Abstract
Drugs of abuse exert biphasic motor activity effects, which seem to be associated with their motivational effects. In the case of ethanol, heterogenous rat strains appear to be particularly sensitive to the sedative effects of the drug. In contrast, ethanol’s activating effects have been consistently reported in rats genetically selected for ethanol affinity. Heightened ethanol affinity and sensitivity to ethanol’s reinforcement are also observed in non-selected rats during early ontogeny. In the present study we examined psychomotor effects of ethanol (1.25 and 2.5 g/kg) in 8, 12 and 15-day-old pups. Motor activity in a novel environment was assessed 5–10 or 15–20 minutes following drug treatment. Rectal temperatures and latency to exhibit the righting reflex were recorded immediately after locomotor activity assessment. Ethanol exerted clear activating effects at 8 and 12 days of age (Experiments 1a and 1b) and to a lesser extent at 15 days. At this age ethanol enhanced locomotor activity in the first testing interval (Experiment 1b) and suppressed locomotion at 15–20 minutes (Experiment 1a). Ethanol-mediated motor impairment was more pronounced in the youngest group (PD 8) than in the older ones. Blood ethanol concentrations were equivalent in all age groups. The present study indicates that preweanling rats are sensitive to ethanol’s stimulating effects during the second postnatal week, and suggest that specific periods during early ontogeny of the rat can provide a valuable framework for the study of mechanisms underlying ethanol’s stimulation and reinforcement effects.
Introduction
Adult heterogeneous rats typically don’t show locomotor activating effects after peripheral ethanol administration (Erickson and Kochhar, 1985; Chuck et al., 2006; Correa et al., 2003; Masur et al., 1986). These activating effects of ethanol are, however, observed in adult rats selectively bred for ethanol affinity. Alcohol-preferring rats show increased locomotor activity after being administered low ethanol doses during adolescence (Rodd et al., 2004) or adulthood (Waller et al, 1986). Ethanol’s activating effects have also been observed in Sardinian alcohol-preferring rats (Agabio et al., 2001; Colombo et al., 1998), University of Chile B rats (Quintanilla, 1999) and Alko-alcohol rats (Paivarinta and Korpi, 1993), which share the characteristic of voluntarily consuming relatively high amounts of ethanol. Yet Sardinian alcohol-nonpreferring, University of Chile A or Alko-nonalcohol rats are not sensitive to ethanol’s psychostimulant effects. As a whole, these findings suggest an association between genetic predisposition to consume ethanol and susceptibility to stimulant effects of the drug.
In contrast with adult rats, preweanling heterogeneous rats are sensitive to biphasic (stimulant and depressant) locomotor effects of ethanol within the course of the state of intoxication. When employing a relatively high ethanol dose (2.5 g/kg) pups show locomotor activation soon after administration of the drug. When blood ethanol concentrations (BECs) reach peak values, ethanol suppresses motor activity (Arias et al., 2008). This time course of ethanol’s activation properties coincides with the observation of biphasic motivational (positive and negative) effects observed in tests of ethanol reinforcement in preweanling rats subjected to relatively high ethanol doses (2 g/kg; Molina et al., 2007): relatively neutral stimuli presented during the rising phase of the blood ethanol curve acquired positive hedonic properties, but stimuli presented 30 minutes following ethanol administration, acquired aversive properties (Molina et al., 2007). The apparent coincidence between biphasic ethanol’s motivational and locomotor effects derived from an ontogenetic analysis suggests that these effects of ethanol may be related mechanistically.
Recent studies consistently indicate that during early stages in development there are important changes in the sensitivity to ethanol’s reinforcing properties and ethanol consumption. Before postnatal day 10 (PD10) pups seem to be more sensitive to ethanol’s rewarding effects and less sensitive to its aversive properties (Arias and Chotro, 2006; Chotro and Arias, 2007; Chotro et al., 2007). Specifically, 7 and 8-day-old rats acquired appetitive conditioned responses to ethanol even when employing a high ethanol dose (3.0 g/kg) that promotes conditioned taste aversion learning in older pups (Arias and Chotro, 2006; Chotro and Arias, 2007). Ethanol ingestion is higher in PDs 8 and 12 than later in the preweanling period or in adulthood (Sanders and Spear, 2007; Truxell and Spear, 2004; Truxell et al., 2007). Finally, Hunt and collaborators reported that 10-day-old pups are less sensitive to the aversive effects of ethanol than 16-day-old pups (Hunt et al., 1991). If ethanol’s motor effects (activation and sedation) are associated with the reinforcement (appetitive and aversive) properties of the drug, it is plausible that sensitivity to the motor effects of ethanol vary as a function of age during this preweanling period. According to the preceding observations, it should be expected that younger rats are more sensitive to ethanol’s activating effects than older preweanling rats.
In the present study we examined sensitivity to ethanol’s locomotor activating effects in a genetically heterogeneous rat strain (Sprague-Dawley) during the second postnatal week of life. In Experiments 1a and 1b, psychomotor effects of moderate-to-high ethanol doses (1.25 and 2.5 g/kg, respectively) were assessed in 8, 12 and 15-day-old pups. Two additional dependent variables representing possible disruptive effects of the state of intoxication (righting reflex and rectal temperatures), were also evaluated. Blood ethanol concentrations derived from these ethanol doses were also assessed for each age group (Experiment 2).
EXPERIMENT 1a
According to a recent study, a relatively high ethanol dose (2.5 g/kg ethanol) induces locomotor stimulant effects in 13-day-old Sprague-Dawley rats when behavioral assessments are conducted soon after ethanol administration (Arias et al., 2008). In the present experiment we examined acute sensitivity to ethanol’s activating effects during the second postnatal week of life. Pups were tested 5–10 or 15–20 minutes after ethanol administration in a novel environment. The reason we selected these two post-administration intervals is to avoid marked sedative effects of ethanol that we observed at a similar age when ethanol in blood reached peak values (30 minutes after ethanol administration; see Arias et al., 2008). We considered that the testing intervals selected would give us a better opportunity to observe differences in sensitivity to ethanol’s stimulating effects across age.
Eight, 12 and 15-day old pups were assessed in terms of locomotor activity after receiving 1.25 or 2.5 g/kg ethanol. A pharmacological control group (pups given only vehicle, i.e., 0.0 g/kg ethanol) was also included in the present experiment. The rationale for including these ages is again that during the second postnatal week of life there are substantial changes in ethanol ingestion and apparent sensitivity to the motivational effects of the drug (Arias and Chotro, 2006; Hunt et al., 1991; Sanders and Spear, 2007).
Immediately after behavioral assessment, rectal temperature and latency to perform the righting reflex were recorded. Temperature is an important factor that can modulate locomotion in preweanling rats (Goodrick, 1975). Warmer ambient temperatures reduce locomotion in the infant rat. The increased locomotor activity observed in preweanling rats tested in isolation may reflect a behavioral response to regulate body temperature (Campbell and Raskin, 1978; Goodrick, 1975). On the other hand, ethanol-induced loss of the righting reflex has been considered an index of motor impairment (Dudek and Phillips, 1990), which obviously may compete with the stimulant effects of ethanol during a locomotor activity test. Since ethanol reduces body temperature and induces loss of the righting reflex, these dependent variables were taken into consideration in the present study. Given age-related differences in sensitivity to stimulant effect of ethanol, we wanted to determine their relationship to the motor impairment or hypothermic effects of ethanol.
