SUMMARY
“Oncogene addiction” describes an unexplained dependency of cancer cells on a particular cellular pathway for survival or proliferation. We report that differential attenuation rates of pro-survival and pro-apoptotic signals in oncogene-dependent cells contribute to cell death following oncogene inactivation. Src-, BCR-ABL-, and EGF receptor-dependent cells exhibit a similar profile of signal attenuation following oncogene inactivation characterized by rapid diminution of phospho-ERK, -Akt, and –STAT3/5, and a delayed accumulation of the pro-apoptotic effector, phospho-p38 MAPK. These findings implicate a transient imbalance in survival and apoptotic oncogenic outputs in the apoptotic response to oncogene inactivation. Moreover, these observations implicate a common profile of signal attenuation for multiple oncogenes, and suggest that “addiction” associated with apoptosis reflects an active, not a passive process.
SIGNIFICANCE
The phenomenon of “oncogene addiction” has now been well documented in multiple mouse tumor models and cancer cell lines. Moreover, oncogene addiction may account for the dramatic clinical responses reported in some cancer patients treated with targeted kinase inhibitors. However, a molecular mechanism to explain oncogene addiction has been elusive. Our findings suggest that differential decay rates of pro-survival and pro-apoptotic signals emanating from an oncoprotein, such as an activated kinase, can contribute to tumor cell death following acute inactivation of an oncogene upon which they have become dependent. Our findings represent the first experimental studies that attempt to provide a molecular mechanism for oncogene dependency, and they may have important implications for the therapeutic use of targeted kinase inhibitors.
INTRODUCTION
“Oncogene addiction” is a term that was first coined by Bernard Weinstein to describe the apparent acquisition of dependency by tumor cells on a single oncogenic activity (Weinstein, 2000; Weinstein, 2002; Weinstein et al., 1997). This phenomenon has been most clearly illustrated in several different transgenic mouse models of tumorigenesis, and is characterized by the proliferative arrest, differentiation, and/or apoptosis of tumor cells upon the acute inactivation of an oncogene that initially contributed to the tumor phenotype. For example, in a leukemic model in which inducible transgenic Myc overexpression causes T cell and myeloid leukemias, switching off the Myc oncogene causes tumor cells to undergo growth arrest, differentiation, and apoptotic cell death (Felsher and Bishop, 1999). Similarly, in a transgenic model of BCR-ABL-induced leukemia, switching off the transgene results in rapid apoptosis of leukemic cells (Huettner et al., 2000). The oncogene addiction phenomenon appears to apply to solid tumors as well, since in a model of conditional transgenic H-Ras-induced mouse melanomas, turning off the activated Ras gene causes massive apoptosis within tumors (Chin et al., 1999).
In addition to these transgenic oncogene models, cell culture studies of human cancer cells have further substantiated the concept that tumor cells can become dependent on a single oncogenic pathway for their sustained proliferation or survival. For example, human pancreatic cancer cell lines harboring a mutationally activated K-Ras oncogene can be growth inhibited by introducing antisense K-Ras oligonucleotides (Aoki et al., 1997). Similarly, selective kinase inhibitors that target either the BCR-ABL fusion kinase, such as imatinib (Gleevec) (Druker et al., 1996; Gambacorti-Passerini et al., 1997) or gefitinib (Iressa) or erlotinib (Tarceva) which target the EGF receptor kinase (Mukohara et al., 2005) can efficiently kill a subset of cultured tumor cells that express those oncogenes. Such findings seem to indicate that many tumor cells, despite the accumulation of multiple genetic alterations, retain dependency on a limited number of oncogenes that initially drove them to a malignant phenotype.
The apparent dependency on individual oncogenes exhibited by tumor cells potentially reveals an “Achilles’ heel”, or vulnerable point, within such cells that renders them susceptible to the activities of anti-tumor agents that selectively target these oncogene products (Weinstein, 2002). Indeed, examples of dramatic clinical response have been observed in a subset of BCR-ABL-positive chronic myelogenous leukemia patients treated with imatinib (O’Dwyer et al., 2003). Similarly, a subset of patients with non-small cell lung cancer, where mutationally activated or amplified EGF receptors are sometimes observed, exhibit striking clinical responses to gefitinib and erlotinib (Lynch et al., 2004; Paez et al., 2004; Pao et al., 2004). It is believed that such responses similarly reflect the phenomenon of oncogene addiction, thereby highlighting its importance in the context of cancer therapeutics that target activated oncoproteins.
Despite accumulating evidence (largely derived from transgenic mouse models, cell culture studies of human cancer cell lines, and clinical studies of targeted kinase inhibitors) that oncogene addiction is a widespread and important phenomenon, a molecular mechanism to explain it has yet to be clearly elucidated. It has been suggested that the “circuitry” of a cancer cell has somehow been corrupted such that it acquires a dependency on signaling pathways that are not normally required in the cell from which the tumor cell was derived (Weinstein, 2002). This could certainly be true, although it has been difficult to prove this experimentally. We have recently proposed a related but distinct hypothesis to explain oncogene addiction (Sharma et al., 2006). We propose that differential attenuation rates among the multiple pro-apoptotic and pro-survival signals emanating from an activated oncogene result in a transient imbalance in signaling. This imbalance results from the fact that survival signals are relatively short-lived following acute inactivation of the oncogene, while pro-apoptotic signals persist sufficiently long to produce an apoptotic outcome. To test this theoretical model experimentally, we have examined the attenuation kinetics of known survival effectors following the inactivation of the Src, BCR-ABL, and EGF receptor oncogenes in several cell culture models associated with oncogene dependency. We determined that a common profile of signal attenuation involving the ERK, Akt, and STAT3/5 proteins is associated with oncogene inactivation in each of these cases, and that in each setting, a temporally delayed accumulation of at least one pro-apoptotic effector, phospho-p38 MAP kinase is observed. Based on these and additional findings described below, we conclude that a shared mechanism of differential signal attenuation can contribute to the oncogene addiction phenomenon in multiple different contexts.
RESULTS
Modeling oncogene addiction in cells dependent on the BCR-ABL oncogene
To model the phenomenon of oncogene addiction in cultured cells, we took advantage of a previously described cell line, TonB210.1, in which the BCR-ABL oncoprotein that is detected in the vast majority of human chronic myelogenous leukemias (CML), is expressed in a doxycycline-regulated manner (Klucher et al., 1998). TonB210.1 cells were derived from an interleukin-3 (IL-3)-dependent hematopoietic cell line (BAF3), and expression of the BCR-ABL protein in these cells renders them IL-3 independent in a cell viability assay. As previously reported (Klucher et al., 1998), these cells undergo massive apoptotic cell death within 48–72 hours following disruption of BCR-ABL expression (not shown). This dependency on maintained BCR-ABL survival signaling appears to model the well established clinical response to ABL kinase inhibitors seen in some CML patients.
We observed that levels of BCR-ABL protein in TonB210.1 cells begin to decline within 8 hours of doxycycline treatment (Fig. 1), and this is accompanied by a concomitant decrease in the phosphorylation of several major phospho-tyrosine-containing proteins (including BCR-ABL itself), as revealed by immunoblotting of cell lysates with a phospho-tyrosine antibody (Fig. 1). In these doxycycline-treated cells, morphologic indications of apoptosis become apparent within 8 hours of treatment, and cleaved PARP, a marker of apoptosis, can also be detected at this time point (data not shown). Between 24 and 48 hours following treatment, massive apoptosis becomes evident and the vast majority of cells are dead within 72 hours. Thus, as previously described, these cells are highly dependent on BCR-ABL expression for their survival.
