Abstract
The major risk factors for esophageal adenocarcinoma are gastroesophageal reflux disease (GERD) and Barrett esophagus, a squamous-to-columnar cell metaplasia that predisposes to malignancy. Adenocarcinomas in Barrett esophagus are thought to arise through a sequence of growth-promoting, genetic alterations that accumulate until the cells have acquired the physiologic hallmarks of cancer proposed by Hanahan and Weinberg. Moreover, GERD and Barrett esophagus are associated with chronic esophagitis, and inflammation is a well known risk factor for cancer formation. The cell that gives rise to Barrett metaplasia is not known. It has been proposed that the metaplasia may arise from a change in the differentiation pattern of stem cells that either reside in the esophagus or are recruited to the esophagus from the bone marrow. Alternatively, it is possible that Barrett metaplasia develops through the conversion of one differentiated cell type into another. Regardless of the cell of origin, Barrett metaplasia ultimately must be sustained by stem cells, which might be identified by intestinal stem cell markers. An emerging concept in tumor biology is that cancer stem cells are responsible for sustaining tumor growth. If Barrett cancers develop from Barrett stem cells, then a therapy targeted at those stem cells might prevent esophageal adenocarcinoma. This report reviews the risk factors for Barrett esophagus and esophageal adenocarcinoma, the mechanisms by which genetic alterations might contribute to carcinogenesis in Barrett esophagus, and the role of stem cells in the development of Barrett metaplasia and adenocarcinoma.
Keywords: Barrett esophagus, Esophageal adenocarcinoma, Metaplasia, Stem Cells
1. Introduction
Cancer of the esophagus is the world’s eighth most common malignancy, affecting approximately 500,000 individuals worldwide each year [1]. It is one of the most deadly gastrointestinal tumors, with 5-year mortality rates that exceed 80% [2]. There are the two major histological types of esophageal cancer, squamous cell carcinoma and adenocarcinoma, and their epidemiological features differ considerably. Squamous cell carcinoma of the esophagus has a predilection for black and Asian populations and, worldwide, more than 80% of esophageal cancers are squamous cell carcinomas. In contrast, esophageal adenocarcinoma affects white populations predominantly.
In the United States over the past several decades, the frequency of squamous cell carcinoma has declined while there has been a dramatic rise in the frequency of esophageal adenocarcinoma [2]. Indeed, the incidence of esophageal adenocarcinoma has increased by more than 600% since 1975 [3]. The major risk factors for esophageal adenocarcinoma are gastroesophageal reflux disease (GERD) and Barrett esophagus, the condition in which an abnormal, intestinal-type epithelium replaces the squamous epithelium that normally lines the esophagus [4;5]. This report will review the risk factors for esophageal adenocarcinoma, and discuss the molecular biology of Barrett esophagus and esophageal adenocarcinoma.
2. Risk Factors for Esophageal Adenocarcinoma
2.1. GERD and Barrett Esophagus
GERD and its sequela Barrett esophagus are the major risk factors for esophageal adenocarcinoma [4;5]. GERD is thought to be the factor that both injures the esophageal squamous epithelium and provides the abnormal milieu necessary for healing of the reflux esophagitis through metaplasia rather than through the regeneration of squamous epithelium. The specialized intestinal metaplasia of Barrett esophagus appears to be more resistant to acid-peptic damage than the native squamous epithelium but, for reasons that are not clear, is predisposed to carcinogenesis. Indeed, the large majority of esophageal adenocarcinomas appear to arise from this specialized intestinal metaplasia [5]. Adenocarcinomas in Barrett esophagus develop through a sequence of genetic alterations which eventually endow cells with unlimited proliferative capacity [6]. These alterations are reviewed in detail below.
2.2. Obesity
Obesity has been established as a strong risk factor for esophageal adenocarcinoma. A recent systematic review of the literature found a positive association between body mass index (BMI) and the risk of esophageal adenocarcinoma [7]. Other recent data has found a stronger association of esophageal adenocarcinoma with central (abdominal) obesity than with BMI alone. In a nested case-control study, the strong association between esophageal adenocarcinoma and abdominal obesity did not diminish when adjusted for overall BMI [8]. A strong association between central obesity and Barrett esophagus also has been reported [9]. Central obesity may predispose to GERD by increasing pressure within the abdomen. In addition, obesity may alter circulating levels of pro-proliferative factors so as to promote esophageal carcinogenesis. For example, the insulin resistance that may accompany obesity results in elevated serum levels of insulin-like growth factors, which are pro-proliferative. Obesity also is associated with elevated levels of the pro-proliferative hormone leptin, and with decreased levels of the anti-proliferative hormone adiponectin [10]. It has been proposed that the rising frequency of obesity in Western countries may underlie the rising incidence of esophageal adenocarcinoma.
