l-glutamine, and 50 U IL-2.
BIRC5 expression was decreased after clinical treatment of ATL with Zenapax and bortezomib
If BIRC5 plays an important role in ATL cell survival in vivo, then a clinical response to treatment might be expected to correlate with a decrease in BIRC5 expression and reduced malignant cell burden. During the course of this study, patient ATL2 was treated with Zenapax (humanized monoclonal antibody that binds to IL-2Rα) for 6 weeks. At the conclusion of the treatment, PBLs were retested by flow cytometry and Affymetrix gene expression analysis. Flow cytometric analysis demonstrated that 63% of the malignant cells were eliminated as determined by staining for 7G7, a second epitope on IL-2Rα that is not blocked by Zenapax binding. In the Zenapax-treated cells, the RNA expression level of BIRC5 was decreased 10-fold (Figure 6A). It is important to note that BIRC5 expression is determined on an equivalent amount of RNA isolated from control or treated cells. Thus, the decrease in BIRC5 expression is not the result of the decrease in the number of circulating tumor cells. We also observed that TCF4 was down-regulated in Zenapax-treated cells (data not shown). A similar, albeit less dramatic, decrease in BIRC5 expression was detected in leukemia patient ATL3 after treatment with Zenapax (Figure 6A). Interestingly, the decrease in number of malignant cells in the peripheral blood of patient ATL3 was also not as dramatic as in ATL2. These results suggest that Zenapax treatment decreases BIRC5 expression, which correlates with decreased viability of ATL cells.
Figure 6.
BIRC5 expression after drug treatment. (A) Graph comparing the relative expression of BIRC5 in ATL2 and ATL3 before and after (−, +) Zenapax (humanized anti–IL-2Rα) treatment. (B) Expression of BIRC5 in PBLs in lymphoma (ATL8), acute stage (ATL8a), and 3 and 72 hours (ATL8b and 8c) after bortezomib treatment (1.3 mg/m2 twice weekly for 2 weeks). (C) The level of expression of antiapoptotic proteins BIRC5 (Abcam), XIAP (Cell Signaling Technology, Danvers, MA), and Bcl-xL (Cell Signaling Technology) were examined by Western blot analysis in whole-cell extracts (50 μg) from ATL patient 8. Actin levels were determined as a loading control. Also shown are the levels of IκBα (EMD Chemicals, Gibbstown, NJ). (D) Total RNA from untreated (−) or treated (+) cells with 10 nM bortezomib was analyzed on Apoptosis GEarrays (Superarray Bioscience). BIRC5 expression in untreated samples was set at 1.
A decrease in BIRC5 expression was also observed in patient ATL8 after bortezomib treatment. Two years after diagnosis of ATL8 with lymphoma, the patient's PBL count elevated from normal to 40 000 cells/μL (Table 1, ATL8 and ATL8a). The patient was treated with bortezomib to rapidly decrease the tumor cell burden in the peripheral blood. To assess the effect of treatment, PBLs were drawn at pretreatment (ATL8a) and at 3 and 72 hours after treatment (ATL8b, ATL8c). Affymetrix analyses showed significant changes in BIRC5 gene expression through disease progression and treatment (Figure 6B). First, BIRC5 expression was dramatically increased in the acute leukemia sample (lane 2 vs lane 1) consistent with a role in leukemic progression. Second, by 72 hours after treatment, BIRC5 expression was dramatically decreased (lane 4). Concomitant with a drop in BIRC5 expression (72 hours after treatment), a 60% reduction in lymphocyte cell count in the peripheral blood was observed (Table 1). It is important to point out that BIRC5 expression is determined on an equivalent amount of RNA isolated from control or treated cells. The decrease in BIRC5 expression, therefore, is not the result of the decrease in the number of circulating tumor cells. Thus, a correlation between BIRC5 expression and decreased cell viability was observed.