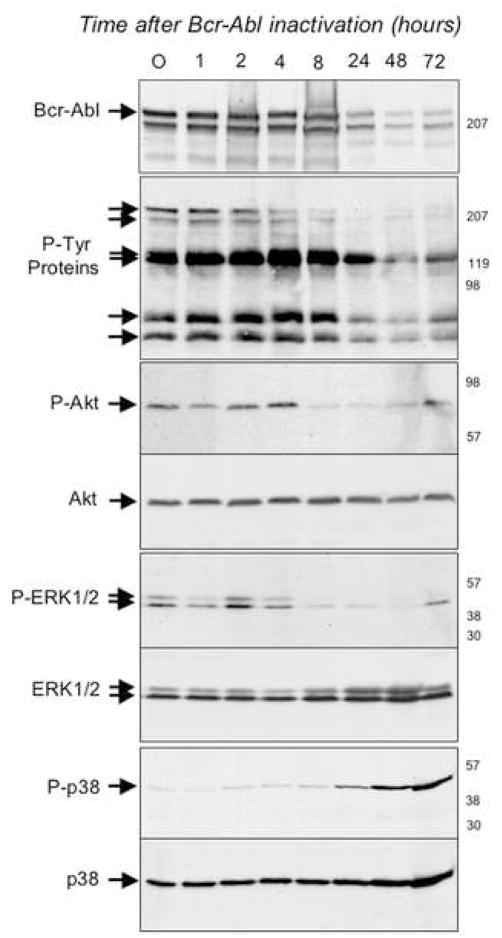
Figure 1. Modulation of signaling following acute Bcr-Abl inactivation in TonB210.1 cells.
Bcr-Abl was induced in TonB210.1 cells by doxacycline treatment for 18h. Bcr-Abl was then inactivated by the removal of doxacycline and at various times post-inactivation of Bcr-Abl cells were harvested and their proteins were analyzed by SDS-PAGE followed by Western blotting using antibodies directed against Bcr; phospho-tyrosine; phospho-Akt (P-Akt); Akt; phospho-ERK1/2 (P-ERK1/2); ERK1/2; phospho-p38 (P-p38); p38. Times post-inactivation of Bcr-Abl (in hours) are indicated at the top of each lane, the relative migration of the relevant proteins are indicated on the left-hand side of each autoradiogram and to the right of each autoradiogram are indicated the relative migration of molecular weight standards. In the second panel, arrows point to several unidentified proteins whose tyrosine phosphorylation appears to decrease upon Bcr-Abl inactivation.
To determine the kinetics of signal attenuation associated with known downstream mediators of cell survival in this setting, we examined the phosphorylation status of Akt, STAT-5, and ERK1,2 (Steelman et al., 2004). As shown in figure 1 (and data not shown), each of these proteins is phosphorylated for as long as BCR-ABL protein is active (up to 4 hours). At 8 hours post-doxycycline treatment, concomitant with BCR-ABL inactivation, the phosphorylation of each of the proteins is substantially diminished (Fig. 1 and data not shown). Thus, these 3 “survival effectors” are each rapidly inactivated following disruption of BCR-ABL activity. We also examined the phosphorylation status of the p38 MAP kinase, an established downstream target of BCR-ABL that has been implicated in pro-apoptotic signaling in some contexts (Bandyopadhyay et al., 2004). Interestingly, while phospho-p38 MAP kinase levels are barely detectable prior to BCR-ABL inactivation, a clear increase in phospho-p38 is observed within 24 hours of doxycycline treatment, suggesting that a temporally delayed activation of p38 contributes to the apoptotic response that follows acute inactivation of BCR-ABL (Fig. 1).
Modeling oncogene addiction in cells dependent on a mutationally activated EGF receptor
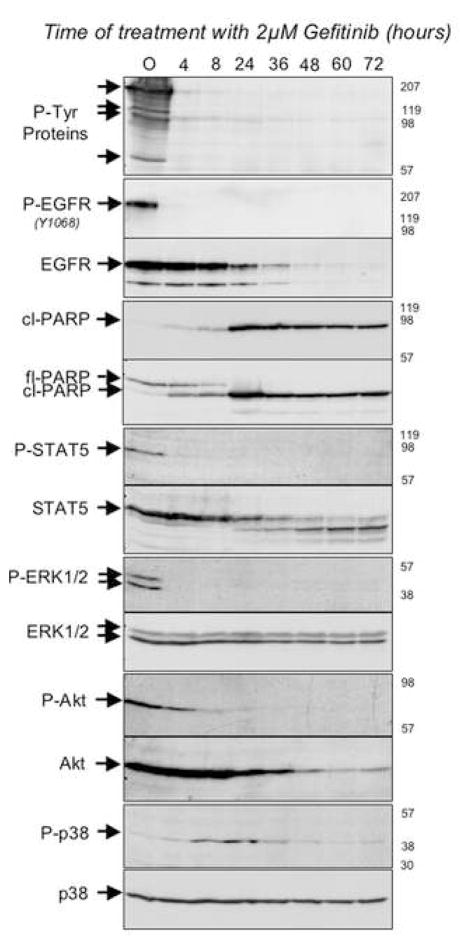
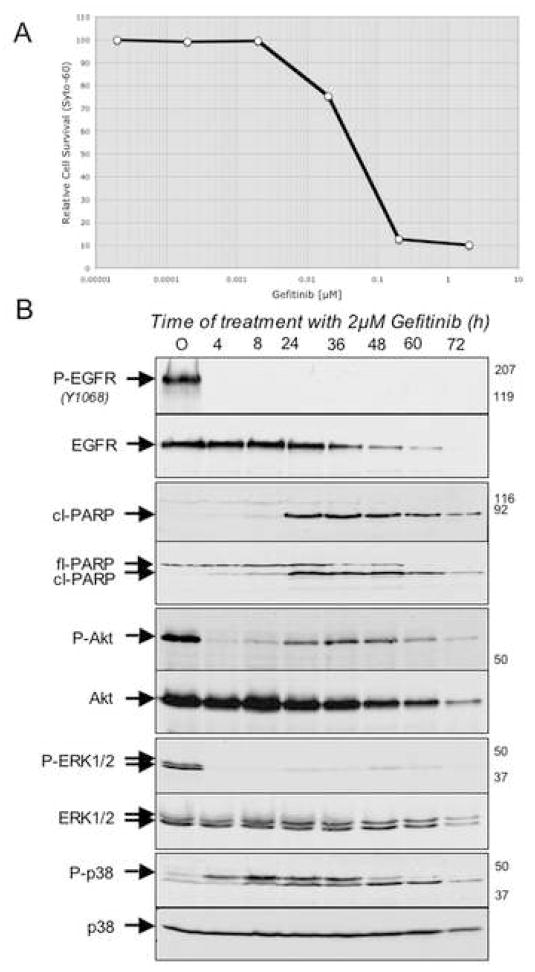
We next wanted to determine whether the inactivation of survival effectors and subsequent accumulation of activated p38 that was observed upon BCR-ABL inactivation could extend to other cell culture models of oncogene dependency. Therefore, we took advantage of another BAF3-derived cell line in which expression of a mutationally activated form of the EGF receptor that has been detected in a subset of non-small cell lung cancers confers IL-3-independent cell survival (Jiang et al., 2005). In these cells, treatment with the selective EGF receptor kinase inhibitor, gefitinib, results in the virtual complete inactivation of EGFR autophosphorylating activity within 4 hours, and massive apoptotic cell death within 72 hours, as previously reported (Jiang et al., 2005) (Fig. 2 and data not shown). As was seen in the TonB210.1 cells upon inactivation of BCR-ABL, cleaved PARP can be detected in these cells within 24 hours of EGFR inactivation (Fig. 2). Moreover, within 4 hours of gefitinib treatment, levels of phospho- STAT5, ERK1,2, and Akt are all substantially reduced (Fig. 2), and between 8 and 24 hours, phospho-p38 levels accumulate (Fig. 2G). Thus, models of oncogene dependency driven by either BCR-ABL or EGFR appear to involve a shared mechanism of signal attenuation following oncogene inactivation that involves the relatively rapid decay of survival signaling followed by a delayed activation of the p38 pro-apoptotic kinase.
Figure 2. Modulation of signaling following acute EGFR (L858R) inactivation in BaF3-EGFR(L858R) cells.
