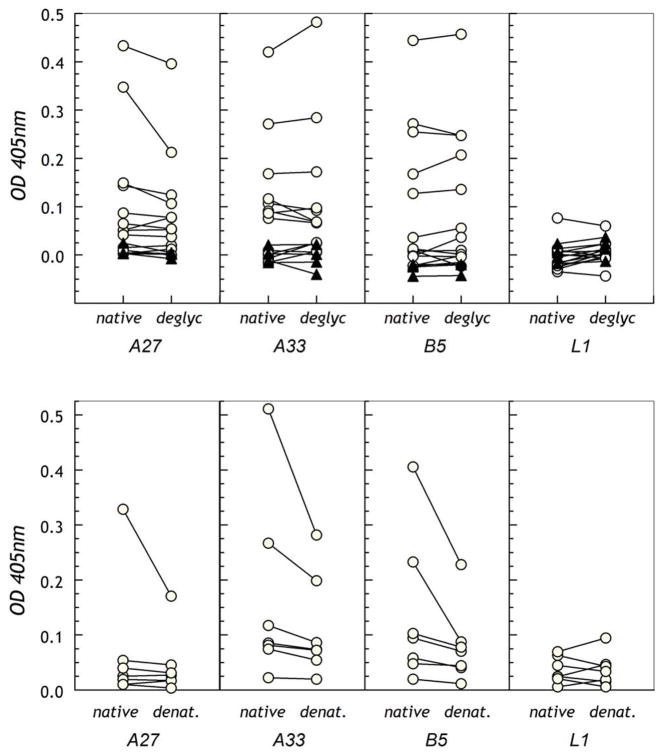
Figure 7. Optimal antigenic reactivity of sera requires post-translationally-modified or properly-folded target proteins.
A. Untreated or endoglycosidase F1-treated proteins were immobilized on uncoated 384-well micro-ELISA plates and reactivity of purified IgG was assessed. B. Untreated or TFA-treated proteins were immobilized on ELISA plates for testing of reactivity of IgG from selected vaccinated individuals. In both panels, the dotted line indicates the cut-off for positivity where 0.05–0.1 is considered borderline and above 0.1 significantly positive.