Abstract
Little is known about prostaglandin F2α in cardiovascular homeostasis. Prostaglandin F2α dose-dependently elevates blood pressure in WT mice via activation of the F prostanoid (FP) receptor. The FP is expressed in preglomerular arterioles, renal collecting ducts, and the hypothalamus. Deletion of the FP reduces blood pressure, coincident with a reduction in plasma renin concentration, angiotensin, and aldosterone, despite a compensatory up-regulation of AT1 receptors and an augmented hypertensive response to infused angiotensin II. Plasma and urinary osmolality are decreased in FP KOs that exhibit mild polyuria and polydipsia. Atherogenesis is retarded by deletion of the FP, despite the absence of detectable receptor expression in aorta or in atherosclerotic lesions in Ldlr KOs. Although vascular TNFα, inducible nitric oxide enzyme and TGFβ are reduced and lesional macrophages are depleted in the FP/Ldlr double KOs, this result reflects the reduction in lesion burden, as the FP is not expressed on macrophages and its deletion does not alter macrophage cytokine generation. Blockade of the FP offers an approach to the treatment of hypertension and its attendant systemic vascular disease.
Keywords: hypertension, renin, PGF2α receptor, juxtaglomerular granular cell, water metabolism
Control of hypertension has contributed to a decline of cardiovascular morbidity and mortality. Therapies have targeted neurohumoral mechanisms, such as the sympathoadrenal and renin-angiotensin-aldosterone systems (RAAS) as well as downstream effectors and volume control. Elevated blood pressure (BP) cosegregates with clinical cardiovascular events and randomized trials have revealed the efficacy of antihypertensive drugs to reduce the risk of stroke and myocardial infarction (1). Angiotensin II activates and up-regulates NADPH oxidase (2), augmenting oxidant stress and vascular dysfunction. Both pharmacological and genetic disruption of elements of the RAAS decreases BP and retards atherogenesis (3–5).
Prostaglandins (PGs) also contribute to BP homeostasis. Elevation of BP complicates the use of nonsteroidal anti-inflammatory drugs and relates to the degree of inhibition of cyclooxygenase (COX)-2 and the selectivity with which it is attained (6). Genetic and pharmacological manipulations suggest that products of COX-1 may elevate BP (7), although the impact of manipulating the PG cascade is conditioned by genetic background in mice (8). Prostacyclin (PGI2) is a potent renin secretagogue (9), and its biosynthesis is increased markedly in pregnancy, a high-renin but hypotensive condition; its biosynthesis is depressed in pregnancy-induced hypertension (10). Deletion of its I prostanoid receptor (the IP) reduces BP in renoprival models of high-renin hypertension in rodents (11). PGI2 is also a vasodilator and promotes sodium excretion; indeed, salt-sensitive hypertension characterizes IP-KO mice in some genetic backgrounds (12).
PGF2α is derived mainly from COX-1 in the female reproductive system, where it is required for normal parturition in mice (13, 14). The F prostanoid receptor (the FP) is also expressed abundantly in the kidney, particularly in the distal convoluted tubules and cortical collecting ducts (CCD) (14). Renal elaboration of PGF2α is augmented by salt (15), and activation of the FP modulates water absorption in the collecting ducts in vitro by inhibiting the action of vasopressin (15). Both PGF2α and thromboxane A2 mediate lipopolysaccharide-induced tachycardia (16). However, the importance of PGF2α in vivo in fluid/electrolyte homeostasis and BP regulation remains to be defined.
In the present study, we determine that PGF2α elevates BP, accelerates atherosclerosis, and restrains water intake in rodents. This result occurs despite the absence of detectable FP expression in normal aorta or atherosclerotic lesions. Antagonists of the FP, mechanistically distinct from direct renin inhibitors (5, 17), may emerge as therapies in the treatment of both hypertension and its attendant vascular disease.
Results
BP Is Reduced and the RAAS Is Depressed in FP−/− Mice.
The effect of FP deficiency on BP in normolipidemic (both on normal chow and following salt depletion) and hyperlipidemic (Ldlr−/−/FP−/−) mice was assessed by both tail-cuff measurement and telemetry. Resting systolic pressure was lower in normolipidemic FP−/− mice (normal diet, 109.8 mm Hg ± 3.3, n = 11; salt depletion, 107.7 mm Hg ± 3.4, n = 11) compared with WT controls (normal diet, 125.3 mm Hg ± 3.2, n = 11; P < 0.001; salt depletion, 121.0 mm Hg ± 3.3, n = 16; P < 0.01) (Fig. 1A) and in hyperlipidemic Ldlr−/−/FP−/− mice [103.3 mm Hg ± 3.26, n = 16, 12 weeks high-fat diet (HFD); 115.2 mm Hg ± 1.53, n = 16, 24 weeks HFD] compared with Ldlr−/− controls (120.1 mm Hg ± 3.16, n = 16, P < 0.0001, 12 weeks HFD; 124.2 mm Hg ± 3.18, n = 16, P < 0.05, 24 weeks HFD) (Fig. 1 B and C). The difference in BP between FP−/− mice and controls was sustained throughout the 24-h period in the telemetric studies. Tachycardia, reactive to hypotension, did not differ between the groups. Neither BP nor heart rate differed between normolipidemic and hyperlipidemic mice.
