Abstract
Purpose
To investigate the effects of ranibizumab on retinal morphology in patients with neovascular age-related macular degeneration (AMD) using optical coherence tomography (OCT) quantitative subanalysis.
Methods
Data from 95 patients receiving intravitreal ranibizumab for neovascular AMD were collected. StratusOCT images were analyzed using custom software entitled “OCTOR” which allows precise positioning of pre-specified boundaries on every B-scan. Changes in thickness/volume of the retina, subretinal fluid (SRF), subretinal tissue (SRT), and pigment epithelial detachments (PEDs) at week 1, and at months 1, 3, 6 and 9 post-treatment were calculated.
Results
Total retinal volume reached its nadir at month 1, with an average reduction of 0.43 mm3 (P<0.001). By month 9, this initial change had been reduced to a mean reduction of 0.32 mm3 (P=0.0011). Total SRF volume reached its lowest level by month 1, with an average reduction of 0.24 mm3 (P<0.001). This reduction lessened subsequently, to 0.18 mm3 by month 9. There was an average 0.3 mm3 decrease in total PED volume by month 1 (P<0.001), and this later declined further, to 0.45 mm3 by month 9 (P=0.0014). Total SRT volume was reduced by an average of 0.07 mm3 at month 1 (P=0.0159) and subsequently remained constant.
Conclusions
Although neurosensory retinal edema and SRF, showed an early reduction to nadir following initiation of ranibizumab therapy, the effect on the retina was attenuated over time, suggesting a possible tachyphylaxis. PED volume showed a slower but progressive reduction. Manual quantitative OCT subanalysis may allow a more precise understanding of anatomic outcomes and their correlation with visual acuity.
Keywords: optical coherence tomography, quantitative image analysis, age-related macular degeneration
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in the developed world among people over the age of 50 years.1 The most common form of AMD resulting in severe visual loss is characterized by the development of choroidal neovascularization (CNV).2 Current treatment options for this neovascular form of AMD include thermal laser photocoagulation, photodynamic therapy with verteporfin, pegaptanib (Macugen, OSI-Eyetech, Inc., Melville, NY), ranibizumab (Lucentis, Genentech, Inc., South San Francisco, CA) and the off-label use of agents such as intravitreal bevacizumab (Avastin, Genetech).3-7 The efficacy of these treatments is primarily determined by assessing visual acuity outcomes, however, fluorescein angiography (FA) and optical coherence tomography (OCT) measurements are often used as secondary outcome parameters.8
StratusOCT (Carl Zeiss Meditec, Dublin, CA) software provides automated detection of the inner and outer retinal boundaries and, as a result, is commonly used in clinical trials to provide quantitative information regarding central retinal thickness.8, 9 StratusOCT is also widely used for qualitative assessment to establish the presence of retinal cysts, subretinal fluid (SRF), pigment epithelial detachments (PEDs), and other morphologic characteristics.9 However, many of these additional features, visible on OCT, cannot be quantified by existing StratusOCT software algorithms. Furthermore, the limited quantitative information that is available, is frequently flawed due to inaccurate detection of the inner and outer boundaries of the retina.10-12
In an effort to both improve the accuracy of retinal thickness measurements and to obtain quantitative information regarding other morphologic characteristics, we developed a software tool entitled “OCTOR” that allows the user to draw the boundaries of all structures of interest manually.13 Grading rules and conventions for delineating OCT morphologic features in neovascular AMD, as well as the reproducibility of this approach, have been previously reported.14
Ranibizumab, a recombinant monoclonal antibody fragment designed to neutralize all known active forms of vascular endothelial growth factor A (VEGFA), is the first treatment for neovascular AMD that not only prevented visual acuity loss, but also improved visual acuity, in large proportions of patients in phase III clinical trials.6, 15 To date, clinical trials of ranibizumab have reported StratusOCT-derived measures of central retinal thickess as their only quantitative OCT data.8, 9 In this report, we use OCTOR-generated OCT subanalysis to provide quantitative information regarding the longitudinal effects of intravitreal ranibizumab on the morphology of the retina in patients with neovascular AMD.
Materials and Methods
Data Collection
Ranibizumab was approved for use in CNV secondary to AMD by the U.S. Food and Drug administration on June 30th, 2006. For this retrospective study, data from all patients receiving their initial intravitreal injections of ranibizumab, between July 2006 and September 2007 at the Doheny Eye Institute, were collected and reviewed. Approval for data collection and analysis was obtained from the institutional review board of the University of Southern California. The research adhered to the tenets set forth in the Declaration of Helsinki.
