Abstract
This paper describes the development of the B3LYP localized orbital correction model which improves the accuracy of the B3LYP thermochemical predictions for compounds containing transition metals. The development of this model employs a large data set containing 36 experimental atomic energies and 71 bond dissociation energies. B3LYP calculations were carried out on these systems with different basis sets. Based on an electronic structure analysis and physical arguments, we built a set of 10 parameters to correct atomic data and a set of 21 parameters to correct bond dissociation energies. Using the results from our biggest basis set, the model was shown to reduce the mean absolute deviation from 7.7 to 0.4 kcal∕mol for the atomic data and from 5.3 to 1.7 kcal∕mol for the bond dissociation energies. The model was also tested using a second basis set and was shown to give relatively accurate results too. The model was also able to predict an outlier in the experimental data that was further investigated with high level coupled-cluster calculations.
INTRODUCTION
Transition metals play a key role in catalysis and are involved in a number of important industrial processes and biological reactions. It is therefore essential for electronic structure methods to handle these elements correctly and efficiently. Yet, electronic structure methods encounter several pitfalls in dealing with metal-containing systems. First, the number of electrons already present in the metal and the high coordination number observed in typical metal-containing compounds imply that the smallest systems of practical interest are already quite large to be handled efficiently with accurate wave-function methods such as CCSD(T). A second point is that transition metals, unlike organic systems, manifest a large number of low-lying excited states, sometimes inducing a true multireference character in the wavefunction, but in all cases presenting wavefunction-based electron correlation methods with formidable problems. Finally, basis set convergence for transition metals is significantly less well understood than it is for lighter atoms [where complete basis set (CBS) extrapolation methods are well developed and yield demonstrably good convergence], and a number of other effects, such as relativistic corrections, must be taken into account. In combination, these features make it exceptionally difficult to perform benchmark calculations for transition metals; in fact, arguably true benchmarks (accurate to ∼1 kcal∕mol) are not available even for diatomic transition metal species in the current literature.
This state of affairs has prompted a search for alternatives, of which density functional theory (DFT) is the obvious candidate. DFT has shown important successes in handling metal-containing systems as can be testified by the number of inorganic and bioinorganic papers published in the last decade using these methods. Yet, little is really known about the precise accuracy of various DFT based approaches for transition metals, in contrast to the situation for first or second row elements where extensive tests of thermodynamic predictions have been performed against accurate experimental data and converged wavefunction-based benchmarks.1, 2 DFT calculations on first-row transition metals attempting to assess accuracy have been carried out in the past but were generally limited to relatively small or medium sized test sets,3, 4, 5, 6, 7, 8, 9, 10 although a few papers with larger test sets have appeared in recent years.2, 11, 12 Moreover, only few research groups have tried to improve DFT by including transition metals in their training sets.10
In a previous paper,13 we have described a novel approach to understanding and correcting the errors in DFT energetics for molecules composed of atoms in the first and second rows of the Periodic Table. The paper identifies the principal errors in gradient corrected and hybrid DFT as arising from an inaccurate treatment of nondynamical correlation errors in atoms and molecules, particularly large errors are made in treatment of different atomic hybridization states for delocalized, for singly occupied orbitals, and for ionic bonds. Using an empirical parametrization based on localized electron pairs (associated with bonds or lone pairs), the errors for the B3LYP localized orbital corrections (B3LYP-LOC) functional for the 222 molecules in the Pople G3∕99 test set14 are reduced from 4.8 to 0.8 kcal∕mol, an accuracy that is competitive with G3 theory, a high level ab initio methodology incorporating QCISD(T) level calculations as its most costly component. These results represent the best yet achieved for any DFT method, and the first in which virtually no significant outliers in atomization energies can be observed for the G3 data set, despite the presence of many large, complex molecules which exhibit errors in the 10–20 kcal∕mol range in a standard B3LYP treatment. We have further extended the method to ionization potentials and electron affinities in Ref. 15, with similar success, in the process identifying a number of new sources of error, the most important of which is a systematic error in treating the interaction of two unpaired electrons with parallel spins on the same atom.
While these results suggest that the fundamental physics behind the DFT-LOC approach are essentially correct, and that the method will have substantial practical utility, the present formulation is incomplete in a number of important dimensions: treatments of transition states, excited states, extended states (e.g., solids), and transition metals have not yet been addressed. While in principle there is no barrier to addressing any of these dimensions, in practice each represents a formidable problem, not the least of which is due to the lack of a reliable, and sufficiently large, experimental database against which the methodology can be parametrized.
As the second major objective of the present paper, we develop an initial parametrization of B3LYP-LOC for the first-row transition metal series. Our initial model is based on a wide range of gas phase experimental data for excitation energies, ionization potentials, and bond energies. Typical results for current DFT functionals on these sorts of systems yield errors in the range of 3–15 kcal∕mol with average mean absolute deviations (MADs) on the order of 5–10 kcal∕mol for diverse data sets. We show that qualitatively, the dominant sources of errors for transition metals are analogous to those identified in Ref. 13 for first and second row atoms and molecules. The quantitative model that we have developed, while lacking in the precision of B3LYP-LOC in Ref. 13, represents a large improvement as compared to existing methods; reducing the MAD of B3LYP for a large database (106 experimental energies) from 6.1 to 1.2 kcal∕mol.
While these results are encouraging, they do not represent a complete treatment of transition metal–containing systems. Gas phase data exist primarily for metals either in their neutral state or as singly charged cations; however, higher oxidation states are also commonly found in systems of practical interest (e.g., chemical or biological systems containing Cr, Mn, Fe, or Co). It is also the case that our data set primarily contains compounds in which the metal has a small coordination number; again, practically important systems typically involve higher coordination numbers. Extending our model to these higher oxidation states and coordination numbers is possible, but validating any such extension will require the exploration of different types of data sets (e.g., spin crossover data), an endeavor that we reserve for another publication. Our belief is that a great deal can be learned from studying the small, low oxidation state systems investigated herein, despite the noise in the experimental data, and the challenge of handling high spin, low oxidation state, low coordination number compounds, in which the chemical bonding can take on a number of unusual forms. As in the case of first and second row compounds, it is possible to identify systematic patterns in the errors, relate these to physical concepts involving nondynamical electron correlation, and develop a model in which the ratio of adjustable parameters to data points is reasonable. This model can then serve as a starting point for considering the other classes of transition metal–containing systems discussed above.
The paper is organized as follows. Section 2 describes the methodologies used for the calculations and assembles the transition metal–containing data sets to be investigated. Section 3 discusses our B3LYP results for these molecules for the various relevant thermodynamic quantities, and a detailed analysis of the various electronic structures obtained is carried out. In Sec. 4, we build an explicit B3LYP-LOC model for the largest basis set used in our study which is based on the quadruple-ζ basis set developed by Weigend et al.,16 we also develop a model for the widely used LACV3P**++ basis which employs pseudopotentials. To an even greater extent than for first and second row elements, some of the errors in the DFT treatment of metal-containing systems arise from basis set incompleteness, relativistic effects (including spin-orbit coupling), and other issues not directly related to the exchange-correlation functional; empirical corrections in principle can absorb some fraction of these errors as well as correlation errors, enabling quite reasonable improvements to be obtained with the smaller, as well as larger, basis set, and in the absence of explicit treatment of relativity. Hence, in our approach, the empirical correction model is explicitly basis set dependent (although the architecture of the corrections is shown to work robustly for the two different basis sets we investigate).
The construction of a B3LYP-LOC model for our data set, described in detail in Sec. 4, requires a careful analysis of the bonding in a wide range of small transition metal species. As previous papers over the past several decades have noted, this is a highly nontrivial task in many cases. Calculating corrections in the LOC formalism requires assignment of electrons to various types of bonds, lone pairs, singly occupied orbitals, etc., and also making some estimate of the dimensions of the orbitals as compared to bond lengths. While we cannot claim that our assignments are always optimal (due to the difficulty of the problem and the large amount of data we have investigated), we have at least endeavored to make our arguments along these lines transparent. Our basis sets, geometries, energies, and wavefunctions are available as supplementary material17 for those wishing to pursue their own investigations. While many of these systems have been studied previously with DFT methods using different functionals or (in some cases) other ab initio methods, the results reported herein represent, to our knowledge, one of the largest set of DFT calculations carried out using several different widely used basis sets. These data sets and the accompanying analysis should therefore be useful to other investigators regardless of whether they pursue an approach related to DFT-LOC. Finally, in Sec. 5, we discuss various aspects of the results and suggest future directions of research. The present paper has to be regarded as an initial effort, the relevance of which will need to be tested by application of the parameters, and more generally the basic ideas, to larger, more complex, higher coordination transition metal–containing species. We believe that, although these latter species have significant differences in electronic structure as compared to the coordinatively unsaturated, low ionization state compounds considered here, the insights obtained are nevertheless going to be very relevant when proceeding to the next stages of the project. But modifications and improvements of the method will likely be necessary as further aspects of transition metal electronic structure are encountered.
METHODOLOGY
Test set
Our test set is limited to the first-row transition metals excluding Zn. We mainly focused on these metals because they are the metals most commonly found in biology and are also the most common at the surface of the earth. Zn was excluded because it has a slightly different behavior, since its 3d shell is generally filled for the metal and also for its common ion Zn2+. We intend to build a DFT-LOC model for Zn in a subsequent publication.
To assemble our experimental data set, we retained complexes for which gas phase experimental data were available for all or almost all transition metals considered. These constraints were imposed because computational calculations are more consistent with gas phase experimental data (solvent effects are not necessarily well modeled and will introduce errors unrelated to the functional), and we wanted to have the data for all first-row transition metals so that the origin of the DFT errors would be more easily interpreted, as we could see the variation of the error according to the nature of the metal.
The first component of our test set consists of 36 atomic data, including excitation energies of the metals and the monocations, and also includes the first and second ionization potentials. The second component of the test set comprises 71 metal-ligand dissociation energies (M–H, M–CH3, M–O, M+–H, M+–CH3, M+–CH2, M+–O, and M+–OH), which contains both neutral and monocationic species. It should be noticed that even trying to diversify the test set as much as we could, the experimental data are rather scarce2 and impose therefore a strong limitation on the coverage of transition metal–containing compounds. Particularly, the coordination number and oxidation state of the metals are not the ones most commonly found in typical chemical and biological applications. But even if the average error obtained on this data set would not necessarily accurately reflect the errors that would be expected in such applications, the relative simplicity of the molecules is helpful in trying to track down the origin of the errors that are observed, and many if not most of the insights obtained can be expected to be transferable to other systems.
The experimental data for the excitations and ionization potentials were obtained from spectroscopic data.18 “Experimental” electronic bond dissociation energies (De) were derived from the bond dissociations energies (D0) essentially obtained from the work of Armentrout and co-workers.19De was obtained by correcting D0 for thermochemical effects whenever required and then by subtracting computed contribution from the zero point energies (ZPEs). All these corrections were obtained from vibrational frequency calculations at the 6-31G* level (vide infra).
Regarding the accuracy, our experimental data are quite diverse. We have a very high accuracy for all neutral and monocationic atomic data (excitation energies and ionization potentials). Regarding the dissociation energies, the distribution of estimated accuracies is very broad with an average experimental error around 3 kcal∕mol. Some inaccurate data (larger than 4 kcal∕mol) were nevertheless included so that experimental data for all the complexes of the different metals will be available. The computational errors must be analyzed in light of the reported experimental error bars to get a better idea of the real deficiency of DFT methods. To supplement the experimental data, we have also carried out a number of CCSD(T) calculations which we believe are approaching benchmark quality, with estimated errors of ∼2–3 kcal∕mol. While these calculations pose considerable challenges themselves, the initial efforts along these lines, as reported in the present paper, appear promising, and further work in this direction is indicated.
Computational methodology
All calculations were carried out with the B3LYP functional20 which is one of the most commonly used functionals for metal-containing systems.21 Future work will investigate the interesting question of whether alternative functionals can provide better results, as has been suggested in a number of recent papers.2, 9, 10, 12, 22 Our success with the B3LYP-LOC correction scheme for first and second row systems, along with the broad literature employment of B3LYP, motivated our choice in this initial study. Geometries were optimized for different spin states and different starting configurations with the Los Alamos LACVP (Ref. 23) basis on the metals and the 6-31G* basis on the other elements. The geometries with the lowest energies were retained and frequency calculations were carried out at the same level. The results of frequency calculations were used to correct experimental D0 from thermochemical effects and to compute the ZPE contributions with scaled frequencies (a factor of 0.9806 was used as advised by Scott and Radom24). The experimental electronic dissociation energies (De) were subsequently derived from the experimental dissociation energies (D0) by removing the contribution from the ZPE. Then single point calculations were done using three larger basis sets. The LACV3P** and LACV3P++** were used for the metals,25 whereas the other elements were treated with the 6-311G** and 6-311++G** basis sets, respectively. To approach the basis convergence limit, we also tested a modified quadruple-ζ basis set recently developed by Weigend et al.16 from which the g polarization functions were removed [QZVP(-g)].
All DFT calculations were carried out within an unrestricted framework with the JAGUAR suite of programs,26 without using any symmetry constraints. Different initial guesses were used to ensure that the right ground state wave function was found. We also investigated different possible states of our complexes when no experimental evidence or no related theoretical work reported the ground spin state. For those cases, the energies reported are the lowest one for all the spin states investigated.
Coupled-cluster calculation was carried out to check the level of accuracy of the experimental data, and the DFT calculations, against high level ab initio results. All these restricted CCSD(T) calculations were performed with MOLPRO (Ref. 27) using cc-pVXZ-DK (X=T,Q,5) basis sets28 for main group elements. In order to achieve the highest accuracy possible, we also correlated the 3s and 3p electrons and we therefore used cc-pwCVXZ-DK (X=T,Q,5) basis sets on the metal atoms. Indeed, it has been shown that the 3s and 3p electron correlation plays a significant role for the accurate prediction of bond dissociation energies.29 To achieve chemical accuracy, we also extrapolate our results to the infinite basis set limit using the 1∕X3 dependence of the residual correlation energy on the basis set size as proposed by Halkier et al.30 We therefore use Eq. 1 to get the extrapolated energy,
| (1) |
where is the extrapolated energy and are the correlation energies calculated on basis X and Y, respectively (in the above formula, we assume X>Y). Relativistic effects may also have significant effects for transition metal compounds. Scalar relativistic effects were included through the Douglas–Kroll approximation31 and the results were also corrected for spin-orbit coupling32 effects. ZPE corrections were also included as described for the DFT calculations.
The electronic configurations, bonding orbitals, and natural populations were derived from the electronic structures obtained with the LACV3P** basis set, using the NBO program.33NBO analysis is a technique for studying hybridization and colvalency in molecules based on the eigenvector of the first order density matrix. It produces localized orbitals that closely match the Lewis structures usually employed by chemists.33 The size of the orbitals was determined by measuring the second moment of Boys’ localized orbitals34 as described in Ref. 13.
COMPUTATIONAL RESULTS WITH B3LYP
Atoms
Excitation energies of neutral atoms
The calculated and experimental excitation energies reported Table 1 are derived from the electronic transition between configurations 3dn4s2→3dn+14s1 and not necessarily starting from the atomic ground state. These experimental excitation energies have also been J-averaged explaining why Ni is reported in our table as having a 3d94s1 ground state configuration, whereas the 3d84s2 configuration is experimentally the true ground state.
Table 1.