BaF3-EGFR(L858R) cells constitutively express the L858R activating mutation in EGFR which renders the BaF3 cells independent of IL-3. EGFR (L858R) was inactivated by treating cells with 2μM of Gefitinib and at various times post-treatment cells were harvested and their proteins were analyzed by SDS-PAGE followed by Western blotting using antibodies directed against phospho-tyrosine (P-Tyr proteins); phospho-EGFRY1068 (P-EGFR); EGFR; cleaved PARP (cl-PARP); full-length PARP (fl-PARP); phospho-STAT-5 (P-STAT5); STAT-5; phospho-ERK1/2 (P-ERK1/2); ERK1/2; phospho-Akt (P-Akt); Akt; phospho-p38 (P-p38); p38. Times post-treatment with Gefitinib (in hours) are indicated at the top of each lane, the relative migration of the relevant proteins are indicated on the left-hand side of each autoradiogram and to the right of each autoradiogram are indicated the relative migration of molecular weight standards. In the top panel, arrows point to several unidentified proteins whose tyrosine phosphorylation appears to decrease upon v-src inactivation.
Modeling Src kinase dependency using a temperature-sensitive switch
Like BCR-ABL and EGFR kinases, the Src kinase has also been implicated in human tumorigenesis, and can also transduce pro-survival as well as pro-apoptotic activities. This signaling duality of Src has been most clearly demonstrated using a fibroblast cell line in which a temperature-sensitive activated c-Src mutant (Maroney et al., 1992) is expressed to produce a malignant phenotype associated with morphologic transformation (Johnson et al., 2000). When cultured in 10% serum at 39.5 degrees, the non-permissive temperature (Src is inactive), these cells exhibit a relatively flat, non-transformed appearance in culture. When the temperature is shifted to 35 degrees, the permissive temperature, these cells rapidly become spindle-shaped and refractile, exhibiting clear characteristics of Src-transformed fibroblasts (Fig. 3A,B). When these cells are shifted to 39.5 degrees, and the serum concentration is reduced to 0.2%, substantial levels of apoptosis are observed within two hours of the temperature shift (Fig. 3C,D). Significantly, the parental fibroblasts from which these Src-transformed cells were derived do not undergo apoptosis in reduced serum, even when shifted to 39.5 degrees (data not shown). Thus, this cell culture model appears to represent an additional setting in which an oncogene addiction-like phenomenon is involved.
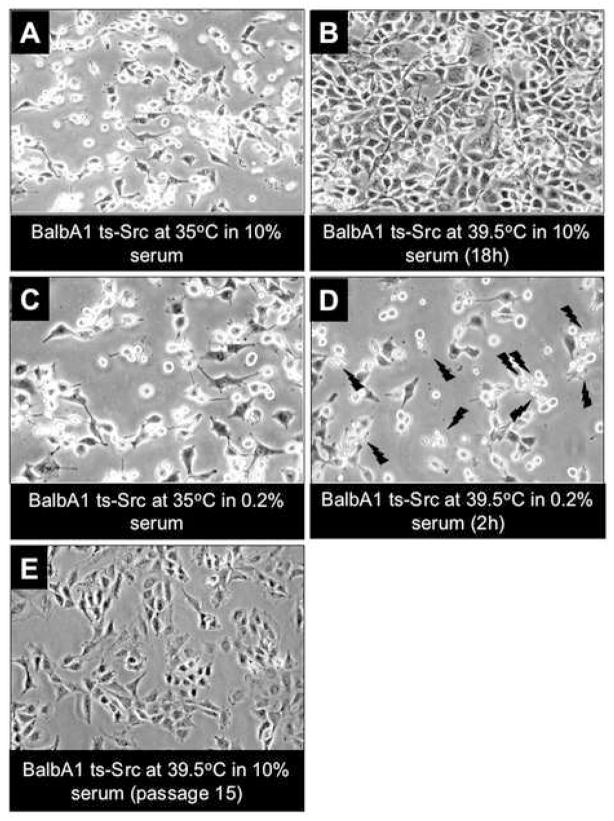
Figure 3. Morphological alterations induced by changes in serum and temperature in Balb-A1 ts-Src cells.
A: Balb-A1 ts-Src cells growing in 10% serum containing medium at 35°C display the typical transformed morphology of Src-transformed cells.
B: Overnight incubation at 39.5°C in the presence of 10% serum causes the cells to revert to a normal morphology.
C: Shown at a slightly higher magnification are Balb-A1 ts Src cells growing over-night in 0.2% serum containing medium at 35°C.
D: When cells shown in panel C were shifted to 39.5°C (in 0.2% serum) cellular apoptosis was evident (indicated by jagged arrows) within two hours after the temperature shift.
E: Morphology of Balb-A1 ts-Src cells maintained continuously at 39.5°C in the presence of 10% serum, after 15 passages.
By examining phospho-proteins in these cells following acute Src inactivation, we determined that within 30 minutes of Src inactivation, levels of phosphotyrosinated proteins rapidly begin to decline (Fig. 4A), cleaved PARP begins to accumulate (Fig. 4A), and levels of phospho-STAT3, -Akt, and -ERK1,2 are substantially diminished (Fig. 4A). Moreover, levels of phospho-p38 begin to increase within 1 hour of Src inactivation, and continue to increase until about 6 hours following temperature-shift (Fig. 4A).
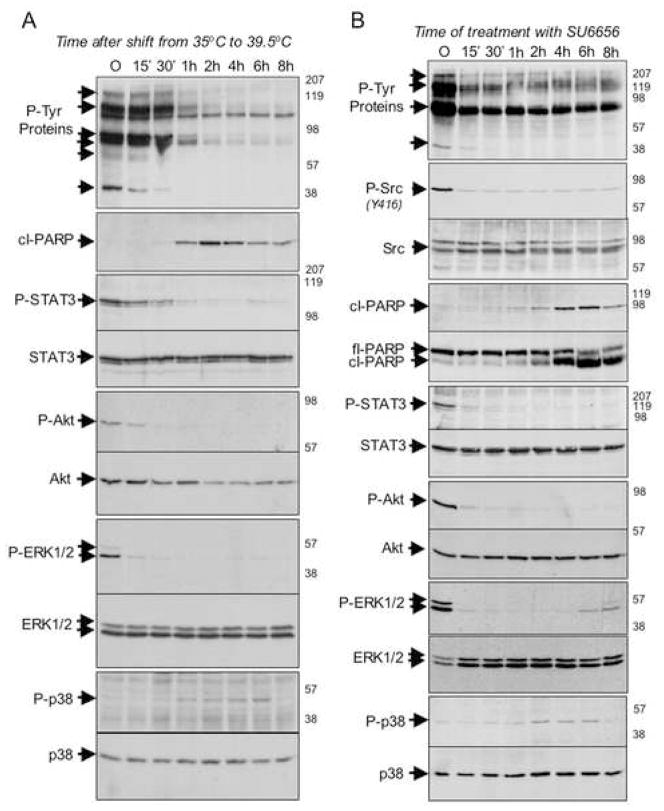
Figure 4. Modulation of signaling following acute Src inactivation in Balb-A1 ts-Src cells and Ψ2 v-Src 3T3 cells.
A: The ts-Src oncoprotein was inactivated in serum-starved Balb-A1 ts-Src cells by shifting cells to 39.5°C. At various times post-inactivation of Src, cells were harvested and their proteins were analyzed by SDS-PAGE followed by Western blotting using antibodies directed against phospho-tyrosine (P-Tyr proteins); cleaved PARP (cl-PARP); phospho-STAT-3 (P-STAT3); STAT-3; phospho-Akt (P-Akt); Akt; phospho-ERK1/2 (P-ERK1/2); ERK1/2; phospho-p38 (P-p38); p38. Times post-inactivation of Src (in minutes and hours) are indicated at the top of each lane, the relative migration of the relevant proteins are indicated on the left-hand side of each autoradiogram and to the right of each autoradiogram are indicated the relative migration of molecular weight standards.