To gain further insight into the cellular factors involved in sensitivity to bortezomib, we examined expression of several antiapototic factors. Indeed, we found that, as with the mRNA level, the level of BIRC5 protein decreased with bortezomib treatment (Figure 6C top panel). In contrast, the antiapototic factors XIAP and Bcl-xL did not decrease (Figure 6C). As expected, treatment with bortezomib caused an increase in IκBα levels, indicating blockage of the proteasome.
To determine whether bortezomib inhibited BIRC5 expression directly, we analyzed BIRC5 expression after treatment of cells with bortezomib in vitro. The expression of 112 apoptosis-related genes was analyzed in MT2 and C81 cells in response to 10 nM bortezomib. One of the most consistent and notable changes detected was a dramatic down-regulation of BIRC5 on treatment with bortezomib (Figure 6D). The decrease in BIRC5 signal intensity between the untreated and bortezomib-treated cells was most pronounced in the HTLV-1–infected MT2 cells, which showed an average decrease of 37-fold in 2 separate experiments. The fold change in HTLV-1–infected C8166 cells was consistent, but less dramatic, approximately 2-fold. In contrast, housekeeping genes, such as GAPDH or the biotinylated artificial sequence control, had no significant changes in expression level (data not shown). These results demonstrate that bortezomib treatment of HTLV-1 cells decreases BIRC5 expression.
Genes down-regulated in ATL leukemia cells
We also analyzed genes that were down-regulated in ATL leukemia cells compared with control PBLs. Approximately 191 genes identified as decreased 4-fold or more (Table S3) were analyzed using the Pathway Studio, version 4.0 program. Of interest, at the center of the down-regulated pathway array was interferon-γ (IFN-γ), which has both antiviral activity and antiproliferative effects on transformed cells and can potentiate the antiviral and antitumor effects of the type I interferons (Figure 7).43–45 The decrease in IFN-γ expression is interesting because studies examining type 1 cytokine expression in BLV infection of cattle found a significant decrease in IFN-γ to correlate with disease progression.46 Further, in a study examining the role of IFN-γ in tumorigenesis in Tax-transgenic mice, Mitra-Kaushik et al reported that IFN-γ−/− mice show accelerated tumor onset, dissemination, and death compared with IFN-γ−/+ or +/+ mice.47 In view of these studies, it is interesting to speculate that, although Tax may initially increase IFN-γ production in CD4+ lymphocytes,48 this production is blocked as tumor cells progress, contributing to tumorigenesis.
Figure 7.
Schematic of molecular interactions using Pathway Studio, version 4.0, for genes decreased in leukemia samples more than 4-fold below CD4+. The central gene is IFN-γ. The dotted lines represent regulation of function between 2 genes. The shape of a given protein is indicative of its functional class as shown in the legend. Also included in the legend is the definition of the lines connecting 2 genes.
We also observed that STAT4 expression was approximately 11-fold lower in leukemia cells compared with CD4+ cells. STAT4 is a critical mediator of proinflammatory immune responses and functions through signal transduction and activation of transcription.49 STAT4-deficient mice demonstrated that STAT4 is required for most IL-12 biologic responses, including IFN-γ production.49,50
Also of interest is the observation that BCL-2 is significantly down-regulated (14-fold) in ATL cells. BCL-2 normally forms heterodimers with Bax, Bad, Bak, and Bcl-X(L) to function in antiapoptotic activity.51 Once apoptosis is induced, however, Bcl-2 is proteolytically cleaved. The cleaved protein, lacking the BH4 motif, has proapoptotic activity, causing the release of cytochrome C into the cytosol, promoting further caspase activity.
We also found it interesting that FOS and functionally related genes JUNB, FOSL2, FOSB, and CREM were down-regulated in ATL cells. FOS is a nuclear phosphoprotein that forms a tight but noncovalently linked complex with the JUN/AP-1 transcription factor. In the heterodimer, c-fos and JUN/AP-1 basic regions interact with symmetric DNA half-sites, regulating genes that have an important role in signal transduction, cell proliferation, and differentiation. FOS expression increases on a variety of stimuli, including growth factors, cytokines, neurotransmitters, polypeptide hormones, stress, and cell injury.
