Abstract
Spinules found in brain consist of small invaginations of plasma membranes which enclose membrane evaginations from adjacent cells. Here, we focus on the dynamic properties of the most common type, synaptic spinules, which reside in synaptic terminals. In order to test whether depolarization triggers synaptic spinule formation, hippocampal slice cultures (7 day-old rats, 10–14 days in culture) were exposed to high K+ for 0.5–5 min, and examined by electron microscopy. Virtually no synaptic spinules were found in control slices representing a basal state, but numerous spinules appeared at both excitatory and inhibitory synapses after treatment with high K+. Spinule formation peaked with ~1 min treatment at 37 ° C, decreased with prolonged treatment, and disappeared after 1–2 min of washout in normal medium. The rate of disappearance of spinules was substantially slower at 4 °C. NMDA treatment also induced synaptic spinule formation, but to a lesser extent than high K+ depolarization. In acute brain slices prepared from adult mice, synaptic spinules were abundant immediately after dissection at 4ºC, extremely rare in slices allowed to recover at 28 °C, but frequent after high K+ depolarization. High pressure freezing of acute brain slices followed by freeze-substitution demonstrated that synaptic spinules are not induced by chemical fixation. These results indicate that spinules are absent in synapses at low levels of activity, but form and disappear quickly during sustained synaptic activity. The rapid turnover of synaptic spinules may represent an aspect of membrane retrieval during synaptic activity.
Keywords: hippocampal slice culture, acute brain slices, clathrin, vesicle recycling, electron microscopy
Electron microscopy has provided a structural basis for understanding chemical synapses. Here, we focus on a particular structure revealed by electron microscopy –the synaptic spinule (Tarrant and Routtenberg, 1977) – which consists of a double-membrane invagination inside synaptic terminals. The distribution and structural characteristics of spinules in brain have been described in detail (Sorra et al., 1998; Spacek and Harris, 2004) subsequent to their discovery (Westrum and Blackstad, 1962). Their presence in synapses is highly variable in different experimental systems and their functions remain undetermined. Some studies report spinule formation under pathological or excitatory conditions such as post-mortem effect (Tarrant and Routtenberg, 1977), or long-term potentiation (LTP, Schuster et al, 1990). Two studies report that synaptic spinules form quickly after stimulation. Synaptic spinules appear in bullfrog brain after 5–20 sec of high K+ depolarization (Van Harreveld and Trubatch, 1975). Another study combines electrical recording and simultaneous perfusion fixation to describe formation of spinules in rat hippocampal CA1 after 1 min of stimulation at 40 HZ (Applegate and Landfield, 1988). However, no quantitative morphometry was carried out, and most important, it was left unclear whether spinules are transient structures and if so, how quickly they disappear.
Another major piece of missing information is whether spinules are present in synapses during basal activity. The closest approximation to a baseline for spinule formation derives from a study of bullfrog brains in which synaptic spinules were shown to be rare after cerebral cortex was simply dissected, allowed to recover, and then freeze-substituted for EM analysis (Van Harreveld and Trubatch, 1975). Most other studies on spinules have been carried out on rodent brains fixed by perfusion with aldehydes. However, perfusion fixation can result in unintended procedural delays, which in turn, can lead to synaptic activity and related structural changes (Tao-Cheng et al., 2007). Thus, in the present study, we turned to examining hippocampal slice cultures in order to have better control of the access of fixatives and the activity state of the synapses.
We set out to explore the role of activity in the formation of synaptic spinules, and to find out whether basal levels of synaptic activity can produce spinules in slice cultures as well as in other experimental systems, such as acute brain slices, dissociated neuronal cultures and perfusion-fixed brains. This investigation required careful consideration of procedural factors such as tissue handling and fixation artifacts in the process of spinule formation. Finally, as a first step to elucidate the functional significance of synaptic spinules, we investigated the time course of spinule formation and disappearance during stimulation and after the cessation of excitatory conditions.
EXPERIMENTAL PROCEDURES
Hippocampal slice cultures
The animal protocol used in this study was approved by the NIH Animal Use and Care Committee and conforms to NIH guidelines. On average, one animal was used to generate 6 slice cultures for each experiment, and a total of 19 experiments were carried out. Briefly, the hippocampus was removed from postnatal 6–8 day old Sprague Dawley rats and cut at 250 μm thickness with a Mcllwain tissue chopper. Slices were placed on a Falcon #353090 cell culture inserts in 6-well culture dishes and incubated overnight in media containing HMEM with 12.5 mM Hepes, Hanks salts, 25% horse serum, 1 mM glutamine, 27 mM glucose, 1mM pyruvate, 0.5 mM ascorbate and N3 (a growth factor cocktail), kept in a 5% carbon dioxide incubator at 35 ° C. The media was changed to one including 5% horse serum on the second day, and changed every second day until slices were used between in vitro days 10 – 14.
Treatment of hippocampal slice cultures
For experiments, slice culture inserts in 6-well dishes were placed on a floating platform in a water bath at 37°C. All medium changes were made by first removing the old media, then transferring the inserts into a new well containing 1 ml of medium and adding 1 ml of medium on top to submerge the slices. Slices were washed once with normal incubation medium (124 mM NaCl, 2 mM KCl, 1.24 mM KH2PO4, 1.3 mM MgCl2, 2.5 mM CaCl2, 30 mM glucose in 25 mM HEPES at pH 7.4) before the addition of test medium.
Depolarization treatment was 90 mM KCl (NaCl concentration correspondingly reduced) for 0.5, 1, 1.5, 2, 3, or 5 min. NMDA treatment was 50 μM for 0.5, 1, 2, 3, 5 or 15 min. Some slices were treated for up to 15 min with 250 μM of NMDA. To examine recovery after depolarization, high K+ medium was removed and the dishes were washed 3–4 times in normal incubation medium for a total of 1, 2, 3, 5, 10, 30 and 60 min. In some experiments, recovery after high K+ treatment was performed in ice-cold incubation medium on ice. Experimental controls were processed in parallel, including all the medium changes and washing steps. Some slice cultures were fixed immediately without any treatment to assess their basal state.