For inclusion in the study, eyes were required to have subfoveal CNV secondary to AMD; StratusOCT imaging performed no more than three weeks prior to their first injection; and StratusOCT imaging on at least one follow-up visit. In cases where a patient received ranibizumab treatment in both eyes, only the first eye injected was included. Patients who received intravitreal ranibizumab injections in other institutions prior to their initial treatment in the Doheny Eye Institute, or as participants in a clinical trial, were excluded. Our analysis was not limited to patients who met a minimum follow-up period. Any patient switched to an alternative treatment for neovascular AMD in the study eye, was excluded from further analysis from that point forward. As this was not a prospective study, the dosing or re-treatment strategy for all patients and visits was not standardized. Patients were treated at the discretion of the physician applying a OCT-guided ranibizumab-retreatment protocol similar to that described in the PrONTO study.9
StratusOCT images were collected at 1 week and 1, 3, 6, and 9 months following initial injection of intravitreal ranibizumab. Images were obtained using the Radial Lines protocol of 6 high-resolution B-scans on a single StratusOCT machine. The Fast Macular Scan protocol was used only when photographers were unable to obtain adequate high-resolution images, most commonly in patients with unstable fixation or poor cooperation. Data for each case were exported to disk using the export feature available in the StratusOCT version 4.0 analysis software.
The number and type of any previous treatments, for CNV secondary to AMD in the study eye, were recorded. The interval between the last treatment and the initial ranibizumab injection was also noted. After the initial treatment, additional injections of ranibizumab were given at the discretion of the treating physician based on response to therapy. The number and timing of these retreatments were recorded for each patient. Other data collected included age and gender, as well as best corrected Snellen visual acuity and angiographic CNV lesion classification at the time of initial intravitreal ranibizumab injection.
Computer-Assisted Grading Software
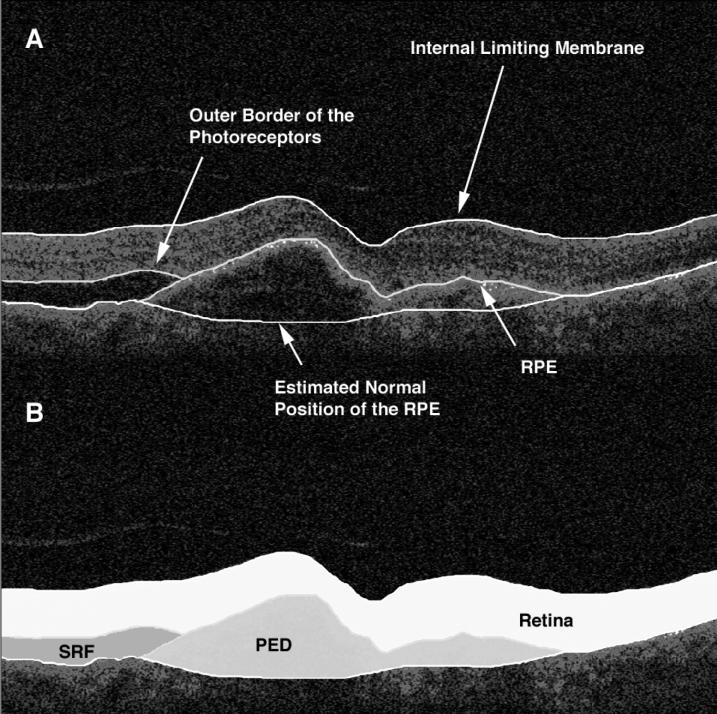
The software program used for OCT analysis (entitled “OCTOR”) was written by Doheny Image Reading Center software engineers to facilitate viewing and manual grading. OCTOR is publicly accessible at http://www.driamd.org and has been described and validated in previous reports.13, 14 This software, which effectively operates as a painting program and calculator, imports data exported from the StratusOCT machine and allows the grader to use a computer mouse to draw various boundaries in the retinal cross-sectional images (Fig. 1).
Figure 1.
Optical coherence tomography B-scan of an eye demonstrating subretinal fluid (SRF) accumulation and pigment epithelial detachment (PED). The clinically relevant boundaries (internal limiting membrane, outer photoreceptor border, retinal pigment epithelium [RPE], and the estimated normal position of the RPE layer [A]) are graded using “OCTOR” (computer-assisted manual grading) software, which then computes the volumes of the spaces (retina, SRF, and PED) defined by these boundaries (B).