Fig. 1.
Deletion of FP reduces BP and decreases renin-angiotensin-aldosterone concentration. Systolic BP (SBP) measurements in (A) FP−/− mice on a normal chow diet and after salt depletion by tail cuff and Ldlr−/−/FP−/− on HFD for either (B) 12 weeks by tail cuff or (C) 24 weeks by radiotelemetry. Values represent mean ± SEMs analyzed by unpaired Student t test. *, P < 0.05 versus WT controls (n = 11–16), *, P < 0.05, ***, P < 0.0001 versus Ldlr−/− controls (n = 16–18). (D) Plasma renin concentration (PRC), (E) plasma angiotensinogen I concentration, and (F) plasma aldosterone concentration (PAC) in FP−/− mice under normal and salt-depleted conditions. For salt depletion, mice were subjected to a low-salt diet (0.12% NaCl) and injected with furosemide (25 mg/kg i.p.) every day. Values represent mean ± SEMs analyzed by unpaired Student t test. *, P < 0.05 versus WT control mice (n = 6–9).
Deletion of the FP Depressed Plasma Renin, Angiotensin I, and Aldosterone Concentrations.
Salt depletion activated the RAAS and exacerbated the impact of FP deletion on plasma renin concentration and on angiotensin I (Fig. 1 D and E), but not aldosterone secretion (Fig. 1F), suggesting that other angiotensin-independent factors (e.g., adrenergic control) may be involved in the regulation of aldosterone secretion in response to salt depletion (18). Similar results were obtained in hyperlipidemic mice Fig. S1 A and B.
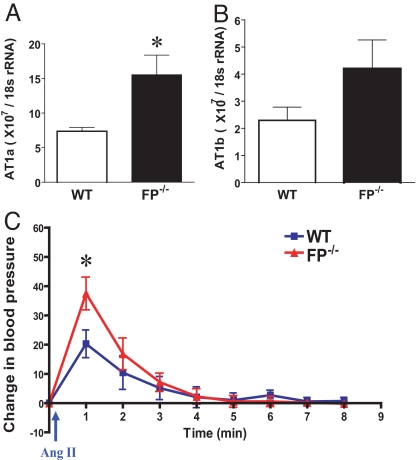
The hypotensive effect of FP deletion occurred despite a compensatory increase in tissue responsiveness to angiotensin II. This peptide regulates BP by binding to angiotensin type 1 receptors (AT1) in the vasculature. mRNA expression of the dominant isoform, AT1a, increased in aorta in the FP−/− mice (7.37 × 107/18S rRNA ± 0.56 vs. 15.54 × 107/18S rRNA ± 2.84, n = 4; P < 0.05) (Fig. 2A) compared with WT controls. A less pronounced increase was observed in AT1b expression (P = 0.13) (Fig. 2B). Correspondingly, the hypertensive response to acute intracarotid infusion of angiotensin II peptide (50 ng/kg) was increased significantly in FP KO mice compared with WT controls (37.5 ± 5.6 vs. 20.3 ± 4.7, n = 7; P < 0.05) (Fig. 2C).
Fig. 2.
Elevated pressor response mediated by angiotensin II in FP−/− mice with up-regulated expression of AT1 gene in aorta. (A) AT1a and (B) AT1b gene expression in aorta by real-time RT-PCR. *, P < 0.01 (n = 4) versus WT, repeated 3 times. (C) BP change after angiotensin II (Ang II) administration (50 ng/kg) compared with baseline in FP−/− and WT litter mates. Mean arterial BP was monitored in mice by pressure transducer connected to a catheter in left artery. Baseline BP is readout before injection. BP changes are depicted as the difference between baseline and after injection at 1-min intervals. *, P < 0.01 versus WT (n = 8–9).
FP Activation Stimulates Renin Secretion by Juxtaglomerular Granular Cells.
Consistent with a previous report (14), intense specific FP signals were detected by using in situ hybridization with an FP antisense probe in epithelial tubules in the renal cortex (i.e., CCD) of WT mice (Fig. 3A); no detectable signal was seen with an FP sense probe. Furthermore, RT-PCR analysis of micro-dissected nephron segments revealed that FP receptors were expressed both in the CCD and in the preglomerular arteriole (Fig. 3B). As decreased renin activity was observed in FP−/− mice, the extension of granulation in afferent arterioles was quantified. The high-contrast regions seen at the end of afferent arterioles represent clusters of renin-positive cells as documented previously (19). The extent of visible granulation (Fig. 3C, red arrows) in the vascular trees at the terminal ends of the arterioles is decreased significantly in FP−/− mice (40% renin positive; P < 0.0001) compared with WT controls (59% renin positive) (Fig. 3D). Kidney renin RNA expression was also down-regulated significantly in FP−/− mice (Fig. S2).