Preparation and treatment of acute brain slices
Adult mice were anesthetized with either halothane or isofluorane before decapitation (five animals were used). Brains were dissected on ice and transferred to ice-cold, pre-oxygenated bicarbonate-type artificial cerebral spinal fluid (ACSF) buffer (124 mM NaCl, 4.4 mM KCl, 26 mM NaHCO3, 1 mM Na2HPO4, 1.3 mM MgSO4, 10 mM glucose, and 2.5 mM CaCl2), and chilled for 5 minutes. The hippocampus was quickly dissected and sliced at a thickness of 250 μm for immediate high-pressure freezing, or at 400 μm for immersion fixation. Slices for immersion fixation were fixed: (1) immediately after slicing (some slices were 250 μm thick); (2) after recovery for 2.5 hr at 28 °C in ACSF in an interface slice chamber (Fine Science Tools, Foster City, CA); or (3) after recovery and treatment with high K+ (90 mM, 2 min). After recovery, the health of the slices was verified with extracellular field potential recording in the stratum radiatum of the hippocampal CA1 region (Miller et al., 2002) and only slices with EPSPs >5 mV were used for experiments.
Chemical fixation and standard processing for EM
Slice cultures were fixed with 2% glutaraldehyde and 2% paraformaldehyde, or 4% glutaraldehyde in 0.1N cacodylate buffer at pH 7.4 for 1–3 hr at room temperature and then stored at 4°C for 1–5 days. Acute brain slices were either fixed by the above methods or directly with 1% osmium tetroxide (OsO4) in 0.1N cacodylate buffer on ice for 1 hr, and then washed and stored in buffer at 4°C.
The fixed slice cultures were brushed off the insert filter and, in parallel with the acute slices, carefully trimmed for sampling from the CA1 region in such a way that slices were sectioned in the plane that retained their top and bottom surfaces for orientation. Aldehyde-fixed samples were post fixed with 1% OsO4 in 0.1N cacodylate buffer for 1 hr on ice. All chemical-fixed samples (aldehyde as well as direct OsO4-fixed slices) were then mordanted en block with 0.25% uranyl acetate in acetate buffer at pH 5.0 overnight at 4°C, washed, dehydrated through graded series of ethanol, and finally embedded in epoxy resins.
Immunogold labeling
Samples were fixed with 4% paraformaldehyde in PBS for 45 min to 1 hr, permeablized with 0.1% saponin, immunolabeled with clathrin antibody (clone X22, ABR, Golden, CO), incubated with secondary antibody conjugated with Nanogold (Nanoprobes, Yaphand, NY), silver enhanced (HQ kit from Nanoprobes), and finally embedded in epoxy resin for thin sectioning (cf. Otmakhov et al., 2004 for slice cultures and Tao-Cheng et al., 2006 for dissociated cultures). Controls for immunolabeling included withholding primary antibody and comparing with other, non-clathrin primary antibodies.
Freeze-substitution of acute brain slices
CA1 areas of the brain slices were extracted with a two-millimeter circular hole-puncher, placed in an aluminum specimen carrier, covered with 1-hexadecene (Sigma-Aldrich), and immediately high pressure (>2100 bar) frozen with a Bal-Tec HPM 010 apparatus (TechnoTrade Intl, Manchester, NH).
The frozen samples were freeze-substituted in a Leica AFS unit (Bannockburn, IL) with 2% OsO4 in acetone, warming from −160 to −10°C over 44 hrs, mordanted in saturated uranyl acetate in acetone overnight, rinsed in acetone and methanol and brought to room temperature, and finally embedded in epoxy resins.
Preparation and treatment of dissociated hippocampal cultures
Electron micrographs from previous studies were used for spinule morphometry in disassociated hippocampal cultures. Neuronal cultures 3–4 weeks old (Tao-Cheng, 2006) or 3–10 days old (Tao-Cheng, 2007) were prepared as described in Dosemeci et al., (2001). During experiments, culture dishes were placed on a floating platform in a water bath maintained at 37°C. Cultures were washed once with normal medium, then treated with the same medium (control), high K+ medium (90 mM K+), or with NMDA (30–250 μM) before fixation.
Perfusion fixation for intact brain
Thin sections from perfusion fixed brains from previous studies (Tao-Cheng et al., 2007) were used for spinule morphometry in fixed intact brain. Briefly, two groups of perfusion fixations were compared: (1) “fast” perfusion fixation, where the elapsed time from opening of the chest cavity to seeing the fixative flowing out of the atrium was less than 100 sec, and (2) “delayed” perfusion fixation, where blood was flushed out for 5–8 min with PBS before fixative was introduced. Brains were prepared for standard electron microscopy.
Morphometry
Sampling from slice cultures
Slice cultures were covered by many layers of glia both on the top (air interface) and the bottom (attached to filter) surfaces. All sections were cut perpendicular to the surfaces of the slice culture, and only the central zone of the slices, where the organization of the neuropil resembles that of the brain, were sampled for morphometry. The CA 1 region was selected for sectioning, and clustered pyramidal neuronal somas were located for orientation (supplemental Fig. 1). Stratum radiatum was characterized by the presence of many large primary dendrites extending from the clustered somas. Counting of spinules was restricted to an area of stratum radiatum immediately adjacent to the neuronal somas (within 50–70 μm from the edge of the clustered somas).
Sampling from acute brain slices
After recovery, acute brain slices presented a sampling challenge similar to that of the slice cultures in that the original cut surfaces manifested many dark neurons and other evidence of cutting damage. There was a gradient of damage extending up to 100 μm into the core of the slice. Only the central zones of slices lying beyond the damaged areas were sampled for morphometry.
Sampling from perfusion fixed adult brains
Proximal stratum radiatum from hippocampal CA1 region, was sampled for spinule counting in perfusion fixed brains (for details of sampling criteria, see Tao-Cheng et al., 2007).
In all instances, sampling data used to count spinules were photographed with a CCD digital camera system (XR-100 from AMT, Danvers, MA) at 10,000X and printed at a final magnification of 37,500X.
Synaptic spinule counts
Spinules appear as double-membrane tubular structures (cf. Spacek and Harris, 2004), and depending on their location, are categorized as axonal, astrocytic, dendritic, or of unknown origin. The present study focused on spinules residing in synaptic terminals marked by synaptic vesicles–these spinules constituted the majority type (87.7 ± 2.7%) of total spinules in high K+ treated slice cultures from 10 experiments. Each synaptic spinule, regardless of its size and orientation, was scored as one entity (arrows in Fig. 1B). Their frequency was calculated as total number of spinules divided by total area sampled, and normalized as # spinules/1000 μm2. Statistical analysis (KaleidaGraph by Synergy Software) was carried out by Student’s t-test (2-sided, unpaired data with unequal variance) with the confidence level set at p <0.01 unless otherwise indicated.