Material and Methods
Subjects
Two hundred fifty Sprague-Dawley pups (123 females and 127 males), representative of 45 litters were utilized. Animals were born and reared at the vivarium of the Center for Developmental Psychobiology (Binghamton University, NY) under conditions of constant room temperature (22±1.0 °C), on a 12-hour light 12-hour dark cycle. Births were examined daily and the day of parturition was considered as postnatal day 0 (PD0). All litters were culled to 10 pups (5 females and 5 males whenever possible) within 48 hours after birth. Rats used in these experiments were maintained and treated in accordance with the guidelines for animal care and use established by the National Institutes of Health (1986) and the guidelines indicated by the Institutional Animal Care and Use Committee (IACUC).
Procedures
Ethanol treatment
On PD 8, 12 or 15, pups were separated from their mothers and quasi-randomly assigned to different groups defined by ethanol treatment (0.0, 1.25 or 2.5 g/kg ethanol). Pups from a given litter were distributed across the different drug conditions, and in no case was more than one subject from the same litter and gender assigned to the same group. Pups were placed in a holding maternity cage (45 × 20 × 20 cm) partially filled with clean wood shavings. The floor of the cage was maintained at 33°C (±1°C) through the use of a heating pad.
One hour later, body weights were individually recorded (± 0.01 g) and pups received an intragastric (i.g.) administration of 1.25 or 2.5 g/kg ethanol (volume administered was equivalent to 0.015 ml per gram of body weight of a 10.5 % v/v or 21 % ethanol solution, respectively). Another group of subjects was administered an equivalent volume of water. Intragastric administrations were performed using a 10-cm length of polyethylene tubing (PE-10 Clay Adams, Parsippany, New Jersey) attached to a 1 ml syringe with a 27 G × 1/2 needle. This tubing was gently introduced through the mouth and slowly pushed into the stomach. The entire procedure took less than 20 seconds per pup.
Locomotor activity assessment
Five or fifteen minutes after ethanol administration, locomotor activity was evaluated in a novel environment consisting of a Plexiglas container (10 × 10 × 12 cm). The floor of this environment was lined with absorbent paper. A fresh piece of paper was employed for each animal. A circuit board (2-cm in width) surrounded the four sides of each chamber. This board had six infrared photo emitters and six infrared photoreceptors. The photo beams crossed the chamber generating a matrix of nine cells that allowed measurement of overall amount of activity. Custom-made software developed by W. Kashinsky served to analyze the number of beams crossed by each subject every 10th of a second. Each activity test had a total duration of 5 min and data were divided into 1-min bins. This measure (number of beams broken) was highly and positively correlated with time walking and wall climbing at specific ages considered in the present study under similar experimental conditions (PD8: rxy = 0.89, n=15; PD12: rxy = 0.85, n=15; PD15: rxy = 0.86, n=15, all ps < 0.0001; rxy represents Pearson’s product-moment correlation coefficient).
Rectal temperature and righting reflex assessment
Immediately after the locomotor activity test, pups were placed in a supine position over a smooth surface. Latency to perform the righting reflex was recorded as the time required for the animal to right itself. After this test, rectal temperature was recorded using a Physitemp Temperature Monitor (TH8 Model, Clifton, NJ) equipped with a rectal probe (RET-3, tip diameter: 0.065 in.). This probe was lubricated with mineral oil kept at room temperature and was then inserted 0.5 cm in the rectum. Temperature recordings were obtained 20 s following insertion of the probe.
Design and Data analysis
The factorial design of the present experiment was defined by the following variables: ethanol treatment (0.0, 1.25 or 2.5 g/kg), age (8, 12 or 15 day old) and time of assessment (5–10 or 15–20 minutes after ethanol administration). In this experiment 11 to 16 pups were included in each group. No significant effect of gender or interaction with the remaining factors was found when evaluating the different dependent variables in the present study. Hence, for the inferential analysis and descriptive presentation of the results, data were collapsed across gender.
The dependent variable considered for the analysis of activity was number of beams broken during the 5-minutes test. As mentioned, this variable is highly and significantly correlated with time walking and wall climbing at each specific age considered in this study. However, the spacing of the light beams is the same size regardless the age of testing, and there are important differences in size from ages 8 to 15. In addition, locomotor activity in water-treated controls significantly differed across age. For these reasons activity data were analyzed in two separate ways. First, a 3 (ethanol treatment) × 2 (time of assessment) between-factor ANOVA was conducted for each specific age. Secondly, an additional analysis with standardized scores was conducted. We standardized activity data from ethanol-treated pups, considering as the group of reference the respective water-control group of the same age tested at the same post-administration interval. Standardized scores (z-scores) were calculated by subtracting the mean of the water-control group (same age and testing interval) from the individual score of ethanol-treated pups, and dividing by the standard deviation (of the corresponding control group). Standardized scores provide a distribution identical to the distribution of raw scores and allow direct comparisons between groups of different age. Negative z scores indicate that pups treated with ethanol were less active than their respective water-treated control. Positive z scores reflect more locomotion than controls. This strategy allowed us to separate changes in locomotion related to alcohol effects from age-related differences in locomotor activity.
These z-scores were analyzed by means of a 2 (ethanol treatment: 1.25 or 2.5 g/kg) × 3 (age of testing) × 2 (time of assessment) ANOVA. The same statistical approach was employed for the analysis of the remaining dependent variables included in the study (rectal temperature and latency to perform the righting reflex) since we also observed differences in water-treated controls across age. Significant main effects or interactions indicated by the ANOVAs were further analyzed through post-hoc tests (Duncan’s multiple range test with a Type I error set at 0.05).
Results
Locomotor activity
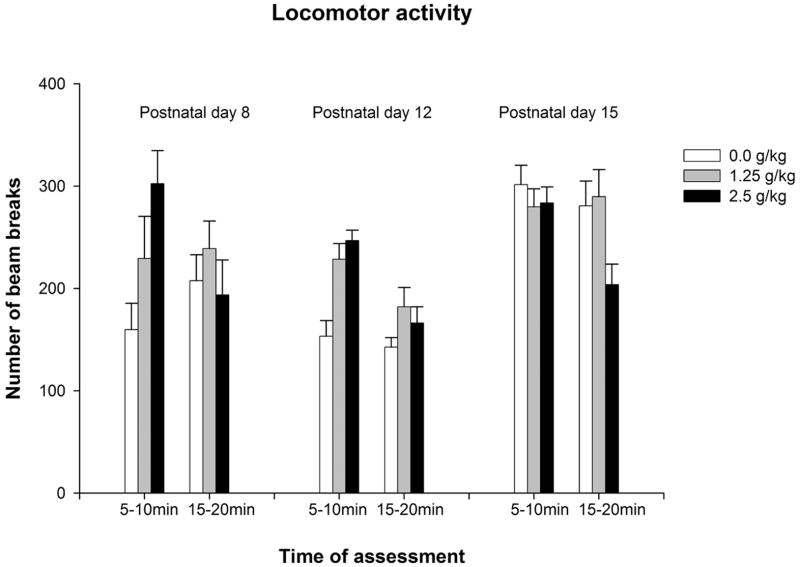
Figure 1a depicts locomotor activity scores as a function of ethanol treatment, time of assessment and age of testing. Ethanol exerted clear stimulant effects in the youngest group (PD8) when the activity test was conducted 5, but not if 15, minutes after ethanol administration. Locomotor activating effects of ethanol were also evident in 12-day-old pups, an effect that was more marked 5 than 15 minutes after drug treatment. In contrast, on PD 15, the stimulant effect of ethanol was no longer observed, and 15 minutes after drug administration ethanol suppressed motor activity. In the youngest group the corresponding ANOVA indicated a significant interaction between ethanol treatment and time of assessment [F(2,75) = 3.35, p < 0.05]. Post-hoc comparisons revealed that pups given 2.5 g/kg showed higher locomotor activity scores than those treated with water at 5–10 minutes. Pups given 1.25 g/kg did not differ from the remaining groups at this post-administration interval.