Fig. 3.
FP mediates renin expression in the kidney. (A) In situ hybridization for FP in mouse kidney sections (10 μm) by using 35S-labeled antisense cRNA probe (red). (Scale bar: 200 μm.) (B) FP mRNA expression detected by RT-PCR in microdissected mouse nephron segments. Controls without addition of reverse transcriptase (RT−) to show no PCR contamination: CCD, proximal convoluted tubule (PCT), preglomerular arteriole (PGA), glomerulus (Glom), and positive control (kidney cortex). (C) Visualization of renin granules in microdissected renal arterial vessels from WT and FP−/− mice. Glomeruli are sheared off in the microdissection process. High-contrast regions, usually seen at the end of the afferent arterioles, represent clusters of renin-positive cells (red arrows), or renin-negative cells (green arrows). (D) Quantification of juxtaglomerular granular (JG) negative and positive afferent arterioles. (E) Effects of latanoprost (Lata), travoprost (Trav), and cicaprost (Cica) on renin mRNA expression in JG-enriched cultured cells. Both FP agonists (latanoprost and travoprost) stimulated an increase in renin mRNA expression in a dose-dependent manner in the WT group, and no effect was observed in FP−/− mice. Each point represents mean ± SEM of 4 cell groups, repeated 3 times. *, P < 0.05 by one-way ANOVA test.
Detection of FP receptors in proximal afferent glomerular arterioles and depression of the RAAS in FP KOs suggested that FP activation may mediate renin secretion in juxtaglomerular granular (JG) cells. To test this hypothesis, cultured JG cells prepared from WT and FP−/− mice were stimulated with either the IP agonist cicaprost (20) or the FP agonists latanoprost (21) and travoprost (22). Both FP agonists, which differ in potency, increased JG renin mRNA expression in a concentration-dependent manner (Fig. 3E) that was abrogated by deletion of the FP. By contrast, the effects of cicaprost as a renin secretagogue were similar in WT and FP−/− mice. However, despite the increase in JG renin, latanoprost, unlike cicaprost, failed to increase JG cell cAMP (Fig. S3), a mediator of renin expression and exocytosis. Furthermore, patch-clamp studies using single WT JG cells in vitro incubated with latanoprost showed no change in membrane capacitance (Fig. S4), suggesting that the FP does not directly mediate renin exocytosis.
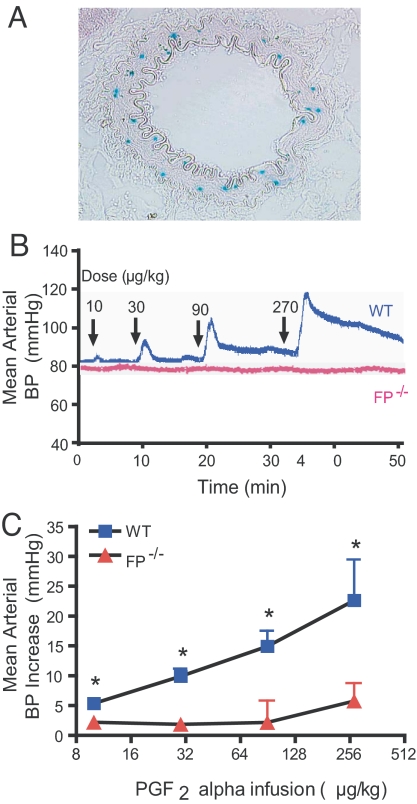
FP Is Expressed in Resistance Arterioles and Elevates BP.
Given that the lacZ gene was knocked into the endogenous FP receptor locus in the FP-targeting allele (13), the receptor expression pattern was monitored by mapping β-gal activity. Scattered activity of β-gal was detectable in the medial smooth muscle layer of renal small arteries in FP+/− mice (Fig. 4A), but was evident in neither FP+/− mouse aorta nor in the atherosclerotic lesions of Ldlr−/−/FP−/− mice (Fig. S5 A–F). PGF2α is a vasoconstrictor in vitro (23), and the dose-dependent elevation of BP in anesthetized WTs evoked by infusion of PGF2α is abrogated in FP−/−mice (Fig. 4 B and C). The increase in BP at the highest rate of infusion in the FP−/−mice likely reflects the weak affinity of PGF2α for EP1 and/or EP3 receptors (24, 25).
Fig. 4.