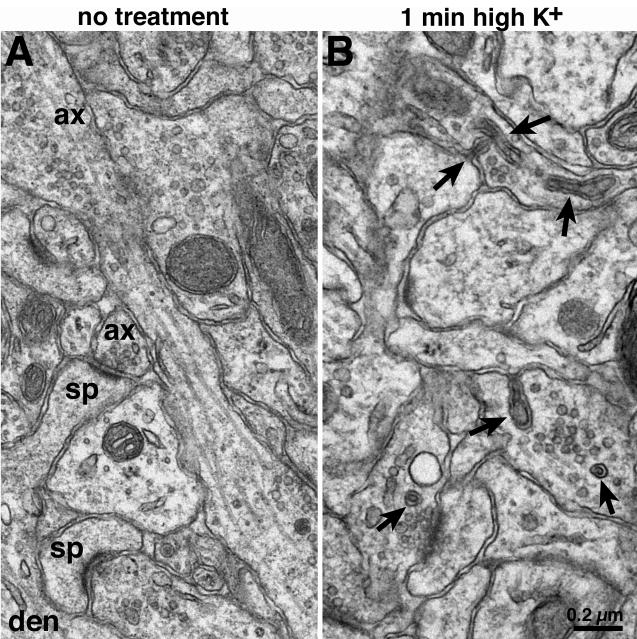
Fig. 1. Sampling of spinules by electron microscopy.
Dendrites (den), spines (sp) and axon terminals (ax) were identified and marked in a slice culture with no treatment (A). Spinules (arrows in B) were seen in a slice culture treated with high K+. Scale bar = 0.2 μm
RESULTS
Synaptic spinules are induced by depolarization
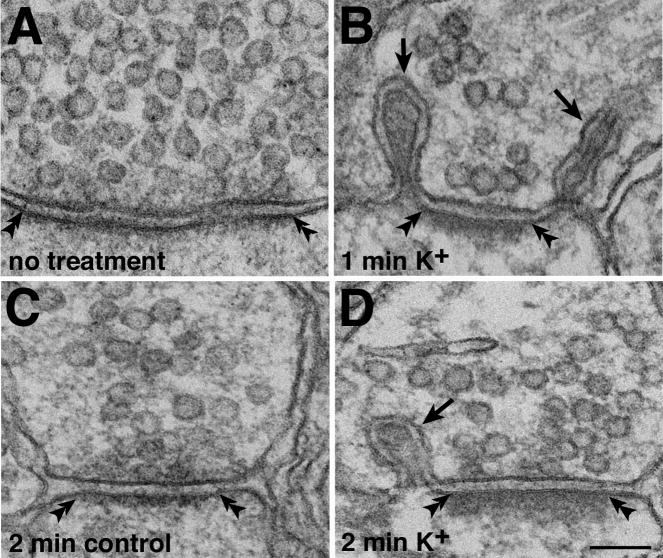
Spinules at synaptic terminals were virtually absent in untreated slice cultures (no synaptic spinules in 1930 μm2 of thin sectioned area, 3 experiments, Fig. 1A, 2A) and in controls run in parallel to experiments (two spinules in 6860 μm2, 11 experiments, Fig. 2C). Excitatory (asymmetric) synapses in these samples have markedly thin postsynaptic densities (PSD, Figs. 2A, C) indicative of a low level of activity (cf. Dosemeci et al., 2001). Spinules appeared, although not at every synaptic profile, after high K+ depolarization (90 mM, 0.5 to 5 min, 13 experiments, arrows in Fig. 2B, D). The percentage of synaptic profiles containing spinules in slice cultures treated with 1 min high K+ ranged from 12–22.5% (3 experiments: 59 synaptic profiles with spinules/493 total, 35/178, and 55/244). PSDs, as expected, became progressively thicker with depolarization and CaMKII clusters (aggregates of tightly clustered CaMKII, Dosemeci et al., 2000) became apparent as high K+ treatment times increased (data not shown), indicative of a high level of activity (cf. Dosemeci et al., 2001). Thus, synaptic spinules are absent in slice cultures in their basal state, but form after depolarization.
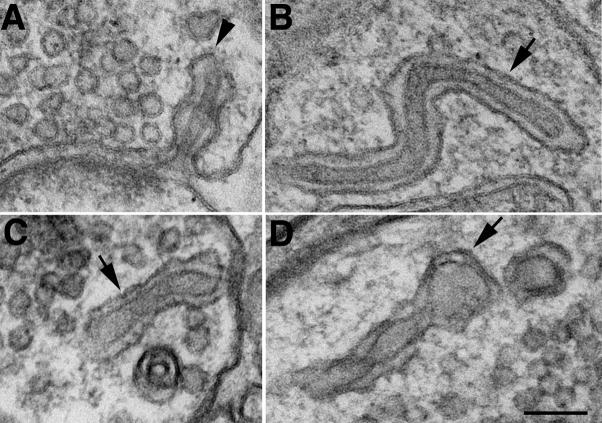
Fig. 2. Depolarization-induced formation of synaptic spinules at excitatory synapses in hippocampal slice cultures.
Synaptic spinules were not found in samples fixed immediately without treatment (A), or in experimental controls (C, 2 min in normal medium). After high K+ depolarization, spinules (arrows in B, D) were found in some synaptic terminal profiles. The postsynaptic densities (PSD, double arrowheads) were relatively thin in A and C compared to those in B and D. Scale bar = 0.1 μm.
Characterization of synaptic spinules
The defining feature of synaptic spinules is their parallel double-membrane appearance. Spinules were also typically near clusters of synaptic vesicles (SV in Fig. 3E). The inner membrane of synaptic spinules can arise from various locations (cf. Sorra et al., 1998; Spacek and Harris, 2004), but a frequent location was immediately adjacent to the PSD (Fig. 2B, D), though they never arose from the membrane lining the PSD itself. The outer membranes of synaptic spinules often showed patches of clathrin-coat, typically at their leading tip (Fig. 3A–C, arrowheads), but it could also appear along the outer tubule (Fig. 3C, double arrowheads). The presence and identity of clathrin was confirmed by immunogold labeling (Fig 3F, G).
Fig. 3. Variations in clathrin coats on spinules.