Discussion
In the largest study of ATL patients by microarray analysis to date, we have compared the gene expression profiles from 32 ATL patients, which include smoldering, chronic, lymphoma and acute leukemia ATL patients. Because of the homogeneity of the cell population and the tight clustering demonstrated by the leukemia patient samples, we largely focused our initial analysis on this group to identify potential therapeutic targets. The increase in BIRC5 expression in ATL cells was of significant interest. First, BIRC5 plays an important role in cell viability and belongs to the inhibitor of apoptosis protein family of proteins. Binding of BIRC5 to, and inhibition of, certain caspases inhibits apoptosis induced by a variety of stimuli.52 Second, BIRC5 is overexpressed in a variety of human cancers. High expression of BIRC5 has been associated with resistance to chemotherapy and poor prognosis of carcinomas of the lung, breast, colon, and esophagus.53,54 Third, among the known inhibitor of apoptosis proteins, BIRC5 has been reported to be overexpressed in ATL and the expression level correlated with a shorter survival of the patients.55–57
Although BIRC5 has been a focal point of several studies related to HTLV-1 and ATL, it is important to note that our results make a significant and critical contribution. Several groups have reported a correlation between HTLV-1–infected cell death and decreased BIRC5 expression. Che et al55 demonstrated that sodium arsenite was able to down-regulate BIRC5 gene expression in HTLV-1–infected cells, inhibiting cell growth and inducing apoptosis. Similarly, Hayashibara et al56 reported that resveratrol induces down expression of BIRC5 in cultured HTLV-1 virus–infected cell lines. In a 2006 study, Dewan et al58 reported that ritonavir, an HIV protease inhibitor, induced apoptosis and inhibited transcriptional activation of NF-κB in cultured ATL cells. Ritonavir inhibited expression of BIRC5, a target of NF-κB. Finally, Sanda et al59 have analyzed the effects of a novel IκB kinase (IKK) inhibitor, 2-amino-6-[2-(cyclopropylmethoxy)-6-hydroxyphenyl]-4-piperidin-4-yl nicotinonitrile (ACHP), on cell growth of cultured ATL leukemic cells. ACHP inhibited the phosphorylation of IκBα and p65, as well as NF-κB DNA binding, and was associated with down-regulation of the NF-κB target genes including BIRC5, inducing cell growth arrest and apoptosis in these cultured cells. Our studies provide the first analysis of BIRC5 gene expression in uncultured ATL patient cells after clinical treatment in vivo with Zenapax or bortezomib. Moreover, we provide evidence that a BIRC5 shRNA inhibited BIRC5 expression and decreased cell viability when introduced into ATL cells. This is the first direct experimental evidence that specifically targeting BIRC5 gene expression decreased viability of ATL cells. Our studies further extend the understanding of BIRC5 expression in ATL cells by linking TCF4 expression to regulation of BIRC5 expression in HTLV-1–transformed cells as well as ATL cells.
Sasaki et al28 recently reported analysis of expression patterns of 12 000 genes in 8 acute ATL patients. Three genes including TSLC1/IGSF4, CAV1, and PGDS were overexpressed more than 30-fold in the uncultured ATL cells. In the present study, we also observed that PGDS and TSLC1/IGSF4 expression was elevated in all the leukemia samples analyzed. Increased expression of PTHLH, CCNB1, and RGS13 was also seen here and by Sasaki et al.28 In contrast to the findings of Sasaki et al,28 we did not see elevated expression of the CAV1 in the majority of the ATL leukemia samples examined. In our analysis, 3 of the 14 ATL patients (ATL8a, ATL18, and ATL32) had elevated levels of CAV1 RNA. The remaining 11 ATL patients had low CAV1 levels similar to that observed in control CD4+ lymphocytes. We have, however, observed that cultured lymphocytes express elevated levels of CAV1. It is possible that the cells analyzed by Sasaki et al28 had been stimulated during lymphocyte purification. Interestingly, Sasaki et al28 did not find elevated BIRC5 levels in their analysis. This could be the result of the difference in probe pair usage in the Hu95A microarray used by Sasaki et al28 compared with the HU133A microarrays we used. The later microarray contains more probe pairs scanning a larger portion of the BIRC5 gene.