Systemic pressor response induced by PGF2α infusion is disrupted in FP−/− mice. (A) FP expression detected in the medial layer of renal resistant artery in FP+/− by β-gal activity (blue). (B) Representative BP tracing from FP−/− and WT litter mates at different doses of PGF2α infusion (arrows). (C) The BP increase after PGF2α administration in FP−/− and WT mice. The BP increase is the difference between peak/trough after injection and baseline. Value are presented as mean ± SEM. *, P < 0.05 (n = 7).
Aberrant Water Metabolism in FP−/− Mice.
Both daily water consumption (WT, 5.181 mL/25 g body weight ± 0.39; FP−/−, 7.621 mL/25 g body weight ± 0.49, n = 10; P < 0.05) and urine output (WT, 1.527 mL/25 g body weight ± 0.12; FP−/−, 1.845 mL/25 g body weight ± 0.11, n = 9; P = 0.08) tended to be greater in FP−/− mice than controls (Fig. S6 A and B, respectively). Moreover, both urinary (WT, 1,888 mOsm/kg*H2O ± 82.40; FP−/−, 1,473 mOsm/kg*H2O ± 67.16, n = 6–9; P < 0.05) and plasma (WT, 313.2 mOsm/kg*H2O ± 3.5; FP−/−, 302.7 mOsm/kg*H2O ± 2.8, n = 6; P < 0.05) osmolality were reduced in FP−/− mice (Fig. S6 C and D, respectively). Plasma osmolality rose significantly to a similar degree in FP−/− and WT mice (Fig. S6D) that were subjected to 24 h water deprivation. However, a mild defect in regulation of renal medullary osmolality was apparent (1,859 ± 84.3 mOsm/kg*H2O in FP−/− vs. 2,329 ± 197.8 mOsm/kg*H2O in WT; n = 12–14; P < 0.05) (Fig. S6E). Activation of the FP inhibits water absorption in the rabbit collecting duct in vitro (15), implicating arginine-vasopressin. The FP was detected in microdissected WT hypothalamus (Fig. S6F), and hypothalamic arginine-vasopressin was up-regulated in the FP−/− mice (Fig. S6G).
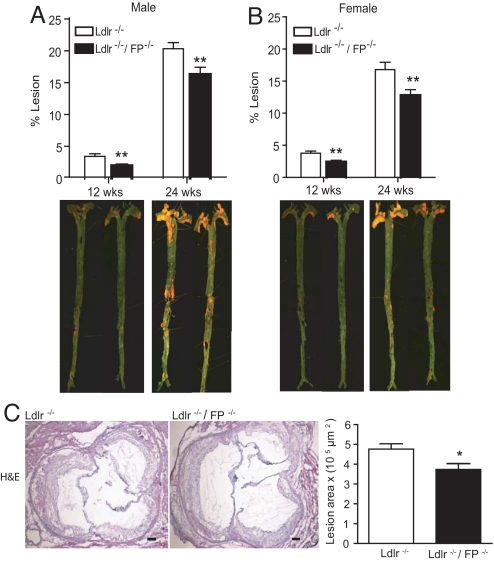
FP Disruption Attenuates Atherogenesis in Ldlr-Deficient Mice.
Total body weight and plasma cholesterol did not differ between Ldlr−/− control and Ldlr−/−/FP−/− mice. Atherosclerotic lesional area, calculated en face, was reduced by FP deletion (males, average 42% reduction; P = 0.006 at 12 wks HFD; average 20% reduction; P = 0.01 at 24 wks HFD; females, average 37% reduction; P = 0.003 at 12 wks HFD; 24% reduction; P = 0.009 at 24 wks HFD) (Fig. 5 A and B). Lesional burden, as assessed by cross-sectional analysis of the aortic root, was also reduced in Ldlr−/−/FP−/− mice (3.7 × 105 μm2 ± 0.2 vs. 4.7 × 105 μm2 ± 0.2; P = 0.02) (Fig. 5C). Both in situ and X-gal staining of aortas and aortic root sections failed to detect FP expression in either normal or hyperlipidemic mice (Fig. S2).
Fig. 5.
Reduced atherosclerotic lesional development in FP−/− mice. Atherosclerotic lesion area measured by en face staining in male (A) and female (B) Ldlr−/− and Ldlr−/−/FP−/− mice at 12 and 24 weeks on HFD. (C) Aortic root sections (8 μm) from male mice on HFD for 24 weeks were stained by using H&E, with the lesional area calculated. (Scale bar: 100 μm.) Values are presented as mean ± SEM and analyzed by unpaired Student t test, *, P < 0.05 (n = 8–10); **, P < 0.01 (n = 12–16) versus Ldlr−/− control mice.