Figure 1.

Figure 1a: Locomotor activity (number of beams broken) as a function of ethanol treatment (0.0, 1.25 or 2.5 g/kg), post-administration interval of assessment (5–10 or 15–20 min) and age (8, 12 or 15 day-old). Vertical lines illustrate standard errors of the means.
Figure 1b: The z-score activity values as a function of ethanol treatment (1.25 or 2.5 g/kg), post-administration interval of assessment (5–10 or 15–20 min) and age (8, 12 or 15 day-old). Vertical lines indicate standard errors of the means.
At 12 days, the corresponding ANOVA indicated significant main effects of ethanol treatment and time of assessment [F(1,82) = 14.76, p < 0.005, and F(2,82) = 9.79, p < 0.005]. The interaction between these factors almost achieved significance [F(2,82) = 2.77, p = 0.06]. Post-hoc analyses revealed that pups given ethanol (1.25 or 2.5 g/kg) moved more than water-treated controls, an effect driven mainly by the stimulating effect of ethanol at 5–10 minutes after ethanol administration.
The ANOVA conducted with activity data from the oldest group (PD 15) indicated a significant main effect of ethanol treatment [F(2,75) = 3.24, p < 0.05]. The interaction between ethanol treatment and time of assessment was close to significance [F(2,75) = 2.50, p = 0.08]. According to the post-hoc analyses, the higher ethanol dose suppressed motor activity at this age. This effect was mainly observed in the last testing interval (15–20 minutes).
The analysis conducted with the standardized scores revealed significant main effects of age and time of assessment [F(2,157) = 22.08, p < 0.0001 and F(1,157) = 11.03, p < 0.005, respectively]. The interaction ethanol treatment by time of assessment also achieved significance, F(1,157) = 6.84, p < 0.001. Post-hoc comparisons indicated that, regardless the ethanol dose (1.25 or 2.5 g/kg), z-scores from P12 were significantly higher than those from the remaining ages (see Figure 1b). In addition, z-scores from 8-day-old pups were also significantly higher than those from 15-day-old pups, indicating that the ethanol’s activating effect was more marked at 8 and 12 days than at 15 days, when ethanol suppressed locomotor activity. Finally, regardless the age, z-scores from pups given 2.5 g/kg ethanol were higher than those from pups give 1.25 g/kg ethanol when pups were tested 10–15 minutes, but not 5–10 minutes after ethanol administration.
Righting reflex
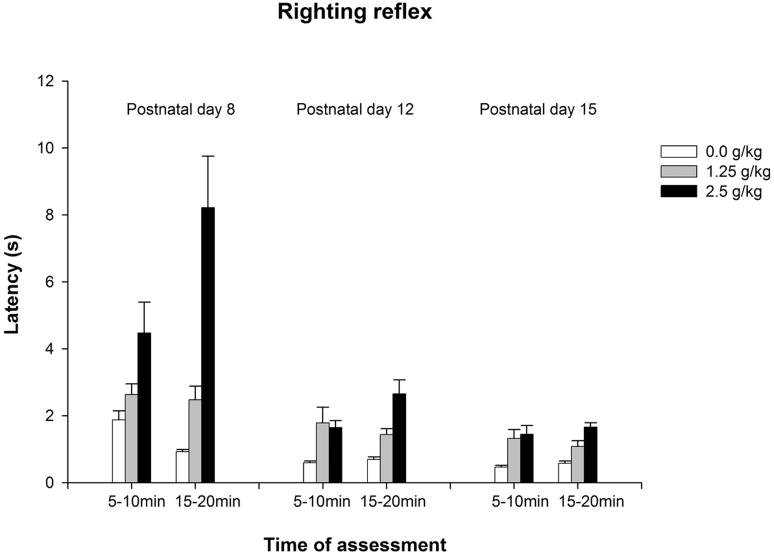
Figure 2a depicts latency to right as a function of age, ethanol treatment and time of evaluation. Motor impairment induced by ethanol was more evident in the younger rats, especially in those given the highest ethanol dose. The corresponding ANOVAs detected significant effects of ethanol treatment at each age [PD8: F(2,75) = 20.18, p < 0.0001; PD12: F(2,82) = 12.86, p < 0.0001; PD15: F(2,75) = 15.16, p < 0.0001]. The interaction ethanol treatment × time of assessment achieved significance only in the youngest group [PD8: F(2,74) = 4.76, p < 0.05]. Post-hoc analyses revealed that at PD8, 5 minutes after ethanol administration the larger ethanol dose (2.5 g/kg) significantly increased latency to right, while twenty minutes after ethanol treatment latencies obtained with 2.5 g/kg were significantly higher than those obtained with the remaining ethanol doses (0.0 and 1.25 g/kg). At PD12 and PD15, pups given ethanol (1.25 or 2.5 g/kg) showed higher latencies than water treated controls.
Figure 2.
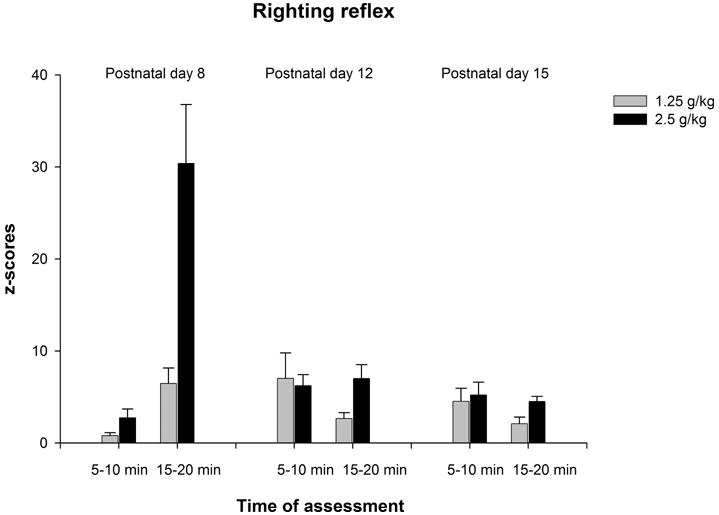
Figure 2a: Latency (seconds) to perform the righting reflex as a function of age (8, 12 or 15 day-old), post-administration time (5–10 or 15–20 min) and ethanol treatment (0.0, 1.25 or 2.5 g/kg ethanol). Vertical lines illustrate standard errors of the means.
Figure 2b: Standardized scores corresponding to latency to perform the righting reflex as a function of age (8, 12 or 15 day-old), post-administration time (5–10 or 15–20 min) and ethanol treatment (1.25 or 2.5 g/kg ethanol). Vertical lines illustrate standard errors of the means.