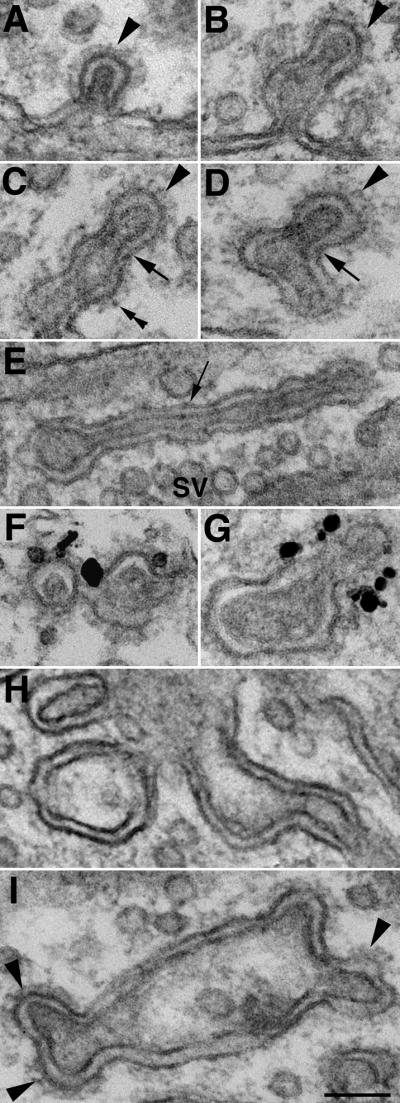
All images are from slice cultures treated with high K+. Clathrin coating was often at the leading front of spinules (arrowheads in A, B, C, D). Arrows in C, D point to the narrow pinch-waist which lacked clathrin coat. One elongated spinule (E) had stretches of relatively smooth membranes (arrow) in the middle portion without the pinch-waist appearance. Black grains in (F, G) represent silver enhanced immunogold labeling of clathrin. Panels H, I show large double-membrane structures which are irregular in shape and with clathrin coating only at a few locations (arrowheads in I). SV – synaptic vesicles. Scale bar = 0.1 μm.
The size and shape of spinules were highly variable. The length ranged from ~80 nm (Fig 3A) to as long as ~500 nm (Fig. 3E). Many spinules had a periodic (~80 nm) pinch-waist appearance (Fig. 3C, D) as if they could be fissured into individual units as suggested by the lack of clathrin at the waist (arrows in 3C, D). In this type of spinule, at its widest point, the diameter of the outer (axonal) membrane was typically ~ 75 nm, and that of the inner (dendritic) membrane was~ 35 nm (Fig. 3A–C). However, many other spinules were relatively straight, without the pinch-waist appearance, and without a clathrin coat (arrow on left in Fig. 2B; arrow in 3E). In fact, the pinch-waist appearance was closely related to the presence of the clathrin coats. In addition to these typical tubular spinules, there were also some irregularly shaped, double-membrane structures (Fig. 3G, H, I) with diameters larger than the tubular ones. These larger structures usually had smooth outer membranes (Fig. 3H), sometimes punctuated by clathrin-coated protuberance (arrowheads in 3I). Regardless of the size and shape of the spinules, the gap between the two membranes remained relatively uniform at ~20 nm (Fig. 3A–I), which is typical of intercellular spaces in brains fixed with conventional glutaraldehyde fixative.
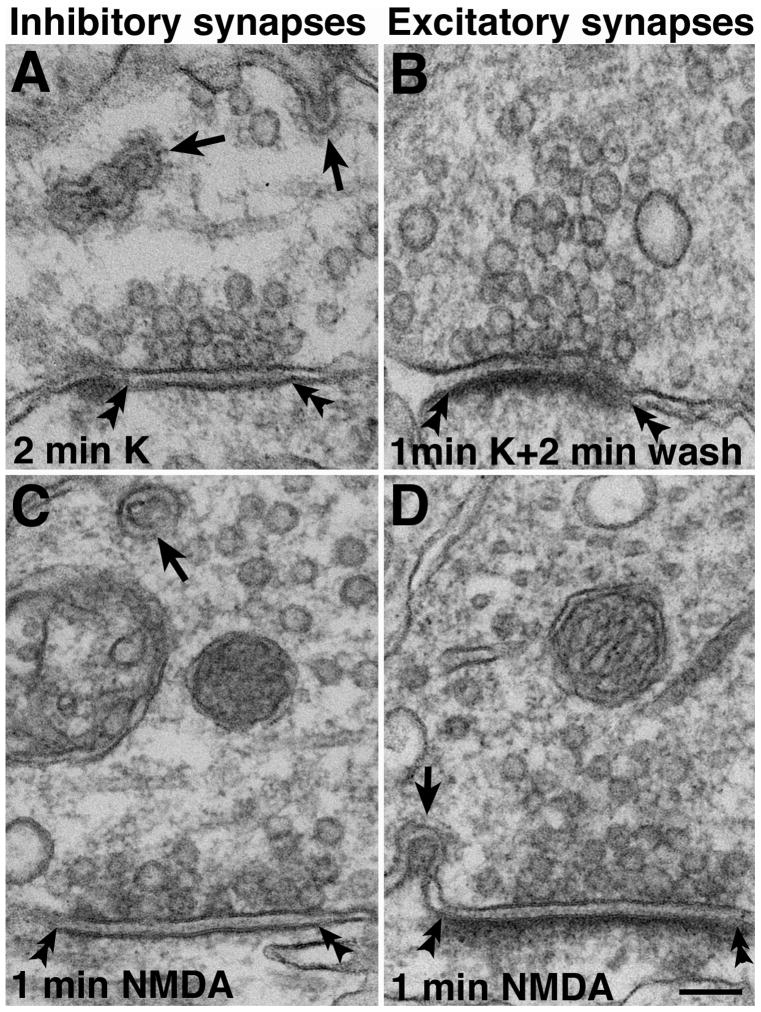
Spinules are also formed in inhibitory synapses (Fig. 4A) after depolarization. Inhibitory synapses are identified by their location on a soma or larger dendrites, and by their lack of a prominent PSD even after high K+ depolarization. The detailed structure of spinules at inhibitory synapses (arrows in Fig. 4A) was identical to that of excitatory synapses.