Surface macrophage staining (CD68) as a percentage of total lesional area at the aortic root (average 46%) was reduced (n = 8, P = 0.0007) (Fig. 6 A and B) in Ldlr−/−/FP−/− mice. There was no observable difference in the immunostaining of the other cellular and matrix markers tested (smooth muscle cell marker α-actin, Fig. S7A; endothelial cell marker PECAM-1, Fig. S7B; collagen, Fig. 7C). Correspondingly, lesional expression of the inflammatory cytokines, TNFα, TGFβ, and inducible nitric oxide enzyme was reduced in Ldlr−/−/FP−/− mice at 24 weeks on the HFD (Fig. 6 C–E). This result raised the possibility that PGF2α might regulate macrophage cytokine production directly and thereby influence atherogenesis. However, FP deletion did not alter basal or lipopolysaccharide-stimulated cytokine production ex vivo (TNFα, Fig. S8A; TGFβ, Fig. S8B; IL-6, Fig. S8C; IL-12, Fig. S8D; inducible nitric oxide enzyme, Fig. S8E) in peritoneal macrophages from Ldlr−/−, nor was FP expression detectable by real-time RT-PCR in cultured peritoneal macrophages as previously reported (26). Thus, the reduction in lesional cytokines might result from, rather than mediate, the retardation of atherogenesis in Ldlr−/−/FP−/− mice.
Fig. 6.
Reduced macrophage accumulation and inflammatory cytokine profile in aorta from FP−/− mice. (A) Representative aortic root sections (8 μm) from Ldlr−/− and Ldlr−/−/FP−/− mice immunostained by using rat anti-mouse CD68. (Scale bar: 100 μm.) (B) Quantification of CD68-positive staining (brown) to total lesion area in Ldlr−/− control and Ldlr−/−/FP−/− male mice on HFD for 24 weeks. Real-time RT-PCR expression analysis of inflammatory cytokines TNFα (C), TGFβ (D), and inducible nitric oxide enzyme (iNOS) (E) normalized to 18S rRNA in whole aortas from Ldlr−/− control and Ldlr−/−/FP−/− mice on HFD for 12 and 24 weeks. Values are presented as mean ± SEM and analyzed by unpaired Student t test. ***, P < 0.0001 versus Ldlr−/− control mice for immunostaining (n = 8–10). *, P < 0.05; **, P < 0.01; and ***, P < 0.0001 versus Ldlr−/− control mice for quantitative RT-PCR expression (n = 6).
Discussion
Previous studies have established that PGI2 and PGE2 are renin secretagogues, acting via the IP and the EP2 and EP4 receptors, respectively (11, 27). Despite an increase in AT1-mediated responsiveness to exogenous angiotensin II, reflective of suppression of the endogenous RAAS, basal BP is reduced by FP deletion in WT and Ldlr−/− mice. Suppressing the RAAS is sufficient to lower BP in rodents. Previous studies have demonstrated that disruption of the renin (28), angiotensinogen (29), angiotensin-converting enzyme (30), and AT1a (31) all reduce BP. Thus, PGF2α may act via the FP to modulate BP by regulating expression of renin; further experiments will address this possibility.
Nonsteroidal anti-inflammatory drugs elevate BP variably in humans, and the impact of gene disruptions in the PG pathway on BP in rodents is complex and conditioned by genetic background. Deletion of the IP reduces BP in renoprival models of high-renin hypertension in mice (11). However, besides regulating renin release, both PGE2 and PGI2, predominantly products of COX-2, are vasodilators and promote urinary sodium excretion; salt-sensitive hypertension is evident in both EP2−/− and IP−/− mice (12, 15). By contrast, deletion of the EP1 receptor for PGE2 reduces BP (32) and deletion of either the EP1 or the TP receptor for thromboxane (i.e., thromboxane A2) results in a diminished hypertensive response to infused angiotensin II (33, 34). Here, deletion of the FP results in hypotension coincident with depression of the RAAS and occurs despite an augmented response to infused angiotensin II. Furthermore, despite renal tubular expression of the FP and a natriuretic response to PGF2α infusion (35), urinary electrolyte excretion and the response to an aldosterone receptor antagonist is unaltered in FP−/− mice. Thus, the mechanism by which deletion of this receptor reduces BP is quite distinct from that observed with other prostanoids.
PGs modulate the impact of sympathoadrenal activation on both renin synthesis and exocytosis, particularly under conditions of renoprival stress, such as hemorrhage or salt deprivation (36). Synthesis and secretion of renin is controlled at a cellular level by multiple second messengers including Ca2+, cAMP, and cGMP (37). Activation of the Gs coupled EP2, EP4, or IP receptors by PGE2 or PGI2 in JG cells increases intracellular cAMP (38). Here, FP receptors were detected in the proximal afferent arteriole where JG cells are located and FP agonists dose-dependently induced renin expression in cultured JG cells. However, this finding did not coincide with an increase in JG cell cAMP, and patch-clamp studies using single JG cells treated with FP agonists exhibited no change in membrane capacitance, suggesting that the FP does not directly mediate renin exocytosis. High concentrations of PGF2α can also bind to and activate Gi- or Gq-coupled EP1 and EP3 receptors (24, 25), which are coexpressed in JG cells (39, 40). However, we did not observe a significant increase of renin expression in JG cells treated with PGF2α as reported previously (41). JG cells develop from afferent arteriole vascular smooth muscle cells by a reversible metaplastic transformation, which is characterized by numerous granular vesicles and an epithelioid cell shape (42, 43). Their number is subject to homeostatic regulation (37). FP receptor expression is marked in afferent arterioles where renin granular cells were decreased in the FP−/− mice. Thus, rather than acting directly, activation of the FP appears to regulate JG cell differentiation and consequent renin expression, explaining low RAAS activity in FP−/− mice. Moreover, FP−/− mice exhibited modest polyuria and polydipsia with evidence of a mild defect in regulation of renal medullary osmolality on water deprivation. A similar defect in concentrating ability has been reported in mice lacking the homologous EP1 receptor (15).