After the grader draws the required layers in each of the 6 B-scans, the software calculates the distance in pixels between the manually drawn boundary lines for each of the various defined spaces. Using the dimensions of the B-scan image, the calculated pixels are converted into micrometers to yield a thickness measurement at each location. The thickness at all unsampled locations between the radial lines is then interpolated based on a polar approximation to yield a thickness map analogous to the StratusOCT output data. After interpolation, thickness values are converted into volumes (mm3) by multiplying the average thickness measurement by the sampled area. The interpolation algorithim, intergrader reliability, and intragrader reproducibility have previously been validated.13, 14
Analogous to the StratusOCT software, OCTOR provides a report showing the calculated thickness/volume values for the 9 Early Treatment Diabetic Retinopathy Study macular subfields. The means and standard deviations of the foveal center point thickness are also calculated. In contrast to the StratusOCT output, OCTOR provides separate maps for the various macular spaces (e.g. retina, subretinal fluid, subretinal tissue, pigment epithelial detachment).
Grading Procedure
OCT scans were analyzed by certified OCT graders at the Doheny Image Reading Center who were masked to any related clinical information at the time of grading. All OCT scans included in this study met reading center criteria for sufficient image quality including the absence of significant artifactitious variations in signal intensity or generalized reductions in signal strength. Boundaries drawn in each of the 6 OCT B-scans included the internal limiting membrane, outer border of the photoreceptors, borders of subretinal fluid and subretinal tissue (if present), inner surface of the RPE and estimated normal position of the RPE layer (in cases of RPE elevation). All boundaries were drawn in accordance with the standard OCT grading protocol of the Doheny Image Reading Center.14
After completion of the grading, OCTOR was used to calculate output parameters for the various spaces: retina, subretinal fluid, subretinal tissue, and pigment epithelial detachment. In addition, the combined parameters, inner retinal surface height from the retinal pigment epithelium (IHRPE) and inner retinal surface height from the choroid (IHC), were also calculated.
Statistical Methods
The mean and standard deviation of the foveal center point (FCP) thickness, as well as the total volume (subfields 1-9), were calculated for each space in each case. Volume was measured in cubic millimeters while thickness was measured in micrometers.
The change from baseline in thickness and volume measurements was calculated at each available follow-up visit. To analyze these changes, a paired t test or Wilcoxon signed rank test was performed, depending on whether the data were normally distributed. For each paired statistical test, casewise deletion of missing data was done in case one variable had a missing value. P values < 0.05 were considered statistically significant. Statistical analysis was performed using commercially available software (Intercooled Stata for Windows, Version 9, Statacorp LP, USA).
Results
Patient Enrollment and Follow-Up
140 patients received their initial intravitreal injections of ranibizumab in the Doheny Eye Institute between July 2006 and September 2007. 95 patients, receiving their initial intravitreal injections of ranibizumab, met the inclusion criteria for the study. 45 patients were excluded from the study, for the following reasons: 12 patients had received intravitreal ranibizumab in another institution prior to their initial treatment in the Doheny Eye Institute; 5 patients had received intravitreal ranibizumab as part of a clinical trial prior to their initial treatment in the Doheny Eye Institute; 15 patients were excluded for lack, or unavailability, of StratusOCT imaging within the three week period prior to their initial injection; 6 patients were excluded for lack of StratusOCT imaging at any follow-up visit; 6 patients were excluded for receiving intravitreal injections of ranibizumab for conditions other than AMD; 1 patient's fellow eye was excluded.
10 patients received treatment with intravitreal bevacizumab at some point following their initial treatment with intravitreal ranibizumab, and consequently their subsequent StratusOCT images were excluded from our analysis.
StratusOCT images were available for analysis at follow-up time points as follows: 95 patients at baseline, 15 patients at Week 1; 80 patients at Month 1; 84 patients at Month 3; 58 patients at Month 6; 37 patients at Month 9.