The ANOVA conducted with z-scores indicated significant main effects of age, F(2,157) = 6.35, p < 0.005, time of assessment, F(1.157) = 10.11, p < 0.005, and ethanol treatment, F(1,157) = 15.09, p < 0.0005. The following interactions also achieved significance: Age by time of assessment, F(1,157) = 18.93, p < 0.0001, age by ethanol treatment, F(2,157) = 7.12, p < 0.005, time of assessment by ethanol condition, F(1,157) = 11.88, p < 0.001, and age by time of assessment by ethanol condition, F(2,157) = 4.87, p < 0.01. Post-hoc analyses indicated that z-scores from 8-day-old pups given 2.5 g/kg and tested 20 minutes after ethanol treatment were significantly higher than those from 8-day-old pups treated with the same ethanol dose but tested 10 minutes after drug treatment. In addition, z-scores from 8-day-old pups given 2.5 g/kg and tested 20 minutes after ethanol treatment also were significantly higher than pups from the remaining groups of age (PD12 and PD15) given the same ethanol treatment and tested in the same post-administration interval (see Figure 2b).
Rectal temperature
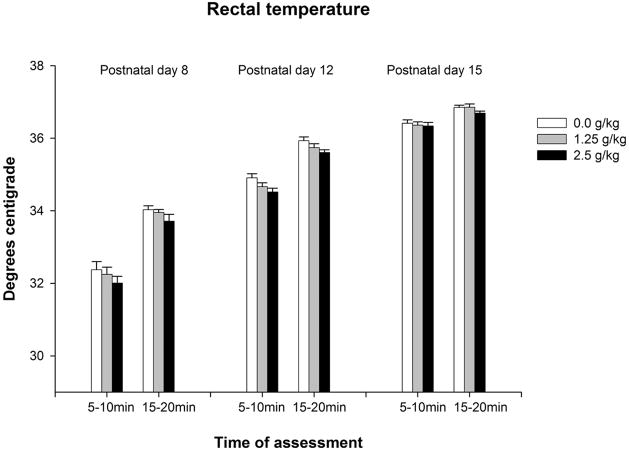
The ANOVAs conducted with rectal temperatures revealed a significant main effect of time of assessment in each age group [PD8: F(1,75) = 146.57, p < 0.0001; PD12: F(1,82) = 163.37, p < 0.0001; PD15: F(1,75) = 36.08, p < 0.0001]. In addition, a significant main effect of ethanol treatment was detected in 12-day-old pups, F(2,82) = 6.04, p < 0.005. The corresponding post-hoc analyses indicated that rectal temperatures were significantly lower 10 minutes than 20 minutes after ethanol administration. Finally, 12-day-old pups given ethanol dose (1.25 or 2.5 g/kg) showed lower temperature than their corresponding water-treated controls (see Figure 3a).
Figure 3.
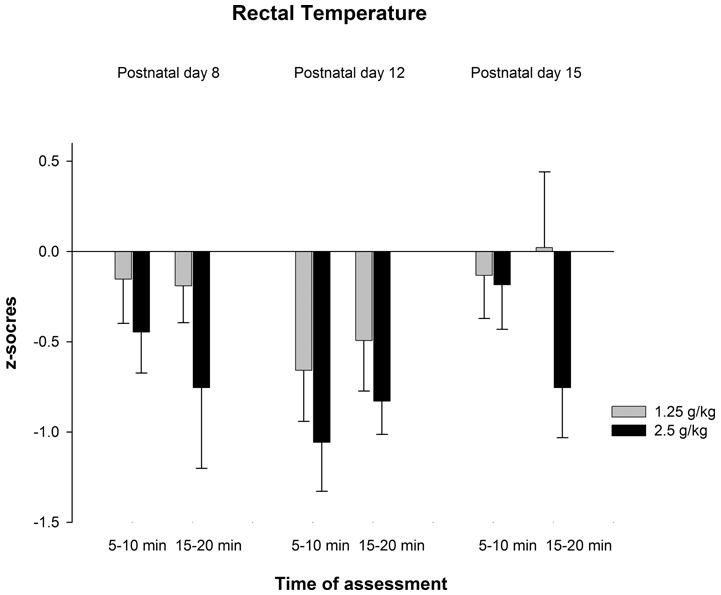
Figure 3a: Rectal temperature in preweanling rats as a function of age (8, 12 or 15 day-old), post-administration time (5–10 or 15–20 min) and ethanol treatment (0.0, 1.25 or 2.5 g/kg ethanol). Vertical lines illustrate standard errors of the means.
Figure 3b: The z-score values corr3esponding to rectal temperature as a function of ethanol treatment (1.25 or 2.5 g/kg), post-administration interval of assessment (5–10 or 15–20 min) and age (8, 12 or 15 day-old). Vertical lines indicate standard errors of the means.
The analysis of the standardized temperature scores indicated significant main effects of ethanol treatment [F(1,157) = 5.87, p < 0.05] and age [F(2,157) = 3.37, p < 0.05]. Post-hoc analyses indicated that z-scores from pups given 2.5 g/kg ethanol were significantly lower than those from pups given 1.25 g/kg ethanol. In addition, z-scores from 12-day-old pups were significantly lower than those from 15-day-old pups (Figure 3b).
In summary, results from the present experiment indicate that during the second postnatal week of life pups are highly sensitive to the ethanol’s stimulating effects, and also that there may be age-related differences in sensitivity to ethanol’s motor effects across age. On PDs 8 and 12, ethanol induced clear behavioral stimulation when compared with their water-treated controls. In contrast, in the oldest age group (PD 15) ethanol suppressed locomotion. Sensitivity to motor impairment induced by ethanol also varied as a function of age, with greater sensitivity in the youngest group (PD 8) than the older ones (PD 12 and PD 15). The hypothermic effects of ethanol were only detected in 12-day-old rats, not at the remaining ages.
EXPERIMENT 1b
One potential limitation of Experiment 1a existed for the index of locomotor activity (number of beams broken). This measure does not provide much information about the quality of the motor effects induced by ethanol in the preweanling rat. As mentioned (see methods section of Experiment 1a) the number of beams broken in the apparatus utilized in Experiment 1a is highly correlated with total time the pups spend wall climbing and walking. But this is not sufficient to conclude that ethanol increases distance traveled in preweanling rats, as it does, for example, in adult mice (Boehm et al., 2008). In addition, the open field utilized in Experiment 1a is relatively small and the 15-day-old rats had more limited space than younger infants in which to demonstrate movement. For that reason we decided to run an additional experiment in an alternative open field to corroborate the observations from Experiment 1a, and also to gain additional information related to the quality of behavior that is enhanced by ethanol at similar ages.
Materials and Methods
Subjects
One hundred and ninety nine Sprague-Dawley pups (91 females and 108 males), representative of 25 litters were utilized. Rearing and housing conditions were similar to those described in Experiment 1a.
Procedures
The design of Experiment 1b and the procedures employed were exactly the same as those described in Experiment 1a. The only difference was the apparatus utilized to measure locomotor activity. The activity test was conducted in a Plexiglas open field (42 × 42 × 30 cms; VersaMax Animal Activity Monitoring System, Accuscan Instruments, Columbus, OH, USA). In this apparatus, locomotion was detected by interruption of eight pairs of intersecting photocell beams evenly spaced along the walls of the testing environment. This equipment was situated in sound-attenuating box chambers (53 × 58 × 43 cms) equipped with a light and fan for ventilation and background noise. Consecutive photocell beam interruptions were translated to distance traveled in cm by the VersaMax. This dependent variable takes into account the path the animal takes, and is a more accurate indicator of ambulatory activity than number of beams broken. Activity data were collected during 5 minutes at the same post-administration intervals (5–10 and 15–20 minutes) as in Experiment 1a.