Hypertension facilitates atherogenesis, probably in part by causing endothelial dysfunction (44, 45). Given our failure to detect FP in atherosclerotic (or normal) aorta, the reduction in BP may have contributed to the impact of FP deletion on atherogenesis; however, the relationship between BP and atherogenesis in mice is complex (46). Reduction of BP with either angiotensin or adrenergic receptor blockade also restrains atherogenesis in mice (5). TP, angiotensin, and adrenergic receptors are all detectable in the vascular smooth muscle cells of large blood vessels and/or in lesional macrophages. Indeed, PGF2α may act as an incidental ligand at the TP in hamster aorta (47). Direct renin inhibition appears to retard atherogenesis by depleting lesional macrophage renin (48). Here, the mechanism by which atherogenesis is restrained is quite distinct: the FP is undetectable in either aortic smooth muscle cells or in lesional macrophages. Indeed, FP deletion fails to modulate macrophage cytokine release. Thus, whereas lesional macrophages are depleted and some inflammatory cytokines depressed in Ldlr−/−/FP−/− mice, this effect reflects, rather than causes, the reduction in plaque burden. Hence, restraint of atherogenesis in Ldlr−/−/FP−/− mice correlates with a reduction in systemic BP and the impact of FP deletion on RAAS activation in the kidney. It remains to be determined whether disordered renal RAAS mediates the effects of FP deletion on either BP or atherogenesis.
The present studies suggest that antagonism of the FP may afford a strategy for the control of BP and its attendant vascular disease.
Materials and Methods
Animal Husbandry.
All animals were housed and procedures carried out according to the guidelines of the Institutional Animal Care and Usage Committee of the University of Pennsylvania. All of the FP−/− (13) and litter-mate control mice for experiments are on a C57BL6 background. For atherosclerosis study, mice at 5–7 weeks of age were placed on a high-fat Western-style diet (88137, 0.7% NaCl; Harlan Teklad) for a period of 12 or 24 weeks.
BP Measurement by Tail Cuff and Telemetry.
Systolic BP was measured in conscious mice (age- and sex-matched) by using a computerized noninvasive tail-cuff system (Visitech Systems) and PA-C20 telemetry probes (Data Sciences International) as described elsewhere (33).
En Face Quantification of Atherosclerosis Lesion Area.
Formalin-fixed aortas were cleaned of adventitial fat, opened longitudinally, and stained with Sudan IV (Sigma-Aldrich). The stained aorta was pinned onto black wax for quantification. Images were photographed and digitized by using the Image Pro analysis system (Phase 3 Imaging Systems). Cross-sectional analysis of lesion area burden was also carried out on H&E-stained 8-μm aortic root sections by using Phase 3 Imaging Systems. Total lesion area >300 μm of the aortic root was measured every 96 μm, with average lesional area derived from 3–4 sections.
Statistical Analysis.
Data are expressed as mean ± SEM. Analyses were performed initially by ANOVA and, if appropriate, by subsequent pair-wise comparisons. Distribution-free approaches were used to avoid assumptions as to the normality of the distributions of the variables involved. Differences were considered statistically significant at P < 0.05. Prism 4.0 software (GraphPad InStat 3) was used for all of the calculations.
For additional information, see SI Methods.
Supplementary Material
Acknowledgments.
We thank S. Narumiya (Kyoto University, Sakyo-ku, Kyoto, Japan) for providing FP KO mice. We also thank J. Zhen and W. Yan for excellent technical assistance. The study was supported by National Institutes of Health Grants HL-083799, HL-62250 (to G.A.F), and HL-066223 (to E.M.S.), and American Heart Association Jon Holden DeHaan Scientist Development Grant 0730314N (to Y.Y.). G.A.F. is the McNeill Professor in Translational Medicine and Therapeutics.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811834106/DCSupplemental.