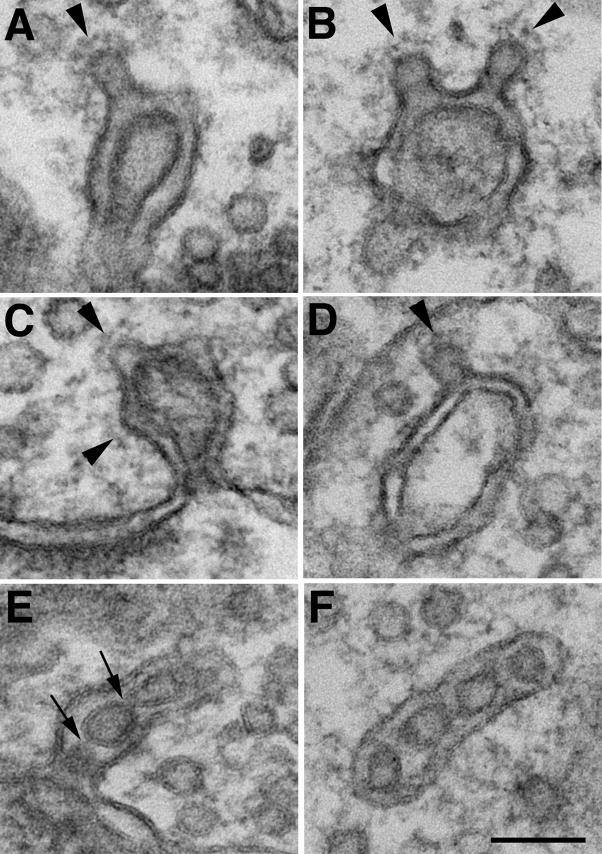
Fig. 4. Synaptic spinule formation and recovery.
(A) - After high K+ depolarization, synaptic spinules (arrows) were also found in inhibitory synapses which lack prominent PSDs (double arrowheads). (B) - Spinules disappeared quickly after cessation of stimulation and a few min of returning to normal medium. The PSD is still thickened in this excitatory synapse. (C) - Spinule (arrow) in an inhibitory synapse after NMDA treatment. (D) - Spinule (arrow) in an excitatory synapse after NMDA treatment. The PSD is visibly thickened. Scale bar = 0.1 μm.
Synaptic spinules form and disappear quickly after high K+ treatment
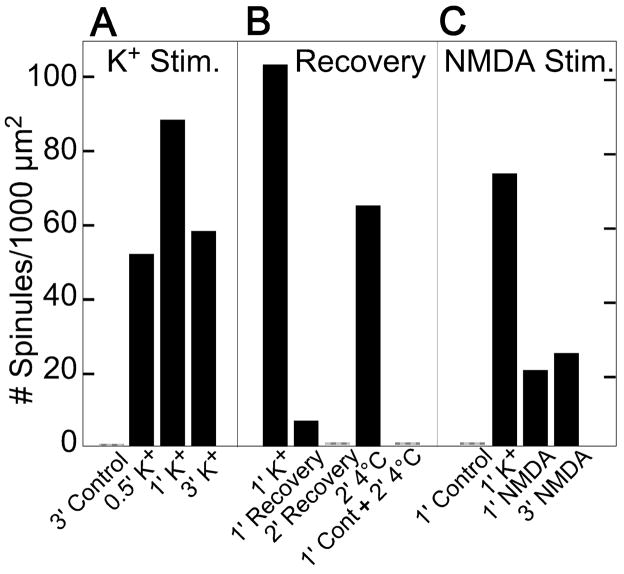
Slice cultures were examined at different intervals after high K+ depolarization to document the time course of spinule formation (3 experiments). Synaptic spinules formed as early as 30 sec following depolarization and their number peaked at ~1 min of exposure to high K+. Longer treatment in high K+ resulted in decreased numbers of synaptic spinules (Fig. 5A).
Fig. 5. Time course of synaptic spinule formation and disappearance.
Bar graphs within each group (A, B, and C) were from sister slice cultures carried out in parallel experiments. Data in each group were generated from one representative experiment (one slice culture per bar), and the total areas of thin section examined in each group were 2800, 4090 and 3025 μm2, respectively. Samples were exposed to high K+ (90 mM) medium for indicated intervals, then they were either fixed immediately (A) or allowed to recover in normal medium for indicated intervals at 37 °C or 4 °C before fixation (B). (C) - Samples were fixed immediately after high K+ or NMDA (50 μM) treatment at indicated intervals. Consistency among different experiments is indicated by number of spinules formed (ranging from ~70 – 105/1000 μm2) after 1 min high K+ in the three separate experiments.
The number of synaptic spinules induced by high K+ treatment decreased substantially within minutes of washing in normal medium (3 experiments, Fig. 5B, 2nd and 3rd bar), and returned to control levels after 10 min. After 1–5 min of normal medium wash following the high K+ treatment, the PSDs remained thicker (Fig. 4B) than those in controls, indicating that synaptic spinules disappear faster than the PSD thickenings. The rate of disappearance of synaptic spinules was slowed considerably when the washing buffer was at 4°C (3 experiments, Fig. 5B, 4th bar).
NMDA induces synaptic spinule formation
Slice cultures were treated with NMDA (50 μM) for various time intervals in order to investigate whether synaptic spinule formation is mediated by NMDA receptors (4 experiments). PSDs consistently thickened after 1–2 min of NMDA treatment (Fig. 4D), to a similar extent to that occurring after 1–2 min of high K+ depolarization, indicating a postynaptic effect mediated by NMDA receptors (cf. Dosemeci et al., 2002). However, these NMDA-treated slice cultures manifested fewer spinules than those after high K+ depolarization (Fig. 5C), and the number of spinules did not decrease with NMDA treatment time. Spinules were also found in inhibitory synapses after NMDA treatment (Fig. 4C).
Synaptic spinules in acute brain slices
Numerous synaptic spinules appeared in mouse hippocampal slices fixed immediately after dissection at 4°C (13 slices from 5 animals), and they might have either a pinch-waist (Fig. 6A) or a straight (Fig. 6B) appearance. Again, clathrin coating was associated with the pinch-waist appearance (arrow in Fig. 6A).
Fig. 6. Synaptic spinules in acute brain slices.
Spinules (arrows) in acute slices prepared from adult mouse hippocampus and fixed immediately with glutaraldehyde (A, B), 1% OsO4 (C), or high pressure freezing followed by freeze-substitution (D). Scale bar = 0.1 μm.
Synaptic spinules were rare in slices allowed to recover in oxygenated chambers for 2.5 hrs at 28°C, but increased when the recovered slices were treated with high K+ (four slices for each condition from two animals). The number of spinules from one representative experiment (consisting of slices made from the same animal) was 280/1000 μm2 immediately after dissection, 2.5/1000 μm2 after recovery, and 80/1000 μm2 upon high K+ depolarization. The observation that synaptic spinules are virtually absent in recovered slices but are induced by high K+ depolarization was confirmed in archived micrographs of rat hippocampal slices (Tao-Cheng et al., 2002).
Synaptic spinule formation in depolarized slices typically occurred in zones deep in the middle of the slice that lacked damaged tissue, but not in the superficial zones close to the cut surface, where extracellular space substantially widened during the recovery period.