Baseline Characteristics
Of the 95 patients included in our analysis, 59 (62%) were female, while 36 (38%) were male. The mean age of patients was 81 years (standard deviation [SD] = 7), while the median age was also 81 years (range, 55 to 96 years). 43 (46%) patients had undergone prior treatment for CNV in their study eye: 25 (26%) had received previous intravitreal injections of bevacizumab, 15 (16%) had undergone previous photodynamic therapy with verteporfin, 4 (4%) had undergone previous thermal laser photocoagulation, and 9 (9%) had received previous intravitreal pegaptanib. Patients who had previously received intravitreal bevacizumab had a mean of 2.3 injections (range, 1-5 injections), with the last injection occurring a mean of 81 days (range, 19-264 days), before the first ranibizumab injection. Mean visual acuity was 20/129 at baseline, 20/103 at week 1, 20/112 at month 1, 20/115 at month 3, 20/115 at month 6, and 20/126 at month 9. At baseline, the neovascular lesions were categorized by fluorescein angiography as predominantly classic (17 eyes, 18%), minimally classic (15 eyes, 16%) and as occult with no classic (57 eyes, 60%). Angiographic classification at baseline was unavailable for 6 eyes (6%).
Treatment with Ranibizumab
The mean and median number of injections of intravitreal ranibizumab for the study period were 3.3 (SD 2.0) and 3.0 (range, 1-10 injections), respectively. The number of injections between baseline and each study visit, as well as, the time between most recent re-injection and each study visit was recorded and is summarized in Table 1.
Table 1.
Treatment Data
| Month 3 | Month 6 | Month 9 | ||
|---|---|---|---|---|
| SD = standard deviation | ||||
| Number of injections prior to study follow-up | ||||
| Mean ± SD | 1.8 ± 0.6 | 3.1 ± 1.2 | 4.4 ± 1.8 | |
| Median | 2 | 3 | 4 | |
| Range | 1-3 | 1-6 | 1-10 | |
| Time between last re-injection and study follow-up (days) | ||||
| Mean ± SD | 46.9 ± 20.1 | 64.9 ± 42.8 | 84.3 ± 53.3 | |
| Median | 37 | 42 | 77 | |
| Range | 8-105 | 23-168 | 21-245 | |
Morphologic Outcome using OCTOR Analysis
Change in OCT parameters as calculated by OCTOR after manual grading are summarized in Table 2.
Table 2.
Change in Optical Coherence Tomography (OCT) Parameters for the Various Spaces and Subfields, from Baseline, provided by “OCTOR” (Computer-Assisted Optical Coherence Tomography Grading Software), and by Stratus OCT Automated Analysis
| Mean Change from Baseline†: | Week1 | Month 1 | Month 3 | Month 6 | Month 9 | |
|---|---|---|---|---|---|---|
| OCTOR Retina | FCP Thickness (μm) | −55.8 ± 87.89* | −44.83 ± 91.54** | −30.52 ± 94.5** | −30.55 ± 78.11* | −16.95 ± 133.25 |
| Total Volume (mm3) | −0.37 ± 0.53** | −0.43 ± 0.78** | −0.33 ± 0.88** | −0.28 ± 0.63** | −0.32 ± 1.07** | |
| Subretinal Fluid | Total Volume (mm3) | −0.11 ± 0.15** | −0.24 ± 0.50** | −0.24 ± 0.47** | −0.19 ± 0.47** | −0.18 ± 0.48** |
| Subretinal Tissue | Total Volume (mm3) | −0.03 ± 0.15 | −0.07 ± 0.3* | −0.05 ± 0.38 | −0.06 ± 0.34 | −0.06 ± 0.5 |
| Pigment Epithelial Detachment | Total Volume (mm3) | −0.23 ± 0.6 | −0.3 ± 0.87** | −0.18 ± 1.03** | −0.35 ± 0.93** | −0.45 ± 1.12** |
| Height from RPE | FCP Thickness (μm) | −69.27 ± 92.15** | −87.74 ± 95.53** | −67.61 ± 118.08** | −58.28 ± 84.92** | −52.76 ± 142.61** |
| Total Volume (mm3) | −0.51 ± 0.64** | −0.88 ± 1.34** | −0.63 ± 1.19** | −0.54 ± 1.03** | −0.54 ± 1.44** | |
| Height from Choroid | FCP Thickness (μm) | −130.07 ± 143.69** | −90.56 ± 136.76** | −69.52 ± 175.65** | −75.78 ± 130.30** | −68.79 ± 205.51** |
| Total Volume (mm3) | −0.74 ± 0.86** | −1.03 ± 1.57** | −0.8 ±1.87** | −0.86 ± 1.54** | −1.01 ± 2.10** | |
| StratusOCT Retina | FCP Thickness (μm) | −82.47 ± 82.58** | −73.09 ± 93.43** | −59.82 ± 102.32** | −55.38 ± 85.27** | −44.68 ± 131.55* |
| Total Volume (mm3) | −0.60 ± 0.59** | −0.63 ± 1.26** | −0.55 ± 1.43** | −0.47 ± 0.94** | −0.56 ± 1.43** | |
Mean change from baseline calculated by subtracting value at baseline from value at follow-up for each case, and then taking the mean value across all cases.