Excitation energies of atoms (kcal∕mol) from experiment, errors (kcal∕mol) against experiment (theory-experiment) obtained by other authors with high level ab initio methods and the errors obtained with B3LYP for our different basis sets. Experimental values quoted in Ref. 6, taken from Ref. 36. The QCISD(T) calculations have been done with a modified basis set based on Wachters.
| Expt.a | Sc D(s2) → F(s1) 33.0 | Ti F(s2) → F(s1) 18.7 | V F(s2) → D(s1) 5.8 | Cr D(s2) → D(s1) −23.1 | Mn S(s2) → D(s1) 49.6 | Fe D(s2) → F(s1) 20.1 | Co F(s2) → F(s1) 9.7 | Ni F(s2) → D(s1) −0.7 | Cu D(s2) → S(s1) −34.4 | MAD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Errors | QCISD(T)b | 1.4 | 0 | −0.9 | −2.3 | 2.1 | −0.2 | −2.1 | −3.5 | −8.3 | 2.3 |
| LACV3P** | −14.4 | −9.4 | −5.1 | −6.2 | −8.8 | −7 | −2.4 | 2.3 | 1.6 | 6.4 | |
| LACV3P++** | −14.6 | −10 | −6.7 | −8.1 | −12.9 | −9.8 | −6.7 | −2.2 | −3.6 | 8.3 | |
| QZVP(−g) | −16.2 | −13.9 | −11 | −8.8 | −17.1 | −14.8 | −12.1 | −8.7 | −7.1 | 12.2 | |
Overall, we can see that the excitation energies are underestimated for the atoms. Thus, the B3LYP functional generally overbinds the 3dn+14s1 configuration relative to the 3dn4s2, as has been previously observed for other functionals.6, 35 This can be understood for the first four metals of the row (Sc→Cr) as being due to an overestimation of the nondynamical correlation for the excited state (the energy is thus found to be too low) due to the fact that two singly occupied 3d and 4s orbitals are generated from the doubly occupied 4s orbital. The number of parallel spin-spin interactions that are created due to the excitation increases as the atomic number of the first four metals increases, leading to a striking progression in which the error systematically diminishes, thus indicating that adding a parallel spin errors opposes overbinding (as is the case for first and second row atoms). In the case of the last six metals, the number of singly and doubly occupied orbitals is constant but we are going from a doubly occupied 4s to a doubly occupied 3d. The relative errors for this transfer are complicated to analyze, but one can argue that with a complete basis set the overbinding of the singly occupied 4s orbital will be larger than that for the 3d orbital [as is observed for Cu with the QZVP(-g) basis, in which parallel spin corrections do not play a role]. Here, the parallel spin errors increase the overbinding as atomic number decreases because more parallel spin interactions are created upon excitation as atomic weight diminishes in the series, again in agreement with earlier work on first and second row atoms.
It can be noticed that high level methods such as QCISD(T) do not show the same systematic underestimation of the excitation energy. First, QCISD(T) is more accurate than B3LYP. For copper, deviation as large as 8.3 kcal∕mol is observed but this is due to the fact that relativistic effects were not taken into account in the calculation. Nonetheless, even with corrections for relativistic effects, deviation as large as 4 kcal∕mol are not uncommon.37
Excitation energies of the monocations
In case of the monocation excitation energies (Table 2), the resulting patterns are similar to those observed for the neutral excitations, but the details are different due to the different starting occupation numbers. When we develop the B3LYP-LOC model, we will use the same parameters, and physical model, to explain the excitation energies of the monocations as are used for the neutral atoms. Thus, this data set serves as a crosscheck for the explanations described above for the neutral excitations. The results, shown below, demonstrate that exceptionally good agreement is obtained for both data set with a single set of parameters.
Table 2.
Excitation energies of monocations (kcal∕mol) from experiment, errors (kcal∕mol) against experiment (theory-experiment) obtained by other authors with high level ab initio methods and the errors obtained with B3LYP for our different basis sets. Experimental values quoted in Ref. 4, taken from Ref. 38, T, W are the TZV and a modified spdf Wachters basis sets, respectively.
| Expt.a | Sc+ D(s1) → F(s0) 13.8 | Ti+ F(s1) → F(s0) 2.3 | V+ D(s0) → F(s1) 7.6 | Cr+ S(s0) → D(s1) 35.1 | Mn+ S(s1) → D(s0) 41.7 | Fe+ D(s1) → F(s0) 5.8 | Co+ F(s0) → F(s1) 9.9 | Ni+ D(s0) → F(s1) 24.9 | Cu+ D(s0) → D(s1) 64.8 | MAD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Errors | CCSD(T) (T)b | 2.8 | 2.4 | −1.2 | 3.3 | 14.2 | 9.7 | −9.1 | −8.7 | 3.1 | 6.1 |
| QCISD(T) (W)c | 2.1 | 0.9 | 0.5 | 1.6 | 2.6 | −0.3 | 2.3 | 4.2 | 9.9 | 4.0 | |
| LACV3P** | −7.7 | −2.8 | −0.2 | 2.5 | −7.3 | −4.8 | 0.7 | −4.2 | −3.7 | 10.7 | |
| LACV3P++** | −7.7 | −2.6 | −0.3 | 2.4 | −7.7 | −4.9 | 1.5 | −3.2 | −2.3 | 10.6 | |
| QZVP(−g) | −9.9 | −7.7 | 5.4 | 3.3 | −13.6 | −10.4 | 7.0 | 3.4 | 0.7 | 7.0 | |
The literature results obtained with CCSD(T) or QCISD(T) do not present the systematic errors observed with B3LYP, although significant, apparently random, errors are observed in both references cited above. Moreover large deviations are observed between CCSD(T) and QCISD(T) calculations for Mn+ until Cu+. These discrepancies are troubling given that the methods are expected to give similar results. The basis set may be the main cause of the problem; it is likely that very large basis sets and CBS extrapolation of some sort should be used to obtain reliable results on metal containing compounds when carrying out CCSD(T) and QCISD(T) calculations.
Ionization potentials of neutral atoms
The numbers reported in Table 3 for the first ionization potential correspond to the removal of the 4s electron from the ground state of the atoms (except in the case of Ni whose ground state depends whether or not experimental values are J-averaged).
Table 3.
First ionization potentials (kcal∕mol) from experiment, errors (kcal∕mol) against experiment (theory-experiment) obtained by other authors with high level ab initio methods and the errors obtained with B3LYP for the different basis sets. Experimental values quoted in Ref. 40; taken from Ref. 36. W is a modified spdf Wachters basis set.
| Expt.a | Sc ds2 → ds 151.3 | Ti d2s2 → d2s 157.5 | V d3s2 → d3s 162.8 | Cr d5s → d5 155.9 | Mn d5s2 → d5s 171.3 | Fe d6s2 → d6s 182.2 | Co d7s2 → d7s 190.9 | Ni d8s2 →d8s 199.9 | Cu d10s →d10 178.0 | MAD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Errors | QCISD(T) (W)b | −2.1 | −2.5 | −2.8 | −2.8 | −3.5 | −3.9 | −3.9 | −0.2 | −6 | 3.1 |
| LACV3P** | 0.1 | 0.6 | 1.1 | 5.5 | 2.9 | 4.8 | 4.5 | 4.1 | 6 | 3.3 | |
| LACV3P++** | 0 | 0.5 | 1 | 7.4 | 2.7 | 4.7 | 4.6 | 4.2 | 10 | 3.9 | |
| QZVP(−g) | −0.6 | −0.5 | 0 | 5.8 | 1.5 | 0.8 | 1 | 0.7 | 7.1 | 2 | |
We can see that the ionization potentials are in very good agreement with experiment, in the case of the large basis set, for all transition metals except Cr+ and Cu+ for which B3LYP gives too high energies (overestimates). We attribute this observation to the fact that for these two cases the ground states of the atoms are 4s1 and ionization leads to removing an unpaired 4s electron, whereas for the other metals a paired 4s electron is removed. Based on previous work and the discussions above, we expect that an unpaired electron in an atom is calculated by B3LYP to be more overbound than an electron in a doubly occupied orbital; hence, the ionization potential of the former should be overestimated compared to the latter. This expectation is consistent with the QZVP(-g) results in the table above.
Ionization potentials of the monocations
It can first be noticed that the second ionization potentials are consistently overestimated by B3LYP (Table 4). A similar tendency was previously observed for the first ionization potentials of the first and second row elements and to a lesser extent with the first transition metal ionization potentials discussed above. For this series, the ionization potential involves the removal of an electron from either a singly occupied 4s or 3d orbital. Again, removal of an electron from a singly occupied orbital should lead to overestimation of the ionization potential by B3LYP as is observed.
Table 4.
Second ionization potentials (kcal∕mol) from experiment and the errors obtained with B3LYP for the different basis sets. Experimental values quoted in Ref. 41; taken from Refs. 42, 43, 44, 45.
| Expt.a | Sc+ Ds1 → Ds0 295.2b | Ti+ Fs1 → Fs0 313.1b | V+ Ds0 → Fs0 337.1c | Cr+ Ss0 → Ds0 380.2b | Mn+ Ss1 → Ss0 360.7b | Fe+ Ds1 → Ds0 373.3b | Co+ Fs0 → Fs0 394.0b | Ni+ Ds0 → Fs0 419.0d | Cu+ Ds0 → Ds0 468.0e | MAD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Errors | LACV3P** | 4.7 | 6.2 | 7.4 | 10.1 | 7.4 | 8.9 | 10.1 | 6.7 | 7.2 | 7.6 |
| LACV3P++** | 4.7 | 6.1 | 7 | 9.8 | 7 | 8.4 | 10.5 | 7.2 | 8.3 | 7.7 | |
| QZVP(−g) | 6 | 6.4 | 12.3 | 10.6 | 6.3 | 7 | 14.8 | 12.4 | 11 | 9.6 | |
Neutral molecular systems
The experimental results for neutral molecular systems display much larger errors compared to the atomic data set. On average, error bars of 3.1 kcal∕mol are estimated by the experimental groups for the dissociation energies. But important variations are present since 50 dissociation energies in the data set have an estimated experimental error lower than 3.0 kcal∕mol. The least accurate data (for Mn–CH3, V–CH3, Cu–O) have, respectively, uncertainties of 17, 9.0, and 7.2 kcal∕mol, respectively. The big error bars for the value of Mn–CH3 is due to the fact that only lower and upper limits are given experimentally. Generally, the M–CH3 molecules have relatively large error bars on their bond energies (average error of 6.3 kcal∕mol). All these data were nevertheless included in our data set given that metal bond energies are relatively scarce and that the experimental accuracy is in any case limited. A critical comparison of the theoretical results in regard to the experimental errors will be essential to accurately assess the performances of the B3YP functional. In what follows, we begin by discussing the neutral molecules, and then proceed to monocations.
M–H compounds (hydrides)
It can be noticed that large deviations between DFT calculations and experiment in the range of 6–10 kcal∕mol are observed for the hydride data set (see Table 5). Generally, for copper, surprisingly good agreement is obtained for all basis sets. This molecule has its 3d subshell filled and forms a sigma bond with the hydrogen. From an electronic point of view, this bond is therefore relatively similar to a C–H or a C–C bond. Moreover, the metal hydrogen distance (1.5 Å) is also consistent with the C–C bond distance. Based on our previous study,13 we would expect a B3LYP error for this bond energy in the range of −1.96–0.37 kcal∕mol. The errors obtained (−1.8 to −0.1 kcal∕mol) support this analysis.
Table 5.
Bond dissociation energies at 0 K (D0) and electronic bond dissociation energies (ZPE exclusive) (De) for the hydride complexes (kcal∕mol) from experiment and the errors (theory-experiment) obtained by other authors and obtained with B3LYP for our different basis sets. Experimental values for D0 taken from Ref. 46, 47, 48, 49, 50, 51. W, is a modified spdf Wachters basis set.
| ScH | TiH | VH | CrH | MnH | FeH | CoH | NiH | CuH | MAD | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | Expt. | 47.5a | 48.0b | 49.1c | 44.5d | 29.3d | 36.6d | 45.8e | 58.7f | 60.8f | ||
| De | Expt. | 49.9 | 50.2 | 51.3 | 46.7 | 31.3 | 39 | 48.2 | 61.2 | 63.3 | ||
| Errors | De | MCPF (W)g | 2.5 | −2.7 | 2.4 | 2.4 | 7.2 | −0.5 | −3.4 | 0.8 | −2.6 | 2.7 |
| LACV3P** | 5.5 | 4.8 | 12.1 | 7.2 | 3.2 | 11.9 | 6.3 | 6.7 | −0.1 | 6.4 | ||
| LACV3P++** | 5.8 | 5 | 12.7 | 6.3 | 3.5 | 12.8 | 8.5 | 4.5 | −1.8 | 6.8 | ||
| QZVP(−g) | 8.2 | 9.3 | 16.8 | 8.2 | 8.1 | 17.7 | 14.7 | 4.5 | −0.5 | 9.8 |
Interestingly, VH and FeH display errors approximately twice as large as the other molecules. The electronic structures of both compounds do not show any peculiarity compared to the other compounds of this data set and the modified coupled pair functional (MCPF) calculations display similar errors to those observed for the other elements. These results suggest that B3LYP has some specific issue in modeling these electronic structures.
The electronic structures for all these compounds are very similar. A sigma bond is generally formed between the metal and the hydrogen. This bond is slightly polarized toward the hydrogen as shown by the natural bond orbital (NBO) analysis (see Table S1 in Supplementary Materials17). This bonding scheme is reminiscent of the bonding in a polarized molecule such as water. The bonds are relatively similar for all the series but some peculiarities can be observed. The NBO analysis shows no alpha-spin bonding part for Mn–H. Moreover, the bond distance significantly increases and the bond dissociation energy is significantly lower than for the other compounds. These facts suggest that the bond is weaker in MnH than in the other compounds but a beta-spin bonded orbital still remains and so this compound is partly bonded. The ScH and CuH bonds are also peculiar since the bond analysis shows that both molecules have sigma bonding with no spin polarization since the alpha- and beta-spin parts are equivalent. Yet, the Cu–H and Sc–H bonds differ by the atomic hybridization of the function on the metal contributing significantly to the orbital. The orbital contribution in Sc–H is sd3 hybridized, whereas for Cu–H it is almost a pure s atomic orbital (see Table S1). Two groups of bonds can also be observed based on the hybridization of their alpha-spin components. Fe–H to Ni–H have an alpha-spin bonding with an almost pure s character, whereas Ti–H to Cr–H have an alpha-spin orbital with a dominant contribution from the 3d atomic orbitals (see Table S1).
M–CH3 compounds (Methyls)
The B3LYP errors for M–CH3 compounds are relatively high on average (∼10 kcal∕mol) (see Table 6). First, it can be noticed that the errors for ScCH3, VCH3, and MnCH3 are extremely large (∼15–20 kcal∕mol). The error for NiCH3 is relatively small and the bond dissociation energy for CuCH3 is significantly underestimated. The last point is surprising given the kinship between the electronic structures of CuH and CuCH3. An important difference between the two bonds arises because the Cu–C bond (1.9 Å) is significantly longer than the Cu–H (1.5 Å) bond (see Table S2 in Supplementary Materials17). As explained in our previous study of organic systems,13 having a substantially longer bond length leads to a greater underestimation of the nondynamical correlation of the electron pair in the bond and thus explains why the bond dissociation energy of Cu–CH3 is significantly underestimated compared to Cu–H.
Table 6.
Bond dissociation energies at 0 K (D0) and electronic bond dissociation energies (ZPE exclusive) (De) for the methyl complexes (kcal∕mol) from experiment and the errors (theory-experiment) obtained by other authors and obtained with B3LYP for our different basis sets. Experimental values for D0 taken from Refs. 49, 53, 54, 55 and quoted in Ref. 57. L2 is the LAN2DZ basis set.
| ScCH3 | TiCH3 | VCH3 | CrCH3 | MnCH3 | FeCH3 | CoCH3 | NiCH3 | CuCH3 | MAD | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | Expt. | 30.7a | 44.7a | 35.7a | 36.8b | 11.4c | 35.6e | 44.6e | 53.6d | 56.6d | ||
| De | Expt. | 32.8 | 47.7 | 38.2 | 39.2 | 13.8 | 39.2 | 47.7 | 56.7 | 59.8 | ||
| Errors | De | MCPF (L2)e | 14.6 | −7.9 | 5.7 | −3.0 | 14.2 | −7.5 | −11.4 | −8.2 | −11.4 | 9.3 |
| De | LACV3P** | 19.5 | 4.0 | 16.3 | 5.7 | 12.9 | 5.4 | 1.2 | 1.4 | −5.8 | 8.0 | |
| LACV3P++** | 19.0 | 3.7 | 16.1 | 4.1 | 12.7 | 5.8 | 2.1 | −2.0 | −8.6 | 8.2 | ||
| QZVP(−g) | 20.4 | 6.8 | 19.6 | 5.6 | 15.4 | 9.7 | 5.4 | −2.6 | −8.2 | 10.4 |
The MCPF calculations presented in Table 6 (the only high level ab initio results we were able to find in the literature for these systems) also display large errors in the bond dissociation energies and the discrepancies cannot be explained by the experimental error bars despite their large values. No systematic trend is observed in the errors of the method as opposed to the B3LYP error. Results of this type illustrate the challenge of using high level ab initio calculations as benchmarks for metal-containing systems. The errors could be due to a number of factors, including the large experimental error bars, incomplete basis set convergence, or other deficiencies in the electronic structure calculations.