B: V-src oncoprotein was inactivated in serum-starved Ψ2 v-Src 3T3 cells by the addition of 3μM of SU-6656, a src-specific inhibitor. At various times post-inactivation of Src, cells were harvested and their proteins were analyzed by SDS-PAGE followed by Western blotting using antibodies directed against phospho-tyrosine (P-Tyr proteins); activated phospho-src (P-Src); total src; cleaved PARP (cl-PARP); full-length PARP (fl-PARP); phospho-STAT-3 (P-STAT3); STAT-3; phospho-Akt (P-Akt); Akt; phospho-ERK1/2 (P-ERK1/2); ERK1/2; phospho-p38 (P-p38); p38. Times post-inactivation of Src (in minutes and hours) are indicated at the top of each lane, the relative migration of the relevant proteins are indicated on the left-hand side of each autoradiogram and to the right of each autoradiogram are indicated the relative migration of molecular weight standards. In the top panel, arrows point to several unidentified proteins whose tyrosine phosphorylation appears to decrease upon v-src inactivation.
To verify independently that the observed signaling changes following temperature shift truly reflect the consequences of Src-inactivation, analogous studies were performed using a selective small molecule inhibitor of the Src kinase, SU-6656 (Blake et al., 2000). 3T3 mouse fibroblasts that had been transformed with a viral form of activated Src begin to exhibit apoptotic characteristics within 2 hours of treatment with SU-6656, and exhibit similar changes in the phosphorylation of STAT3, Akt, ERK1,2, and p38 proteins as seen in cells transformed by the temperature-sensitive Src mutant upon shift to the non-permissive temperature (Fig. 4B). Thus, the regulated phosphorylation of these proteins exhibits a kinetic profile upon Src inactivation that is analogous to that seen upon acute disruption of the BCR-ABL and activated EGFR kinases. Significantly, the signaling changes and apoptotic effects of Src inhibition with SU-6656 are only revealed in the absence of serum, consistent with a serum-provided survival signal that protects cells from the apoptotic output from oncogenic Src acutely following its inactivation (Figures S1,S2). Moreover, NIH3T3 cells expressing endogenous wild-type Src are unaffected by SU-6656 treatment even in the absence of serum, indicating that the observed apoptotic response is dependent on an output from oncogenic Src (Figure S3). Taken together, these findings suggest that a shared signaling cascade may contribute to “addiction” to Src, BCR-ABL, and EGFR kinases in cell culture models.
Testing the model in a human tumor cell line
The three models of oncogene dependency described above are somewhat artificial in that they involve the introduction of a single oncogene into a cell type that may not correspond closely with the cell type that is malignantly transformed by the oncogene in the context of human tumorigenesis. Therefore, we performed a similar analysis of a human tumor-derived cell line that exhibits features associated with oncogene addiction. Specifically, we used the PC-9 human non-small cell lung cancer line, which harbors an activating mutation within the EGF receptor gene, and has been found to be exquisitely sensitive to the effects of gefitinib in a cell viability assay (Ono et al., 2004). Thus, 2 μM gefitinib kills the vast majority of these cells within 72 hours of treatment (Fig. 5A), with levels of phospho-EGFR rapidly depleted within 4 hours of treatment (Fig. 5B) and levels of cleaved PARP detectable within 8 hours of treatment (Fig. 5B). We observed that levels of phospho-Akt, and phospho-ERK1,2 in PC-9 cells are substantially diminished within 4 hours of gefitinib exposure (Fig. 5B). Notably, phospho-Akt levels partially recover prior to apoptosis, consistent with the signal attenuation model described in the next section. Moreover, levels of phospho-p38 are observed to increase significantly beginning at 4 hours following gefitinib treatment, peaking by 8 hours, and persisting for 36 hours (Fig. 5B). Hence, a profile of signal attenuation among these proteins is seen in gefitinib-treated human lung cancer cells that appears to be quite similar to the profile observed in each of the three models described above.
Figure 5. Modulation of signaling following acute EGFR inactivation in PC-9 cells.
A: A dose-response curve for cell killing by Gefitinib in sub-confluent PC-9 cells following 72 of treatment with the inhibitor.
B: Activated EGFR in PC-9 cells was inactivated by the addition of 2μM of Gefitinib. At various times post-EGFR inactivation, cells were harvested and protein lysates were analyzed by SDS-PAGE followed by Western blotting using antibodies directed against phospho- EGFRY1068 (P-EGFR); total EGFR; cleaved-PARP (cl-PARP); full-length PARP (fl-PARP); phospho-Akt (P-Akt); Akt; phospho-ERK1/2 (P-ERK1/2); ERK1/2; phospho-p38 (P-p38); p38. Times post-inactivation of EGFR (in hours) are indicated at the top of each lane, the relative migration of the relevant proteins are indicated on the left-hand side of each autoradiogram and to the right of each autoradiogram are indicated the relative migration of molecular weight standards.
Oncogene addiction may reflect the timing of signal attenuation
Since we observed that acute inactivation of Src, BCR-ABL, and EGFR oncogenes results in the rapid diminution of several cell survival effectors and the subsequent engagement of at least one pro-apoptotic effector, we wanted to examine a hypothesis that the apoptotic response to oncogene inactivation in these settings actually reflects a transient imbalance between pro-survival and pro-apoptotic signaling. To test this hypothesis, we utilized the temperature-sensitive Src-transformed cells, in which Src activity can be reversibly regulated. We reasoned that, if apoptosis in these cells following Src inactivation results from sustained pro-apoptotic signaling downstream of Src following rapid loss of pro-survival signals, it might be possible to “protect” these cells from apoptosis following Src inactivation by culturing them for a period of time in serum (the survival factor) at 39.5 degrees prior to reducing serum to levels that would otherwise cause apoptosis in these cells.
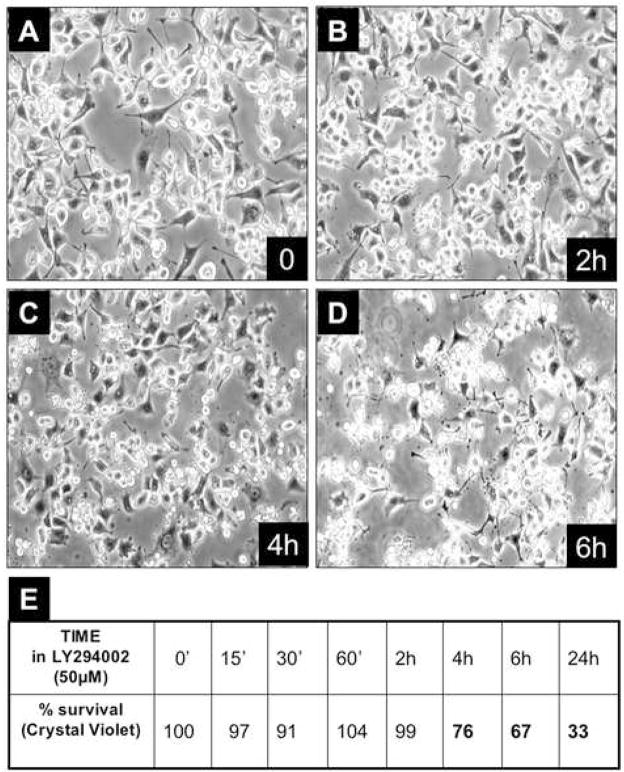
First, we determined that inactivation of the PI-3kinase/Akt pathway using the PI-3 kinase inhibitor, LY294002, in cells maintained at 35 degrees in reduced serum (0.2%) causes a high percentage of cells to undergo apoptosis (Fig. 6), indicating that serum is providing a PI-3-kinase-dependent cell survival signal in this context. Then, we observed that when the Src-TS-transformed cells are initially propagated at 35 degrees (Src is activated) and then shifted to 39.5 degrees (Src is inactivated), they can be maintained in the presence of 10% serum indefinitely (Fig. 3E). Moreover, the cells can then be transferred to low serum concentrations while remaining viable and without indications of apoptosis (data not shown). This finding clearly indicates that these Src-transformed cells are not absolutely dependent on Src activity for their survival and that they can be manipulated in such a way as to suppress the apoptotic response that can follow acute Src inactivation.
Figure 6. Effect of LY282004 on Balb-A1 ts-Src cells at 35°C in 0.2% serum.