2.3. Dietary Factors
Investigators from Glasgow have proposed that increased intake of dietary nitrate may be contributing to the rising incidence of esophageal adenocarcinoma. Most dietary nitrate is in green, leafy vegetables. Ingested nitrate is absorbed by the small intestine and is largely excreted unchanged in the urine. However, approximately 25% of the dietary nitrate is concentrated by the salivary glands and secreted into the mouth where bacteria on the tongue reduce the recycled nitrate (NO3−) to nitrite (NO2−). When swallowed nitrite encounters acidic gastric juice, the nitrite is converted rapidly to nitric oxide (NO), a toxic molecule. After nitrate ingestion, high levels of NO have been demonstrated in the distal esophagus and proximal stomach. NO can be genotoxic and, potentially, carcinogenic. Following World War II, Western countries sharply increased their use of nitrate-based fertilizers, resulting in an increased concentration of nitrates in vegetables. It is conceivable that this has contributed to the rising frequency of esophageal adenocarcinoma [11;12].
Despite this attractive theory, the bulk of available epidemiological data suggest that a diet high in fruits and vegetables may protect against Barrett and adenocarcinoma of the esophagus. Furthermore, a recent prospective study conducted by the National Cancer Institute found no apparent association between total fruit and vegetable intake and the incidence of esophageal adenocarcinoma [13]. On subgroup analysis, furthermore, a significant inverse association was found between spinach intake (a potential source of dietary nitrates) and adenocarcinoma of the esophagus [13].
2.4. Helicobacter pylori
H. pylori has been classified by the World health Organization’s International Agency for Research on Cancer as a group I (definite) carcinogen for adenocarcinoma of the distal stomach [14]. In contrast, a recent meta-analysis has confirmed that there is a significant inverse relationship between esophageal adenocarcinoma and H. pylori infection [15]. A similar inverse relationship between H. pylori and Barrett esophagus also has been reported [15;16]. Thus, H. pylori appears to protect against the development of Barrett and adenocarcinoma of the esophagus. Although the exact mechanism for this protective association is not clear, it has been suggested that H. pylori infections which decrease gastric acid secretion may prevent the development of GERD, which is an undisputed risk factor for esophageal adenocarcinoma.
2.5. Alcohol and Tobacco Use
Unlike esophageal squamous cell cancer where alcohol and tobacco use are strong risk factors, a number of studies have found that there is only a moderate association of tobacco use with esophageal adenocarcinoma and no clear association with alcohol use. A recent, prospective study conducted by the National Cancer Institute has found that smokers have a 55% increased risk of esophageal adenocarcinoma, whereas alcohol use (defined as >3 alcoholic beverages per day) does not appear to influence the risk for this tumor [17].
3. Molecular Pathogenesis of Esophageal Adenocarcinoma Arising in Barrett Esophagus
Metaplasia, the process in which one adult cell type replaces another, is one way in which tissues respond to chronic inflammation [18]. Although the metaplastic cells may be more resistant to the inflammatory insult than the native cells, the metaplasia also may predispose to malignancy. In the esophagus, chronic inflammation due to GERD results in the replacement of reflux-damaged squamous epithelium by specialized intestinal metaplasia, which appears to be more resistant to reflux-mediated injury. Unfortunately, Barrett esophagus is predisposed to develop esophageal adenocarcinoma.
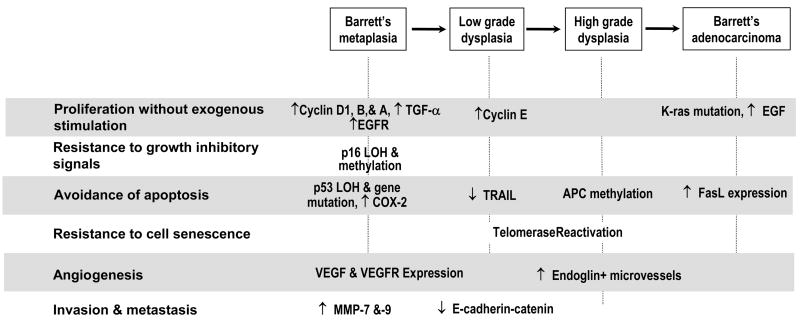
As benign, metaplastic Barrett cells progress to carcinoma, they accumulate a number of genetic alterations that give them growth advantages. These alterations eventually endow the cells with the essential physiological hallmarks of cancer cells including the ability to proliferate without exogenous stimulation, to resist growth-inhibitory signals, to avoid triggering apoptosis, to resist cell senescence, to develop new vascular supplies (angiogenesis), and to invade and metastasize [19]. The sections below focus on how genetic alterations acquired during the neoplastic progression of Barrett esophagus result in the acquisition of each of the cancer hallmarks (Figure 1).