As expected, the electronic structures of the M–CH3 compounds are very similar to those calculated for M–H. We observed the same reduced bonding for Mn and the peculiar bonding scheme for Sc and Cu (See tables S1 and S2 in Supplementary Materials17). Again, the same change in the hybridization of the alpha-spin bonding orbital is observed between TiCH3→CrCH3 and FeCH3→NiCH3.
MO compounds (oxides)
Experimental bond dissociation energies for the oxides give rise to some controversy. For instance, CrO bond dissociation energy has been reported to be 101.6 kcal∕mol by Pedley and Marshall58 (Table 7) but, in another study, Kang and Beauchamp reported a value around 110.0 kcal∕mol with a better accuracy.59 In a first attempt to compare the experimental and the theoretical values, we observed that using the value of Beauchamp et al. would lead to a constant underestimation of the bond dissociation energies by ∼10 kcal∕mol for all methods including CCSD(T). This inconsistency between all the methods and the experimental disagreement leads us to prefer the value given by Marshall et al. as the experimental reference.
Table 7.
Bond dissociation energies at 0 K (D0) and electronic bond dissociation energies (ZPE exclusive) (De) for the metal oxides (kcal∕mol) from experiment and the errors (theory-experiment) obtained by other authors and obtained with B3LYP for our different basis sets. Experimental values for D0 taken from Refs. 58, 60, 62, 63, 64 and quoted in Ref. 64. W-aug is modified Wachters(f) aug cc-pVTZ basis set.
| ScO | TiO | VO | CrO | MnO | FeO | CoO | NiO | CuO | MAD | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | Expt. | 160.3a | 158.4a | 148.5a | 101.6a | 88.3b | 96.2c | 90.9b | 89.1b | 63.5d | ||
| De | Expt. | 161.8 | 159.9 | 150 | 102.7 | 89.6 | 97.5 | 92.1 | 90.4 | 64.4 | ||
| Errors | D0 | D0 CCSD(T)(W-aug)e | −1.2 | −0.7 | −4.2 | −2.4 | −6.2 | −12 | −6.9 | −2.7 | −2.2 | 4.3 |
| De | LACV3P** | −6.1 | −6.9 | −7.6 | −9.6 | −5.9 | −7.6 | −10.6 | −8.7 | −2.8 | 7.3 | |
| LACV3P++** | −7 | −7.8 | −8.3 | −11.6 | −5.6 | −7 | −9.7 | −11.5 | −5.3 | −8.2 | ||
| QZVP(−g) | −0.4 | 0.4 | 2.5 | −2 | 5.1 | 5.1 | 1 | −5.3 | −0.5 | 2.5 |
CuO is also a controversial case since experimental values ranging from 62.0 to 65.7 kcal∕mol have been reported.58, 60, 61 Since no clear trend was observed with the ab initio methods, we use the intermediate value of 63.5 kcal∕mol which is generally, the reference value.61
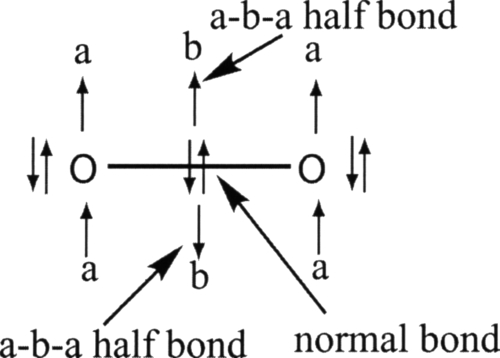
The electronic structures of oxides show that, for most compounds, the bonding between the metal and the oxygen most closely approximates a triple bond. One bond is of sigma type and the two others are pi-bonds. CuO is significantly different since it has only a single sigma bond (see Table S3.1 in Supplementary Materials17) polarized toward the oxygen. Yet, it should be noticed that the electronic structures of the other compounds are quite diverse despite the fact that they can all be claimed to possess a triple bond. ScO, TiO, and VO all have a clear triple bond polarized toward the oxygen. CrO has a very similar electronic structure but it lacks one alpha bonding component. The beta component forms what we shall call an a-b-a half bond. It corresponds to an electronic structure where one electron is in a lone pair orbital on one center, a beta electron is in bonding orbital, and another alpha electron is in a lone pair on the other atomic center. This kind of bonds is quite common with metals but is also encountered in molecules such as O2. O2 actually has two such half bonds and is better represented with this half bond as displayed on Fig. 1 than with the usual Lewis structure. Indeed, the structure, represented Fig. 1, shows both the diradicaloid character of O2 (ground state is a triplet) and the fact that its net bond order is 2. The Lewis structure, on the other hand, just accounts for the double bond.
Figure 1.
Electronic structure displaying two a-b-a half bonds. This representation points out the fact that each oxygen center bears some radical character and that the total bond order of the molecule is 2 (1+2⋆1∕2). Each a-b-a half bond actually displays only one unpaired electron and not three since the beta electron can actually be paired with one of the alpha electon localized on the oxygen. This explains why the system of three electrons is described altogether as an a-b-a half bond. Extended NBO analysis and a sligthly different representation of dioxygen molecules is given in Ref. 66.
MnO, FeO; CoO, and NiO all have similar electronic structures with one full bond and two a-b-a half bonds.
Complexes with monocations
M+–H compounds (hydrides)
The performances of B3LYP methods are relatively good for the metal-cation hydrides compare to the neutral hydrides (see Tables 5, 8) since the MAD range from 3.5 to ∼4.5 kcal∕mol depending on the basis set. Reasonably good results are obtained with CCSD(T) with a MAD of 2.9 kcal∕mol. Interestingly, B3LYP results for FeH+ and NiH+ appear to be systematically incorrect since errors in the range of 4–10 kcal∕mol are observed whereas the CCSD(T) results are in good agreement with experiment (0.4 and −1.8 kcal∕mol, respectively).
Table 8.
Bond dissociation energies at 0 K (D0) and electronic bond dissociation energies (ZPE exclusive) (De) for the cation-metal hydrides (kcal∕mol) from experiment and the errors (theory-experiment) obtained by other authors and obtained with B3LYP for our different basis sets. Experimental values for D0 taken from Refs. 67, 68, 69, 70, 71, 72, 73 and quoted in Ref. 57. W is the Wachters basis set.
| ScH+ | TiH+ | VH+ | CrH+ | MnH+ | FeH+ | CoH+ | NiH+ | CuH+ | MAD | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | Expt. | 56.3a | 53.3b | 47.3c | 31.6d | 47.5e | 48.9f | 45.7g | 38.6h | 21.2a | ||
| De | Expt. | 58.8 | 55.8 | 49.8 | 33.9 | 49.8 | 51.3 | 48.2 | 41.1 | 23.2 | ||
| Errors | D0 | GVBh | −1.1 | 0.7 | −3.7 | −7.3 | −7.9 | −1.9 | −2.1 | −2.9 | −0.3 | 3.1 |
| De | MCPF (W)i | −5.8 | −5.3 | −4.7 | −3.8 | −9.7 | −3.7 | −8.5 | −5.6 | ⋯ | 5.9 | |
| CCSD(T) (W)j | −2.5 | −2.4 | −0.4 | −3.4 | −6.6 | 0.4 | −4.8 | −1.8 | −4.3 | 2.9 | ||
| De | LACV3P** | 2.7 | 2.4 | 4.8 | 2.5 | −1.1 | 6.6 | −0.6 | 6.8 | 4.1 | 3.5 | |
| LACV3P++** | 3.0 | 2.5 | 5.0 | 2.6 | −1.1 | 6.9 | −1.0 | 6.4 | 3.8 | 3.6 | ||
| QZVP(−g) | 4.9 | 6.0 | 3.1 | 3.9 | 1.8 | 9.8 | −2.9 | 4.1 | 4.5 | 4.5 |
The MH+ compounds have very similar electronic structures since they all have a single sigma bond as in the case of MH (see Table S4 in Supplementary Materials17). Yet, CrH+ and CuH+ appear to be special cases since their bond dissociation energy is very low and the spin population on the hydrogen atom is relatively important. These facts suggest that these compounds do not have a real bond but are just coupled electrostatically.
M–CH3 (Methyls)
The bond dissociation energies calculated with B3LYP are in relatively good agreement with experiment with MADs generally around 3.2–3.6 kcal∕mol (see Table 9). Generally, it seems that B3LYP has a tendency to overestimate the bond dissociation energies of the methyl complexes.
Table 9.
Bond dissociation energies at 0 K (D0) and electronic bond dissociation energies (ZPE exclusive) (De) for (kcal∕mol) from experiment and the errors (theory-experiment) obtained by other authors and obtained with B3LYP for different basis sets. Experimental values for D0 taken from Refs. 19, 54, 55, 76, 77, 78, 79, 80. L2, W are the LAN2DZ and Wachters basis sets, respectively.
| MAD | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | Expt. | 57.7a | 52.3b | 48.6c | 29.0d | 50.0e | 56.0f | 47.5g | 43.8g | 28.5h | ||
| De | Expt. | 60 | 56.8 | 52.8 | 31.5 | 53.1 | 59.4 | 50.8 | 46.9 | 30.9 | ||
| Errors | De | QCISD(T) (L2)j | −3.6 | −0.8 | −7.5 | −6.8 | −9.1 | −7.6 | −2.7 | −12.7 | −16.6 | 7.5 |
| MCPF (L2)j | −8.5 | −6 | −10 | −4.6 | −12.2 | −8.5 | −5.2 | −10 | −5.5 | −7.8 | ||
| MCPF (W)k | −9.4 | −10.9 | −13.3 | −9 | −12.9 | −9.5 | −8.5 | −12.2 | ⋯ | 10.7 | ||
| CCSD(T) (W)k | −7.4 | ⋯ | −10.4 | −5.3 | −11.4 | −7.6 | −5.6 | −6.8 | ⋯ | 7.8 | ||
| De | LACV3P** | 1.3 | 2.5 | −1.5 | 4.9 | −2.6 | 3.8 | 4.3 | 4.2 | 7.4 | 3.6 | |
| LACV3P++** | 0.7 | 2 | −1.9 | 4.3 | −3 | 3.6 | 3.2 | 3.3 | 6.6 | 3.2 | ||
| QZVP(−g) | 2.3 | 5.4 | −3.9 | 5.6 | −0.3 | 5.9 | 1.5 | 1.1 | 6.8 | 3.6 |
Surprisingly, the B3LYP values seem to be substantially better than the CCSD(T) or QCISD(T) values reported on the same systems.3, 5 But the basis sets used for these calculations were relatively small and the results strongly depend on that. Very large basis sets are probably required for CCSD(T) calculations to be able to recover most of the correlation energy.
The electronic structures of the compounds are similar to their hydride counterparts since they also have a sigma bond. and also have small dissociation energies and spin populations relatively high on the carbon. This suggests that these compounds do not possess an actual bond.
M+–CH2 compounds (methylenes)
The performances of B3LYP on the complexes of the metals with methylene are similar to those observed with the methyl with a MAD ranging from 3.7 to 4.3 kcal∕mol (Table 10). Thus and have very similar behaviors. Yet, while B3LYP has a tendency to overestimate the bond dissociation energies of the methyl complexes, it generally underestimates the bond dissociation energies of the methylene complexes, particularly in case of Sc, Ti, and V.
Table 10.
Bond dissociation energies at 0 K (D0) and electronic bond dissociation energies (ZPE exclusive) (De) for (kcal∕mol) from experiment and the errors (theory-experiment) obtained by other authors and obtained with B3LYP for our different basis sets. Experimental values for D0 taken from Refs. 49, 51, 82, 83, 84, 85, 86, 87. W and W+f are Wachters basis sets and Wachters basis set augmented by f function, respectively.
| MAD | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | Expt. | 97.6a | 92.4b | 78.7c | 52.6d | 69.4e | 82.6f | 74.7g | 73.9h | 62.6h | ||
| De | Expt. | 99.5 | 94.5 | 81.2 | 55.1 | 72.2 | 85.5 | 77.9 | 77.1 | 65.7 | ||
| Errors | De | MCPF (W)i | −23.1 | −18.3 | −15.0 | −15.2 | −26.3 | −24.9 | −10.3 | −11.1 | −12.7 | 17.4 |
| CCSD(T) (W)i | −18.8 | −14.5 | −10.7 | −8.7 | −16.1 | −14.6 | −5.0 | −8.4 | −11.5 | 12.0 | ||
| MCPF (W)j | −26.5 | −21.1 | −18.0 | −14.6 | −25.8 | −27.5 | −20.7 | −21.8 | ⋯ | 22.0 | ||
| CCSD(T) (W)j | −21.6 | −18.2 | −16.3 | −13.3 | −27.1 | −25.4 | −16.6 | −17.8 | ⋯ | 19.5 | ||
| CCSD(T) (W+f)k | −4.1 | −10.2 | −0.8 | −4.8 | −13.3 | −13.9 | 5.2 | −7.3 | −11.0 | 7.8 | ||
| RMR CCSD(T) (W+f)k | −6.0 | −9.3 | −0.7 | −4.2 | −11.5 | −13.6 | 4.4 | −8.3 | −11.0 | 7.7 | ||
| De | LACF3P** | −13.1 | −10.4 | −5.4 | 1.2 | −2.7 | −1.1 | 1.7 | −0.1 | 0.3 | 4.1 | |
| LACV3P++** | −13.8 | −11.1 | −6.3 | 0.6 | −3.3 | −1.4 | 0.8 | −1.0 | −0.4 | 4.3 | ||
| QZVP(−g) | −10.9 | −7.0 | −7.5 | 2.9 | 0.4 | 1.8 | −0.2 | −1.9 | −0.6 | 3.7 |
Surprisingly the CCSD(T) results reported by Ricca and Bauschlicher81 or by Blomberg et al.5 are particularly bad with MADs around 12.0 and 19.5 kcal∕mol, respectively, and systematically underestimate the bond dissociation energies. The results recently reported by Li and Paldus29 with the same basis set are in somewhat better agreement with the experiment (MAD of 7.8 kcal∕mol), although these results must be viewed as qualitatively inadequate as well. Their results also show that the CCSD(T) and the RMR CCSD(T) are consistent and thus the CCSD(T) method should give accurate results even on systems for which large multireference character is expected such as these methylene complexes. Interestingly, the discrepancies between the results of the different authors seem to arise from the fact that including the correlation of the 3s and 3p electrons, or not, gives rise to important differences in the final bond dissociation energies, as explained by Li and Paldus.29 Thus including the 3s and 3p electron correlation seems to be important if we want to reproduce accurately bond dissociation energies with CCSD(T).
In spite of the better agreement with experiment obtained by Li and Paldus, we can see that their CCSD(T) results also underestimate the bond dissociation energies and that the B3LYP results for the same basis set are actually better. The average errors obtained with the CCSD(T) methods are still quite large (7.8 kcal∕mol). As has been noted previously, factors such as convergence to the complete basis set limit or relativistic corrections may be responsible for the discrepancy. Further investigation of the CCSD(T) protocol here will clearly be necessary.
Regarding the electronic structure, all these compounds display a double bond except in case of Cu, which has a single sigma bond. Indeed a similar pattern as the one observed for the metal oxides is also observed with the methylene complexes since Sc, Ti, and V all have two full bonds. Cr, Mn, Fe, Co, and Ni have one bond and an a-b-a half bond. All these bonds are generally polarized toward the carbon atom with the exception of Ti and V, both of which have an alpha-spin bond strongly polarized toward the metal.