Design and Data analysis
Activity data were analyzed following the strategy described in Experiment 1a.
Results
Figure 4a represents distance traveled by preweanling rats as a function of ethanol treatment, time of assessment and age. The ANOVA conducted with activity data from 8-and 12-day-old rats indicated in both cases significant main effects of ethanol treatment [PD8: F(2,56) = 11.18, p < 0.0001; PD12: F(2,61) = 11.23, p < 0.0005], and time of assessment [PD: F(1,55) = 4.30, p < 0.05; PD12: F(2,61) = 8.30, p < 0.01]. Post-hoc analyses revealed that at both ages distance traveled by pups given ethanol (1.25 or 2.5 g/kg) was longer than in the water-treated controls. In addition, pups also covered a longer distance in the second post-administration interval than in the first one.
Figure 4.
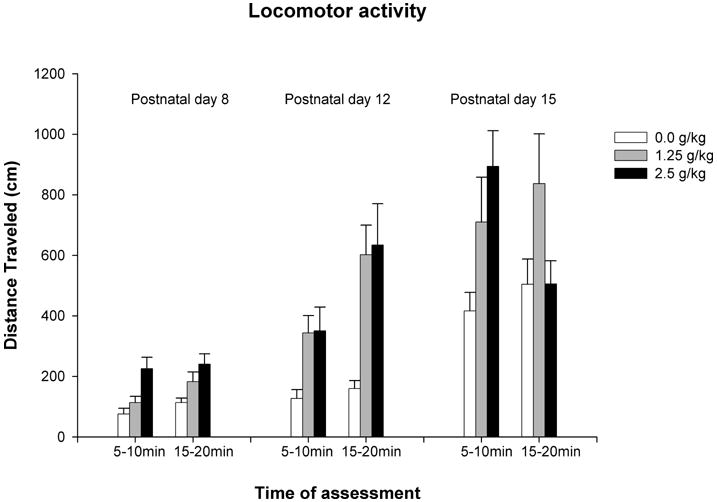
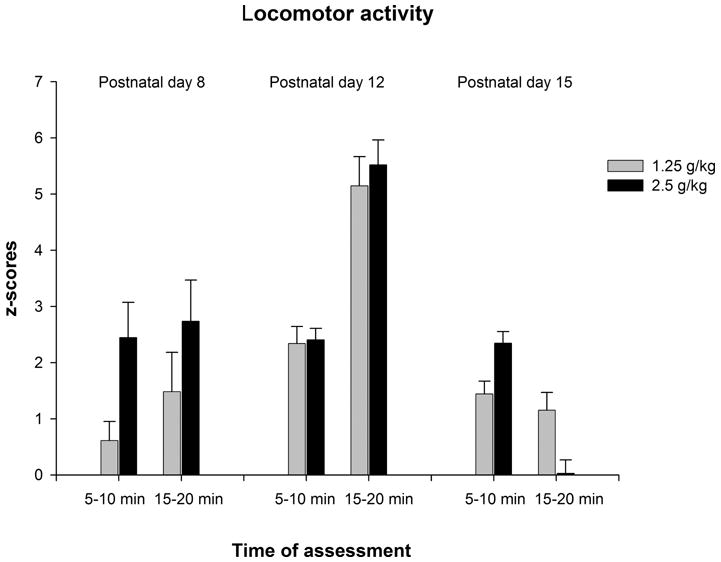
Figure 4a: Distance traveled (cm) during the 5-minutes test as a function of ethanol treatment (0.0, 1.25 or 2.5 g/kg), post-administration interval of assessment (5–10 or 15–20 min) and age (8, 12 or 15 day-old). Vertical lines illustrate standard errors of the means.
Figure 4b: The z-score activity values as a function of ethanol treatment (1.25 or 2.5 g/kg), post-administration interval of assessment (5–10 or 15–20 min) and age (8, 12 or 15 day-old). Vertical lines indicate standard errors of the means.
The ANOVA conducted with data scores obtained from 15-day-old rats indicated a significant main effect of ethanol treatment [F(2,64) = 4.05, p < 0.05] as well as the interaction between ethanol treatment and time of assessment [F(2,64) = 3.19, p < 0.05]. Post-hoc analysis indicated that pups given 2.5 g/kg covered a longer distance than water treated controls when infants were tested 5–10 minutes after ethanol administration, while pups given 1.25 g/kg did it when they were tested 15–20 minutes after ethanol administration. In addition, distance traveled in pups given 2.5 g/kg was longer in the first post-administration interval than in the second one.
The ANOVA conducted with the z-scores revealed a significant main effect of age [F(2,122) = 12.38, p < 0.001] which interacted with time of assessment [F(2,122) = 7.58, p < 0.001]. Post-hoc analyses revealed that in the second period of assessment (15–20 min) z-scores from 12-day-old rats were significantly higher than those from the remaining groups, and that z-scores from 8-day-old rats were also higher than those at 15 days of age (see Figure 4b).
Results from Experiment 1b corroborated that preweanling rats are highly sensitive to the stimulating effect of ethanol, increased the generality of this effect by testing in a different apparatus, and indicated that ethanol-induced stimulation can be expressed during infancy in terms of distance traveled. In addition, according to the analysis of the standardized values, this effect seems to be more marked in 8- and 12-day-old rats than in 15-day-olds, which is congruent with what was observed in Experiment 1a. However, in Experiment 1b the oldest group showed ethanol-mediated behavioral stimulation in the first testing interval, and also unlike Experiment 1a, we found no motor suppressive effects of ethanol in the last period of evaluation.
EXPERIMENT 2
Experiment 2 was conducted to determine BECs in 8, 12 and 15 day-old pups resulting from the intragastric administration of 1.25 or 2.5 g/kg ethanol. Blood ethanol levels were determined at 7.5 or 17.5 min post-administration time, time points that coincide with the middle point of the activity tests conducted in Experiments 1a and 1b. The goal of the experiment was to determine if age-related differences in ethanol’s locomotor and motor impairment effects observed in Experiment 1 could be explained by age-related differences in BECs.
Material and Methods
Subjects
Ninety-five Sprague-Dawley pups (44 females and 51 males) representative of 29 litters were utilized. Rearing and housing conditions were similar to those described in Experiment 1a.
Procedures
On PD 8, 12 or 15 pups were separated from their mothers and assigned to a given ethanol treatment (i.g. administration of either 1.25 or 2.5 g/kg ethanol). Ethanol was administered following exactly the procedures employed in Experiment 1a.
Pups were sacrificed 7.5 or 17.5 minutes after receiving the corresponding ethanol dose. Trunk blood was obtained following decapitation. Blood samples were collected using a heparinized capillary tube. They were immediately centrifuged (6.000 rpm; Micro-Haematocrit Centrifuge, Hawksley & Sons LTD, Sussex, England) and stored at −70 °C. BECs were determined using an AM1 Alcohol Analyzer (Analox Instruments, Lunenburg, MA). Calculation of BECs was made by oxidizing ethanol to acetaldehyde in the presence of ethanol oxidase. The apparatus measures the rate of oxygen required by this process, which is proportional to ethanol concentration. BECs were expressed as milligrams of ethanol per deciliter of body fluid (mg/dl = mg%).