In a separate study, Tsukasaki et al27 analyzed the expression pattern in chronic versus acute ATL patients by analyzing 3 matched chronic and acute ATL samples from the same patient, as well as PBLs from a typical chronic ATL and an acute phase patient. Among the approximately 200 genes up-regulated in uncultured acute ATL samples, the authors highlighted immunophilin PPIB, a member of a conserved class of proteins important for T-cell activation. RNA-binding proteins YB-1 and nucleolin were also reported to be up-regulated, both of which bind to the JNK response element and stabilize IL-2 mRNA.
To distinguish the genes noted in our study as unique to ATL and not representative of a set of genes deregulated in CD4+ malignancies, we compared the gene set we observed in ATL leukemia patients with another T-cell malignancy. Sezary syndrome is a leukemic variant of cutaneous T-cell lymphoma in which neoplasic CD4+ skin-homing memory T cells or Sezary cells are found in the skin, lymph nodes, and peripheral blood. Sezary syndrome has similar clinical and pathologic features to ATL. When we compare overexpressed genes in ATL to Sezary syndrome, only 20 genes were found to be shared by both diseases.60 Notably, BIRC5, CDC2, and LYN/SYK genes were not increased in Sezary cells. In contrast, RANK/L and PTHLH were increased for both ATL and Sezary syndrome. This suggests that, although some genes are overexpressed in both tumors, there are also distinguishing gene sets for each T-cell malignancy.
An effective clinical treatment regimen for ATL patients has not been realized. Our studies have identified several genes that could be targeted in the treatment of ATL. Among the potential targets is BIRC5, an inhibitor of apoptosis. BIRC5 has been successfully targeted in other cancers by drugs, such as oxaliplatin, sodium arsenite, adenovirus vectors encoding siRNAs, kinase inhibitors, and resveratrol.61 In view of these studies, it may make sense to consider combining general therapeutic regimens, such as Zenapax, with a targeted therapy for proteins such as BIRC5. By adding the targeted intervention, the 2 therapeutic agents could act via different mechanisms of action and manifest additive or synergistic efficacy in the treatment of ATL.
Supplementary Material
Acknowledgments
The authors thank Diana O'Hagan, Wendy Gao, and Leslie Moses for their assistance in the management of the clinical care of these patients and Keven Huang for help with graphics.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.A.P.-M. and J.N.B. designed and analyzed data, were the principal investigators, and wrote the paper; M.R. and C.A.P.-M. performed the research; K.D. performed experiments in Figure 5; J.T. and M.-J.L. contributed to the design and analysis; and D.O., J.C.M., J.E.J., and T.A.W. contributed to the writing and provided patient samples.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John N. Brady, Laboratory of Cellular Oncology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: bradyj@mail.nih.gov.