P<0.05
P<0.01
Effect on the Neurosensory Retina (Fig. 2)
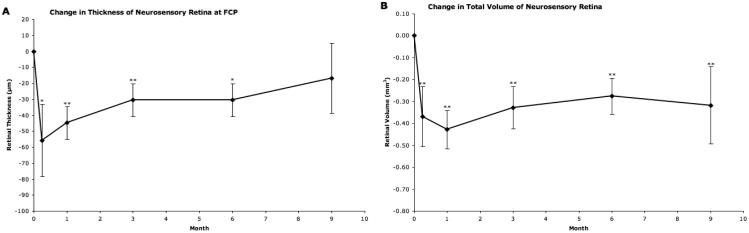
Figure 2.
Neurosensory retina outcomes as provided by “OCTOR” (computer-assisted optical coherence tomography grading software). A, Mean change from baseline in thickness of the neurosensory retina at the foveal center point (FCP). B, Mean change from baseline in total volume of the neurosensory retina. Vertical lines, 1 standard error of the mean. *P<0.05, **P<0.01.
The retinal space showed an average 44.83 μm decrease from baseline in FCP thickness by month 1 (P<0.001). This initial decrease lessened during the remainder of the study, with an average reduction (compared to baseline) of 30.55 μm at month 6 (P=0.0178), and 16.95 μm at month 9 (P=0.2642). The total retinal volume reached its nadir at month 1, with an average reduction of 0.43 mm3 (P<0.001). By month 9, this initial change had been reduced to a mean reduction of 0.32 mm3 (P=0.0011).
Effect on Subretinal Fluid (Fig. 3)
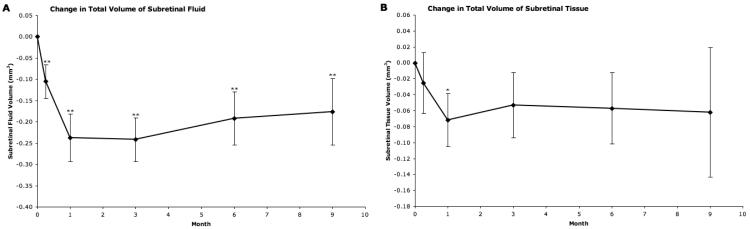
Figure 3.
Subretinal outcomes as provided by “OCTOR” (computer-assisted optical coherence tomography grading software). A, Mean change from baseline in total volume of subretinal fluid. B, Mean change from baseline in total volume of subretinal tissue. Vertical lines, 1 standard error of the mean. *P<0.05, **P<0.01.
The total volume of subretinal fluid reached its lowest level by month 1, with an average reduction from baseline of 0.24 mm3 (P<0.001). This reduction was maintained at month 3, but lessened subsequently, with an average reduction of 0.18 mm3 by month 9.
Effect on Subretinal Tissue (Fig. 3)
The total volume of subretinal tissue was reduced by an average of 0.07 mm3 by month 1 (P=0.0159) and this reduction appeared to be maintained through the remainder of the study period, although subsequent changes compared with baseline were not statistically significant.
Effect on Pigment Epithelial Detachment (Fig. 4)
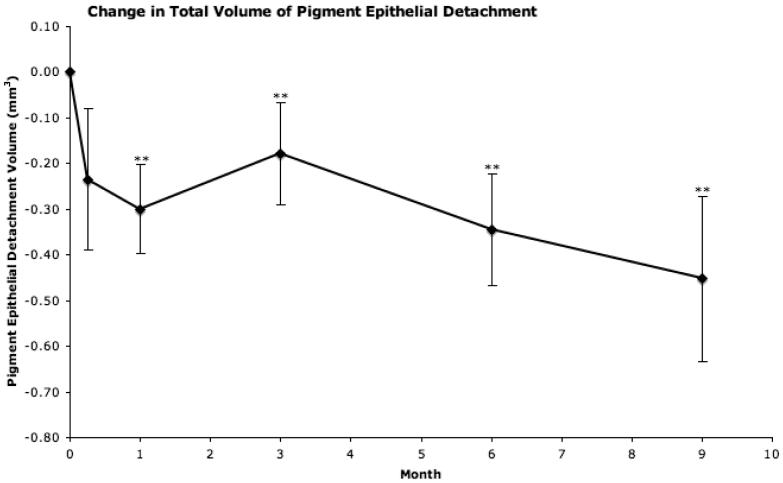
Figure 4.