Data analysis
Blood ethanol concentration was analyzed by means of a 2 (ethanol treatment: 1.25 or 2.5 g/kg) × 3 (age of testing: PD 8, PD 12 or PD15) × 2 (time of assessment: 7.5 or 17.5 min) between-factor ANOVA. Significant main effects or interactions were further analyzed through post-hoc tests (Duncan’s multiple range test with a Type I error set at 0.05).
Results
The ANOVA revealed significant effects of the main factors ethanol treatment, F(1, 83) = 1168.69, p < 0.0001, and time of assessment, F(1, 83) = 556.83, p < 0.0001. As could be expected, the 2.5 g/kg ethanol dose generated significantly higher BECs than the 1.25 g/kg ethanol dose. This factor did not interact with any of the remaining variables included in the analysis. In addition, BECs were significantly higher at 17.5 minutes than at 7.5 minutes after ethanol administration (see Table 1).
Table 1.
Blood ethanol concentration (mg %) as a function of ethanol treatment (1.25 or 2.5 g/kg), post-administration time (7.5 or 17.5 min) and age (8, 12 or 15 day-old). Values represent mean ± standard errors of the means.
| PD 8 | PD 12 | PD 15 | ||||
|---|---|---|---|---|---|---|
| 7.5 min | 17.5 min | 7.5 min | 17.5 min | 7.5 min | 17.5 min | |
| 1.25 g/kg | 49.36 ± 1.02 | 114.66 ± 4.17 | 50.18 ± 1.85 | 121.51 ± 3.38 | 55.95 ± 0.88 | 122.98 ± 2.30 |
| 2.5 g/kg | 90.05 ± 3.75 | 212.74 ± 8.99 | 85.73 ± 3.26 | 227.51 ± 9.32 | 91.72 ± 3.32 | 215.41 ± 8.03 |
According to the present experiment, blood ethanol levels were equivalent across age at both post-administration times (7.5 and 17.5 minutes). Age-related differences in the acute effects of ethanol observed in Experiment 1 cannot be explained in terms of age-related differences in ethanol absorption and metabolism.
Discussion
In the present study ethanol exerted locomotor stimulant effects in 8- and 12-day old pups. At these ages, both ethanol doses (1.25 and 2.5 g/kg ethanol) increased locomotor activity. In contrast, this activating effect of ethanol was not consistently observed on PD 15. At this age ethanol significantly decreased motor activity in Experiment 1a, and only increased locomotor activity in the first post-administration time in Experiment 1b (but with lesser magnitude than in younger infants). This difference across experiments in the motor effects induced by ethanol in 15-day-old rats may be due to differences in the testing chambers employed, since locomotor activity patterns induced by various drugs can be affected by the spatial structure of the testing environment (Eilam et al., 2003; Paulus and Geyer, 1997). In addition, in Experiment 1a and 1b we used subjects from different litters, and we did observe important variations across litters in the sensitivity to this effect of ethanol. Also, the dependent variable analyzed in Experiment 1a (number of photo beams broken) was different than in Experiment 1b (distance traveled), which may also contribute to the differences across experiments. Despite these differences, the overall ontogenetic profile observed in both experiments indicates a greater sensitivity to ethanol-mediated stimulation in 8- and 12-day-old infants than in 15-day-olds. This differential sensitivity to ethanol’s activating effects across age cannot be explained by age-related differences in the pharmacokinetic profile of the drug. According to results obtained in Experiment 2, blood ethanol levels did not differ across age.
Previous studies from our laboratory suggest that during the second postnatal week of life the younger the infant the less sensitive it is to the aversive effects of ethanol (Hunt et al., 1991; Arias et al., 2006). In addition, younger rats seem to be more predisposed to display heightened ethanol intake patterns and to acquire conditioned acceptance of ethanol (Arias et al., 2006; Sanders and Spear, 2007; Truxell and Spear, 2004; Truxell et al., 2007). In other words, the ontogenetic profile associated with the motivational properties of ethanol seems to coincide with the ontogenetic profile of ethanol’s capability to induce the stimulation effects observed in the present study.
In Experiment 1a ethanol-mediated hypothermia was observed only in 12-day-old rats. It was expected that hypothermic effects of ethanol would be more marked in 15-day-old pups given 2.5 g/kg ethanol and tested in the later post-administration interval, because at this age these conditions reduced locomotor activity (at least in Experiment 1a). The implication is that at these ages, hypothermia and locomotor suppressive effects are relatively independent measures, since ethanol’s activating effects seem to be more marked in 12 than in 15 day old rats. Other studies have reported disassociation between ethanol-induced motor suppressive and hypothermic effects. For example, Pohorecky et al. (1981) found that 2 g/kg ethanol reduced body temperature in adult rats kept at room temperature (22 degrees), but not in rats maintained at 35 degrees. In contrast, motor activity was depressed by ethanol more in animals maintained at higher temperature (35 degrees) than in those kept at room temperature. In this study swim performance was impaired more in animals not showing hypothermia (Pohorecky and Rizek, 1981). In addition, in our study it seems that intragastric procedures alone reduced rectal temperatures, since they were higher in all pups tested 20 minutes than in those tested 10 minutes after ethanol administration, even in water controls. This effect could be masking a greater hypothermic effect of ethanol. Finally, Hunt et al. (1991) reported that in animals kept at room temperature (21 degrees) ethanol-induced hypothermia seems to be more marked at PD10 than at PD 16. In our experiment, also conducted at room temperature, we detected an effect of ethanol upon rectal temperatures in 12-day-old, but not in 15-day-old, rats. However, we did not observe ethanol-induced hypothermia in 8-day-old rats. The lack of effect in the youngest group may be due to the fact that, at this age, pups were relatively unable to regulate their body temperature when kept at room temperature during the activity test, which in turn may preclude observation of a specific effect of ethanol upon body temperature.
The ontogenetic differences observed in locomotor activity as a function of ethanol treatment cannot be explained by differential motor impairment effects induced by ethanol across age. The youngest rats (PD 8) had higher levels of ethanol-mediated locomotor activation than the oldest rats (PD 15), yet ethanol induced greater motor impairment at PD8 than at PD15. These results show a clear dissociation between ethanol’s effects on stimulation and motor impairment during the preweanling period, suggesting that different mechanisms underlie these effects of ethanol. Motor impairment induced by ethanol seems to be mediated by GABA(A) receptors from the cerebellar granule cells (Hanchar et al. 2005; Carta et al., 2004) whereas locomotor activating effects of ethanol have been mainly associated with the mesocorticolimbic dopaminergic pathway (e.g. Boehm et al., 2002).
In the present study we did not observe gender differences in response to ethanol. However we have to take with caution the lack of interaction between sex and the remaining independent variables, considering that the number of males or females (between 5 and 10) included in each group may not be sufficient to detect them statistically. There is little basis on which to expect sex differences in response to ethanol at the age tested in the present experiments, but the number of previous tests is limited and further research will be required to assess this issue conclusively in preweanling rats. Other studies conducted with adult rats (Maudsley reactive rats) observed greater ethanol-induced locomotor stimulation in female than in male subjects (Erickson et al., 1985).
As mentioned, adult heterogeneous rats do not typically show increased locomotion after being administered with ethanol. What seems to predominate in these strains instead is dose-response suppression of locomotion (Chuck et al., 2006; Correa et al., 2003; Masur et al., 1986). The differential sensitivity to this effect of ethanol in infants when compared to adult rats may be due to important ontogenetic differences in metabolic and neurochemical systems associated with locomotion and ethanol’s activating and motivational effects. During the ontogenetic period selected for this study locomotion develops rapidly. On PD8 rats are able to stand on all fours and to take a few steps, but fully mature locomotor patterns appear a few days later (on about PD 15; Altman et al., 1975; Ba and Seri, 1995; Gramsbergen, 1998). This rapid development has been associated with maturation of spinal circuitry and mesencephalic structures that exert control of locomotion (Ba and Seri, 1995). According to our results the larger stimulating effects of ethanol were observed at those ages in which this function (locomotion) was relatively immature.