References
- 1.Franchini G, Nicot C, Johnson JM. Seizing of T cells by human T-cell leukemia/lymphoma virus type 1. Adv Cancer Res. 2003;89:69–132. doi: 10.1016/s0065-230x(03)01003-0. [DOI] [PubMed] [Google Scholar]
- 2.Azran I, Schavinsky-Khrapunsky Y, Aboud M. Role of Tax protein in human T-cell leukemia virus type-I leukemogenicity. Retrovirology. 2004;1:20. doi: 10.1186/1742-4690-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagai M, Osame M. Human T-cell lymphotropic virus type I and neurological diseases. J Neurovirol. 2003;9:228–235. doi: 10.1080/13550280390194028. [DOI] [PubMed] [Google Scholar]
- 4.Takenouchi N, Yao K, Jacobson S. Immunopathogenesis of HTLV-I associated neurologic disease: molecular, histopathologic, and immunologic approaches. Front Biosci. 2004;9:2527–2539. doi: 10.2741/1414. [DOI] [PubMed] [Google Scholar]
- 5.Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 6.Murphy EL, Hanchard B, Figueroa JP, et al. Modelling the risk of adult T-cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type I. Int J Cancer. 1989;43:250–253. doi: 10.1002/ijc.2910430214. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto T, Ohno Y, Tsugane S, et al. Multi-step carcinogenesis model for adult T-cell leukemia. Jpn J Cancer Res. 1989;80:191–195. doi: 10.1111/j.1349-7006.1989.tb02289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatta Y, Koeffler HP. Role of tumor suppressor genes in the development of adult T cell leukemia/lymphoma (ATLL). Leukemia. 2002;16:1069–1085. doi: 10.1038/sj.leu.2402458. [DOI] [PubMed] [Google Scholar]
- 9.Sakashita A, Hattori T, Miller CW, et al. Mutations of the p53 gene in adult T-cell leukemia. Blood. 1992;79:477–480. [PubMed] [Google Scholar]
- 10.Bazarbachi A, Hermine O. Treatment of adult T-cell leukaemia/lymphoma: current strategy and future perspectives. Virus Res. 2001;78:79–92. doi: 10.1016/s0168-1702(01)00286-6. [DOI] [PubMed] [Google Scholar]
- 11.Pawson R, Richardson DS, Pagliuca A, et al. Adult T-cell leukemia/lymphoma in London: clinical experience of 21 cases. Leuk Lymphoma. 1998;31:177–185. doi: 10.3109/10428199809057597. [DOI] [PubMed] [Google Scholar]
- 12.Shimoyama M, Ota K, Kikuchi M, et al. Major prognostic factors of adult patients with advanced T-cell lymphoma/leukemia. J Clin Oncol. 1988;6:1088–1097. doi: 10.1200/JCO.1988.6.7.1088. [DOI] [PubMed] [Google Scholar]
- 13.Tsukasaki K, Ikeda S, Murata K, et al. Characteristics of chemotherapy-induced clinical remission in long survivors with aggressive adult T-cell leukemia/lymphoma. Leuk Res. 1993;17:157–166. doi: 10.1016/0145-2126(93)90061-o. [DOI] [PubMed] [Google Scholar]
- 14.Taylor GP, Matsuoka M. Natural history of adult T-cell leukemia/lymphoma and approaches to therapy. Oncogene. 2005;24:6047–6057. doi: 10.1038/sj.onc.1208979. [DOI] [PubMed] [Google Scholar]
- 15.Waldmann A, Goldman CK, Bongiovanni KF, et al. Therapy of patients with human T-cell lymphotrophic virus I-induced adult T-cell leukemia with anti-Tac, a monoclonal antibody to the receptor for interleukin-2. Blood. 1988;72:1805–1816. [PubMed] [Google Scholar]
- 16.Waldmann TA, White JD, Goldman CK, et al. The interleukin-2 receptor: a target for monoclonal antibody treatment of human T-cell lymphotrophic virus I-induced adult T-cell leukemia. Blood. 1993;82:1701–1712. [PubMed] [Google Scholar]