M+–OH compounds (hydroxyls)
From the analysis of the distances and the experimental bond dissociation energies, M+–OH seems to be similar to M+=CH2 but with a much greater variation in the bond dissociation energies. We could thus expect to have the equivalent of a double bond for these compounds. In fact, the electronic structure shows an important variability (see Table S7.1) with no bonds detected in the NBO analysis of Sc–OH+ to one bond and an a-b-a half bond, strongly polarized toward the oxygen in case of V–OH+, Mn–OH+, or Co–OH+. The fact that Sc–OH+ has no bond detected is striking given that this compound also has the largest bond dissociation energy (see Table 11). Interestingly, the spin population on the oxygen atom is relatively close to 0 for Sc to Cr and subsequently increases until ∼0.55e for Ni. In case of Sc, this effect is very strong and suggests that we have a charge transfer phenomenon leading to a species best described as Sc2+ and OH− interacting through an ionic bond. The charge transfer seems to be total for Sc through Cr but only partial in the case of Mn through Ni since some nontrivial spin populations are observed on the oxygen for those compounds.
Table 11.
Bond dissociation energies at 0 K (D0) and electronic bond dissociation energies (ZPE exclusive) (De) for M–OH+ (kcal∕mol) from experiment and the errors (theory-experiment) obtained by other authors and obtained with B3LYP for our different basis sets. CuOH+ is not reported since no experimental data are available for this complex. Experimental values for D0 taken from Refs. 57, 59, 88.
| ScOH+ | TiOH+ | VOH+ | CrOH+ | MnOH+ | FeOH+ | CoOH+ | NiOH+ | MAD | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | Expt. | 119.2a | 111.2a | 103.8a | 73.0b | 79.0c | 87.5c | 71.7c | 56.3c | ||
| De | Expt. | 121.0 | 113.3 | 105.5 | 75.1 | 80.9 | 89.5 | 73.8 | 58.3 | ||
| Errors | D0 | CCSD(T) (AANO)d | −1.7 | −2.3 | −8.2 | ⋯ | ⋯ | ⋯ | −6.6 | −5.6 | 4.9 |
| De | LACV3P** | 3.2 | 1.7 | −5.4 | −9.0 | −6.4 | −3.3 | −3.2 | 4.5 | 5.5 | |
| LACV3P++** | −0.7 | −2.3 | −8.9 | −11.8 | −9.0 | −5.7 | −6.3 | 1.3 | 6.1 | ||
| QZVP(−g) | 2.0 | 1.8 | −7.3 | −6.3 | −1.7 | 1.6 | −3.8 | 3.8 | 4.3 |
M+O compounds (oxides)
Generally, the B3LYP errors for these systems are relatively large except in case of CuO+ and it appears that the bond dissociation energies are systematically underestimated. For CuO+, the errors are relatively small and are in the range from −2.7 to +0.1 kcal∕mol depending on the basis set used (see Table 12).
Table 12.
Bond dissociation energies at 0 K (D0) and electronic bond dissociation energies (ZPE exclusive) (De) for M–O+ (kcal∕mol) from experiment and the errors (theory-experiment) obtained by other authors and obtained with B3LYP for our different basis sets. CuO+ is not reported since no experimental data are available for this complex. (D) is the Dolg∕cc-pVTZ basis set. Experimental values for D0 taken from Refs. 90, 91, 92.
| ScO+ | TiO+ | VO+ | CrO+ | MnO+ | FeO+ | CoO+ | NiO+ | CuO+ | MAD | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | D0 Expt. | 164.0a | 158.7a | 134.0a | 85.8b | 68.0b | 81.4b | 75.9b | 64.1b | 31.1c | ||
| De | De Expt. | 165.5 | 160.2 | 135.5 | 86.9 | 69.0 | 82.5 | 76.9 | 65.0 | 31.8 | ||
| Errors | D0 | MRSDCI (D)d | −12.6 | −13.2 | 1.0 | −15.0 | −23.1 | −11.4 | −0.6 | −8.4 | −13.5 | 11.0 |
| De | MRMP (D)d | −20.9 | −14.1 | −1.3 | −13.3 | −13.2 | 6.3 | 7.3 | −0.6 | 5.1 | 9.1 | |
| LACV3P** | −10.4 | −13.1 | −11.3 | −22.7 | −12.4 | −10.6 | −13.9 | −15.5 | −0.9 | 12.3 | ||
| LACV3P++** | −12.0 | −14.8 | −12.7 | −23.9 | −13.6 | −11.3 | −15.3 | −17.0 | −2.7 | 13.7 | ||
| QZVP(−g) | −4.4 | −4.7 | −5.4 | −13.6 | −4.2 | −0.7 | −10.0 | −11.1 | 0.1 | 6.0 |
As in the case of the MO data set, the electronic structures of the MO+ compounds suggest the presence of a triple bond (see Table S8.1 in Supplementary Materials17). In fact, only Sc, Ti, and V display a true triple bond, substantially polarized toward the oxygen. CrO+ has a double bond and one a-b-a half bond. MnO+ has a single bond and one a-b-a half bond, whereas FeO+ and CoO+ have a single bond and two a-b-a half bonds (see Table S8.1 in Supplementary Materials17). NiO+ and CuO+ both have three a-b-a half bonds. This bonding scheme reflects the trend observe in the bond dissociation energy with a decrease in energy from Sc to Cu.
B3LYP-LOC MODEL
In this section, we present the B3LYP-LOC model to correct the thermochemistry of metal atoms and a series of small, metal-containing molecules. Since B3LYP errors depend on the basis set used, we can infer that the B3LYP-LOC model will be partly basis set dependent. However, since this model was built on physical arguments, it should best apply at the infinite basis set limit. We therefore decided to use the biggest basis set we have [QZVP(-g)] to build our initial B3LYP-LOC model. To test the basis set dependency of B3LYP-LOC, we will also fit the parameters to the LACV3P++** basis set results since this basis set has been widely employed by us and other groups in the studies of metal containing compounds. We do not explicitly make corrections for basis set error or for relativity; however, such corrections are present, being absorbed into the localized atomic and bond correction parameters that are an intrinsic component of the methodology. How effective such heuristic approximations are must be judged by the results.
In the first section below, we will build the model to correct the energies of our atomic data set. We will see that the same physical arguments as those developed for atomic data of main group elements15 also apply to the first transition metal row. In a second part, we will show how based on the physical arguments previously developed for atomization energies13 and on our electronic structure analysis of the compounds in our molecular data set, we have developed a model to correct molecular dissociation energies of first-row transition metals. Finally, we will investigate whether modern CCSD(T) calculations may be used to help design more accurate B3LYP-LOC models.
Atomic excitation and ionization energies
The atomic data set can be divided into two main groups of physical processes. The excitation energies involve the transfer of one electron in one orbital to another orbital, whereas the ionization energies involve the removal of an electron from one orbital. As we develop a model similar to what was developed for main group atom ionization potentials, we will first discuss the B3LYP-LOC model for correcting ionization energies.
In Ref. 15, we have shown that B3LYP ionization energies for atoms can be corrected by taking into account the nature of the orbital from which the electron is removed (2s, 2p, 3s, or 3p) and its occupancy (singly or doubly occupied). This scheme derived from the fact that in Ref. 13, it was argued that the B3LYP errors correlate with the size of the orbitals and thus different corrections should be expected for 2s or 3p orbitals since their sizes are different. It was also argued that the error depends on the occupancy of the orbital because the B3LYP was shown to partly model the intraorbital nondynamical correlation with the self-interaction term. But for a singly occupied orbital there is no nondynamical correlation in reality, whereas the self-interaction error is still at work. This effect therefore leads B3LYP to overbind electrons in singly occupied orbitals. In the case of doubly occupied orbitals, the situation is more complex and the sign of the B3LYP error depends on the relative size of the orbital compared to the space available for nondynamical correlation.
The last important set of parameters was derived from the observation that the B3LYP errors on ionization potentials for the removal of electrons coming from orbitals of the same nature (3p, for instance) and having the same occupation numbers (like in B, C, or N) were actually quite different. It was hypothesized that this was due to a systematic error of B3LYP in modeling the interaction of two unpaired electrons with parallel spins. The model subsequently developed supported this hypothesis.
For the corrections of the B3LYP errors on ionization potentials of the atoms of the first transition metal row, we proposed to follow the same scheme. In this case, we end up having four different situations.
-
(1)
Removal of an electron from a singly occupied 4s orbital.
-
(2)
Removal of an electron from a doubly occupied 4s orbital.
-
(3)
Removal of an electron from a singly occupied 3d orbital.
-
(4)
Removal of an electron from a doubly occupied 3d orbital.
During these different operations, the number of unpaired spin interactions will be modified. Two kinds of parallel spin interactions may be listed.
-
(1)
Interaction between an electron in a 4s and one in a 3d orbital.
-
(2)
Interaction between two electrons both in 3d orbitals.
As was argued in Ref. 15, the correction parameters for parallel spin interactions are expected to be transferable and will also be transferable to the calculation of excitation energies (as opposed to ionization potentials).
No B3LYP-LOC parameters have been previously developed for electronic excitations for the main group elements. Yet, the excitation of an electron appears to be close in nature to the ionization process and, in fact, ionization can be viewed as an excitation of a bound electron toward the continuum states. Therefore the same scheme as the one used for ionization potentials should be used for excitation energies with one major difference, which is that in the case of excitation energies, we need to take into account the nature and occupation of the orbital to which the electron is excited. We therefore have again four cases.
-
(1)
An electron is transferred from a doubly occupied 4s orbital to a singly occupied 3d orbital. Thus, a doubly occupied 3d orbital is formed in the excited state (denoted 4s2→3d2).
-
(2)
An electron is transferred from a singly occupied 4s orbital to an empty orbital 3d (denoted 4s1→3d1).
-
(3)
An electron is transferred from a doubly occupied 4s orbital to an empty orbital 3d (denoted 4s2→3d1).
-
(4)
An electron is transferred from a singly occupied 4s orbital to a singly occupied 3d orbital (denoted 4s1→3d2).
For the corrections for the ionization potentials and electron affinities, we did not use parameters of the same value, and opposite sign, as the physical processes would have suggested, because the anion wave functions (and ipso facto the densities) are generally quite different from their neutral or cationic counterparts (due to the electron repulsion introduced by the extra electron). Since in the case of the excitations described above the wave functions do have (more or less) the same nature, we consider our parameters as applying reversibly. Thus, the transfer of an electron from a singly occupied 3d orbital toward an empty 4s orbital (3d1→4s1) will be considered to have the same correction parameter with the opposite sign, as compared to the transfer from a 4s singly occupied to an empty 3d orbital (4s1→3d1).
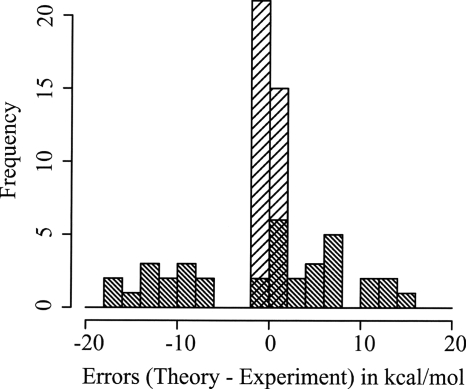
The values and the assignment of the parameters for all energies present in our atomic data set are presented in Tables 13, 14. We can see that the performance of this model is very good since the MAD goes from 7.7 kcal∕mol with B3LYP to 0.42 kcal∕mol with B3LYP-LOC. The standard deviation is also relatively small in the B3LYP-LOC model (0.51 kcal∕mol) compared to B3LYP (9.22 kcal∕mol). Thus, the model not only improves the performances but also removes the outliers (see Fig. 2). Indeed, all the values from B3LYP-LOC are within 1.0 kcal∕mol from the experimental values. Given the high accuracy of our atomic data set, we can consider that B3LYP-LOC fulfills the conditions for chemical accuracy for the atomic data set.
Table 13.
Values of the B3LYP-LOC model for first transition metal row atomic data after fitting.
| Parameters | Values (kcal∕mol) QZVP(−g) | Values (kcal∕mol) LACV3P++** |
|---|---|---|
| Excitation energies | ||
| 4s2→3d2 | 7.09 | −1.92 |
| 4s1→3d1 | 12.67 | 9.15 |
| 4s2→3d1 | 19.98 | 16.23 |
| 4s1→3d2 | 0.95 | −5.03 |
| Ionization potential | ||
| 4s1 | −7.10 | −7.95 |
| 4s2 | 0.26 | −1.83 |
| 3d1 | −20.81 | −17.63 |
| 3d2 | −10.06 | −6.06 |
| Parallel spin interactions | ||
| 3d↔3d spin-spin | −2.67 | −2.63 |
| 4s↔3d spin-spin | −0.24 | −0.24 |
Table 14.
Parameter assignments for excitation energies and ionization potentials for the atomic data set. The relative errors (theory-experiment) for B3LYP and B3LYP-LOC are given for the QZVP(−g) basis set as well as the MAD.
| B3LYP QZVP(−g) Errors (kcal∕mol) | Excitation | Removal | Parallel spin interactions | B3LYP-LOC QZVP(−g) error (kcal∕mol) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4s2→3d2 | 4s1→3d1 | 4s2→3d1 | 4s1→3d2 | 4s1 | 4s2 | 3d1 | 3d2 | 3d↔3d spin-spin | 4s↔3d spin-spin | |||
| Excitation energies (Te) | ||||||||||||
| Sc D(s2)→F(s1) | −16.2 | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1 | 2 | 0.67 |
| Ti F(s2)→F(s1) | −13.9 | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 2 | 3 | 0.06 |
| V F(s2)→D(s1) | −11.0 | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 3 | 4 | −0.01 |
| Cr D(s2)→S(s1) | −8.8 | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 4 | 5 | −0.73 |
| Mn S(s2)→D(s1) | −17.1 | 1 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | −4 | 4 | −0.30 |
| Fe D(s2)→F(s1) | −14.8 | 1 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | −3 | 3 | −0.41 |
| Co F(s2)→F(s2) | −12.1 | 1 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | −2 | 2 | −0.11 |
| Ni F(s2)→D(s1) | −8.7 | 1 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | −1 | 1 | 0.85 |
| Cu D(s2)→S(s1) | −7.1 | 1 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | −0.03 |
| Sc+ D(s1)→F(s0) | −9.9 | ⋯ | 1 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1 | −1 | 0.36 |
| Ti+ F(s1)→F(s0) | −7.7 | ⋯ | 1 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 2 | −2 | 0.08 |
| V+ D(s0)→F(s1) | 5.4 | ⋯ | −1 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | −3 | 3 | 0.03 |
| Cr+ S(s0)→D(s1) | 3.3 | ⋯ | −1 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | −4 | 4 | 0.41 |
| Mn+ S(s1)→D(s0) | −13.6 | ⋯ | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | ⋯ | −4 | −5 | −0.72 |
| Fe+ D(s1)→F(s0) | −10.4 | ⋯ | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | ⋯ | −3 | −4 | −0.51 |
| Co+ F(s0)→F(s1) | 7.0 | ⋯ | ⋯ | ⋯ | −1 | ⋯ | ⋯ | ⋯ | ⋯ | 2 | 3 | −0.04 |
| Ni+ D(s0)→F(s1) | 3.4 | ⋯ | ⋯ | −1 | ⋯ | ⋯ | ⋯ | ⋯ | 1 | 2 | −0.69 | |
| Cu+ D(s0)→D(s1) | 0.7 | ⋯ | ⋯ | −1 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1 | −0.49 | |
| Ionization potentials | ||||||||||||
| Sc (s2d1)→(s1d1) | −0.6 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | 1 | −0.55 |
| Ti (s2d2)→(s1d2) | −0.5 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | 2 | −0.67 |
| V (s2d2)→(s1d2) | 0.0 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | 3 | −0.45 |
| Cr (s1d5)→(d5) | 5.8 | ⋯ | ⋯ | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | ⋯ | −5 | −0.10 |
| Mn (s2d5)→(s1d5) | 1.5 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | 5 | 0.55 |
| Fe (s2d8)→(s1d8) | 0.8 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | 4 | 0.09 |
| Co (s2d2)→(s1d7) | 1.0 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | 3 | 0.53 |
| Ni (s2d8)→(s1d8) | 0.7 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | 2 | 0.49 |
| Cu (s1d10)→(d10) | 7.1 | ⋯ | ⋯ | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | −0.02 |
| Sc+ (s1d1)→(s0d1) | 6.0 | ⋯ | ⋯ | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | ⋯ | −1 | −0.87 |
| Ti+ (s1d2)→(s0d2) | 6.4 | ⋯ | ⋯ | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | ⋯ | −2 | −0.22 |
| V+ (s0d4)→(s0d3) | 12.3 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1 | ⋯ | −3 | ⋯ | −0.53 |
| Cr+ (s0d5)→(s0d4) | 10.6 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1 | ⋯ | −4 | ⋯ | 0.53 |
| Mn+ (s1d5)→(s0d5) | 6.3 | ⋯ | ⋯ | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | ⋯ | −5 | 0.36 |
| Fe+ (s1d6)→(s0d6) | 7.0 | ⋯ | ⋯ | ⋯ | ⋯ | 1 | ⋯ | ⋯ | ⋯ | ⋯ | −4 | 0.85 |
| Co+ (s0d8)→(s0d7) | 14.8 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1 | 2 | ⋯ | −0.58 |
| Ni+ (s0d0)→(s0d8) | 12.4 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1 | 1 | ⋯ | −0.37 |
| Cu+ (s0d10)→(s0d0) | 11.0 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1 | ⋯ | ⋯ | 0.96 |
| MAD (kcal∕mol) | 7.66 | 0.42 | ||||||||||
Figure 2.