A–D: Serum-starved, Balb-A1 ts-Src cells were treated with 3μM LY294002 and at various times post-treatment (0, 2h, 4h, 6h) cell morphology was documented by photomicrography.
E: Cell survival was quantitated by crystal violet staining and tabulated.
Signal attenuation following oncogene activation may be temporally orchestrated via a phosphatase activity
The findings described above suggest that the kinetics of signal attenuation following acute inactivation of these various oncogenes contributes to the apoptotic response, and that this could result from a transient imbalance in pro-survival and pro-apoptotic phosphorylation-mediated signaling. Moreover, the fact that each of these three oncogenic kinases (Src, BCR-ABL, and EGF receptor) has been reported to promote activation of the p38 MAP kinase (Bandyopadhyay et al., 2004; Harper et al., 2002; Turkson et al., 1999), and yet this kinase exhibits an apparent delayed activation upon oncogene inactivation, raises the possibility that relief from a phosphatase activity that suppresses levels of active, phospho-p38 contributes to its delayed accumulation in these settings. In other words, we hypothesize that while “steady-state” phospho-p38 levels appear to be relatively low in each of these cells prior to oncogene inactivation, this may reflect a dynamic balance between p38 phosphorylation and phosphatase-mediated de-phosphorylation in which the phosphatase activity predominates.
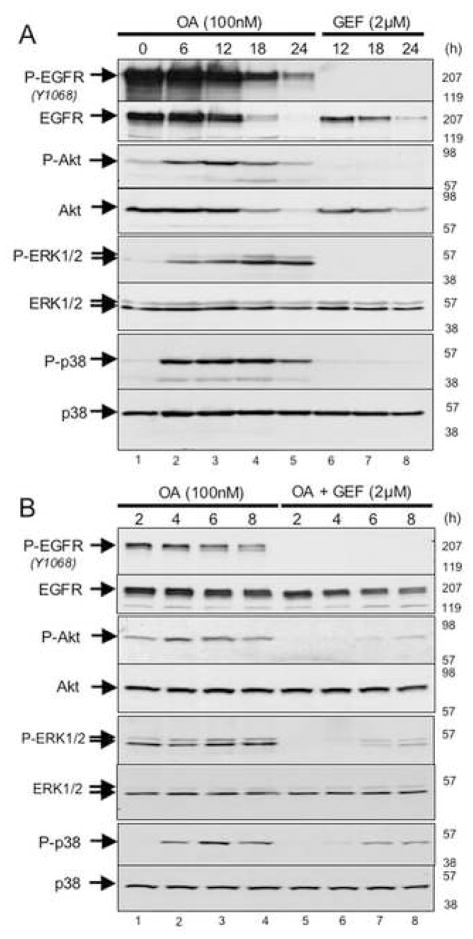
To test this hypothesis, we treated the EGFR-transformed BAF3 cells with the phosphatase inhibitor, okadaic acid, which inhibits the PP2A phosphatase that has been previously shown to regulate phospho-p38 levels (Lee et al., 2003). As shown, okadaic acid treatment of these cells is sufficient to cause the accumulation of phospho-Akt, -ERK, and –p38 within 6 hours of treatment (Fig. 7A; lanes 1–4), suggesting that the activation of these kinases is under negative regulatory control by an okadaic acid-sensitive phosphatase. Note that levels of phospho-EGFR eventually begin to decline, commensurate with the onset of apoptotic cell death in the population (Fig. 7A; lanes 4 and 5). Then, we determined that the activation of these kinases by okadaic acid is largely suppressed by co-treatment with the EGFR inhibitor, gefitinib (Fig. 7B). Together, these observations suggest that a balance between EGFR kinase activity and an okadaic acid-sensitive phosphatase activity (possibly PP2A) contributes to the net phosphorylation status of several of the downstream mediators of EGFR-dependent pro-survival as well as pro-apoptotic signaling responses. These findings also implicate cellular phosphatases in orchestrating the timing of signal attenuation following acute inactivation of oncogenic kinases, such as the EGF receptor.
Figure 7. Effect of okadaic acid and gefitinib either singly or in combination on modulation of signaling in BaF3-EGFR (l858R) cells.
BaF3-EGFR(L858R) cells were treated with either 100nM okadaic acid (OA) alone (lanes 1–5 in panel A; lanes 1–4 in panel B), gefitinib (GEF) alone (lanes 6–8 in panel A) or 100nM okadaic Acid together with 2μM gefitinib (lanes 5–8 in panel B). At various periods of time post-treatment (as indicated at the top of each lane) cells were harvested and their proteins were analyzed by SDS-PAGE followed by Western blotting using antibodies directed against phospho-EGFRY1068 (P-EGFR); EGFR; phospho-Akt (P-Akt); Akt; phospho-ERK1/2 (P-ERK1/2); ERK1/2; phospho-p38 (P-p38); p38. The relative migration of the relevant proteins are indicated on the left-hand side of each autoradiogram and to the right of each autoradiogram are indicated the relative migration of molecular weight standards.
DISCUSSION
Oncogene addiction has now been well documented in several experimental cancer models and appears to play an important role in the clinical response to various targeted cancer therapies that have been recently developed. However, a clear molecular mechanism to explain the phenomenon of oncogene addiction has been somewhat elusive. Here, we have tested a specific hypothesis that oncogene addiction is associated with differences in the attenuation rates of multiple competing pro-survival and pro-apoptotic signals emanating from a single oncoprotein source. In three different cell culture models of oncogene dependency, we have observed a similar profile of rapid signal attenuation of several key survival effectors, and a temporally delayed accumulation of at least one pro-apoptotic effector, phospho-p38 MAP kinase. This signaling profile is also observed in a human lung cancer cell line, PC-9, which harbors an activating EGF receptor mutation and is efficiently killed by a pharmacological concentration of gefitinib that exhibits efficacy in a subset of treated patients. These observations suggest that a common signaling cascade associated with coordinated timing of signal attenuation may underlie the apoptotic response to acute oncogene inactivation in the context of several different oncogenes and may also contribute to the clinical response to kinase inhibitors in a subset of cancer patients.
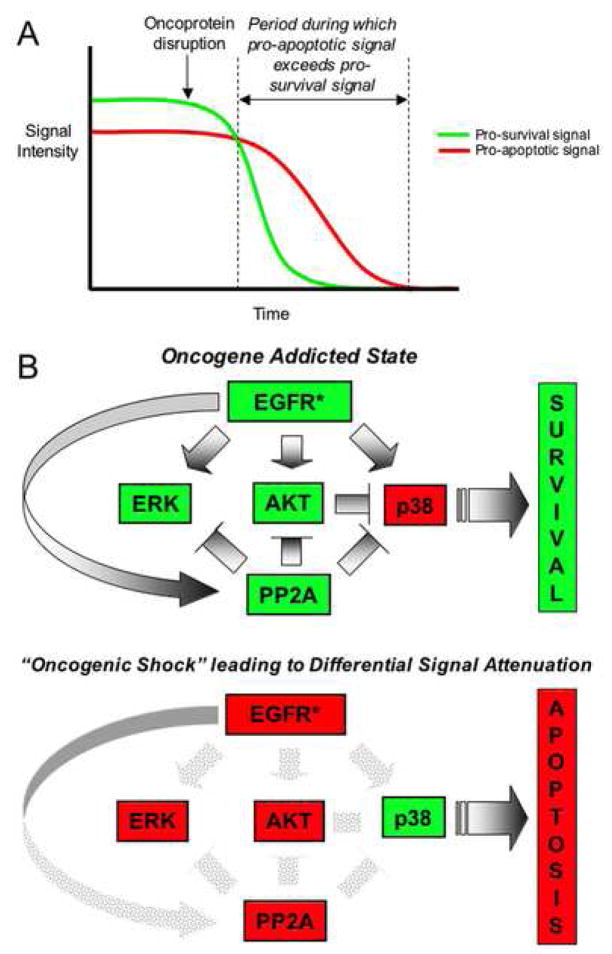
In this model of differential signal attenuation (Fig. 8), we propose that, upon disruption of oncogenic activity, a temporary imbalance in pro-apoptotic and pro-survival signals results from the fact that at least some of the established survival factors, such as ERKs, Akt, and STAT3/5 undergo relatively rapid inactivation, whereas pro-apoptotic signals can persist long enough to drive an apoptotic outcome in the absence of counter-acting survival signals. This model implies that a temporal window exists during which lingering “unchecked” pro-apoptotic signals emanating from an oncoprotein that has recently been inactivated cause the cell to pass a commitment point toward apoptosis. In fact, experimental studies have demonstrated that commitment to an apoptotic outcome can occur within only a few hours following the initiation of an apoptotic signal (Brunet et al., 1998). Our observation (especially in the PC-9 system) that phospho-AKT levels are rapidly inactivated for a brief period in lung cancer cells treated with the EGF receptor kinase inhibitor gefitinib, and then recover prior to apoptosis (Fig. 5B), is consistent with a model wherein a transient loss of pro-survival signaling is sufficient to shift the survival-death balance and to produce an apoptotic outcome. In addition, we have determined that transient transfection of cultured NIH3T3 cells with an activated Ras mutant that is specifically defective for interaction with the PI-3 kinase/Akt survival pathway causes apoptosis in a substantial fraction of transfected cells, whereas activated Ras that retains this interaction does not cause apoptosis (Figure S4).