Figure 1.
Major genetic alterations acquired by metaplastic Barrett cells during progression to esophageal adenocarcinoma. The approximate histological stage at which each genetic change has been recognized is depicted.
3.1. Proliferation without Exogenous Stimulation
In general, it is the expression of oncogenes that allows cells to proliferate without exogenous stimulation. Proto-oncogenes are normal cellular genes that promote cell growth. Oncogenes are proto-oncogenes that have become overactive as the result of mutation. Examples of oncogenes implicated in the development of esophageal adenocarcinoma are cyclins D1, E, B1, and A. Cyclins D1 and E, along with their cyclin-dependent kinases (cdks), regulate the pivotal G1 to S transition point in the cell cycle. Cyclin A is expressed during the S and G2 phases, whereas cyclin B1 acts to control the G2 to M transition. Increased nuclear expression of cyclin D1 protein has been detected in biopsy specimens of non-dysplastic Barrett metaplasia, suggesting that it may play an early role in carcinogenesis [20]. In contrast, overexpression of cyclin E has been found in dysplastic Barrett epithelium and in adenocarcinomas, but not in non-dysplastic Barrett esophagus [21]. Expression of cyclin B1 has been detected in non-dysplastic and dysplastic Barrett esophagus as well as in Barrett adenocarcinomas [22]. Cyclin A expression has been found to increase as the metaplasia progresses through dysplasia to adenocarcinoma [23].
In addition to the direct activation of oncogenes, alterations in growth factors, growth factor receptors, or the signaling pathways that mediate growth factor-receptor interactions can also allow cells to proliferate without exogenous stimulation. For example, increased expression of epidermal growth factor (EGF), transforming growth factor-α (TGF-α) and EGF receptor (EGFR, also called ErbB-1) have been found in esophageal adenocarcinomas [24;25]. Increased expression of TGF-α and the EGFR have been found to occur early in non-dysplastic Barrett epithelium [24;25]. The role of the oncogenic form of the normal EGFR family member erbB-2 (also called HER2 or Neu) in esophageal adenocarcinoma progression remains controversial. However recent data suggest that HER2 amplification may be associated with a worse outcome for esophageal adenocarcinoma [26;27]. Downstream of tyrosine kinase receptors like EGFR are the Ras proteins (including H-ras and K-ras,), which play a central role in the regulation of cell proliferation. K-ras mutations have been reported in 11–40% of esophageal adenocarcinomas [28;29].
3.2. Resistance to Growth-Inhibitory Signals
Tumor suppressor genes are normal genes that usually function to restrain cell growth. Cells can acquire the ability to resist growth inhibitory signals by inactivating tumor suppressor genes through one or a combination of three mechanisms including mutation of the gene, loss of heterozygosity (LOH, which is a deletion of the chromosomal region containing the gene), or promoter methylation (attachment of methyl groups to the promoter region of genes). Tumor suppressor genes implicated in the progression of Barrett metaplasia to cancer have shown inactivation by all of these mechanisms.
Examples of tumor suppressor genes implicated in the development of esophageal adenocarcinoma include p16, p53, p14ARF, p27, and the adenomatous polyposis coli (APC) gene. p16 and p53 proteins normally act to block cell cycle progression at the G1 to S transition and, therefore, inactivation of the p16 or p53 gene enables unregulated cell growth. Allelic loss of 9p21, the chromosomal locus for p16, and methylation of the p16 promoter have been reported in 45–54% of esophageal adenocarcinomas [30;31]. Moreover, p16 mutation, LOH or promoter methylation has been detected in non-dysplastic, Barrett metaplasia in approximately 80% of cases, suggesting that genetic alterations of p16 are among the earliest events in the neoplastic progression of Barrett esophagus [32].
Inactivation of p53 by LOH at its 17p locus along with mutation of the remaining allele has been found in approximately 50–90% of esophageal adenocarcinomas [33;34] Non-malignant cells of specialized intestinal metaplasia also can develop p53 LOH and mutation, but rarely in the absence of a preexisting alteration of p16 [35]. Decreased expression of p14ARF, a key regulator of p53 protein, has been found as Barrett metaplasia progresses to adenocarcinoma, with 75% of these cancers exhibiting undetectable levels of p14ARF [36]. Loss of protein expression for p27, an inhibitor of cyclin E activity, has been observed in 83% of esophageal adenocarcinomas, and has been correlated with aggressive tumor behavior and poor patient outcomes [37]. Methylation of APC has been found in over 80% of cases of Barrett esophagus with high grade dysplasia or adenocarcinoma, and in approximately 40 % of patients with non-dysplastic Barrett metaplasia [38;39].