Histograms representing the error distributions (theory-experiment) for B3LYP (high density hatchings) and B3LYP-LOC (low density hatchings) for the QZVP(-g) basis set.
In case of the LACV3P++** basis set, we observe that B3LYP-LOC still gives reasonable results after refitting even if the efficiency is less impressive than with the QZVP(-g) basis set. Indeed, the MAD goes from 5.42 to 1.54 kcal∕mol. Thus, as expected, it appears that with the biggest basis B3LYP-LOC gives better results than with a lower level basis set, even if the performance of the small basis is actually better with B3LYP. The basis sets have an important impact on the computed energies and errors due to the use of small basis sets may actually cancel errors from the functional itself. In the case of B3LYP-LOC corrections, these error cancellations are not effective anymore and it introduces new errors even after having refitted the parameters.
Yet, even if the parameters derived for OZVP(-g) and LACV3P++** basis sets are rather different they follow the same general trends (see Table 13). The most important deviations are found for the 4s2→3d2 and the 4s1→3d2 excitation energies. Interestingly, we can see that the parallel spin-spin interaction parameters are almost identical for both basis sets. Apparently, the error in the parallel spin-spin interaction integrals has very little basis set dependence. The results make sense if one assumes that the exchange integrals depend primarily on the short to medium range component of the wavefunction, whereas the self-interaction error in singly occupied orbitals leading to overbinding (for example) can have a strong dependence on the tail of the wavefunction (where differences in the basis sets would be quite substantial). It is also interesting to notice that the 4s-3d parallel spin-spin interaction appears to be very small for both basis sets after fitting. In fact, this parameter could be eliminated without significantly reducing the effectiveness of the model. However, it also does not appear to lead to any sort of instabilities in the fitting process, so for the present we have chosen to retain it.
Regarding the validity of our statistics, we can see that our atomic data set contains 36 values and we have fitted a total of 10 parameters. We thus have on average 3.6 data points for one fitted parameter. The ratio of parameters to data points is thus not particularly large, and overfitting is then a potential problem. Yet, we must emphasize that our parametrization scheme, based on physical arguments, strictly follows the one developed for first and second row elements. The results obtained following exactly the same rules end up giving extraordinarily good results, particularly for the larger quadruple zeta basis set. A number of observations can be made supporting the validity of the underlying physical assumptions. Firstly, within a specified type of transition (and varying the metal within that transition), the relative value of the corrections for each metal depends on only one (global) parameter, the unpaired spin interaction, and this leads to remarkably accurate rendering of these differences, just as in the case of first and second row atoms. Secondly, the parameter trends as a function of the type of transition are uniformly in accord with the physical reasoning laid out in Refs. 13, 15. Finally, the fact that the larger all electron basis set yields substantially more accurate results than the smaller one again suggests that the correct physics has been captured by the model. Both fits, after all, have the same ratio of adjustable parameters to data points, yet the error in the QZ fit is qualitatively smaller than that from the smaller basis set, despite starting from a larger average error. These observations can only be explained by hypothesizing that the specific nondynamical correlation errors posited by the LOC model dominate the QZ data, whereas in the case of the smaller basis, other errors are intermixed and are not as well modeled by the parametrization.
In the analysis of bond dissociation energies that follow, we use the experimental atomic data directly to correct errors due to atomic state hybridization changes in different types of chemical interactions. However, given the accuracy of the model for correcting atomic energies [particularly for the all electron QZVP(-g) basis], little or no degradation in the quality of the results would be observed if the fitted parameters above were to be used instead. We expect that it will, in fact, be necessary to use these parameters when treating spin and charge state changes in more complex metal-containing complexes, as will be explored in future work.
B3LYP-LOC model for bond dissociation energies
In this section, we will present the parametrization scheme of B3LYP-LOC used to correct bond dissociation energies of molecules containing first-row transition metals. In our previous study on atomization energies of first and second row atoms,13 three main classes of corrections were required. A first class, corrections for atomic hybridization states, was aimed at correcting the changes in the hybridization of the lone pairs and singly occupied orbitals in the molecules. The second class, bond corrections, fixed the deficiencies of B3LYP in modeling the nondynamical correlation of the bonds. The last class, the environmental corrections, was set up to take into account the effects exerted by the neighboring atoms on the nondynamical correlation present in a localized orbital. In the following part, we will see how these different classes of parameters can also be applied to transition metal–containing molecules.
Atomic corrections
The atomic configuration embedded in a molecule is generally different from the configuration found in the bare atom because of the rehybridization that occurs when the bond is formed. This effect is commonly found with organic molecules where the main group elements mix different atomic orbitals to form the molecular orbitals. This process not only alters the bonding orbitals but also the lone pairs and singly occupied orbitals localized on a specific atom. This description also applies for coordination and organometallic compounds, but one pitfall with bonds involving metals is that their hybridization is very variable and not as well defined as it is when encountered for main group elements. For instance, the carbon atom is well known to adopt mainly sp, sp2, or sp3 hybridized states, whereas we have seen that it is common to find a metal with partial hybridization like 4s0.843d3.45. This diversity in the hybridization of first-row transition metals is probably due to the fact that they have multireference characters and thus different hybridization states are at play at the same time. It is important to take this diversity into account when defining the B3LYP-LOC corrections.
Thus, to quantitatively correct for this effect, we followed the same scheme as the one already used by Bauschlicher et al.56, 75 We basically rely on the metal hybridization number given by the NBO analysis and calculated from the 3d population the percentage of excited state present in the configuration of the metal inside the molecule. In the case of CoH, the hybridization for the metal given by NBO is 4s0.93d7.8. The configuration of the ground state is 4s23d7 and the first excited state is 4s13d8. Thus from the 3d population, it appears that the configuration of the metal is a combination of 80% of the first excited state and 20% of the ground state. We must therefore correct this molecule for 80% of the error generated by B3LYP for the first excitation energy. This method assumes that the electronic structure given by B3LYP is essentially correct and that the error predominantly affects the energies. This assumption is generally well verified, and we see few dependencies of the hybridization numbers with the basis sets for instance. As we are limited by the number of points present in our molecular data set, the excitation energy parameters were not fitted and were directly taken from the experimental values in the atomic data set. However, as we pointed out above, use of the atomic correction parameters would yield very similar results.
This scheme was applied to all molecules present in the data set except to M+–OH. Actually, we have seen in the analysis (see Sec. 3C4) that the electronic structures in this series can be best understood in term of an electron transfer from the metal to the oxygen, yielding in effect a coordination complex between M2+ and OH−. In such a configuration, the previous analysis, based on 3d population and excitation is no longer valid. Instead, we postulated that the atomic error was dominated by the degree of charge transfer from the metal to the ligand and thus by the degree of ionization of the atom. To quantify the degree of charge transfer, we used the natural population analysis (NPA) spin population (Mulliken population gives similar results) on the oxygen [see Supplementary Materials, Table S7.2 (Ref. 17)]. For instance, for Sc+–OH, the spin population on the oxygen is ∼0.0. Thus a full electron has been transferred from the metal to the oxygen, and we must correct our molecule for the error made by B3LYP on the second ionization potential. Similarly, Fe+–OH has a NPA spin population of 0.37, suggesting that the molecule should be corrected for only 63% of the error made by B3LYP on the ionization potential.
To balance the calculation, we must also take into account the error due to B3LYP in the electron affinity of OH. A calculation with the QZVP(-g) basis set shows that the B3LYP error for the electron affinity of OH is −7.09 kcal∕mol (theory-experiment). This error correction was included for all M+–OH compounds and scaled according to the degree of electron transfer. The last point that appears to be important for the atomic corrections of M+–OH compounds is that we observed a large basis set superposition error (BSSE) when the dissociation energies are computed relative to M2+ and OH−. This basis set superposition appears in our thermodynamic cycle and must therefore be included and scaled in our corrections. The BSSE for the calculation with the dication has a value between 6 and 7 kcal∕mol depending on the metal and generally almost canceled the error in the electron affinity of OH. We also carried out some tests which indicated that the BSSE in the case of the monocation is negligible (less than 0.5 kcal∕mol) and therefore no correction was included for molecules others than the hydroxyls.
Finally, we should note that if one is restricted to making only corrections for the atomic hybridization states (as, for example, has been done by other workers56, 75), the net improvement in the rms error is relatively small, diminishing from 5.3 to 5 kcal∕mol for the QZVP (g) basis. It is necessary to implement all three types of corrections (atomic, bond, environmental) in order to achieve the qualitative reduction in the error that we report below.
Bond corrections
The bond correction parameters are the most difficult to set up for metal-containing systems. We need to keep the number of parameters relatively small to avoid overfitting, given the limited size of the data set that is available, but the bonds in coordination and organometallic compounds are relatively diverse and may therefore require a relatively large number of parameters to achieve high accuracy. We thus need to cluster the bonds of the different compounds (enabling each cluster to be fitted with only one parameter) in such a way that it will have a minor impact on the final accuracy. To help us in this task, we will first rely on an analysis of the relative size of the bond orbital compared to the bond length. Then, we will review the different families of compounds in our data set and assign the different bond parameters by combining the relative orbital to bond length size and details of the electronic structure.
Analysis of the size of the orbitals relative to bond length.
In our previous article,13 we have shown that the size of the orbital relative to bond length was one of the major determinants for the error made by B3LYP in estimating the error in the nondynamical correlation associated with a given pair of electrons in a bond. In particular, we have seen that when the size of a doubly occupied localized bond is “big” relative to the interatomic distance, then the nondynamical correlation will be overestimated, whereas when it is small the nondynamical correlation is underestimated and the molecule is therefore underbound. A metric was defined in that paper to be able to quantify the difference between the bond length and the localized orbital size. This metric corresponds to the second moment (S) of Boys’ localized orbital minus the square of half the bond distance (L2). This metric was only applied to single bonds since sigma and pi bonds have considerable differences in shapes and the interaction between multiple orbitals may be too complex to be captured by this simple metric. We will thus focus now on our molecular compounds having only sigma bonds between the metal and the ligand such as M–H, M–CH3, M+–H, and M+–CH3.
The electronic structure of the compounds was discussed in paragraph III, and the values of the distances and the second moment of the localized orbitals are given in the Supplementary Materials.17 In a first attempt, our aim is to cluster different molecules together in such a way that a common parameter could correct all of them. One good way to perform this operation is to use the quantity S-L2, called the relative size of the orbital, and to plot it so that we can detect molecules having similar relative sizes and thus similar parameters. This plot is presented in Fig. 3 by taking for the size S, the average between the sizes of the alpha-spin and beta-spin localized orbital (since all our calculations are spin unrestricted).
Figure 3.
Plot of the second moment of the localized bond orbital (S) minus the square root of half the bond length (L2) (relative size) according to the nature of the compounds with single bond. The proposed clusters, based on the relative sizes of the orbital, are circled. A constantly increasing value was used to order compounds on the x-axis. This extra degree of freedom was used for convenience in clustering.
As we can see in Fig. 3, the neutral compounds (M–H and M–CH3) have relative sizes that correlate with their position in the Periodic Table. The first elements in the period (Sc, Ti, V) have relative sizes lower than the late elements (Co, Ni, Cu). This trend is actually consistent with the fact that the radius of the atoms decreases along the period. To cluster these compounds we first observe that M–CH3 have relative sizes more spread than M–H, and we may thus suspect that in spite of having similar electronic structures (both have only one sigma bond) they may have different correction parameters. Since we were limited by the number of data in our molecular set, we decided to divide each groups into two subgroups. In the case of M–H compounds, a natural cluster based on the relative size appears to be ScH, TiH, and VH (see Fig. 3). The remaining elements are consistent with a second cluster. In the case of M–CH3, based on the relative size of the orbitals, three clusters naturally appears: Sc–CH3, Ti–CH3, and V–CH3 in one cluster, Mn–CH3 and Cr–CH3 in a second and the remaining elements in a third. Since the number of clusters should be limited, we merged the first two clusters (see Fig. 3). It is quite possible that the Mn and Cr compounds should actually have different parameters; while we lack the data at present to conclude this definitively, it is something that should be investigated further in future work.
Regarding the ionic compounds (M+–H,M+–CH3), the relative sizes of the orbitals follow a different trend than for the neutral elements. The relative size generally decreases from Sc to Cr. Then, with Mn, it increases to reach a value higher than with Sc and then decreases again to Cu. Generally, we observe a big gap between the relative sizes of Cr and Cu and their predecessors. Moreover, the relative sizes of MH+ and M+–CH3 compounds are very similar and less spread than in the case of the neutral elements. This configuration leads us to cluster both types of compounds together. We therefore grouped CrH+, CuH+, CrCH3+, and together while the remaining elements are grouped together (see Fig. 3). We thus can see that the relative size of orbitals allowed us to define some clusters for compounds with single bond. Based on these data and the electronic structure analysis presented previously, we can construct the B3LYP-LOC model.
Parametrization of the model.
Neutral hydrides. For the neutral hydrides, the analysis of the relative sizes has shown that these compounds may be clustered into two groups: Sc to V and Cr to Cu. The electronic structure analysis (see Sec. 3B) has shown that all the compounds in this series have similar sigma bonds with the exceptions of MnH, which has only a beta-spin bonding orbital and ScH and CuH, which have a sigma bond but with different hybridizations than the other compounds. Ideally, some idiosyncratic parameters should be defined for ScH, MnH, and CuH but since we are limited in the number of parameters and since the difference in the hybridization should be partly taken into account by the atomic hybridization corrections (vide supra), ScH, MnH, and CuH were left within their corresponding clusters in Fig. 3. Thus we have one bond parameter for ScH, TiH, and VH and one for CrH, MnH, FeH, CoH, NiH, and CuH.
Neutral methyls. The neutral methyls have electronic structures similar to the hydrides. The analysis of the relative sizes of the orbitals has also highlighted two clusters in this series. The first cluster, Sc–CH3 to Mn–CH3, has orbital size similar to the group of ScH, suggesting that both groups could be merged. The least squares fitting supports this hypothesis, and we thus assign the same correction parameter to the group of Sc–CH3 as to the group of ScH. The second cluster (FeCH3–CuCH3) has also relative sizes consistent with those found in the cluster CrH–CuH, suggesting merging them as well. But the fitting did not support this hypothesis probably because in the second cluster, the d shell is at least half filled and this might trigger different behaviors when a hydrogen is bound than when a methyl is bound since the bond distances are significantly different. Moreover, it should be noticed that in case of the Sc–CH3 to Mn–CH3 cluster, experimental values suffer from large error bars. If these values present a systematic error, our ability to cluster them with the ScH cluster may also be an artifact. This point should be investigated in the future when more accurate bond dissociation energies will be available for these complexes [perhaps via converged CCSD(T) calculations].