Lack of synaptic spinules in dissociated hippocampal cultures
Examination of archives of images of dissociated cultures (Tao-Cheng, 2006) showed that spinules are extremely rare in 3–4 week old cultures either in control samples, or after high K+ depolarization. After examining a total area of more than 8500 μm2, only one double-membrane structure was seen in a 3 week old sample treated with a high dose of NMDA (250 μM for 15 min). While it was labeled with clathrin antibody (supplemental Fig. 2A), it was not located in a synaptic terminal. On the other hand, spinule-like structures were occasionally found in archived micrographs of untreated young cultures (3–6 days in vitro, Tao-Cheng, 2007), but these were, again, not located in synaptic terminals (supplemental Fig. 2B).
Synaptic spinules in perfusion fixed brains
In order to see if spinules were affected by perfusion fixation, the numbers of synaptic spinules were counted from archived micrographs of “fast” and “delayed” perfusion fixed mouse brains (Tao-Cheng et al., 2007, three and four animals, respectively). Many more (~ 5 fold) synaptic spinules (130/1000μm2) were found in delayed than in fast perfusion-fixed samples (27/1000 μm2).
Synaptic spinules are not artifacts of aldehyde fixation
In order to avoid the strong cross-linking effects of aldehyde fixatives, we performed direct OsO4 fixation as well as direct freezing followed by freeze-substitution. Our goal was to determine whether spinules could be artifacts caused by aldehyde fixative cross-linking adjacent membranes. Synaptic spinules were indeed seen after direct OsO4 fixation (Fig. 6C, 3 slices from 3 animals) and direct high pressure freezing without any chemical fixation (Fig. 6D, 4 slices from the same 3 animals). The fine structure and dimensions of these spinules were very similar to those seen after aldehyde fixation. One major difference was that the clathrin coat is less prominent after direct OsO4 fixation or direct freezing followed by freeze-substitution. Also, synaptic spinules seen here in mouse brains were smaller than those in bullfrog brains that were similarly freeze-substituted (Van Harreveld and Trubatch, 1975).
Fate of synaptic spinules
Synaptic spinules are typically situated among synaptic vesicles inside the terminals, but are not common elsewhere along the axon. Since some synaptic spinules are known to be free-standing (Eckenhoff and Pysh, 1979; Spacek and Harris, 2004), the lack of spinules outside of synaptic terminals suggests that if spinules move out of synapses, they do not do so in recognizable form.
Small coated protrusions, similar in size to neighboring synaptic vesicles, occasionally originated from spinules (arrowheads in Fig. 7A, B, C, D), and the inner tubules of spinules sometimes appeared to be vesiculating (Fig. 7E). Occasionally, elongated, membrane-bound structures with dimensions similar to spinules, but containing multiple vesicles (Fig. 7F) occurred in synapses. These observations are suggestive of transitional structures, as if parts of the outer tubule membranes were resolved by coated vesicle budding, while other parts of spinules become multivesicular bodies to be transported out of the terminal and degraded in lysosomes.
Fig. 7. Possible fate of synaptic spinules.
(A, B, C, D) – Small, clathrin-coated protrusions (arrowheads) can bud off from spinules of various sizes. A and B are from glutaraldehyde-fixed acute brain slices, C and D are from slice cultures treated with 30 sec of high K+ (C), and with 1 min of NMDA (D). (E) - The inner tubule of a spinule is vesiculated (arrows). (F) – An elongated membrane-bound structure with a row of vesicles. Scale bar = 0.1 μm.
DISCUSSION
The present study demonstrates that the formation of synaptic spinules results from heightened synaptic activity. We focused on spinule formation in organotypic hippocampal slice cultures because their structure and circuitry closely resembles that of intact brains, and they are amenable to controlled experiments. No attempt was made to block basal activity in control slice cultures, so some synaptic activity is likely to be present (Echevarria et al., 2000; Mohajerani et al., 2005; Yu et al., 2007). Despite the basal level of synaptic activity, synaptic spinules are virtually absent in control slice cultures, but are consistently induced by depolarization in high K+. Thus, we conclude that synaptic spinules are not present during basal activity, at least in slice cultures, and that their formation requires sustained high levels of activity.
The activity-dependence of spinule formation may explain the highly variable spinule frequencies observed with different experimental models and procedures. Acute brain slices fixed immediately after preparation are expected to contain highly activated synapses due to stimulation arising from the dissection and, indeed, these slices show numerous spinules. The fact that dissections are typically done at cold temperature may contribute to the abundance of spinules because cold substantially slows the disappearance of spinules. Synaptic spinules are virtually absent in slices that were allowed to recover, an observation consistent with results from rapid frozen and freeze-substituted bullfrog brain (Van Harreveld and Trubatch, 1975). Moreover, the present study samples from areas deep (100 μm) in the slice where tissues still closely resemble that of an intact brain. Our results strongly suggest that spinules are absent in newly quiescent synapses in recovered acute brain slices.
Perfusion fixation has been the method of choice for EM studies of the brain (Palay et al, 1962), but if not monitored carefully, may produce unexpected structural changes (Tao-Cheng et al., 2007). When spinules in two groups of perfusion fixed brains (fast vs delayed) were compared, the delayed perfusion-fixed brains had many more spinules. We assume that the spinules as well as other signs of activation form under the ischemia-like excitatory conditions produced during the delay. However, the fast perfusion-fixed brains also had a detectable number of synaptic spinules, which could be attributed to excitation induced by perfusion fixation if the surgical procedures or the fixatives themselves are still not fast enough to capture the native levels of synaptic activity in living brains. Thus, perfusion fixation may not be an optimal method for evaluating synaptic spinule formation in brains of living animals. This limitation leaves open the possibility that the spinules in fast perfusion-fixed brains reflect a background level of spinule formation in the resting brains. Indeed, resting brains show higher spontaneous synaptic activity than resting slice cultures (Echevarria et al., 2000).
We have previously reported two types of structural changes in neurons after depolarization: PSD thickening (Dosemeci et al., 2001) and CaMKII clustering (Tao-Cheng et al., 2002). Here, we show that depolarization also induces synaptic spinule formation, but its time course and progression differs from that of PSD thickening and CaMKII clustering. Synaptic spinule formation in slice cultures peaks at 1 min of high K+ treatment and then decreases, while PSD thickening and CaMKII clustering continue to increase. Thus, spinule formation appears to be the earliest indicator for heightened synaptic activity, occurring prior to detectible PSD thickening and CaMKII clustering.