3.3. Avoidance of Apoptosis
Normal cells have the ability to destroy themselves through the process of apoptosis, a genetically regulated, innate form of cell suicide. This process prevents the survival of cells that have sustained cancer-promoting injuries that might threaten the organism. The cellular apoptotic machinery can be triggered by a number of factors including DNA damage, death receptor activation, and metabolic abnormalities. Once activated, the apoptotic machinery leads to cell death through activation of an executioner pathway [40]. Tumor cells must find ways to avoid apoptosis if they are to survive.
In addition to its tumor suppressor activity, p53 protein also functions as an initiator of apoptosis. Esophageal adenocarcinoma cells can avoid apoptosis by inactivating p53. Apoptosis also can be initiated when death receptors on the cell surface bind with ligands such as Fas-ligand (FasL) and TNF-related apoptosis inducing ligand (TRAIL)[41;42]. The Fas death receptor normally is found on gut epithelial cells, whereas lymphocytes express both Fas death receptor and FasL. Esophageal adenocarcinoma cells have been found to express FasL, which can bind the Fas receptor on the surface of tumor-killing lymphocytes, thereby destroying the lymphocytes that might attack the cancer cells [43]. Recently, expression of TRAIL has been found to decrease progressively as metaplastic Barrett epithelium develops dysplasia and carcinoma [42].
Synthesis of an agent that blocks apoptosis is another mechanism whereby cancer cells avoid their own destruction. For example, esophageal adenocarcinomas can exhibit increased expression of cyclooxygenase-2 (COX-2), which has been shown to decrease apoptosis rates in esophageal adenocarcinoma cells in vitro, [44;45] COX-2 overexpression also has been detected in benign Barrett metaplasia, and COX-2 expression has been found to increase as the cells progress to dysplasia and carcinoma [46].
3.4. Resistance to Cell Senescence
Senescence, like apoptosis, is an innate mechanism that limits the proliferation of normal cells. As cells undergo successive divisions, their telomeres, which are short repetitive DNA sequences located at the ends of chromosomes, undergo progressive shortening. Once the telomeres shorten to a critical length, the cell enters senescence, a permanent state of growth arrest. Therefore, for cells to replicate indefinitely (i.e. to become immortal), telomere length must be maintained. Telomerase is the enzyme responsible for the synthesis and maintenance of telomeres [47]. High levels of telomerase expression have been found in esophageal adenocarcinoma, whereas low expression levels are found in non-dysplastic Barrett epithelium [48]. Moreover, a marked increase in telomerase expression occurred during the transition from low grade to high grade dysplasia in Barrett epithelium [48].
3.5. Development of New Vascular Supplies (Angiogenesis)
For tumors to grow, they must form new blood vessels to provide nutrients and eliminate metabolic waste products. Vascular endothelial growth factors (VEGFs) are potent promoters of angiogenesis. VEGF expression has been found to be significantly increased in esophageal adenocarcinomas compared to dysplastic and metaplastic Barrett esophagus and normal esophageal mucosa [49]. Endoglin is a receptor for transforming growth factor β1, which preferentially binds to endothelial cells involved in tumor angiogenesis [49]. In esophageal adenocarcinoma, the number of tumor microvessels that stain with endoglin has been found to correlate significantly with angiolymphatic invasion, lymph node metastasis, and overall prognosis [49]. Moreover, Barrett epithelium with high grade dysplasia contains a significantly greater number of endoglin-staining microvessels than Barrett with low grade dysplasia [49].
3.6. Invasion and Metastasis
To invade and metastasize, tumor cells must lose their cell-cell adhesion and acquire the ability to degrade the extracellular matrix. Cadherins are a large family of adhesion molecules that are located on the cell surface, where they bind to cadherins on the surface of neighboring cells. The cadherins are anchored in place by binding to catenins, which are attached to the cell cytoskeleton. Loss of cell-cell adhesion by failure of cadherins to interact with either the catenins or with other cadherins can predispose to invasion and metastasis. As the degree of dysplasia in Barrett epithelium increases, there is a decrease in membranous E-cadherin and β-catenin and an increase in the cytoplasmic and nuclear location of these proteins [50;51].
Matrix metalloproteinases (MMPs) are a family of proteolytic enzymes that mediate the destruction of the extracellular matrix, allowing for tumor invasion and spread. MMPs-7 and -9 have been found to be increased in non-dysplastic Barrett esophagus, with even higher levels found in dysplastic Barrett mucosa and esophageal adenocarcinoma [52;53].