A variety of receptors involved in ethanol’s activating effects, such as opioid (Pastor and Aragon, 2006; Pastor et al, 2005; Pastor et al., 2005), GABA B (Bohem et al., 2002), or D2 (Pastor et al., 2005; Phillips et al., 1998; Wood et al, 1999), undergo significant changes in number, relative density and even function during the second postnatal week of life (Georges et al., 1998; Spain et al., 1985; Tarazi and Baldessarini, 2000; Turgeon and Albin, 1994). Another important difference between young and adult rats that can explain the differential sensitivity to ethanol’s activating effects is related to central and peripheral ethanol metabolism. Brain catalase activity in rats progressive falls from gestational day 19 to adulthood. About 50% of the activity of this enzyme decreases during the first two weeks of life (Del Maestro and McDonald, 1987). The catalase system seems to be involved in ethanol’s stimulating and motivational effects (Escarabajal et al., 2000; Nizhnikov et al., 2007). Peripheral ethanol metabolism is faster in older than younger preweanling rats (Hollstedt et al., 1980; Kelly et al., 1987). Peripheral ethanol metabolites, such as acetate, seem to modulate partially the sedative effects of ethanol (Carmichael et al., 1991; McLaughlin et al., 2008). Since peripheral ethanol metabolism is progressively increasing during the preweanling period and into adolescence, it is plausible that the sedative effects of ethanol are more marked in adult rats than in preweanlings, and these effects could be masking the stimulating effects of ethanol. This hypothesis is congruent with the fact that central ethanol administration can induce stimulating effects in adult heterogeneous rats (Correa et al., 2003). Hence, characteristics of the central and hepatic metabolic systems during the preweanling period of the rat may help to explain changes in sensitivity to ethanol’s activating effects during this ontogenetic period. Future research will be needed to analyze the specific weight of these neurochemical and metabolic systems, or interactions between them, upon locomotor and motivational effects of ethanol in preweanling heterogeneous rats.
Acknowledgments
This work was supported by Supported by grants from the NIAAA (AA11960, AA013098, AA015992) and the NIMH (MH035219) to NES and the Agencia Nacional de Promocion Cientifica y Tecnologica (PICT 05-14024) to JCM, Postdoctoral fellowship from Ministerio de Educacion y Ciencia from Spain to CA, as well as Fundacion Antorchas, Argentina, CONICET (PIP 6485) and FONCyT (PICT 05-38084) to E.C.M (this study has been conducted during the period corresponding to the Doctorate Program in Biological Sciences, Cordoba University). The authors wish to express their gratitude to Teri Tanehaus and Heather Murphy for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agabio R, Carai MA, Lobina C, Pani M, Reali R, Vacca G, et al. Alcohol stimulates motor activity in selectively bred Sardinian alcohol-preferring (sP), but not in Sardinian alcohol-nonpreferring (sNP), rats. Alcohol. 2001;23(2):123–126. doi: 10.1016/s0741-8329(00)00144-0. [DOI] [PubMed] [Google Scholar]
- Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Anim Behav. 2006;23(4):896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Ethanol-induced preferences or aversions as a function of age in preweanling rats. Behav Neurosci. 2006;120(3):710–718. doi: 10.1037/0735-7044.120.3.710. [DOI] [PubMed] [Google Scholar]
- Arias C, Molina JC, Mlewski EC, Pautassi RM, Spear N. Acute sensitivity and acute tolerance to ethanol in preweanling rats with or without prenatal experience with the drug. Pharmacol Biochem Behav. 2008;89(4):608–622. doi: 10.1016/j.pbb.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bâ A, Seri BV. Psychomotor functions in developing rats: ontogenetic approach to structure-function relationships. Neurosci Biobehav Rev. 1995;19(3):413–425. doi: 10.1016/0149-7634(94)00042-y. [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Goldfarb KJ, Serio KM, Moore EM, Linsenbardt DN. Does context influence the duration of locomotor sensitization to ethanol in female DBA/2J mice? Psychopharmacology (Berl) 2008;197(2):191–201. doi: 10.1007/s00213-007-1022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Piercy MM, Bergstrom HC, Phillips TJ. Ventral tegmental area region governs GABA(B) receptor modulation of ethanol-stimulated activity in mice. Neuroscience. 2002;115(1):185–200. doi: 10.1016/s0306-4522(02)00378-0. [DOI] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24(15):3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BA, Raskin LA. Ontogeny of behavioral arousal: the role of environmental stimuli. J Comp Physiol Psychol. 1978;92(1):176–184. doi: 10.1037/h0077423. [DOI] [PubMed] [Google Scholar]
- Carmichael FJ, Israel Y, Crawford M, Minhas K, Saldivia V, Sandrin S, et al. Central nervous system effects of acetate: contribution to the central effects of ethanol. J Pharmacol Exp Ther. 1991;259(1):403–408. [PubMed] [Google Scholar]
- Chotro MG, Arias C. Ontogenetic difference in ethanol reinforcing properties: the role of the opioid system. Behav Pharmacol. 2007;18(7):661–666. doi: 10.1097/FBP.0b013e3282f00754. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C, Laviola G. Increased ethanol intake after prenatal ethanol exposure: studies with animals. Neurosci Biobehav Rev. 2007;31(2):181–191. doi: 10.1016/j.neubiorev.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Chuck TL, McLaughlin PJ, Arizzi-LaFrance MN, Salamone JD, Correa M. Comparison between multiple behavioral effects of peripheral ethanol administration in rats: sedation, ataxia, and bradykinesia. Life Sci. 2006;79(2):154–161. doi: 10.1016/j.lfs.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Vacca G, Gessa GL. Stimulation of locomotor activity by voluntarily consumed ethanol in Sardinian alcohol-preferring rats. Eur J Pharmacol. 1998;357(2–3):109–113. doi: 10.1016/s0014-2999(98)00560-3. [DOI] [PubMed] [Google Scholar]
- Correa M, Arizzi MN, Betz A, Mingote S, Salamone JD. Open field locomotor effects in rats after intraventricular injections of ethanol and the ethanol metabolites acetaldehyde and acetate. Brain Res Bull. 2003;62(3):197–202. doi: 10.1016/j.brainresbull.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Del Maestro R, McDonald W. Distribution of superoxide dismutase, glutathione peroxidase and catalase in developing rat brain. Mech Ageing Dev. 1987;41(1–2):29–38. doi: 10.1016/0047-6374(87)90051-0. [DOI] [PubMed] [Google Scholar]
- Dudek BC, Phillips TJ. Distinctions among sedative, disinhibitory, and ataxic properties of ethanol in inbred and selectively bred mice. Psychopharmacology (Berl) 1990;101(1):93–99. doi: 10.1007/BF02253724. [DOI] [PubMed] [Google Scholar]
- Eilam D, Dank M, Maurer R. Voles scale locomotion to the size of the open-field by adjusting the distance between stops: a possible link to path integration. Behav Brain Res. 2003;141(1):73–81. doi: 10.1016/s0166-4328(02)00322-4. [DOI] [PubMed] [Google Scholar]