The present study is the first to document the rapid turnover of synaptic spinules after cessation of stimulation, by showing that spinules disappear within 1 min of washout of the stimulant. A previous study examining perfusion-fixed brains reported increases in number of spinules up to 48 hr after LTP induction (Schuster et al, 1990). These additional spinules need not be the same ones persisting throughout LTP. More spinules might be forming and turning over due to the heightened levels of activity resulting from LTP, and might also be more readily induced upon additional excitatory stimulation from the perfusion fixation.
The defining structural feature of spinules – their parallel double membranes –suggests that the two membranes may be physically linked. In dissociated cell cultures, neuronal elements are typically not as tightly apposed as in the brain. This lack of close apposition may account for the absence of synaptic spinule in our dissociated hippocampal neuronal cultures. Indeed, cell adhesion molecules may play a role in spinule formation, and their distribution in dissociated cultures may be different from that in intact brain. Future immunolabeling studies might identify specific molecular components of the two apposed membrane of synaptic spinules.
Alternatively, fixatives could cross-link adjacent membranes. It occurred to us that spinule formation might depend on the presence of cross-linking fixatives in the extracellular space at the exact moment that invagination of the synaptic axonal membrane or the evagination of the dendritic membrane is induced. A strong cross-linker and slow-working fixative like glutaraldehyde (Smith and Reese, 1980) might artificially create double membrane structures in highly activated synapses if it cross-linked adjacent membranes during membrane recycling. We tested the role of aldehyde fixatives in producing spinules by using direct OsO4 fixation, which is faster and less cross-linking than aldehyde fixation. The presence of synaptic spinules after direct OsO4 fixation is confirmed in acute brain slices, as expected based on previous work with rat brain (Westrum and Blackstad, 1962). We ultimately turned to direct freezing, followed by freeze-substitution to avoid chemical fixation entirely, and demonstrated the presence of synaptic spinules in mouse brain under these conditions as well. Thus, spinules are not fixation artifacts, though it remains an open question whether their formation is initiated by the invaginating synaptic terminal, or by the evaginating spine thrusting into the synapse.
Spinule formation does not appear to be dependent on a particular postsynaptic receptor or cell type, since spinules form in both excitatory (glutamatergic) and inhibitory (GABAergic) synaptic terminals. In addition to high K+ depolarization, NMDA also induces spinule formation, which could be taken to mean that direct postsynaptic (dendritic) activation initiates spinule formation. Alternatively, the effect of NMDA may be indirect, via activated neurons in the CA3 regions initiating spinule formation at their synaptic terminals in the CA1 region. In this instance, presynaptic terminals, rather than postsynaptic elements, would play a leading role in spinule formation.
Close examination of synaptic spinule structure and dynamics indicate that its time course is within the range of the time course of formation and resolution of clathrin-coated vesicles in presynaptic terminals induced by synaptic activity (Miller and Heuser, 1984). The presence of a clathrin coating at the leading tips of synaptic spinules suggest that clathrin-mediated endocytosis of axonal membranes could be a driving force to initiate spinule formation. Indeed, clathrin has been shown to mediate the internalization of gap junctions (Piehl et al., 2007). In this instance, dynamin and myosin-V1 are also involved in this clathrin-mediated process of turning over large, double-membrane structures.
Clathrin-mediated endocytosis may not be the only force driving spinule formation. Another aspect of axonal membrane recycling at synapses, giant vacuole formation, depends on bulk endocytosis induced by intense synaptic activity (Clayton et al., 2008). This process also occurs with a rapid time course consistent with that of synaptic spinule formation. Giant vacuoles resolve quickly when the internalized membranes are retrieved by coated vesicles (reviewed in Royle and Lagnado, 2003). Indeed, our images of large, extended spinules showing no clathrin coats at all, as well as other images of clathrin-coated small protrusions budding off of them is compatible with the view that some spinule formation parallels giant vacuole formation at synapses, and that the clathrin coating occurs later in order to recycle these internalized membranes. Similar small protrusions were also seen at bullfrog spinules (Van Harreveld and Trubatch, 1975).
Synaptic vesicle recycling requires dynamin 1 (Newton et al., 2006), a GTPase that is an essential component of the final step in endocytosis (reviewed in Hinshaw, 2000). Dynamin 1-null cortical neuronal cultures have extensive synaptic spinules in terminals that are highly active (Hayashi et al., 2008). Numerous clathrin-coated protrusions, similar in size and shape to the ones we report here, are located on the outer membrane of these spinules in dynamin 1-null synapses. Lack of dynamin-1 appears to prevent these clathrin-coated protrusions from recycling, which may also inhibit the turnover of spinules.
We have demonstrated that synaptic spinule formation is induced by heightened synaptic activity, and that spinule invaginations form and disappear rapidly. These new findings must inform any speculation about roles for synaptic spinules. For instance, spinule formation during synaptic activation might quickly remove excess axonal plasma membranes and simultaneously reduce spine volumes. It is possible that spinules have a role in adjusting synaptic size in face of intense activity.
Supplementary Material
Low magnification images showing pyramidal neuronal somas (nuclei marked by * in B) in hippocampal CA1 region from two 11 day old slice cultures. Sampling of spinules was restricted to stratum radiatum.
Spinule-like structures were rarely seen in dissociated hippocampal neurons. (A) shows one such double-membrane structure labeled with clathrin antibody (depicted by black grains). (B) is from a 3 day old culture. Both entities were not associated with synapses. Scale bar = 0.1 μm.
Acknowledgments
We thank Drs. M. W. Brightman and N. Otmakhov for helpful discussions and Virginia Crocker for assistance with the EM immunogold labeling. This research was supported by the Intramural Research Program of the NIH, NINDS.