Neutral oxides. The oxides generally have triple bonds between the metal and the oxygen as we have seen in our electronic structure analysis, and so we cannot apply the criteria on the relative orbital sizes. We thus rely only on the electronic structure to define the parameters. First, we have noticed that CuO has a simple bond with hybridization similar to what was found for CuH. Since these structures were similar, we assign the same correction parameter for CuO as for CuH. The results of the fitting procedure are consistent with this hypothesis. Then, we noticed that ScO, TiO, and VO all have three bonding orbitals. The NBO analysis (see Table S3.1 in Supplementary Materials17) shows that all three bonds have hybridized d orbitals from the metal. We assign one parameter for one of each of these bonds (thus we will have three times the parameter to correct the triple bonds in ScO, TiO, and VO). In the case of CrO, the bonding scheme is slightly different since an alpha-spin bonding orbital is not present anymore giving rise to an a-b-a half bond. Moreover, one of the bonding orbitals in CrO is now a hybridized sd. We assign one parameter for the a-b-a half bond and one parameter for the sd hybridized bond. MnO has one sd hybridized bond and two a-b-a half bonds. These bonds are assumed to be similar to the one found in CrO and thus no new parameter is required. Then FeO, CoO, and NiO all have a bond whose hybridization is dominated by the s atomic orbital (∼s3d). A new parameter is introduced for this type of bond. These compounds also have two a-b-a half bonds. The parameter defined for CrO and MnO is assumed to be the same for those cases so that no new parameter is required.
Cationic hydrides. We now turn our discussion toward the cations. For the cation hydrides, we have seen that the relative (to bond length) sizes of the orbitals are very similar and all compounds were clustered together other than CrH+ and CuH+ which have larger relative sizes. The electronic structure analysis showed that all compounds have a single bond other than CrH+ and CuH+ which are apparently not bonded. So, we defined one bond parameter which is applied to correct all cationic hydrides, excepting CrH+ and CuH+ which are assessed as not having a formal chemical bond and hence do not have a bond correction applied to them. We did not use the bond parameter defined for neutral hydrides because the cationic species have a slightly different behavior than the neutral species and the higher charge of the system is obviously going to change the error produced by B3LYP.
Cationic methyls. The cationic methyls have the same electronic structure as in case of the cationic hydrides with, again, and not bonded. Since, the relative size of the orbitals is also similar to the one obtained for the hydrides, we hypothesized that the same parameter could be used than for the cationic hydrides. So we assigned the same bond parameter for , , , , , , and than for ScH+, TiH+, VH+, MnH+, FeH+, CoH+, and NiH+.
Cationic methylenes. Cationic methylenes display a double bond except in case of . Actually, the structures of cationic methylenes parallel those observed for metal oxides, Sc, Ti, and V, form two full bonds with methylene whereas Cr, Mn, Fe, Co, and Ni form one bond and an a-b-a half bond. Sc has two bonds polarized toward the carbon, whereas Ti and V have one bond polarized toward the carbon and one polarized toward the metal (see Table S6.1 in Supplementary Materials17). We thus define one parameter for a normal bond (polarized toward the carbon) and one for the reversed polarization (polarized toward the metal). For Cr, Mn, Fe, Co, and Ni, we have a normal bond plus an a-b-a half bond for which a new parameter is defined. Since has a single sigma bond very similar to CuH, we hypothesized that the same parameter could be used. The least squares fitting confirmed this hypothesis.
Cationic hydroxyls. The electronic structure analysis has shown that these compounds display a charge transfer from the metal to the ligand and that we therefore have no real bonding since the dominant part in the energies should be the electrostatic interaction. Yet, the presence of the positive charge of the metal is expected to distort the lone pair located on the hydroxyl anion, and this effect should give rise to an error in the estimation of the interaction by B3LYP. This is an example of the rather common kind of interaction in coordination complexes often referred to as dative bonding. Since this effect is expected to be more dependent on the charge on the metal than on the exact nature of the metal, we assume that the parameter to correct for dative bonds should be transferable. We thus have only one bond parameter for the hydroxyl series.
Cationic oxides. As for the neutral oxides, cationic oxides generally display triple bonds. ScO+, TiO+, and VO+ have identical electronic structure with three d-hybridized bonding orbitals polarized toward the oxygen. One parameter was defined to correct one d-hybridized bonding orbital. CrO+ has two d-hybridized bonding orbitals and one a-b-a half bond for which a new parameter was defined. MnO+ has one bonding orbital that is s-hybridized and is strongly polarized toward the oxygen. So a new parameter was introduced for the polarized s-hybridized bonding orbital. It also has an a-b-a half bond for which the previous parameter was kept. FeO+, CoO+, and NiO+ have similar structures with one strongly polarized s-hybridized orbital and two a-b-a half orbitals. The parameters applying to MnO+ were reused.
Environment corrections
We have previously shown13 that environmental effects have also to be taken into account by developing a correction parameter scheme. Two main environmental effects have been used when developing B3LYP-LOC for main group elements. One was aimed at correcting the base bonding orbitals for the effects the neighboring atoms have on it. In our case since our data set only contains very small molecules most of which are actually diatomics, we were not able to investigate environmental corrections of this type. The second set of corrections provided corrections to unpaired electrons localized on one atom but able to delocalize over the neighboring atoms. Because of the delocalization, the orbital size increases and the nondynamical correlation calculated by B3LYP is going to be overestimated resulting in an overbound compound. As unpaired electrons are commonly found in compounds with transition metals, these corrections need to be included in our parametrization.
To guide our parametrization, we must first rely on some physicochemical background to determine when unpaired electron delocalization is possible. For delocalization, we first note that the unpaired electron orbital must have significant overlapping with atomic orbitals on the neighboring atoms. This has implication for metals because in the case of a diatomic molecule, we can have good overlap only with the 4s, 3dz2, 3dxz, and 3dyz of the metal. The other d orbitals will be much less effective in delocalizing their single electron on the neighboring atoms. A second aspect is that positive charges on metals should prevent to some extent delocalization of unpaired electrons.
From these arguments, we can conclude that compounds with triple bonds will have very small errors due to spin delocalization. Thus, we did not set up spin delocalization parameters for the oxides with the exception of CuO, since this compound only has a single bond. For cationic methylenes, the presence of a positive charge on the metal and the fact that we have a double bond between the metal and the carbon atom will lead to a very small contribution from spin delocalization. Thus no spin delocalization parameter was setup for the methylenes. This assumption was a posteriori supported by the results of the least squares fitting. Spin delocalization parameters were not included for the hydroxyls either. As we have seen, these compounds exhibit a charge transfer and the resulting +2 charge on the metal will prevent any delocalization of the unpaired electrons. Normally, the cationic hydrides and methyls should not have spin delocalization parameters since the metal atom is charged. But, we observed exceptions. First, we have seen that CrH+, CuH+, CrCH3+, and did not have any bond correction parameters since no bond was detected. Yet, the s and dz2 orbitals of the metal are going to overlap with the orbitals on the ligand, orbitals that are otherwise corrected with the bond parameters. Thus, for these cases we defined spin delocalization parameters. Since these parameters are expected to depend much more on the nature of the ligand than on the nature of the metal, one parameter was defined for CrH+ and CuH+ and another for and .
Based on a survey of the experimental data, we also observed that generally Sc and Ti display irregular behaviors regarding spin delocalization. A possible physical explanation for this idiosyncratic behavior is the fact that the effective screening of their nuclear charge is more effective than for the other transition metals and their electrons are therefore less tightly bound. As a consequence, these elements would be more prone to polarization and to spin delocalization. For the singly charged molecules such as ScH+, , TiH+, and ; it could mean that a spin delocalization is required despite the fact that the molecule is singly charged. A single spin delocalization parameter was therefore allocated to these molecules. This remark does not affect the previous molecules containing Sc and Ti, since the strong ability of Sc and Ti to polarized is largely counterbalanced by the charge of the metal and∕or the inability to delocalize due to multiple bonding.
The neutral hydrides and methyls are subject to spin delocalization since they are singly bonded and are not charged. Only ScH, CuH, ScCH3, and CuCH3 are not subject to this correction since they are close shell. Least squares fitting shows that it is possible to attribute a common parameter for both the hydrides and the methyls. A common parameter was thus defined for VH to NiH and for VCH3 to NiCH3. Since Ti is a special case, another parameter was set up for TiH and TiCH3.
Results and analysis of the B3LYP-LOC model
The B3LYP-LOC bond and spin delocalization parameters were fitted against our B3LYP errors for the QZVP(-g) basis set; the results for these parameters are presented in Tables 16, 17. Atomic corrections, based on experimental data, are presented in Table 15; these do not involve any adjustable parameters, the correction is computed based on analysis of the wavefunction and the experimental data enumerated below.
Table 16.
Values of the B3LYP-LOC bond correction parameters for the QZVP(−g) and LACV3P++** basis sets (values are given in kcal∕mol). ScCH3 was excluded from the fitting procedure (see text).
| Bond corrections | QZVP(−g) values | LACV3P++** values |
|---|---|---|
| Cr–H; Mn–H; Fe–H; Co–H; Cu–H; Cu–O; Cu+–CH2 | 0.8 | −1.1 |
| Fe–CH3; Co–CH3; Ni–CH3, Cu–CH3 | −6.7 | −6.7 |
| Sc–H; Ti–H; V–H; Sc–CH3; Ti–CH3; V–CH3; Cr–CH3; Mn–CH3 | 6.1 | 3.5 |
| Sc+–H; Ti+–H; V+–H; Mn+–H; Fe+–H; Co+–H; Ni+–H; Sc+–CH3 | 2.9 | 1.2 |
| Ti+–CH3; V+–CH3; Mn+–CH3; Fe+–CH3; Co+–CH3; Ni+–CH3 | ||
| Sc–O; Ti–O; V–O; Cr–O (full bond correction∕ bond∕hybridized d) | −0.7 | −3.1 |
| Cr–O; Mn–O (sp hybridized) | 7.4 | −15.0 |
| Cr–O; Mn–O; Fe–O; Co–O; Ni–O; (a-b-a half bond) | −4.0 | 3.3 |
| Fe–O; Co–O; Ni–O (normal) | 4.7 | −17.3 |
| Sc+–OH; Ti+–OH; V+–OH; Cr+–OH Mn+–OH; Fe+–OH; Co+–OH; Ni+–OH dative bond | 6.5 | −1.0 |
| Sc+–CH2; Ti+–CH2; V+–CH2; Cr+–CH2; Mn+–CH2; Fe+–CH2; Co+–CH2; Ni+–CH2 (full bond) | −6.9 | −8.0 |
| Ti+–CH2; V+–CH2 (alpha-spin polarized) | −0.7 | −1.3 |
| Cr+–CH2; Mn+–CH2; Fe+–CH2; Co+–CH2; Ni+–CH2 (a-b-a bond) | 6.6 | 6.2 |
| Sc+–O; Ti+–O; V+–O (one full d hybridized bond) | −2.1 | −5.0 |
| Cr+–O; Mn+–O; Fe+–O; Co+–O; Ni+–O (a-b-a bond) | −4.2 | −7.3 |
| Mn+–O; Fe+–O; Co+–O; Ni+–O (alpha s hybridized full bond) | 0.5 | −2.6 |
Table 17.
Values of the B3LYP-LOC unpaired spin delocalization correction parameters for the QZVP(−g) basis set (values are given in kcal∕mol). ScCH3 was excluded from the fitting procedure (see text).
| Unpaired spin delocalization corrections | QZVP(−g) values | LACV3P++** values |
|---|---|---|
| V–H; Cr–H; Mn–H; Fe–H; Co–H; Ni–H; V–CH3; Cr–CH3; Mn–CH3; Fe–CH3; Co–CH3; Ni–CH3 | 6.2 | 6.6 |
| Ti–H; Ti–CH3 | −4.5 | −3.5 |
| Cu–O | 0.5 | −3.8 |
| Cr+–H; Cu+–H | 5.1 | 3.1 |
| Cr+–CH3; Cu+–CH3 | 7.1 | 5.6 |
| Sc+–H; Ti+–H; Sc+–CH3; Ti+–CH3 | −1.3 | −0.9 |
Table 15.
Values of all the B3LYP-LOC atomic correction parameters for the QZVP(−g) and LACV3P++** basis sets (values are given in kcal∕mol).
| Hybridization corrections (partial excitation of the atoms in the molecule) | Ionization corrections | ||||
|---|---|---|---|---|---|
| QZVP(−g) | LACV3P++** | QZVP(−g) | LACV3P++** | ||
| Sc | 16.2 | 14.4 | Sc+ | −6.0 | −4.74 |
| Ti | 13.9 | 9.4 | Ti+ | −6.4 | −6.11 |
| V | 11.0 | 5.1 | V+ | −12.3 | −7.05 |
| Cr | −8.8 | 6.2 | Cr+ | −10.6 | −9.77 |
| Mn | 17.1 | 8.8 | Mn+ | −6.3 | −6.97 |
| Fe | 14.8 | 7.0 | Fe+ | −7.0 | −8.35 |
| Co | 12.1 | 2.4 | Co+ | −14.8 | −10.55 |
| Ni | −8.7 | −2.3 | Ni+ | −12.4 | −7.22 |
| Cu | −7.1 | −1.6 | Cu+ | −11.0 | −8.32 |
| Sc+ | 9.9 | 7.7 | |||
| Ti+ | 7.7 | 2.8 | |||
| V+ | −5.4 | 0.2 | |||
| Cr+ | −3.3 | −2.5 | |||
| Mn+ | 13.6 | 7.3 | |||
| Fe+ | 10.4 | 4.8 | |||
| Co+ | −7.0 | −0.7 | |||
| Ni+ | −3.4 | 4.2 | |||
| Cu+ | −0.7 | 3.7 | |||
The performance of B3LYP-LOC appears to be very good since the MAD goes from 5.32 kcal∕mol for B3LYP to 1.66 kcal∕mol for B3LYP-LOC with ScCH3 excluded. First, we must notice that ScCH3 was not included in this fitting process because in all models tested this experimental value appear as an outlier. Moreover, a close analysis of the experimental values shows that the large difference observed in the dissociation energies of ScH and ScCH3 is not consistent with what is observed for the other complexes of the same families. Indeed, this point has been previously pointed out by Armentrout and Georgiadis94 and Bauschlicher et al.56 It is thus encouraging that the B3LYP-LOC method is able to point out possible experimental errors.
One first test of our model is to see whether the values of the parameters follow the trend expected based on physical principles. For instance, analysis of our bond parameters setup shows that there is a strong transition in the middle of the transition metal series (around Cr, Mn approximately). This effect is consistent with the fact that at these positions in the series, the 3d shell is half filled and, thus, a change in the behavior of the B3LYP error is expected. Based on physical grounds, we can check qualitatively the expected effects on the B3LYP error in these two cases. When the 3d shell is less than half filled, the electrons of the bond have the ability to delocalize freely in the empty orbitals of the metal since there is no Coulomb repulsion. They are therefore not going to use the enhanced space provided by the bond. In this case, B3LYP is expected to overestimate the nondynamical correlation in the bonding orbital and a positive correction parameter is expected for this bonds. This is actually what we observed with the least squares fitting (see Table 16). When the 3d shell is more than half filled all orbitals on the metal are partially occupied and the Coulomb repulsion partly prevents the electrons of the bond to delocalize on the metal. The electrons are thus going to use much more the space in the bond to correlate. In this case, B3LYP is expected to slightly underestimate or to overestimate the nondynamical correlation depending how it compares relative to the “reference orbital” for which B3LYP would give no error (see discussion in Ref. 13). Indeed, this is what is observed with the least squares fitting, since values of +0.8 and −6.7 kcal∕mol are given for the bond parameters of CrH–CuH and FeCH3–CuCH3, compared to the value of +6.1 kcal∕mol obtained in the first half of the period. Of course, the relative values of the parameters and the exact limit between the two behaviors described depend on the intensities of the different phenomena relative to what is happening in the reference orbital.