- 17.Waldmann TA, White JD, Carrasquillo JA, et al. Radioimmunotherapy of interleukin-2R alpha-expressing adult T-cell leukemia with Yttrium-90-labeled anti-Tac. Blood. 1995;86:4063–4075. [PubMed] [Google Scholar]
- 18.Zhang Z, Zhang M, Goldman CK, Ravetch JV, Waldmann TA. Effective therapy for a murine model of adult T-cell leukemia with the humanized anti-CD52 monoclonal antibody, Campath-1H. Cancer Res. 2003;63:6453–6457. [PubMed] [Google Scholar]
- 19.Mone A, Puhalla S, Whitman S, et al. Durable hematologic complete response and suppression of HTLV-1 viral load following alemtuzumab in zidovudine/IFN-α-refractory adult T-cell leukemia. Blood. 2005;106:3380–3382. doi: 10.1182/blood-2005-01-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravandi F, Faderl S. Complete response in a patient with adult T-cell leukemia (ATL) treated with combination of alemtuzumab and pentostatin. Leuk Res. 2006;30:103–105. doi: 10.1016/j.leukres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 21.de La Fuente C, Deng L, Santiago F, et al. Gene expression array of HTLV type 1-infected T cells: up-regulation of transcription factors and cell cycle genes. AIDS Res Hum Retroviruses. 2000;16:1695–1700. doi: 10.1089/08892220050193164. [DOI] [PubMed] [Google Scholar]
- 22.Harhaj EW, Good L, Xiao G, Sun SC. Gene expression profiles in HTLV-I-immortalized T cells: deregulated expression of genes involved in apoptosis regulation. Oncogene. 1999;18:1341–1349. doi: 10.1038/sj.onc.1202405. [DOI] [PubMed] [Google Scholar]
- 23.Ng PW, Iha H, Iwanaga Y, et al. Genome-wide expression changes induced by HTLV-1 Tax: evidence for MLK-3 mixed lineage kinase involvement in Tax-mediated NF-kappaB activation. Oncogene. 2001;20:4484–4496. doi: 10.1038/sj.onc.1204513. [DOI] [PubMed] [Google Scholar]
- 24.Pise-Masison CA, Radonovich M, Mahieux R, et al. Transcription profile of cells infected with human T-cell leukemia virus type I compared with activated lymphocytes. Cancer Res. 2002;62:3562–3571. [PubMed] [Google Scholar]
- 25.Gatza ML, Watt JC, Marriott SJ. Cellular transformation by the HTLV-I Tax protein, a jack-of-all-trades. Oncogene. 2003;22:5141–5149. doi: 10.1038/sj.onc.1206549. [DOI] [PubMed] [Google Scholar]
- 26.Matsuoka M. Human T-cell leukemia virus type I and adult T-cell leukemia. Oncogene. 2003;22:5131–5140. doi: 10.1038/sj.onc.1206551. [DOI] [PubMed] [Google Scholar]
- 27.Tsukasaki K, Tanosaki S, DeVos S, et al. Identifying progression-associated genes in adult T-cell leukemia/lymphoma by using oligonucleotide microarrays. Int J Cancer. 2004;109:875–881. doi: 10.1002/ijc.20028. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki H, Nishikata I, Shiraga T, et al. Overexpression of a cell adhesion molecule, TSLC1, as a possible molecular marker for acute-type adult T-cell leukemia. Blood. 2005;105:1204–1213. doi: 10.1182/blood-2004-03-1222. [DOI] [PubMed] [Google Scholar]
- 29.Jeong SJ, Radonovich M, Brady JN, Pise-Masison CA. HTLV-I Tax induces a novel interaction between p65/RelA and p53 that results in inhibition of p53 transcriptional activity. Blood. 2004;104:1490–1497. doi: 10.1182/blood-2003-12-4174. [DOI] [PubMed] [Google Scholar]
- 30.National Center for Biotechnology Information. [Accessed January 7, 2009];GEO. http://www.ncbi.nlm.nih.gov/geo.