Mean change from baseline in total volume of pigment epithelial detachment as provided by “OCTOR” (computer-assisted optical coherence tomography grading software). Vertical lines, 1 standard error of the mean. *P<0.05, **P<0.01.
There was an average 0.3 mm3 decrease in total PED volume by month 1 (P<0.001), and this decreased further during the study, to an average of 0.45 mm3 by month 9 (P=0.0014).
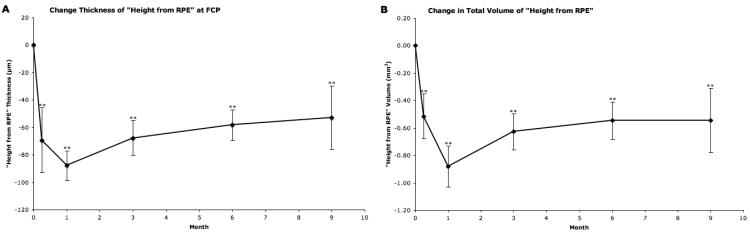
Effect on Inner Retinal Surface Height from the RPE (Fig. 5)
Figure 5.
“Height from RPE” outcomes as provided by “OCTOR” (computer-assisted optical coherence tomography grading software). A, Mean change from baseline in “Height from RPE” at foveal center point (FCP). B, Mean change from baseline in total volume of “Height from RPE”. Vertical lines, 1 standard error of the mean. *P<0.05, **P<0.01.
The inner retinal surface height from the RPE showed an average 87.74 μm decrease from baseline in FCP thickness by month 1 (P<0.001). This initial decrease lessened during the remainder of the study, with an average reduction 52.76 μm at month 9 (P=0.0073). Again, the total macular volume reached its lowest level at month 1, with an average reduction of 0.88 mm3 (P<0.001). By month 9, this initial change had been reduced to a mean level of 0.54 (P<0.001).
Effect on Inner Retinal Surface Height from the Choroid (spanning the distance from the ILM to the base of any PED)
The inner retinal surface height from the choroid at the FCP reached its lowest level by week 1, with an average reduction from baseline of 130.07 μm (P<0.001). This initial decrease lessened during the remainder of the study, with an average reduction of 68.79 μm at month 9 (P=0.0055). In contrast, total volume reached its lowest level at month 1, with an average reduction of 1.03 mm3 (P<0.001). A change of similar magnitude was detected at the study conclusion, with an average reduction of 1.01 mm3 (P<0.001).
Morphologic Outcome Using StratusOCT Analysis
The automated StratusOCT software also provides thickness and volume values. Although these values are said to represent the “retina”, they are often erroneous in CNV cases,12 and typically include the neurosensory retina as well as the subretinal space. The StratusOCT-derived FCP showed an average 73.09 μm decrease from baseline by month 1 (P<0.001). This initial decrease lessened during the remainder of the study, with an average reduction 44.68 μm by month 9 (P=0.0124). The StratusOCT-derived total retinal volume had an average reduction of 0.63 mm3 (P<0.001) at month 1. By month 9, this initial change had been reduced to an average level of 0.56 mm3 (P<0.001).
Discussion
In this retrospective longitudinal study, we performed manual OCT subanalysis with the OCTOR software to evaluate the effects of intravitreal ranibizumab on the morphologic characteristics of the retina in eyes with neovascular AMD. These characteristics were examined over a nine month period and included the neurosensory retina, subretinal fluid, subretinal tissue, and pigment epithelial detachments.