References
- 1.Swales J. Textbook of Hypertension. Boston: Blackwell Scientific; 1994. [Google Scholar]
- 2.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 3.Heo HJ, et al. Endogenous angiotensin II enhances atherogenesis in apoprotein E-deficient mice with renovascular hypertension through activation of vascular smooth muscle cells. Life Sci. 2007;80:1057–1063. doi: 10.1016/j.lfs.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Knowles JW, et al. Enhanced atherosclerosis and kidney dysfunction in eNOS(−/−)Apoe(−/−) mice are ameliorated by enalapril treatment. J Clin Invest. 2000;105:451–458. doi: 10.1172/JCI8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nussberger J, et al. Renin inhibition by aliskiren prevents atherosclerosis progression: Comparison with irbesartan, atenolol, and amlodipine. Hypertension. 2008;51:1306–1311. doi: 10.1161/HYPERTENSIONAHA.108.110932. [DOI] [PubMed] [Google Scholar]
- 6.FitzGerald GA. The choreography of cyclooxygenases in the kidney. J Clin Invest. 2002;110:33–34. doi: 10.1172/JCI16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi Z, Hao CM, et al. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest. 2002;110:61–69. doi: 10.1172/JCI14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang T, et al. Influence of genetic background and gender on hypertension and renal failure in COX-2-deficient mice. Am J Physiol-Renal. 2005;288:F1125–1132. doi: 10.1152/ajprenal.00219.2004. [DOI] [PubMed] [Google Scholar]
- 9.Jackson EK, et al. 6-Keto-prostaglandin E1 is more potent than prostaglandin I2 as a renal vasodilator and renin secretagogue. J Pharmacol Exp Ther. 1981;216:24–27. [PubMed] [Google Scholar]
- 10.Fitzgerald DJ, Entman SS, Mulloy K, FitzGerald GA. Decreased prostacyclin biosynthesis preceding the clinical manifestation of pregnancy-induced hypertension. Circulation. 1987;75:956–963. doi: 10.1161/01.cir.75.5.956. [DOI] [PubMed] [Google Scholar]
- 11.Fujino T, et al. Decreased susceptibility to renovascular hypertension in mice lacking the prostaglandin I2 receptor IP. J Clin Invest. 2004;114:805–812. doi: 10.1172/JCI21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francois H, et al. Prostacyclin protects against elevated blood pressure and cardiac fibrosis. Cell Metab. 2005;2:201–207. doi: 10.1016/j.cmet.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto Y, et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997;277:681–683. doi: 10.1126/science.277.5326.681. [DOI] [PubMed] [Google Scholar]
- 14.Saito O, et al. Expression of the prostaglandin F receptor (FP) gene along the mouse genitourinary tract. Am J Physiol-Renal. 2003;284:F1164–1170. doi: 10.1152/ajprenal.00441.2002. [DOI] [PubMed] [Google Scholar]
- 15.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol. 2008;70:357–377. doi: 10.1146/annurev.physiol.70.113006.100614. [DOI] [PubMed] [Google Scholar]
- 16.Takayama K, et al. Thromboxane A2 and prostaglandin F2alpha mediate inflammatory tachycardia. Nat Med. 2005;11:562–566. doi: 10.1038/nm1231. [DOI] [PubMed] [Google Scholar]
- 17.Brown MJ. Aliskiren. Circulation. 2008;118:773–784. doi: 10.1161/CIRCULATIONAHA.108.787630. [DOI] [PubMed] [Google Scholar]
- 18.Pratt JH. The adrenergic nervous system conversing with the adrenal cortex: new implications for salt-sensitive hypertension. Hypertension. 2006;48:820–821. doi: 10.1161/01.HYP.0000244561.84382.df. [DOI] [PubMed] [Google Scholar]
- 19.Casellas D, Dupont M, Kaskel FJ, Inagami T, Moore LC. Direct visualization of renin-cell distribution in preglomerular vascular trees dissected from rat kidney. Am J Physiol. 1993;265:F151–156. doi: 10.1152/ajprenal.1993.265.1.F151. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong RA, Lawrence RA, Jones RL, Wilson NH, Collier A. Functional and ligand binding studies suggest heterogeneity of platelet prostacyclin receptors. Br J Pharmacol. 1989;97:657–668. doi: 10.1111/j.1476-5381.1989.tb12001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stjernschantz J, Alm A. Latanoprost as a new horizon in the medical management of glaucoma. Curr Opin Ophthalmol. 1996;7:11–17. doi: 10.1097/00055735-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Sharif NA, Davis TL, Williams GW. [3H]AL-5848 ([3H]9beta-(+)-Fluprostenol). Carboxylic acid of travoprost (AL-6221), a novel FP prostaglandin to study the pharmacology and autoradiographic localization of the FP receptor. J Pharm Pharmacol. 1999;51:685–694. doi: 10.1211/0022357991772989. [DOI] [PubMed] [Google Scholar]