List of abbreviations
- PSD
postsynaptic density
- CaMKII
Calcium calmodulin-dependent protein kinase II
- EM
electron microscopy
- NMDA
N-methyl-D-aspartic acid
- LTP
long-term potentiation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Applegate MD, Landfield PW. Synaptic vesicle redistribution during hippocampal frequency potentiation and depression in young and aged rats. J Neurosci. 1988;8:1096–1111. doi: 10.1523/JNEUROSCI.08-04-01096.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EL, Evans GJ, Cousin MA. Bulk synaptic vesicle endocytosis is rapidly triggered during strong stimulation. J Neurosci. 2008;28:6627–6632. doi: 10.1523/JNEUROSCI.1445-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemeci A, Tao-Cheng J-H, Vinade L, Winters CA, Pozzo-Miller L, Reese TS. Glutamate-induced transient modification of the postsynaptic density. Proc Natl Acad Sci U S A. 2001;98:10428–10432. doi: 10.1073/pnas.181336998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemeci A, Vinade L, Winters CA, Reese TS, Tao-Cheng J-H. Inhibition of phosphatase activity prolongs NMDA-induced modification of the postsynaptic density. J Neurocytology. 2002;31:605–612. doi: 10.1023/a:1025735410738. [DOI] [PubMed] [Google Scholar]
- Eckenhoff MF, Pysh JJ. Double-walled coated vesicle formation: evidence for massive and transient conjugate internalization of plasma membranes during cerebellar development. J Neurocytol. 1979;8:623–638. doi: 10.1007/BF01208513. [DOI] [PubMed] [Google Scholar]
- Echevarria D, Albus K. Activity-dependent development of spontaneous bioelectric activity in organotypic cultures of rat occipital cortex. Brain Res Dev Brain Res. 2000;123:151–164. doi: 10.1016/s0165-3806(00)00089-4. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Raimondi A, O’Toole E, Paradise S, Collesi C, Cremona O, Ferguson SM, De Camilli P. Cell- and stimulus-dependent heterogeneity of synaptic vesicle endocytic recycling mechanisms revealed by studies of dynamin 1-null neurons. Proc Natl Acad Sci U S A. 2008;105:2175–2180. doi: 10.1073/pnas.0712171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TM, Heuser JE. Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction. J Cell Biol. 1984;98:685–698. doi: 10.1083/jcb.98.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Yasuda M, Coats J, Jones Y, Martone M, Mayford M. Disruption of dendritic translation of CaMKIIa impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36:507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- Mohajerani MH, Cherubini E. Spontaneous recurrent network activity in organotypic rat hippocampal slices. Eur J Neurosci. 2005;22:107–118. doi: 10.1111/j.1460-9568.2005.04198.x. [DOI] [PubMed] [Google Scholar]
- Newton AJ, Kirchhausen T, Murthy VN. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proc Natl Acad Sci U S A. 2006;103:17955–17960. doi: 10.1073/pnas.0606212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhov N, Tao-Cheng J-H, Carpenter S, Asrican B, Dosemeci A, Reese TS, Lisman J. Persistent Accumulation of Calcium/Calmodulin-Dependent Protein Kinase II in Dendritic Spines after the Induction of NMDA Receptor-Dependent Chemical Long Term Potentiation. J Neurosci Vol. 2004;24:9324–9331. doi: 10.1523/JNEUROSCI.2350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, McGee-Russell SM, Gordon S, Jr, Grillo MA. Fixation of neural tissues for electron microscopy by perfusion with solutions of osmium tetroxide. J Cell Biol. 1962;12:385–410. doi: 10.1083/jcb.12.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehl M, Lehmann C, Gumpert A, Denizot JP, Segretain D, Falk MM. Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process. Mol Biol Cell. 2007;18:337–347. doi: 10.1091/mbc.E06-06-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle SJ, Lagnado L. Endocytosis at the synaptic terminal. J Physiol. 2003;553(2):345–355. doi: 10.1113/jphysiol.2003.049221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster T, Krug M, Wenzel J. Spinules in axospinous synapses of the rat dentate gyrus: changes in density following long-term potentiation. Brain Res. 1990;523:171–174. doi: 10.1016/0006-8993(90)91654-y. [DOI] [PubMed] [Google Scholar]
- Smith JE, Reese TS. Use of aldehyde fixatives to determine the rate of synaptic transmitter release. J Exp Biol. 1980;89:19–29. doi: 10.1242/jeb.89.1.19. [DOI] [PubMed] [Google Scholar]
- Sorra KE, Fiala JC, Harris KM. Critical assessment of the involvement of perforations, spinules, and spine branching in hippocampal synapse formation. J Comp Neurol. 1998;398:225–240. [PubMed] [Google Scholar]
- Spacek J, Harris KM. Trans-endocytosis via spinules in adult rat hippocampus. J Neurosci. 2004;24:4233–4241. doi: 10.1523/JNEUROSCI.0287-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng J-H, Vinade L, Pozzo-Miller L, Reese TS, Dosemeci A. Calcium/calmodulin-dependent protein kinase II clusters in adult rat hippocampal slices. Neuroscience. 2002;115:435–440. doi: 10.1016/s0306-4522(02)00451-7. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng J-H. Activity-related redistribution of presyanptic proteins at the active zone. Neuroscience. 2006;141:1217–1224. doi: 10.1016/j.neuroscience.2006.04.061. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng J-H. Ultrastructural Localization of Active Zone and Synaptic Vesicle Proteins in a Preassembled Multi-vesicle Transport Aggregate. Neuroscience. 2007;150:575–584. doi: 10.1016/j.neuroscience.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng J-H, Gallant PE, Brightman MW, Dosemeci A, Reese TS. Effects of delayed perfusion fixation on postsynaptic density and CaMKII clustering in different regions of the mouse brain. J comp Neurol. 2007;501:731–740. doi: 10.1002/cne.21276. [DOI] [PubMed] [Google Scholar]
- Tarrant SB, Routtenberg A. The synaptic spinule in the dendritic spine: electron microscopic study of the hippocampal dentate gyrus. Tissue and Cell. 1977;9:461–473. doi: 10.1016/0040-8166(77)90006-4. [DOI] [PubMed] [Google Scholar]
- Van Harreveld AV, Trubatch J. Synaptic changes in frog brain after stimulation with potassium chloride. J Neurocytol. 1975;4:33–46. doi: 10.1007/BF01099093. [DOI] [PubMed] [Google Scholar]
- Westrum LE, Blackstad TW. An electron microscopic study of the stratum radiatum of the rat hippocampus (regio superior, CA 1) with particular emphasis on synaptology. J Comp Neurol. 1962;119:281–309. doi: 10.1002/cne.901190303. [DOI] [PubMed] [Google Scholar]
- Yu Z, McKnight TE, Ericson MN, Melechko AV, Simpson ML, Ill BM. Vertically aligned carbon nanofiber arrays record electrophysiological signals from hippocampal slices. Nano Lett. 2007;7:2188–2195. doi: 10.1021/nl070291a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Low magnification images showing pyramidal neuronal somas (nuclei marked by * in B) in hippocampal CA1 region from two 11 day old slice cultures. Sampling of spinules was restricted to stratum radiatum.
Spinule-like structures were rarely seen in dissociated hippocampal neurons. (A) shows one such double-membrane structure labeled with clathrin antibody (depicted by black grains). (B) is from a 3 day old culture. Both entities were not associated with synapses. Scale bar = 0.1 μm.