OCTOR analysis of the neurosensory retina demonstrated that there was a large reduction in total retinal volume as early as one week following initial treatment with intravitreal ranibizumab (Fig. 2). Following this initial significant reduction, the total retinal volume reached a nadir at month 1 and then appeared to increase at subsequent follow-up. The large initial reduction in total retinal volume appeared to be secondary to a significant reduction in intraretinal edema which we suspect was mediated by the anti-permeability effects of ranibizumab. Subsequent increases in the total volume of the retinal space appeared to be secondary to the recurrence of intraretinal cysts and/or the development of diffuse retinal edema. One possible explanation for this apparent subsequent increase in total retinal volume, may be a tachyphylaxis phenomenon, where repeated injections yield decreasing anatomic benefits. Another possible explanation is cystoid degeneration of the retina overlying an older or more chronic CNV lesion. This phenonomenon may also be an artifact of the dosing or retreatment strategy used by the physicians in this study. It is possible that a sustained reduction in retinal thickness may have been observed if therapy was administered monthly (rather than based on OCT findings) in accordance with the primary FDA label. OCTOR analysis of the subretinal space demonstrated similar effects. The subretinal space may be occupied by fluid (hyporeflective) or other material (hyperreflective). Our study demonstrated that there is a significant decrease in subretinal fluid by month 1 of follow-up. In fact, the significant decrease in SRF seemed to occur as early as 1 week after initial treatment. The change in SRF at month 1 was maintained through month 3 but appeared to lessen over the ensuing months.
We assigned the generic label “subretinal tissue” to any hyperreflective material in the subretinal space. SRT decreased significantly at month 1, and this reduction was maintained, although not at statistically significant levels, for the remainder of the study. Interpretation of this finding is complicated as hyperreflective material in the subretinal space may include fibrovascular tissue, hemorrhage, lipid, or thick fibrin. Hemorrhage, lipid or thick fibrin are clinically apparent markers of CNV leakage, more commonly seen in acute phases of neovascular AMD.4 Studies of longer duration may provide more reliable information regarding the evolution of fibrovascular scar tissue over time. Further study will also clarify the relationship between scar formation and the loss of neural tissue from the retina, as well as its association with visual function.
Total volume of the PEDs appeared to decrease slowly in the initial months of the study, however, unlike other morphologic parameters, this decrease was sustained and progressive throughout the study period. Previous studies evaluating qualitative OCT changes following anti-VEGF therapy for neovascular AMD have observed that PEDs appear to regress more slowly than subretinal or intraretinal fluid.7, 9 Our study provides quantitative evidence to support these previous observations. Ranibizumab is an antibody fragment designed, in part, to facilitate penetration of the retina. Penetration throughout the retina may initially be reduced in the context of retinal thickening, SRF, and SRT. Subsequent reductions in these parameters, following treatment, may facilitate penetration through the outer layers of the retina and RPE and thus potentially explain the lagging regression of the PED space. PED subtypes include serous, hemorrhagic, fibrovascular, and drusenoid, with each subtype having its own natural history and prognosis for final visual outcome.16 In this study, manual OCT subanalysis did not distinguish between these subtypes, in part due to the limited characterization of the sub-RPE space afforded by StratusOCT.
Our central retinal thickness findings differ from those of other longitudinal studies of intravitreal ranibizumab using automated StratusOCT measurements.8, 9 In these studies, initial significant decreases in retinal thickness were maintained, or progressed, during the remainder of 12 month study periods. Furthermore, these studies demonstrated a substantially greater magnitude of central retinal thickness change, relative to our findings. These discrepancies may occur, in part, due to the fact that StratusOCT software typically combines the subretinal space with the neurosensory retina in thickness calculations. Therefore, true changes in the thickness of the retina (e.g. increasing intraretinal cysts or diffuse retinal edema) may be masked by changes in the thickness of the subretinal space. In addition, the segmentation of these boundaries by the StratusOCT is frequently erroneous in patients with AMD.12 With manual OCT subanalysis, the subretinal space is quantified separately from retinal thickness, and therefore a more accurate measure of retinal thickness is obtained. The OCTOR software also allows calculation of “Height from the RPE”, a measure of retinal thickness which includes any subretinal material and is analogous to the retinal thickness measurements provided by StratusOCT. When we examined “Height from the RPE”, our findings were closer in magnitude to these prior studies, athough still significantly different. The measurement in prior studies may have also been affected by inaccuracies in the Stratus OCT segmentation. Direct comparison is difficult however, as our study did not exclude patients with permanent structural damage to the foveal center; patients with foveal thickness measurements below a minimum level; or patients who had previously received other forms of treatment for neovascular AMD in their study eye.