- 23.Whittle BJ, Oren-Wolman N, Guth PH. Gastric vasoconstrictor actions of leukotriene C4, PGF2 alpha, and thromboxane mimetic U-46619 on rat submucosal microcirculation in vivo. Am J Physiol. 1985;248:G580–586. doi: 10.1152/ajpgi.1985.248.5.G580. [DOI] [PubMed] [Google Scholar]
- 24.Kiriyama M, et al. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abramovitz M, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 26.Rouzer CA, et al. RAW264.7 cells lack prostaglandin-dependent autoregulation of tumor necrosis factor-alpha secretion. J Lipid Res. 2005;46:1027–1037. doi: 10.1194/jlr.M500006-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Friis UG, et al. Prostaglandin E2 EP2 and EP4 receptor activation mediates cAMP-dependent hyperpolarization and exocytosis of renin in juxtaglomerular cells. Am J Physiol-Renal. 2005;289:F989–997. doi: 10.1152/ajprenal.00201.2005. [DOI] [PubMed] [Google Scholar]
- 28.Yanai K, et al. Renin-dependent cardiovascular functions and renin-independent blood-brain barrier functions revealed by renin-deficient mice. J Biol Chem. 2000;275:5–8. doi: 10.1074/jbc.275.1.5. [DOI] [PubMed] [Google Scholar]
- 29.Kim HS, et al. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci USA. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krege JH, et al. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–148. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- 31.Ito M, et al. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stock JL, et al. The prostaglandin E2 EP1 receptor mediates pain perception and regulates blood pressure. J Clin Invest. 2001;107:325–331. doi: 10.1172/JCI6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan Y, et al. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest. 2007;117:2496–2505. doi: 10.1172/JCI29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francois H, Athirakul K, Mao L, Rockman H, Coffman TM. Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertension. 2004;43:364–369. doi: 10.1161/01.HYP.0000112225.27560.24. [DOI] [PubMed] [Google Scholar]
- 35.Stier CT, Jr, Roberts LJ, II, Wong PY. Renal response to 9 alpha, 11 beta-prostaglandin F2 in the rat. J Pharmacol Exp Ther. 1987;243:487–491. [PubMed] [Google Scholar]
- 36.Schweda F, Friis U, Wagner C, Skott O, Kurtz A. Renin release. Physiology. 2007;22:310–319. doi: 10.1152/physiol.00024.2007. [DOI] [PubMed] [Google Scholar]
- 37.Kurtz A, Wagner C. Cellular control of renin secretion. J Exp Biol. 1999;202:219–225. doi: 10.1242/jeb.202.3.219. [DOI] [PubMed] [Google Scholar]
- 38.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: Structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 39.Morath R, Klein T, Seyberth HW, Nusing RM. Immunolocalization of the four prostaglandin E2 receptor proteins EP1, EP2, EP3, and EP4 in human kidney. J Am Soc Nephrol. 1999;10:1851–1860. doi: 10.1681/ASN.V1091851. [DOI] [PubMed] [Google Scholar]
- 40.Jensen BL, Skouv J, Lundholt BK, Lykkesfeldt AE. Differential regulation of specific genes in MCF-7 and the ICI 182780-resistant cell line MCF-7/182R-6. Br J Cancer. 1999;79:386–392. doi: 10.1038/sj.bjc.6690061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen BL, Schmid C, Kurtz A. Prostaglandins stimulate renin secretion and renin mRNA in mouse renal juxtaglomerular cells. Am J Physiol. 1996;271:F659–669. doi: 10.1152/ajprenal.1996.271.3.F659. [DOI] [PubMed] [Google Scholar]
- 42.Barajas L. Anatomy of the juxtaglomerular apparatus. Am J Physiol. 1979;237:F333–343. doi: 10.1152/ajprenal.1979.237.5.F333. [DOI] [PubMed] [Google Scholar]
- 43.Taugner R, Buhrle CP, Hackenthal E, Mannek E, Nobiling R. Morphology of the juxtaglomerular apparatus and secretory mechanisms. Contrib Nephrol. 1984;43:76–101. doi: 10.1159/000409945. [DOI] [PubMed] [Google Scholar]
- 44.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 45.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 46.Lu H, Cassis LA, Daugherty A. Atherosclerosis and arterial blood pressure in mice. Curr Drug Targets. 2007;8:1181–1189. doi: 10.2174/138945007782403829. [DOI] [PubMed] [Google Scholar]
- 47.Wong SL, et al. Cyclooxygenase-2-derived prostaglandin F2alpha mediates endothelium-dependent contractions in the aortae of hamsters with increased impact during aging. Circ Res. 2009;104:228–235. doi: 10.1161/CIRCRESAHA.108.179770. [DOI] [PubMed] [Google Scholar]
- 48.Lu H, et al. Renin inhibition reduces hypercholesterolemia-induced atherosclerosis in mice. J Clin Invest. 2008;118:984–993. doi: 10.1172/JCI32970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.