Figure 8. Proposed model of oncogene addiction.
A: A graphical depiction of the proposed role of differential signal attenuation in creating a temporal window during which apoptotic signals persist in the absence of pro-survival signals following acute oncogene inactivation to promote cell death.
B: This model depicts the proposed role of differential signal attenuation in the cell death response to acute inactivation of oncogenic EGFR (EGFR*) upon which a tumor cell has become dependent. The upper panel illustrates the oncogene “addicted” state in which cells survive, and the lower panel illustrates the shift in the balance of pro-survival and pro-apoptotic signaling shortly following EGFR inactivation. Red=“off”; Green=“on”.
While there are potentially multiple mechanisms that could contribute to a transient signaling imbalance following oncogene inactivation, the phosphatase inhibitor studies that we have described point to an important role for cellular phosphatases in determining the “steady-state” levels of phosphorylation of the various well studied downstream effectors of many oncogenic kinases. These finding also implicate phosphatases in determining the temporal kinetics of effector activation and deactivation. While okadaic acid is an admittedly blunt tool for elucidating the precise mechanism by which phosphatases might contribute to differential signal attenuation, other reported studies similarly support a role for phosphatase activity in determining the temporal ordering of signal activation and deactivation. For example, previous studies of an apoptosis model in cultured PC12 neural cells that undergo apoptosis upon withdrawal of nerve growth factor revealed a similar temporally coordinated cascade of ERK1,2 inactivation followed by an accumulation of phospho-p38 prior to apoptosis (Xia et al., 1995). In addition, Akt has been reported to negatively regulate p38 activity, indirectly through the ASK1 and MKK3/6 kinases (Ichijo et al., 1997; Kim et al., 2001). Notably, protein phosphatase 5 (PP5), an okadaic acid-sensitive phosphatase, reportedly dephosphorylates ASK1 and inhibits ASK1 signaling (Morita et al., 2001), thereby providing another potential mechanism by which phosphatase activity could contribute to the timing of signal attenuation. These findings, together with our findings, suggest that a shared profile of orchestrated attenuation of pro-survival and pro-apoptotic signals may play an important role in a variety of apoptosis settings.
We recognize that we have only examined a relatively small subset of the numerous downstream effectors that have been previously implicated in the response to activating oncoproteins such as Src, EGF receptor, and BCR-ABL. Consequently, it is a near certainty that any differential signal attenuation mechanisms that may be operating in the models we have examined are more complex than the simple model we have described. Moreover, we note that multiple distinct mechanisms of oncogene addiction may be contributing to this phenomenon in the various contexts in which it has been observed. For example, the proliferative arrest and differentiation responses to oncogene inactivation that have been reported in some settings may involve distinct mechanisms; although, it remains possible that an analogous signaling imbalance of a different nature could be contributing to those outcomes.
The proposed model of oncogene addiction differs somewhat from previous descriptions of the phenomenon in that it implies that the apoptotic outcome in response to oncogene inactivation is an active process that requires pro-apoptotic signals derived from the oncoprotein, as opposed to a passive process in which a cell that is dependent on an oncogene-derived survival signal defaults to an apoptotic death upon inactivation of that oncogene. It is well established that several oncoproteins, including Ras and Src, can produce pro-apoptotic outputs in some contexts (Arber, 1999; Webb et al., 2000). Moreover, most of the well studied oncoproteins, such as EGF receptor, BCR-ABL, and Ras, promote the activation of numerous downstream effector pathways, and in each case, some of these have been linked to pro-apoptotic outcomes depending on the context. Several potential molecular mechanisms to account for a pro-apoptotic outcome have been reported. Thus, Ras can be pro-apoptotic via an interaction with the effector target, Nore1 (Vos et al., 2003). Similarly, the EGF receptor can bind directly to the so-called “death ligand”, FAS/CD95 (Reinehr et al., 2003). In addition, Src can induce apoptosis in B lymphocytes via engagement of the CD20 surface protein (Hofmeister et al., 2000). Thus, it is reasonable to expect that the state of a cell expressing an activated oncoprotein reflects the net balance of multiple diverse signaling pathways that have been engaged.
Accumulating evidence indicates that many tumor cells are poised on the edge of an apoptotic event and that tumor cells have an increased tendency to undergo apoptosis. In most tumor cells, the balance between pro-survival and pro-apoptotic signals clearly favors a survival outcome, however, it is easy to imagine that a temporary imbalance in those pathways in the initial hours following inactivation of the upstream source of those signals could shift cells toward an apoptotic event, particularly if the survival signals dissipate rapidly. Indeed, we have observed that treatment of PC-9 lung cancer cells for as little as 30 minutes (followed by drug washout) is sufficient to kill approximately half of the cells (Figure S5). Moreover, the differential signal attenuation model may also explain the emerging concept that tumor cells in which a kinase has undergone mutational activation or is overexpressed are especially sensitive to the killing effects of drugs that target those kinases. Specifically, the model suggests that increased kinase activity is associated with increased pro-apoptotic (as well as pro-survival) output, and that this excessive pro-apoptotic activity contributes to the cell death observed following acute inactivation of the oncogenic kinase. In support of such a conclusion, we have observed that cultured lung cancer cells that are killed by gefitinib treatment can be more efficiently killed in the presence of 10% serum, which contains EGF, the receptor ligand (Figure S6). This may reflect a role for EGF in driving higher levels of an apoptotic output from mutationally activated EGFR. In addition, this could explain why most normal cells, in which these same kinases are not signaling excessively, are typically not killed by treatment with a drug that targets the kinase. Thus, this differential signal attenuation model raises questions about the appropriateness of the term “addiction” in this setting, which seemingly implies a passive dependency on the oncogenic kinase. As an alternative, we have proposed the term “oncogenic shock” to describe the proposed signaling imbalance that can accompany acute oncogene inactivation, and which ascribes an active pro-apoptotic role to the oncogenic kinase in the consequent cell death response (Sharma et al., 2006). Notably, with such a mechanism, it may not be relevant whether the oncogenic lesion is an initiating event or a late event in tumorigenesis, but rather, its predominance in the tumor may be critical in determining whether targeting it produces a significant clinical response.