The aforementioned clinical trials typically utilize StratusOCT-generated FCS or FCP retinal thickness values as their OCT secondary outcome measures. Even in cases of accurate boundary detection, these parameters may be erroneous, due to failure of scans to pass through the anatomical center of the fovea, or due to the presence of eccentrically positioned neovascular lesions. Therefore, we believe that consideration of the total volume of each morphologic space is preferable to the calculation of thickness at a single point or subfield, and this consideration is facilitated by manual grading with OCTOR software.
Our study has a number of limitations. It is a retrospective longitudinal study and as a result, OCT data is not available at every visit for every patient, and follow-up is not uniform. Furthermore, intravitreal ranibizumab treatment by multiple physicians was assessed, with no pre-specified, standardized retreatment criteria. Another limitation is the relatively small sample size, particularly given the heterogenous morphologic features of neovascular AMD lesions. Nonetheless, this study does suggest that quantitative OCT subanalysis may be of value in monitoring the differential morphologic effects of intravitreal ranibizumab treatment for neovascular AMD over time. Preliminary observations suggest an apparent tachyphylaxis in the effect of an anti-VEGF agent on the thickness/volume of the retina, but this requires further study in a prospective trial with standardized re-treatment guidelines. Future studies may allow us to determine how the differential morphologic effects correlate with visual acuity results as well as to determine which subgroups of patients are likely to have specific morphologic outcomes. Due to time limitations, manual grading with OCTOR is unlikely to be performed in clinical practice, although the ongoing development of accurate, automated OCT segmentation algorithms may increase the relevance of these findings. In the interim, manual quantitative OCT subanalysis may be of value in clinical trials, allowing a more precise understanding of anatomic outcomes.
Acknowledgments
Supported in part by NIH Grant EY03040 and NEI Grant R01 EY014375
Drs. Walsh and Sadda are co-inventors of Doheny intellectual property related to optical coherence tomography that has been licensed by Topcon Medical Systems. However, it is not related to the article's subject matter.
References
- 1.Bressler NM, Bressler SB, Congdon NG, et al. Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch Ophthalmol. 2003;121:1621–1624. doi: 10.1001/archopht.121.11.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–495. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 3.Macular Photocoagulation Study Group Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1991;109:1220–1231. doi: 10.1001/archopht.1991.01080090044025. [DOI] [PubMed] [Google Scholar]
- 4.Barbazetto I, Burdan A, Bressler NM, et al. Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin: fluorescein angiographic guidelines for evaluation and treatment--TAP and VIP report No. 2. Arch Ophthalmol. 2003;121:1253–1268. doi: 10.1001/archopht.121.9.1253. [DOI] [PubMed] [Google Scholar]
- 5.Gragoudas ES, Adamis AP, Cunningham ET, Feinsod M, Guyer DR, Group VISiONCT Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 7.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–372. doi: 10.1016/j.ophtha.2005.11.019. e365. [DOI] [PubMed] [Google Scholar]
- 8.Kaiser PK, Blodi BA, Shapiro H, Acharya NR. Angiographic and optical coherence tomographic results of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:1868–1875. doi: 10.1016/j.ophtha.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 9.Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–583. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Hee MR. Artifacts in optical coherence tomography topographic maps. Am J Ophthalmol. 2005;139:154–155. doi: 10.1016/j.ajo.2004.08.066. [DOI] [PubMed] [Google Scholar]
- 11.Ray R, Stinnett SS, Jaffe GJ. Evaluation of image artifact produced by optical coherence tomography of retinal pathology. Am J Ophthalmol. 2005;139:18–29. doi: 10.1016/j.ajo.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 12.Sadda SR, Wu Z, Walsh AC, et al. Errors in retinal thickness measurements obtained by optical coherence tomography. Ophthalmology. 2006;113:285–293. doi: 10.1016/j.ophtha.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Sadda SR, Joeres S, Wu Z, et al. Error correction and quantitative subanalysis of optical coherence tomography data using computer-assisted grading. Invest Ophthalmol Vis Sci. 2007;48:839–848. doi: 10.1167/iovs.06-0554. [DOI] [PubMed] [Google Scholar]
- 14.Joeres S, Tsong JW, Updike PG, et al. Reproducibility of quantitative optical coherence tomography subanalysis in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:4300–4307. doi: 10.1167/iovs.07-0179. [DOI] [PubMed] [Google Scholar]
- 15.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 16.Zayit-Soudry S, Moroz I, Loewenstein A. Retinal pigment epithelial detachment. Survey of ophthalmology. 2007;52:227–243. doi: 10.1016/j.survophthal.2007.02.008. [DOI] [PubMed] [Google Scholar]