In the less than half filled regime, we also expect the error not to be dependent on the nature of the ligand since we argued that the electrons are not going to use the extra space provided by the bond. Whereas, in the more than half filled regime, the error will depend much more on the nature of the ligand. This fact may explain why we were able to merge the clusters ScH–VH and ScCH3–MnCH3 whereas it was not possible with CrH–CuH and FeCH3–CuCH3 in spite of the fact that in both cases the two clusters have similar relative sizes. The values obtained for CrH–CuH and FeCH3–CuCH3 are consistent with the fact that with the methyls B3LYP is expected to underestimate the nondynamical correlation much more that with the hydrides since the methyl-metal bond allows much more space than the metal-hydrogen bond.
For the cationic hydrides and methyls, we also expect B3LYP to overbind the first elements of the series (Sc–Cr) which is the case since the correction parameter has a value of 2.9 kcal∕mol (see Table 16). But for the second part of the series (Mn–Cu), we surprisingly observe that B3LYP overbinds too. The explanation for this effect is the fact that due to the charge on the metal, the electrons of the bond cannot benefit from the extra space provided by the bond and thus the correction is positive. The least squares fitting provides an intermediate value compared to those observed for the neutral atoms. This is due to the effect of the charge on the metal.
If we now turn to the dative bond, we observe that the high charge on the metal should prevent nondynamical correlation relative to the atoms, leading thus B3LYP to overbind these compounds. This is what is observed with a parameter of +6.5 kcal∕mol (see Table 16).
The parameters for multiple bonds are much more complex to analyze. Correlation in multiple bonds requires the electrons in the various bonds to avoid each other, as well as avoiding the second electron in their own bond. A physical explanation as to how this impacts empirical correction parameters for multiple bonds has not been worked out, even for compounds containing first and second row atoms. What was shown in Ref. 13 was that a relatively small number of parameters provided consistent and reliable corrections for a number of different multiply bonded species. A similar demonstration is not possible for multiply bonded metal species, for one thing because of the lack of sufficient experimental data and also the relatively large error bars on the experimental values, but also because the electronic structures are much more complex than in organic and main group compounds. However, within the transition metal series for each species, some predictive power is exhibited in that the ratio of adjustable parameters to the members of the series is not unreasonable. In fact, three of the parameters [those with absolute value less than 1 kcal∕mol for the QZVP(-g) basis set] can be eliminated from Table 16 without substantially degrading the results for the QZVP(-g) basis set (MAD of 1.72 kcal∕mol), although a similar reduction in parameters creates a considerably larger error (MAD of 2.27 kcal∕mol), as well as some significant outliers, when the LACV3P++** basis set is used.
Regarding the unpaired spin delocalization, it was argued in Ref. 13 that the corresponding parameters should be positive since they are correcting for the B3LYP overbinding due to the orbital size increase resulting from delocalization. The analysis of Table 17 shows that it is not the case. Also in Ref. 13 we observed that triple bonds had negative parameters corresponding to underbinding. Indeed, this effect has a simple explanation. As we mentioned previously, delocalization will be possible when the singly occupied orbital has sufficient overlap with the orbitals on the neighboring atoms. In the case of a triple bond, there is no possibility of delocalization since all overlapping orbitals are already occupied by two electrons. Since no delocalization of the electron is involved, no overbinding is expected in those cases. Yet, an underbinding is observed. This may be the result of the fact that when the bond is formed, the second atom attracts the electron of the bond. As a result, the other lone pairs on the first atom are going to contract and a resulting underbinding by B3LYP is expected. In that way the negative value observed for the triple bond finds an explanation. In the same line, we observe that only Sc and Ti have negative delocalization parameters (see Table 17). The case of Sc is very clear since the singly occupied lone pair has little overlap with the ligand since it is a dx2−y2 or dxy of the metal. In the case of Ti the situation is not so clear since the two singly occupied orbitals are located in one orbital with strong overlap (dxz or dyz) and one with little overlap (dx2−y2 or dxy). The least squares fitting for those cases seems to indicate that the balance is toward the contraction that overwhelms the delocalization. It is also interesting to note that for the cations these effects are limited probably because the perturbation generated by the ligand is limited by the fact that the metal already bears a positive charge.
Another interesting test for our model is to examine its robustness toward a change in the basis set used. Indeed, we have seen that the B3LYP error depends on the basis used to do the calculations. Our assumption was that the major errors would come from B3LYP if a sufficiently large basis set was used, in our case QZVP(-g). If we use the same scheme with a smaller basis set but refitting the parameters, we expect to correct for the errors of the functional and we should thus get improved performances compared to B3LYP alone. If our scheme is too basis set dependent, we may suspect that we are not correcting for the error in the functional but more the error due to the basis set. To see whether it was the case, we tested the same parameter scheme with the LACV3P++** basis set.
The results for LACV3P++** show that the scheme is reasonably robust since we go from a MAD of 6.55 kcal∕mol with B3LYP to a MAD of 1.92 kcal∕mol with B3LYP-LOC. Generally, the parameters obtained for both basis sets follow the same trend; however, some very large deviations are observed in the bonding parameters of the neutral oxides and to a much lower extent for the cationic oxides (see Tables 17, 18). We also noted above the much greater sensitivity to reduction in the number of parameters for this basis set. The conclusion from this analysis is that there is still significant basis set error remaining, particularly for species with multiple bonds and∕or charge transfer, for the LACV3P++** basis, and that the empirical corrections are reducing both types of error simultaneously. The ability to reduce other types of error, in addition to that associated with electron correlation, is in principle a positive feature of the LOC approach, as long as parameters are developed specifically for each basis set (a relatively straightforward endeavor, as the vast majority of the effort is in analyzing the electronic structure); however, the transferability of the corrections to new molecules under these conditions remains to be ascertained.
Table 18.
Best CCSD(T) estimate for the bond dissociation energies D0 (kcal∕mol) calculated for different basis sets and by extrapolation to CBS. The corresponding B3LYP and B3LYP-LOC results are also given.
| D0 Expt. | Experimental error | Best estimate CCSD(T) | B3LYP (QZVP(−g)) | B3LYP-LOC (QZVP(−g)) | |
|---|---|---|---|---|---|
| ScCH3 | 30.7 | 7.0 | 51.5 | 51.1 | 46.9 |
| 57.7 | 3.0 | 55.5 | 60.0 | 55.0 | |
| 50.0 | 2.3 | 47.5 | 49.7 | 44.4 | |
| 56.0 | 3.0 | 55.6 | 61.9 | 55.6 | |
| CoO | 90.9 | 1.4 | 88.8 | 91.9 | 90.1 |
CCSD(T) calculations
The scarcity of experimental data, and the substantial error bars on many of the data points that do exist, suggests that it would be extremely useful, in the development and testing of DFT treatments of transition metals, to have available true benchmark results from high level wavefunction-based ab initio quantum chemistry. As was pointed out above, it appears as though at present, no such true benchmarks (accurate to, e.g., 2 kcal∕mol) exist in the literature. We have used CCSD(T) calculations with large basis sets in a number of previous studies of larger metal complexes to benchmark DFT methods.95 It is unclear, however, whether CCSD(T) can indeed yield 2–3 kcal∕mol accuracy, especially for the small unsaturated species covered here. In what follows, we present a preliminary investigation of the ability of CCSD(T) calculations to serve in this role. As an initial step, we investigate the utility of CCSD(T) results in two ways. Firstly, there is only one highly deviant outlier (error greater than 14 kcal∕mol) in the comparison of B3LYP-LOC result with experiment presented in Table S10 (see Supplementary materials17): the dissociation of ScCH3. As the experimental error bars are particularly large for this system, we proceeded above on the hypothesis that the experimental result was sufficiently in error that it should be removed from the data set. If this is indeed the case, then a high quality CCSD(T) calculation should be much closer to the B3LYP-LOC result than to the experimental result. Secondly, we selected a small number of additional test cases where the experimental error bars are reasonable and where the agreement between B3LYP-LOC and experiment is also respectable. For these cases, if the B3LYP-LOC methodology has improved the reliability of DFT calculation for transition metals, and the CCSD(T) calculations themselves are close to convergence, then the CCSD(T) values should be in good agreement with experiment and B3LYP-LOC.
We used the protocol described in Sec. 2B for the CCSD(T) calculations. The precise accuracy of this protocol is difficult to specify with great confidence, given the lack of highly precise experimental data to compare with, the possible multireference character of transition metal wavefunctions (due to low-lying excited states), etc. But a recent study with a small data set using similar CCSD(T) protocol gave a MAD value for transition metals of 3.1 kcal∕mol.96 We obtained a value of 51.5 kcal∕mol for the D0 for ScCH3. This result is, as suggested above, much closer to the B3LYP-LOC result (47.1 kcal∕mol) than to the experimental result (32.8 kcal∕mol), confirming the original hypothesis that the latter is substantially in error. The question of which of the calculated values is closer to the true experimental result cannot be determined from the small number of CCSD(T) calculations investigated here, but the discrepancy obtained of ∼4 kcal∕mol is quite reasonable given the estimated errors of a few kcal∕mol for both methods.
To make further comparisons of CCSD(T) results to experiment and B3LYP-LOC, we did further calculations on four additional complexes. The results are presented in Table 18. In all four cases, the CCSD(T) results are within a few kcal∕mol of experiment, which is quite encouraging. The largest discrepancy between experiment and B3LYP-LOC is for ; for this case, the B3LYP-LOC results are closer to CCSD(T) than to experiment. The new CCSD(T) calculations are much more accurate than the previous correlated ab initio calculations. This is in large part due to extrapolation to the infinite basis limit, as the results obtained with the smaller cc-pVXZ basis sets are in some cases much less accurate. A great deal of further work will be required to quantify these anecdotal comparisons and derive rigorous statistical assessments of the robustness and accuracy of the various calculations.
CONCLUSION
In this study, we have developed a test set of dissociation energies for compounds of metal from the first transition series and tested the B3LYP functional with different basis sets. Based on this test set, we have developed an empirical B3LYP-LOC scheme for transition metals. We have shown that, based on a careful analysis of the electronic structure and based on physical arguments previously established,13 we were able to reduce the B3LYP error from 5.3 to 1.7 kcal∕mol for the molecules and from 7.7 to 0.4 kcal∕mol for the atomic data. The results for the atomic data are very good and we have reached chemical accuracy with a very simple empirical model using the physical idea that were previously developed.13, 15 The results for the molecules are less impressive, but it must be noticed that the experimental data used in this data set have a much bigger error bar. We have then shown that, despite some basis set dependency of the B3LYP results, the scheme developed here also applies and can give relatively good results. B3LYP-LOC was also able to detect an outlier in the experimental data set which is also interesting because it gives support to the underlying physics used to develop the model and give confidence that the model developed is not just the result of overfitting.
The ratio between the number of parameters and the number of experimental data is still relatively high, but it must be noticed that the diversity of the electronic structures with metal-containing compounds and the scarcity of the experimental data makes this problem intractable given the currently available experimental data set. One interesting way for the future that we have tried to explore is to jointly use experimental data with high level ab initio results [CCSD(T)] so that CCSD(T) can be tested against accurate experimental data and then used to generated accurate value for systems very similar. This method would be very useful to generate very accurate data for different systems with very similar chemistry such as methyl, ethyl propyl complexes and also to detect experimental data that may not be reliable. Further efforts to generate more experimental data, with smaller error bars, would also enable a substantial increase in the confidence level of any DFT-LOC model developed for metal-containing systems.
It will be interesting in the future to apply the B3LYP-LOC corrections to the calculation of barrier heights and to see whether an empirical form could be found that would include and fit to all the B3LYP-LOC corrections already developed. Assuming that such a possibility exists and disregarding the tremendous effort it would require, it would be an excellent mean to introduce real nonlocality into the DFT calculations and it will be done at a very low computational cost. In the case of success, this kind of methods can have a very serious impact in all field of computational chemistry where DFT can be routinely applied.
ACKNOWLEDGMENTS
This work was supported in part by grants to RAF from the NIH (GM40526) and DOE (DE-FG02-90ER14162). D.R. thanks Y. X. Cao for providing the code to calculate the size of the orbitals.
References
- Ghosh A., JBIC, J. Biol. Inorg. Chem. 10.1007/s00775-006-0134-5 11, 671 (2006). [DOI] [PubMed] [Google Scholar]
- Jensen K. P., Roos B. O., and Ryde U., J. Chem. Phys. 10.1063/1.2406071 126, 014103 (2007). [DOI] [PubMed] [Google Scholar]
- Holthausen M. C., Heinemann C., Cornehl H. H., Koch W., and Schwarz H., J. Chem. Phys. 10.1063/1.469541 102, 4931 (1995). [DOI] [Google Scholar]
- Holthausen M. C., Mohr M., and Koch W., Chem. Phys. Lett. 10.1016/0009-2614(95)00535-C 240, 245 (1995). [DOI] [Google Scholar]
- Blomberg M. R. A., Siegbahn P. E. M., and Svensson M., J. Chem. Phys. 10.1063/1.471673 104, 9546 (1996). [DOI] [Google Scholar]
- Yanagisawa S., Tsuneda T., and Hirao K., J. Chem. Phys. 10.1063/1.480546 112, 545 (2000). [DOI] [Google Scholar]
- Koch W. and Hertwig R. H., in Encyclopedia of Computational Chemistry, edited by Schleyer P. (Wiley, New York, 1998), p. 689; [Google Scholar]; Baker J. and Pulay P., J. Comput. Chem. 10.1002/jcc.10280 24, 1184 (2003); [DOI] [PubMed] [Google Scholar]; Lundberg M. and Siegbahn P. E. M., J. Comput. Chem. 10.1002/jcc.20206 26, 661 (2005); [DOI] [PubMed] [Google Scholar]; Barden C. J., Rienstra-Kiracofe J. C., and Schaefer H. F., J. Chem. Phys. 10.1063/1.481916 113, 690 (2000); [DOI] [Google Scholar]; Gutsev G. L. and Bauschlicher C. W., J. Phys. Chem. A 10.1021/jp030146v 107, 4755 (2003); [DOI] [Google Scholar]; Barone V., Adamo C., and Mele F., Chem. Phys. Lett. 10.1016/0009-2614(95)01382-2 249, 290 (1996); [DOI] [Google Scholar]; Barone V. and Adamo C., Int. J. Quantum Chem. 61, 443 (1997); [DOI] [Google Scholar]; Hyla-Kryspin I. and Grimme S., Organometallics 10.1021/om049521b 23, 5581 (2004); [DOI] [Google Scholar]; Conradie J. and Ghosh A., J. Chem. Theory Comput. 3, 689 (2007). [DOI] [PubMed] [Google Scholar]
- Holthausen M. C., J. Comput. Chem. 10.1002/jcc.20279 26, 1505 (2005). [DOI] [PubMed] [Google Scholar]
- Zhao Y. and Truhlar D. G., J. Chem. Phys. 10.1063/1.2202732 124, 224105 (2006). [DOI] [PubMed] [Google Scholar]
- Schultz N. E., Zhao Y., and Truhlar D. G., J. Phys. Chem. A 10.1021/jp0504468 109, 4388 (2005); [DOI] [PubMed] [Google Scholar]; Schultz N. E., Zhao Y., and Truhlar D. G., J. Phys. Chem. A 10.1021/jp0539223109, 11127 (2005). [DOI] [PubMed] [Google Scholar]
- Gutsev G. L., Mochena M. D., Jena P., Bauschlicher C. W., and Partridge H., J. Chem. Phys. 10.1063/1.1788656 121, 6785 (2004). [DOI] [PubMed] [Google Scholar]
- Furche F. and Perdew J. P., J. Chem. Phys. 10.1063/1.2162161 124, 044103 (2006); [DOI] [PubMed] [Google Scholar]; Riley K. E. and Merz K. M., J. Phys. Chem. A 10.1021/jp0705931 111, 6044 (2007). [DOI] [PubMed] [Google Scholar]
- Friesner R. A., Knoll E. H., and Cao Y. X., J. Chem. Phys. 10.1063/1.2263795 125, 124107 (2006). [DOI] [PubMed] [Google Scholar]
- Curtiss L. A., Raghavachari K., Redfern P. C., and Pople J. A., J. Chem. Phys. 10.1063/1.481336 112, 7374 (2000). [DOI] [Google Scholar]
- Knoll E. H. and Friesner R. A., J. Phys. Chem. B 10.1021/jp0619888 110, 18787 (2006). [DOI] [PubMed] [Google Scholar]
- Weigend F., Furche F., and Ahlrichs R., J. Chem. Phys. 10.1063/1.1627293 119, 12753 (2003). [DOI] [Google Scholar]
- See EPAPS Document No.E-JCPSA6-129-633834 for the analysis of the electronic structures, the bond dissociation energies (B3LYP, B3LYP-LOC, experiment) and the geometries and wave functions of the molecules. For more information on EPAPS, see http://www.aip.org/pubservs/epaps.html.