The studies we have performed may also have clinical implications regarding the use of cancer therapies that target oncogenic kinases. For example, co-administration of a drug that promotes cell cycle arrest might suppress apoptosis that would normally be triggered by acute inactivation of an oncogenic kinase, a mechanism that has potentially contributed to the disappointing results observed thus far when EGFR kinase inhibitors are administered together with conventional chemotherapy drugs. These findings also raise the possibility that drugs directed against downstream signaling proteins that shift the balance of kinase-mediated pro-apoptotic and pro-survival signals may also have therapeutic value. Differential signal attenuation may also contribute to the acquisition of drug resistance that frequently develops in patients during treatment with selective kinase inhibitors if genetic or epigenetic changes allow cells to survive during a temporal window between the dissipation of pro-survival and pro-apoptotic signals. These findings also raise the possibility that “on-off cycles”, as opposed to continuous treatment, with targeted kinase inhibitors may be more beneficial to patients by allowing for more “windows of opportunity” for the inhibitors to produce a transient signaling imbalance that leads to apoptotic cell death. Thus, in contrast to traditional chemotherapeutic agents that appear to trigger a DNA damage response, the emergence of small molecules that target specific components of signaling pathways may involve distinct mechanisms of cancer cell death. Further insights into these mechanisms may allow optimal clinical application of these new molecularly targeted agents.
EXPERIMENTAL PROCEDURES
Cell Lines
TonB210.1 cells, expressing the tetracycline inducible Bcr-Abl oncogene was kindly provided by George Q. Daley (Childrens Hospital, Boston) and was maintained as described previously (Klucher et al., 1998). BaF3 EGFR expressing the Gefitinib-sensitizing L858R mutant EGFR was maintained as described previously (Jiang et al., 2005). PC9 cells, expressing the Gefitinib-sensitizing deletion mutation (DE746-A750) were kindly provided by Dr. Kazuto Nishio (National Cancer Center Hospital, Tokyo) and were maintained as described previously (Koizumi et al., 2005). Balb A1-ts src cells expressing the temperature-sensitive v-src oncogene and Ψ2 v-src 3T3 cells expressing the retroviral v-src oncogene were propagated in DMEM containing 10% fetal-bovine serum and penicillin and streptomycin (100 U/ml and 100 g/ml, respectively). NIH 3T3 mouse fibroblasts were maintained in DMEM supplemented with 10% calf serum and penicillin and streptomycin (100 U/ml and 100 g/ml, respectively).
Antibodies
HRP-conjugated anti-phosphotyrosine antibody and rabbit polyclonal antibody against phospho-Akt (Ser 473) were purchased from Biosource. The rabbit polyclonal antibody directed against EGFR was from Santa Cruz Biotechnology. Mouse monoclonal antibodies against Phospho-EGFR (Tyr 1068), Phospho-STAT 5 (Tyr 694), and cleaved PARP as well as rabbit polyclonal antibody against Bcr, phospho-p44/42 ERK kinase (Thr 202/Tyr 204), phospho-Akt (Ser 473), phospho-Src (Tyr 416), phospho-STAT 3 (Tyr 705, full-length PARP, phospho-p38 (Thr 180/Tyr 182) and antibodies directed against their non-phosphorylated counterparts were purchased from Cell Signaling Technology. Anti BrdU antibody was from Becton-Dickinson. Secondary antibodies included HRP conjugated anti-mouse and anti-rabbit antibodies and were purchased from Cell Signaling Technology. The FITC conjugated anti-mouse antibody was from Vector Laboratories.
Inhibitors
Gefitinib was kindly provided be Astra-Zeneca Pharmaceuticals (Macclesfield, UK) and was resuspended in DMSO at a stock concentration of 2mM. Okadaic Acid, LY282004 and the Src-specific inhibitor SU6656 were purchased from Calbiochem and resuspended in DMSO at a stock concentration of 0.1, 50 and 5mM, respectively. All inhibitors were stored in small aliquots at −20°C.
Induction of Apoptosis by Oncogene Inactivation
Cells were plated in their respective maintenance media and used for experimentation when they had reached a confluence of 70%. Apoptosis was induced in each system as described below. At various times after induction of apoptosis, the cells were harvested and the activation state of the relevant signal transducers was assessed by SDS-PAGE followed by Western Blotting.
Bcr-Abl
The Bcr-abl protein was induced in TonB-210.1 cells by incubating them for 18h with 1μg/ml of doxacycline in their growth medium (RPMI-1640 containing Sodium Pyruvate, Penicillin-Streptomycin and 10% fetal bovine serum). Inactivation of Bcr-abl was accomplished by the removal of doxacycline from the growth medium. To this end, cells were collected by centrifugation and washed three times in media without doxacycline, resuspended in media without doxacycline and at various times (0, 1, 2, 4, 8, 24, 48, 72h) cells were harvested for analysis.
Src
Exponentially growing Balb A1-ts src cells growing at 35°C in DMEM containing10% fetal bovine serum (FBS), were washed once with DMEM containing 0.2% FBS and then placed in DMEM containing 0.2% FBS at 35°C for a period of 18h. V-src was inactivated by replacing the medium on the cells with DMEM containing 0.2% FBS that had been pre-warmed to 39.5°C. At various times post-inactivation of src (0, 15m, 30m, 1h, 2h, 4h, 6h and 8h), cells were harvested for analysis.
Exponentially growing Ψ2 v-src 3T3 cells were washed once with serum-free DMEM and then placed in serum free DMEM for a period of 18h. Subsequently, they were treated with 3μM SU6656 (in DMSO) that was added directly to the serum-free growth medium. The untreated controls received an equivalent volume of DMSO. Following treatment with SU6656, cells were harvested at 0, 15m, 30m, 1h, 2h, 4h, 6h and 8h.
EGFR
Exponentially growing BaF3 EGFR (L858R) and PC9 cells were treated with 2μM Gefitinib (in DMSO) that was added directly to the growth medium (RPMI-1640 containing Sodium Pyruvate, Penicillin-Streptomycin and 10% fetal bovine serum). The untreated controls received an equivalent volume of DMSO. Following treatment with Gefitinib, cells were harvested at 0, 4, 8, 24, 36, 48, 60 and 72h. In experiments using BaF3 EGFR (L858R), that involved the use of okadaic acid (OA) shown in figure 8, OA was used at a final concentration of 100nM and was either added either by itself or together with Gefitinib for the appropriate amounts of time as indicated in the figure.
Transfection and Apoptosis Assays
Transfection of NIH 3T3 mouse fibroblasts was performed with an electroporator using reagents and protocols provided by the manufacturer (Amaxa). Cells were transfected with 1μg of vector, H-RasV12 or H-RasV12T35S (White et al., 1995) together with a GFP plasmid provided by Amaxa (to monitor transfection efficiency). 6–8 hours following transfection, cells were washed and transferred to DMEM containing 0.1% FBS. At this time, the transfection efficiency was also assayed by fluorescence microscopy of GFP signal and determined to be approximately 80–90%. Cell number and apoptosis were assayed 40 hours later. The number of cells that adhere to the dish is presented as a percentage of cells relative to vector transfected controls (supplementary data figure 6A). Apoptotic cells were quantitated using fluorescence-activated cell sorting (FACS) according to the manufacturer’s protocol (Becton Dickinson). Cells were incubated with Cell Labeling Reagent BrdU (Amersham-Pharmacia), at 37°C for 30 minutes. Subsequently, all cells (adherent and floating) were collected, washed with PBS and fixed in 80% ethanol. DNA was denatured for 30 minutes with 2M HCl/0.5% Triton X-100 and neutralized with 0.1M NaB4O7·10H2O (pH 8.5), before incubation with anti-BrdU antibody (1:500) and a FITC-conjugated goat anti-mouse secondary antibody (1:50). Cells were stained with 5μg/mL propidium iodide (Sigma) and treated with RNAse A (Sigma) prior to two-dimensional FACS analysis using CELLQUEST software (Becton Dickinson). The number of gated cells in the sub-G1 population is presented as the ratio of apoptotic cells to vector transfected controls (Supplementary data figure 6B).
Cell Harvesting and Protein Analysis
At each time point, cells were placed on ice, scraped in their own media and collected by low-speed refrigerated centrifugation. Cell pellets were then washed once in ice-cold PBS and stored at −80°C until ready for analysis by SDS-PAGE and immuno-blotting using standard procedures.