- Erickson CK, Kochhar A. An animal model for low dose ethanol-induced locomotor stimulation: behavioral characteristics. Alcohol Clin Exp Res. 1985;9(4):310–314. doi: 10.1111/j.1530-0277.1985.tb05550.x. [DOI] [PubMed] [Google Scholar]
- Escarabajal D, Miquel M, Aragon CM. A psychopharmacological study of the relationship between brain catalase activity and ethanol-induced locomotor activity in mice. J Stud Alcohol. 2000;61(4):493–498. doi: 10.15288/jsa.2000.61.493. [DOI] [PubMed] [Google Scholar]
- Gramsbergen A. Posture and locomotion in the rat: independent or interdependent development? Neurosci. Biobehav Rev. 1998;22(4):547–53. [PubMed] [Google Scholar]
- Georges F, Normand E, Bloch B, Le Moine C. Opioid receptor gene expression in the rat brain during ontogeny, with special reference to the mesostriatal system: an in situ hybridization study. Brain Res Dev Brain Res. 1998;109(2):187–199. doi: 10.1016/s0165-3806(98)00082-0. [DOI] [PubMed] [Google Scholar]
- Goodrick CL. Adaptation to novel environments by the rat: effects of age, stimulus intensity, group testing, and temperature. Dev Psychobiol. 1975;8(4):287–296. doi: 10.1002/dev.420080402. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat Neurosci. 2005;8(3):339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstedt C, Rydberg U, Olsson O, Buijten J. Effects of ethanol on the developing rat. I. Ethanol metabolism and effects on lactate, pyruvate, and glucose concentrations. Med Biol. 1980;58(3):158–163. [PubMed] [Google Scholar]
- Hunt PS, Spear LP, Spear NE. An ontogenetic comparison of ethanol-mediated taste aversion learning and ethanol-induced hypothermia in preweanling rats. Behav Neurosci. 1991;105(6):971–983. doi: 10.1037//0735-7044.105.6.971. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Bonthius DJ, West JR. Developmental changes in alcohol pharmacokinetics in rats. Alcohol Clin Exp Res. 1987;11(3):281–286. doi: 10.1111/j.1530-0277.1987.tb01308.x. [DOI] [PubMed] [Google Scholar]
- Masur J, Oliveira de Souza ML, Zwicker AP. The excitatory effect of ethanol: absence in rats, no tolerance and increased sensitivity in mice. Pharmacol Biochem Behav. 1986;24(5):1225–1228. doi: 10.1016/0091-3057(86)90175-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Chuck TL, Arizzi-LaFrance MN, Salamone JD, Correa M. Central vs. peripheral administration of ethanol, acetaldehyde and acetate in rats: effects on lever pressing and response initiation. Pharmacol Biochem Behav. 2008;89(3):304–313. doi: 10.1016/j.pbb.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear N. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41(1):41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizhnikov ME, Molina JC, Spear NE. Central reinforcing effects of ethanol are blocked by catalase inhibition. Alcohol. 2007;41(7):525–534. doi: 10.1016/j.alcohol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals (DHEW Publication No. 86–23) Washington, DC: U.S. Government Printing Office; 1986. [Google Scholar]
- Paivarinta P, Korpi ER. Voluntary ethanol drinking increases locomotor activity in alcohol-preferring AA rats. Pharmacol Biochem Behav. 1993;44(1):127–132. doi: 10.1016/0091-3057(93)90289-6. [DOI] [PubMed] [Google Scholar]
- Pastor R, Aragon CM. The role of opioid receptor subtypes in the development of behavioral sensitization to ethanol. Neuropsychopharmacology. 2006;31(7):1489–1499. doi: 10.1038/sj.npp.1300928. [DOI] [PubMed] [Google Scholar]
- Pastor R, Miquel M, Aragon CM. Habituation to test procedure modulates the involvement of dopamine D2- but not D1-receptors in ethanol-induced locomotor stimulation in mice. Psychopharmacology (Berl) 2005;182(3):436–446. doi: 10.1007/s00213-005-0115-3. [DOI] [PubMed] [Google Scholar]
- Pastor R, Sanchis-Segura C, Aragon CM. Effect of selective antagonism of mu(1)-, mu(1/2)-, mu(3)-, and delta-opioid receptors on the locomotor-stimulating actions of ethanol. Drug Alcohol Depend. 2005;78(3):289–295. doi: 10.1016/j.drugalcdep.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. Environment and unconditioned motor behavior: influences of drugs and environmental geometry on behavioral organization in rats. Psychobiology. 1997;25:327–337. [Google Scholar]
- Phillips TJ, Brown KJ, Burkhart-Kasch S, Wenger CD, Kelly MA, Rubinstein M, et al. Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat Neurosci. 1998;1(7):610–615. doi: 10.1038/2843. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Rizek AE. Biochemical and behavioral effects of acute ethanol in rats at different environmental temperatures. Psychopharmacology (Berl) 1981;72(2):205–209. doi: 10.1007/BF00431658. [DOI] [PubMed] [Google Scholar]
- Quintanilla ME. Effect of low doses of ethanol on spontaneous locomotor activity in UChB and UChA rats. Addiction Biol. 1999;4(4):443–448. doi: 10.1080/13556219971434. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McKinzie DL, Webster AA, Murphy JM, Lumeng L, et al. Low-dose stimulatory effects of ethanol during adolescence in rat lines selectively bred for high alcohol intake. Alcohol Clin Exp Res. 2004;28(4):535–543. doi: 10.1097/01.alc.0000122107.08417.d0. [DOI] [PubMed] [Google Scholar]
- Sanders S, Spear NE. Ethanol acceptance is high during early infancy and becomes still higher after previous ethanol ingestion. Alcohol Clin Exp Res. 2007;31(7):1148–1158. doi: 10.1111/j.1530-0277.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- Spain JW, Roth BL, Coscia CJ. Differential ontogeny of multiple opioid receptors (mu, delta, and kappa) J Neurosci. 1985;5(3):584–588. doi: 10.1523/JNEUROSCI.05-03-00584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci. 2000;18(1):29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Truxell EM, Spear NE. Immediate acceptance of ethanol in infant rats: ontogenetic differences with moderate but not high ethanol concentration. Alcohol Clin Exp Res. 2004;28(8):1200–1211. doi: 10.1097/01.alc.0000134220.34842.18. [DOI] [PubMed] [Google Scholar]
- Truxell EM, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res. 2007;31(5):755–765. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Turgeon SM, Albin RL. Postnatal ontogeny of GABAB binding in rat brain. Neuroscience. 1994;62(2):601–613. doi: 10.1016/0306-4522(94)90392-1. [DOI] [PubMed] [Google Scholar]
- Waller MB, Murphy JM, McBride WJ, Lumeng L, Li TK. Effect of low dose ethanol on spontaneous motor activity in alcohol-preferring and -nonpreferring lines of rats. Pharmacol Biochem Behav. 1986;24(3):617–623. doi: 10.1016/0091-3057(86)90567-8. [DOI] [PubMed] [Google Scholar]
- Wood RD, Shen EH, Chester JA, Phillips TJ. Ontogeny of ethanol-induced locomotor activity and hypothermia differences in selectively bred FAST and SLOW mice. Pharmacol Biochem Behav. 1999;62(2):339–347. doi: 10.1016/s0091-3057(98)00158-0. [DOI] [PubMed] [Google Scholar]