- 31.Kannagi M, Harashima N, Kurihara K, et al. Tumor immunity against adult T-cell leukemia. Cancer Sci. 2005;96:249–255. doi: 10.1111/j.1349-7006.2005.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okada Y, Tsukada J, Nakano K, et al. Macrophage inflammatory protein-1alpha induces hypercalcemia in adult T-cell leukemia. J Bone Miner Res. 2004;19:1105–1111. doi: 10.1359/JBMR.040314. [DOI] [PubMed] [Google Scholar]
- 33.Johnson EN, Druey KM. Functional characterization of the G protein regulator RGS13. J Biol Chem. 2002;277:16768–16774. doi: 10.1074/jbc.M200751200. [DOI] [PubMed] [Google Scholar]
- 34.Creighton CJ, Bromberg-White JL, Misek DE, et al. Analysis of tumor-host interactions by gene expression profiling of lung adenocarcinoma xenografts identifies genes involved in tumor formation. Mol Cancer Res. 2005;3:119–129. doi: 10.1158/1541-7786.MCR-04-0189. [DOI] [PubMed] [Google Scholar]
- 35.Hirose J, Kawashima H, Yoshie O, Tashiro K, Miyasaka M. Versican interacts with chemokines and modulates cellular responses. J Biol Chem. 2001;276:5228–5234. doi: 10.1074/jbc.M007542200. [DOI] [PubMed] [Google Scholar]
- 36.Mauri P, Scarpa A, Nascimbeni AC, et al. Identification of proteins released by pancreatic cancer cells by multidimensional protein identification technology: a strategy for identification of novel cancer markers. FASEB J. 2005;19:1125–1127. doi: 10.1096/fj.04-3000fje. [DOI] [PubMed] [Google Scholar]
- 37.Yoon H, Liyanarachchi S, Wright FA, et al. Gene expression profiling of isogenic cells with different TP53 gene dosage reveals numerous genes that are affected by TP53 dosage and identifies CSPG2 as a direct target of p53. Proc Natl Acad Sci U S A. 2002;99:15632–15637. doi: 10.1073/pnas.242597299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung EJ, Hwang SG, Nguyen P, et al. Regulation of leukemic cell adhesion, proliferation, and survival by beta-catenin. Blood. 2002;100:982–990. doi: 10.1182/blood.v100.3.982. [DOI] [PubMed] [Google Scholar]
- 39.Taniguchi T, Miyazaki T, Minami Y, et al. IL-2 signaling involves recruitment and activation of multiple protein tyrosine kinases by the IL-2 receptor. Ann NY Acad Sci. 1995;766:235–244. doi: 10.1111/j.1749-6632.1995.tb26671.x. [DOI] [PubMed] [Google Scholar]
- 40.Nakayama K, Kamihira S. Survivin an important determinant for prognosis in adult T-cell leukemia: a novel biomarker in practical hemato-oncology. Leuk Lymphoma. 2002;43:2249–2255. doi: 10.1080/1042819021000039956. [DOI] [PubMed] [Google Scholar]
- 41.Tomita M, Kikuchi A, Akiyama T, Tanaka Y, Mori N. Human T-cell leukemia virus type 1 tax dysregulates beta-catenin signaling. J Virol. 2006;80:10497–10505. doi: 10.1128/JVI.00739-06. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Minami Y, Nakagawa Y, Kawahara A, et al. Protein tyrosine kinase Syk is associated with and activated by the IL-2 receptor: possible link with the c-myc induction pathway. Immunity. 1995;2:89–100. doi: 10.1016/1074-7613(95)90081-0. [DOI] [PubMed] [Google Scholar]
- 43.Young HA. Unraveling the pros and cons of interferon-gamma gene regulation. Immunity. 2006;24:506–507. doi: 10.1016/j.immuni.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Wang T, Blatt LM, Seiwert SD. Immunomodulatory activities of IFN-gamma1b in combination with type I IFN: implications for the use of IFN-gamma1b in the treatment of chronic HCV infections. J Interferon Cytokine Res. 2006;26:473–483. doi: 10.1089/jir.2006.26.473. [DOI] [PubMed] [Google Scholar]