- Ralchenko Y., You F. C., Kelleher D. E., Kramida A. E., Musgrove A., Reader J., Wiese W. L., and Olsen K., NIST Atomic Spectra Database, version 3.1.2 ed. (National Institute of Standards and Technology, Gaithersburg, MD, 2007), http://physics.nist.gov/asd3.
- Armentrout P. B. and Kickel B. L., in Organometallic Ion Chemistry, edited by Freiser B. S. (Kluwer Academic, Dordrecht, 1996), p. 1. [Google Scholar]
- Becke A. D., Phys. Rev. A 10.1103/PhysRevA.38.3098 38, 3098 (1988); [DOI] [PubMed] [Google Scholar]; Becke A. D.,J. Chem. Phys. 10.1063/1.464913 98, 5648 (1993); [DOI] [Google Scholar]; Becke A. D., J. Chem. Phys. 10.1063/1.46430498, 1372 (1993); [DOI] [Google Scholar]; Lee C. T., Yang W. T., and Parr R. G., Phys. Rev. B 10.1103/PhysRevB.37.785 37, 785 (1988); [DOI] [PubMed] [Google Scholar]; Vosko S. H., Wilk L., and Nusair M., Can. J. Phys. 58, 1200 (1980). [Google Scholar]
- Siegbahn P. E. M., JBIC, J. Biol. Inorg. Chem. 10.1007/s00775-006-0137-2 11, 695 (2006). [DOI] [PubMed] [Google Scholar]
- Schultz N. E., Zhao Y., and Truhlar D. G., J. Comput. Chem. 10.1002/jcc.20717 29, 185 (2008). [DOI] [PubMed] [Google Scholar]
- Hay P. J. and Wadt W. R., J. Chem. Phys. 10.1063/1.448799 82, 270 (1985). [DOI] [Google Scholar]
- Scott A. P. and Radom L., J. Phys. Chem. 10.1021/jp960976r 100, 16502 (1996). [DOI] [Google Scholar]
- Dunietz B. D., Beachy M. D., Cao Y. X., Whittington D. A., Lippard S. J., and Friesner R. A., J. Am. Chem. Soc. 122, 2828 (2000). [Google Scholar]
- JAGUAR, Jaguar (Schrödinger, Inc., New York, NY, 2005).
- Werner H.-J., Knowles P. J., Lindh R.et al. , MOLPRO, version 2006.1, a package of ab initio programs (Cardiff, UK, 2006).
- Dunning T. H., J. Chem. Phys. 10.1063/1.456153 90, 1007 (1989); [DOI] [Google Scholar]; de Jong W. A., Harrison R. J., and Dixon D. A., J. Chem. Phys. 10.1063/1.1329891 114, 48 (2001); [DOI] [Google Scholar]; Balabanov N. B. and Peterson K. A., J. Chem. Phys. 10.1063/1.1998907 123, 064107 (2005). [DOI] [Google Scholar]
- Li X. and Paldus J., J. Chem. Phys. 10.1063/1.2741265 126, 234303 (2007). [DOI] [PubMed] [Google Scholar]
- Halkier A., Helgaker T., Jorgensen P., Klopper W., Koch H., Olsen J., and Wilson A. K., Chem. Phys. Lett. 10.1016/S0009-2614(98)00111-0 286, 243 (1998); [DOI] [Google Scholar]; Helgaker T., Klopper W., Koch H., and Noga J., J. Chem. Phys. 10.1063/1.473863 106, 9639 (1997). [DOI] [Google Scholar]
- Douglas M. and Kroll N. M., Ann. Phys. 10.1016/0003-4916(74)90333-9 82, 89 (1974); [DOI] [Google Scholar]; Hess B. A., Phys. Rev. A 10.1103/PhysRevA.32.756 32, 756 (1985); [DOI] [PubMed] [Google Scholar]; Hess B. A., Phys. Rev. A 10.1103/PhysRevA.33.3742 33, 3742 (1986). [DOI] [PubMed] [Google Scholar]
- Berning A., Schweizer M., Werner H. J., Knowles P. J., and Palmieri P., Mol. Phys. 10.1080/00268970050177710 98, 1823 (2000). [DOI] [Google Scholar]
- Reed A. E., Curtiss L. A., and Weinhold F., Chem. Rev. (Washington, D.C.) 10.1021/cr00088a005 88, 899 (1988). [DOI] [Google Scholar]
- Foster J. M. and Boys S. F., Rev. Mod. Phys. 10.1103/RevModPhys.32.300 32, 300 (1960); [DOI] [Google Scholar]; Boys S. F., Rev. Mod. Phys. 10.1103/RevModPhys.32.296 32, 296 (1960). [DOI] [Google Scholar]
- Harrison J. G., J. Chem. Phys. 10.1063/1.446076 79, 2265 (1983). [DOI] [Google Scholar]
- Moore C. E., in National Bureau Standard Reference Data Series (United States Government Printing Office, Washington, 1970), Vol. 34. [Google Scholar]
- Raghavachari K. and Trucks G. W., J. Chem. Phys. 10.1063/1.457230 91, 1062 (1989). [DOI] [Google Scholar]
- Moore C. E., in National Standard Reference Data Series (United States Government Printing Office, Washington, 1949), Vol. 467. [Google Scholar]
- Raghavachari K. and Trucks G. W., J. Chem. Phys. 10.1063/1.457005 91, 2457 (1989). [DOI] [Google Scholar]
- Mitin A. V., Baker J., and Pulay P., J. Chem. Phys. 10.1063/1.1563619 118, 7775 (2003). [DOI] [Google Scholar]
- Sansonetti J. E., Martin W. C., and Young S. L., Handbook of Basic Atomic Spectroscopic Data (National Institute of Standards and Technology, Gaithersburg, 2007); [Google Scholar]; http://physics.nist.gov/PhysRefData/Handbook/index.html.
- Sugar J. and Corliss C., J. Phys. Chem. Ref. Data 14, 1 (1985). [Google Scholar]
- Iglesias L., Cabeza M. I., and de Luis B., Pub. Inst. Opt. Madrid No. 47 (1988).
- Shenstone A. G., J. Res. Natl. Bur. Stand. 74A, 801 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar J. and Musgrove A., J. Phys. Chem. Ref. Data 19, 527 (1990). [Google Scholar]
- Kant A. and Moon K. A., High. Temp. Sci. 14, 23 (1981). [Google Scholar]
- Chen Y. M., Clemmer D. E., and Armentrout P. B., J. Chem. Phys. 10.1063/1.461154 95, 1228 (1991). [DOI] [Google Scholar]
- Chen Y. M., Clemmer D. E., and Armentrout P. B., J. Chem. Phys. 10.1063/1.464948 98, 4929 (1993). [DOI] [Google Scholar]
- Sunderlin L. S. and Armentrout P. B., J. Phys. Chem. 10.1021/j100372a042 94, 3589 (1990). [DOI] [Google Scholar]
- Schultz R. H. and Armentrout P. B., J. Chem. Phys. 10.1063/1.459897 94, 2262 (1991). [DOI] [Google Scholar]
- Fisher E. R. and Armentrout P. B., J. Phys. Chem. 10.1021/j100367a083 94, 1674 (1990). [DOI] [Google Scholar]
- Chong D. P., Langhoff S. R., Bauschlicher C. W., Walch S. P., and Partridge H., J. Chem. Phys. 10.1063/1.451044 85, 2850 (1986). [DOI] [Google Scholar]
- Armentrout P. B., ACS Symp. Ser. 428, 18 (1990). [Google Scholar]
- Fisher E. R. and Armentrout P. B., J. Am. Chem. Soc. 10.1021/ja00032a017 114, 2039 (1992). [DOI] [Google Scholar]
- Georgiadis R., Fisher E. R., and Armentrout P. B., J. Am. Chem. Soc. 10.1021/ja00194a016 111, 4251 (1989). [DOI] [Google Scholar]
- Bauschlicher C. W., Langhoff S. R., Partridge H., and Barnes L. A., J. Chem. Phys. 10.1063/1.456998 91, 2399 (1989). [DOI] [Google Scholar]
- Armentrout P. B. and Kickel B. L., in Organometallic Ion Chemistry, edited by Freiser B. S. (Kluwer, Dordrecht, 1996), p. 1. [Google Scholar]
- Pedley J. B. and Marshall E. M., J. Phys. Chem. Ref. Data 12, 967 (1983). [Google Scholar]
- Kang H. and Beauchamp J. L., J. Am. Chem. Soc. 10.1021/ja00284a011 108, 7502 (1986). [DOI] [PubMed] [Google Scholar]
- Watson L. R., Thiem T. L., Dressler R. A., Salter R. H., and Murad E., J. Phys. Chem. 10.1021/j100123a020 97, 5577 (1993). [DOI] [Google Scholar]
- Clemmer D. E., Dalleska N. F., and Armentrout P. B., J. Chem. Phys. 10.1063/1.461403 95, 7263 (1991). [DOI] [Google Scholar]
- Rostai M. and Wahlbeck P. G., J. Chem. Thermodyn. 31, 255 (1999). [Google Scholar]
- Naulin C., Hedgecock I. M., and Costes M., Chem. Phys. Lett. 10.1016/S0009-2614(97)00019-5 266, 335 (1997). [DOI] [Google Scholar]
- Merer A. J., Annu. Rev. Phys. Chem. 10.1146/annurev.physchem.40.1.407 40, 407 (1989). [DOI] [Google Scholar]
- Bauschlicher C. W. and Maitre P., Theor. Chim. Acta 10.1007/BF01113847 90, 189 (1995). [DOI] [Google Scholar]
- Weinhold F. and Landis C. R., Valency And Bonding: A Natural Bond Orbital Donor Acceptor Perspective (Cambridge University Press, Cambridge, 2005). [Google Scholar]
- Elkind J. L., Sunderlin L. S., and Armentrout P. B., J. Phys. Chem. 10.1021/j100345a054 93, 3151 (1989). [DOI] [Google Scholar]
- Elkind J. L. and Armentrout P. B., Int. J. Mass Spectrom. Ion Process. 83, 259 (1988). [Google Scholar]
- Elkind J. L. and Armentrout P. B., J. Phys. Chem. 10.1021/j100272a012 89, 5626 (1985). [DOI] [Google Scholar]
- Elkind J. L. and Armentrout P. B., J. Chem. Phys. 10.1063/1.452138 86, 1868 (1987). [DOI] [Google Scholar]
- Elkind J. L. and Armentrout P. B., J. Chem. Phys. 10.1063/1.449975 84, 4862 (1986). [DOI] [Google Scholar]
- Elkind J. L. and Armentrout P. B., J. Phys. Chem. 10.1021/j100280a054 90, 5736 (1986). [DOI] [Google Scholar]
- Elkind J. L. and Armentrout P. B., J. Phys. Chem. 10.1021/j100282a031 90, 6576 (1986). [DOI] [Google Scholar]
- Ohanessian G. and Goddard W. A., Acc. Chem. Res. 10.1021/ar00179a007 23, 386 (1990). [DOI] [Google Scholar]
- Pettersson L. G. M., Bauschlicher C. W., Langhoff S. R., and Partridge H., J. Chem. Phys. 10.1063/1.453594 87, 481 (1987). [DOI] [Google Scholar]
- Sunderlin L., Aristov N., and Armentrout P. B., J. Am. Chem. Soc. 10.1021/ja00235a013 109, 78 (1987). [DOI] [Google Scholar]
- Sunderlin L. S. and Armentrout P. B., Int. J. Mass Spectrom. Ion Process. 10.1016/0168-1176(89)80064-3 94, 149 (1989). [DOI] [Google Scholar]
- Aristov N. and Armentrout P. B., J. Am. Chem. Soc. 10.1021/ja00268a017 108, 1806 (1986). [DOI] [PubMed] [Google Scholar]
- Fisher E. R., Schultz R. H., and Armentrout P. B., J. Phys. Chem. 10.1021/j100358a027 93, 7382 (1989). [DOI] [Google Scholar]
- Fisher E. R., Sunderlin L. S., and Armentrout P. B., J. Phys. Chem. 10.1021/j100358a026 93, 7375 (1989). [DOI] [Google Scholar]
- Ricca A. and Bauschlicher C. W., Chem. Phys. Lett. 10.1016/0009-2614(95)00971-6 245, 150 (1995). [DOI] [Google Scholar]
- Sunderlin L. S. and Armentrout P. B., J. Am. Chem. Soc. 10.1021/ja00193a015 111, 3845 (1989). [DOI] [Google Scholar]
- Sunderlin L. S. and Armentrout P. B., J. Am. Chem. Soc. 92, 1209 (1988). [Google Scholar]
- Aristov N. and Armentrout P. B., J. Phys. Chem. 10.1021/j100308a024 91, 6178 (1987). [DOI] [Google Scholar]
- Georgiadis R. and Armentrout P. B., Int. J. Mass Spectrom. Ion Process. 10.1016/0168-1176(89)83062-9 89, 227 (1989). [DOI] [Google Scholar]
- Schultz R. H. and Armentrout P. B., Organometallics 10.1021/om00038a049 11, 828 (1992). [DOI] [Google Scholar]
- Haynes C. L. and Armentrout P. B., Organometallics 10.1021/om00020a051 13, 3480 (1994). [DOI] [Google Scholar]
- Clemmer D. E., Aristov N., and Armentrout P. B., J. Phys. Chem. 10.1021/j100105a005 97, 544 (1993). [DOI] [Google Scholar]
- Ricca A. and Bauschlicher C. W., J. Phys. Chem. A 10.1021/jp972531o 101, 8949 (1997). [DOI] [Google Scholar]
- Clemmer D. E., Elkind J. L., Aristov N., and Armentrout P. B., J. Chem. Phys. 10.1063/1.460844 95, 3387 (1991). [DOI] [Google Scholar]
- Fisher E. R., Elkind J. L., Clemmer D. E., Georgiadis R., Loh S. K., Aristov N., Sunderlin L. S., and Armentrout P. B., J. Chem. Phys. 10.1063/1.458906 93, 2676 (1990). [DOI] [Google Scholar]
- Rodgers M. T., Walker B., and Armentrout P. B., Int. J. Mass. Spectrom. 10.1016/S1387-3806(98)14228-8 183, 99 (1999). [DOI] [Google Scholar]
- Nakao Y., Hirao K., and Taketsugu T., J. Chem. Phys. 10.1063/1.1362323 114, 7935 (2001). [DOI] [Google Scholar]
- Armentrout P. B. and Georgiadis R., Polyhedron 10.1016/S0277-5387(00)81783-0 7, 1573 (1988). [DOI] [Google Scholar]
- Carreón-Macedo J.-L. and Harvey J. N., J. Am. Chem. Soc. 10.1021/ja049346q 126, 5789 (2004); [DOI] [PubMed] [Google Scholar]; Carreón-Macedo J.-L. and Harvey J. N., Phys. Chem. Chem. Phys. 10.1039/b513325d 8, 93 (2006); [DOI] [PubMed] [Google Scholar]; Strickland N. and Harvey J. N., J. Phys. Chem. B 10.1021/jp064091j 111, 841 (2007). [DOI] [PubMed] [Google Scholar]
- Deyonker N. J., Peterson K. A., Steyl G., Wilson A. K., and Cundari T. R., J. Phys. Chem. A 10.1021/jp0715023 111, 11269 (2007). [DOI] [PubMed] [Google Scholar]