Supplementary Material
Acknowledgments
We are grateful to members of the Settleman and Haber laboratories, and Michael Fischbach for helpful discussions. We thank Matthew Meyerson for providing the BA/F3-EGFR cells, George Daley for the TonB210.1 cells, and Joan Brugge for the TS-Src plasmid. This work was supported by NIH RO1 CA115830, a V Foundation Award, and a Saltonstall Scholar Award to J. S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki K, Yoshida T, Matsumoto N, Ide H, Sugimura T, Terada M. Suppression of Ki-ras p21 levels leading to growth inhibition of pancreatic cancer cell lines with Ki-ras mutation but not those without Ki-ras mutation. Mol Carcinog. 1997;20:251–258. [PubMed] [Google Scholar]
- Arber N. Janus faces of ras: anti or pro-apoptotic? Apoptosis. 1999;4:383–388. doi: 10.1023/a:1009651406017. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G, Biswas T, Roy KC, Mandal S, Mandal C, Pal BC, Bhattacharya S, Rakshit S, Bhattacharya DK, Chaudhuri U, et al. Chlorogenic acid inhibits Bcr-Abl tyrosine kinase and triggers p38 mitogen-activated protein kinase-dependent apoptosis in chronic myelogenous leukemic cells. Blood. 2004;104:2514–2522. doi: 10.1182/blood-2003-11-4065. [DOI] [PubMed] [Google Scholar]
- Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20:9018–9027. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet CL, Gunby RH, Benson RS, Hickman JA, Watson AJ, Brady G. Commitment to cell death measured by loss of clonogenicity is separable from the appearance of apoptotic markers. Cell Death Differ. 1998;5:107–115. doi: 10.1038/sj.cdd.4400334. [DOI] [PubMed] [Google Scholar]
- Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N, Shen Q, O’Hagan R, Pantginis J, Zhou H, et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400:468–472. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- Gambacorti-Passerini C, le Coutre P, Mologni L, Fanelli M, Bertazzoli C, Marchesi E, Di Nicola M, Biondi A, Corneo GM, Belotti D, et al. Inhibition of the ABL kinase activity blocks the proliferation of BCR/ABL+ leukemic cells and induces apoptosis. Blood Cells Mol Dis. 1997;23:380–394. doi: 10.1006/bcmd.1997.0155. [DOI] [PubMed] [Google Scholar]
- Harper ME, Goddard L, Glynne-Jones E, Assender J, Dutkowski CM, Barrow D, Dewhurst OL, Wakeling AE, Nicholson RI. Multiple responses to EGF receptor activation and their abrogation by a specific EGF receptor tyrosine kinase inhibitor. Prostate. 2002;52:59–68. doi: 10.1002/pros.10069. [DOI] [PubMed] [Google Scholar]
- Hofmeister JK, Cooney D, Coggeshall KM. Clustered CD20 induced apoptosis: src-family kinase, the proximal regulator of tyrosine phosphorylation, calcium influx, and caspase 3-dependent apoptosis. Blood Cells Mol Dis. 2000;26:133–143. doi: 10.1006/bcmd.2000.0287. [DOI] [PubMed] [Google Scholar]
- Huettner CS, Zhang P, Van Etten RA, Tenen DG. Reversibility of acute B-cell leukaemia induced by BCR-ABL1. Nat Genet. 2000;24:57–60. doi: 10.1038/71691. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Jiang J, Greulich H, Janne PA, Sellers WR, Meyerson M, Griffin JD. Epidermal growth factor-independent transformation of Ba/F3 cells with cancer-derived epidermal growth factor receptor mutants induces gefitinib-sensitive cell cycle progression. Cancer Res. 2005;65:8968–8974. doi: 10.1158/0008-5472.CAN-05-1829. [DOI] [PubMed] [Google Scholar]
- Johnson D, Agochiya M, Samejima K, Earnshaw W, Frame M, Wyke J. Regulation of both apoptosis and cell survival by the v-Src oncoprotein. Cell Death Differ. 2000;7:685–696. doi: 10.1038/sj.cdd.4400700. [DOI] [PubMed] [Google Scholar]
- Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucher KM, Lopez DV, Daley GQ. Secondary mutation maintains the transformed state in BaF3 cells with inducible BCR/ABL expression. Blood. 1998;91:3927–3934. [PubMed] [Google Scholar]
- Lee T, Kim SJ, Sumpio BE. Role of PP2A in the regulation of p38 MAPK activation in bovine aortic endothelial cells exposed to cyclic strain. J Cell Physiol. 2003;194:349–355. doi: 10.1002/jcp.10211. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Maroney AC, Qureshi SA, Foster DA, Brugge JS. Cloning and characterization of a thermolabile v-src gene for use in reversible transformation of mammalian cells. Oncogene. 1992;7:1207–1214. [PubMed] [Google Scholar]
- Morita K, Saitoh M, Tobiume K, Matsuura H, Enomoto S, Nishitoh H, Ichijo H. Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. Embo J. 2001;20:6028–6036. doi: 10.1093/emboj/20.21.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukohara T, Engelman JA, Hanna NH, Yeap BY, Kobayashi S, Lindeman N, Halmos B, Pearlberg J, Tsuchihashi Z, Cantley LC, et al. Differential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutations. J Natl Cancer Inst. 2005;97:1185–1194. doi: 10.1093/jnci/dji238. [DOI] [PubMed] [Google Scholar]
- O’Dwyer ME, Mauro MJ, Druker BJ. STI571 as a targeted therapy for CML. Cancer Invest. 2003;21:429–438. doi: 10.1081/cnv-120018235. [DOI] [PubMed] [Google Scholar]
- Ono M, Hirata A, Kometani T, Miyagawa M, Ueda S, Kinoshita H, Fujii T, Kuwano M. Sensitivity to gefitinib (Iressa, ZD1839) in non-small cell lung cancer cell lines correlates with dependence on the epidermal growth factor (EGF) receptor/extracellular signal-regulated kinase 1/2 and EGF receptor/Akt pathway for proliferation. Mol Cancer Ther. 2004;3:465–472. [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr R, Schliess F, Haussinger D. Hyperosmolarity and CD95L trigger CD95/EGF receptor association and tyrosine phosphorylation of CD95 as prerequisites for CD95 membrane trafficking and DISC formation. Faseb J. 2003;17:731–733. doi: 10.1096/fj.02-0915fje. [DOI] [PubMed] [Google Scholar]
- Sharma SV, Fischbach MA, Haber DA, Settleman J. “Oncogenic shock”: Explaining oncogene addiction through differential signal attenuation. Clin Cancer Res. 2006 doi: 10.1158/1078-0432.CCR-06-0096. in press. [DOI] [PubMed] [Google Scholar]
- Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18:189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- Turkson J, Bowman T, Adnane J, Zhang Y, Djeu JY, Sekharam M, Frank DA, Holzman LB, Wu J, Sebti S, Jove R. Requirement for Ras/Rac1-mediated p38 and c-Jun N-terminal kinase signaling in Stat3 transcriptional activity induced by the Src oncoprotein. Mol Cell Biol. 1999;19:7519–7528. doi: 10.1128/mcb.19.11.7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos MD, Martinez A, Ellis CA, Vallecorsa T, Clark GJ. The pro-apoptotic Ras effector Nore1 may serve as a Ras-regulated tumor suppressor in the lung. J Biol Chem. 2003;278:21938–21943. doi: 10.1074/jbc.M211019200. [DOI] [PubMed] [Google Scholar]
- Webb BL, Jimenez E, Martin GS. v-Src generates a p53-independent apoptotic signal. Mol Cell Biol. 2000;20:9271–9280. doi: 10.1128/mcb.20.24.9271-9280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein IB. Disorders in cell circuitry during multistage carcinogenesis: the role of homeostasis. Carcinogenesis. 2000;21:857–864. doi: 10.1093/carcin/21.5.857. [DOI] [PubMed] [Google Scholar]
- Weinstein IB. Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- Weinstein IB, Begemann M, Zhou P, Han EK, Sgambato A, Doki Y, Arber N, Ciaparrone M, Yamamoto H. Disorders in cell circuitry associated with multistage carcinogenesis: exploitable targets for cancer prevention and therapy. Clin Cancer Res. 1997;3:2696–2702. [PubMed] [Google Scholar]
- White MA, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler MH. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.