- 45.Brandacher G, Winkler C, Schroecksnadel K, Margreiter R, Fuchs D. Antitumoral activity of interferon-gamma involved in impaired immune function in cancer patients. Curr Drug Metab. 2006;7:599–612. doi: 10.2174/138920006778017768. [DOI] [PubMed] [Google Scholar]
- 46.Yakobson B, Brenner J, Ungar-Waron H, Trainin Z. Short-termed expression of interleukin-12 during experimental BLV infection may direct disease progression to persistent lymphocytosis. Vet Immunol Immunopathol. 1998;64:207–218. doi: 10.1016/s0165-2427(98)00136-6. [DOI] [PubMed] [Google Scholar]
- 47.Mitra-Kaushik S, Harding JC, Hess JL, Ratner L. Effects of the proteasome inhibitor PS-341 on tumor growth in HTLV-1 Tax transgenic mice and Tax tumor transplants. Blood. 2004;104:802–809. doi: 10.1182/blood-2003-11-3967. [DOI] [PubMed] [Google Scholar]
- 48.Brown DA, Nelson FB, Reinherz EL, Diamond DJ. The human interferon-gamma gene contains an inducible promoter that can be transactivated by tax I and II. Eur J Immunol. 1991;21:1879–1885. doi: 10.1002/eji.1830210815. [DOI] [PubMed] [Google Scholar]
- 49.Kaplan MH. STAT4: a critical regulator of inflammation in vivo. Immunol Res. 2005;31:231–242. doi: 10.1385/IR:31:3:231. [DOI] [PubMed] [Google Scholar]
- 50.Watford WT, Hissong BD, Bream JH, et al. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 51.Basu A, DuBois G, Haldar S. Posttranslational modifications of Bcl2 family members: a potential therapeutic target for human malignancy. Front Biosci. 2006;11:1508–1521. doi: 10.2741/1900. [DOI] [PubMed] [Google Scholar]
- 52.Zangemeister-Wittke U, Simon HU. An IAP in action: the multiple roles of survivin in differentiation, immunity and malignancy. Cell Cycle. 2004;3:1121–1123. [PubMed] [Google Scholar]
- 53.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 54.Duffy MJ, O'Donovan N, Brennan DJ, Gallagher WM, Ryan BM. Survivin: a promising tumor biomarker. Cancer Lett. 2007;249:49–60. doi: 10.1016/j.canlet.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 55.Che XF, Zheng CL, Owatari S, et al. Overexpression of survivin in primary ATL cells and sodium arsenite induces apoptosis by down-regulating survivin expression in ATL cell lines. Blood. 2006;107:4880–4887. doi: 10.1182/blood-2005-08-3423. [DOI] [PubMed] [Google Scholar]
- 56.Hayashibara T, Yamada Y, Nakayama S, et al. Resveratrol induces down-regulation in survivin expression and apoptosis in HTLV-1-infected cell lines: a prospective agent for adult T cell leukemia chemotherapy. Nutr Cancer. 2002;44:193–201. doi: 10.1207/S15327914NC4402_12. [DOI] [PubMed] [Google Scholar]
- 57.Sugahara K, Uemura A, Harasawa H, et al. Clinical relevance of survivin as a biomarker in neoplasms, especially in adult T-cell leukemias and acute leukemias. Int J Hematol. 2004;80:52–58. doi: 10.1532/ijh97.04031. [DOI] [PubMed] [Google Scholar]
- 58.Dewan MZ, Uchihara JN, Terashima K, et al. Efficient intervention of growth and infiltration of primary adult T-cell leukemia cells by an HIV protease inhibitor, ritonavir. Blood. 2006;107:716–724. doi: 10.1182/blood-2005-02-0735. [DOI] [PubMed] [Google Scholar]
- 59.Sanda T, Asamitsu K, Ogura H, et al. Induction of cell death in adult T-cell leukemia cells by a novel IkappaB kinase inhibitor. Leukemia. 2006;20:590–598. doi: 10.1038/sj.leu.2404129. [DOI] [PubMed] [Google Scholar]
- 60.van Doorn R, Dijkman R, Vermeer MH, et al. Aberrant expression of the tyrosine kinase receptor EphA4 and the transcription factor twist in Sezary syndrome identified by gene expression analysis. Cancer Res. 2004;64:5578–5586. doi: 10.1158/0008-5472.CAN-04-1253. [DOI] [PubMed] [Google Scholar]
- 61.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.