4. Contribution of Inflammation and Inflammatory Mediators to the Development of Esophageal Adenocarcinoma
It has been known for more than a century that chronic inflammation can contribute to cancer formation. A number of chronic inflammatory conditions of the gastrointestinal tract, such as ulcerative colitis and chronic pancreatitis, are well known to predispose to carcinogenesis. As noted above, the major risk factors for esophageal adenocarcinoma are GERD and Barrett esophagus, both of which are associated with chronic inflammation. GERD can cause reflux esophagitis, and histological evidence of moderate to severe inflammation along with the expression of the pro-inflammatory cytokines IL-1β, IL-8, and NF-κB (a transcription factor involved in the regulation of pro-inflammatory genes) have been detected in biopsies of Barrett metaplasia [54;55]. Moreover, the infiltrating inflammatory cells are not the only source of pro-inflammatory cytokines, because metaplastic Barrett epithelial cells themselves have been found to express IL-8, IL-1β and IL-10 [54;56]. NF-κB activation and epithelial cell expression of tumor necrosis factor (TNF) -α and its receptor TNFR1 all have been found to increase as metaplastic Barrett mucosa develops dysplastic changes of progressive severity, suggesting that the inflammatory response might be contributing to carcinogenesis [54;57]. Although levels of IL-8 and IL-1β have not been found to be elevated in dysplastic Barrett epithelium, higher expression levels of both cytokines have been detected in esophageal adenocarcinomas [54].
One mechanism whereby inflammation might promote cancer formation is through the production of reactive oxygen species (ROS) that can cause oxidative damage to cellular DNA, proteins, and lipids. Such oxidative damage has been documented in association with GERD in animal models of reflux esophagitis, Barrett esophagus, and esophageal adenocarcinoma as well as in patients with those diseases [58;59]. Lipid membrane peroxidation is an indicator of ROS-mediated cellular injury, and biopsies of inflamed esophageal squamous and Barrett mucosae exhibit higher levels of ROS and lipid peroxidation than uninflamed control tissues [59]. Moroever, Clemons et al. have demonstrated a link between acid-induced ROS and DNA damage in Barrett high-grade dysplasia and esophageal adenocarcinoma cell lines in vitro [60].
One of the defense mechanisms cells have to prevent oxidative damage is the expression of antioxidant enzymes that assist in the removal of ROS. Therefore, low levels of antioxidant enzymes would be expected to enhance ROS-mediated cellular injury. Biopsies of non-dysplastic Barrett metaplasia, Barrett with dysplasia, and esophageal adenocarcinoma all have been shown to express low levels of the antioxidant enzymes glutathione S-transferase and glutathione peroxidase, suggesting that deficiencies in the antioxidant defense system may contribute to inflammation-mediated esophageal carcinogenesis [61;62].
5. Recent Advances in Understanding of the Pathogenesis of Barrett Metaplasia
Barrett metaplasia develops when esophageal squamous epithelium is damaged by GERD. The origin of the stem cells that give rise to the specialized intestinal metaplasia of Barrett esophagus are not known, but have been assumed to reside within the esophagus itself. However, a recent study suggests that stem cells from the bone marrow might contribute to Barrett metaplasia. In this study, female rats had their native bone marrow cells destroyed by a lethal dose of radiation, followed by a bone marrow transplant from male rat donors [63]. The female rats then had an esophagojejunostomy, which results in severe esophagitis and intestinal metaplasia similar to human Barrett esophagus. Nuclear staining for Y chromosome, which must have come from the bone marrow cells of the male donor rats, was found in both squamous cells and metaplastic columnar cells in the esophagi of the female rats. These data suggest that multipotential adult progenitor cells of bone marrow origin contribute to esophageal regeneration and metaplasia in this rat model of reflux esophagitis and Barrett esophagus, and that the progenitor cell for Barrett esophagus could be of bone marrow origin.
Metaplasia also can arise through the conversion of one differentiated cell type into another, a process that generally involves changes in the cell’s pattern of gene expression. CDXs, members of the Caudal gene family, are a homeobox family of transcription factors known to mediate the expression of genes which can determine an intestinal phenotype. The forced expression of Cdx1 or Cdx2 in the gastric epithelial cells of mice leads to intestinal metaplasia in their stomachs [64;65]. In humans, biopsy specimens of specialized intestinal metaplasia have been shown to express both CDX1 and CDX2 [66;67]. Moreover, a number of in vitro studies suggest that acid and bile acids, the major noxious components of gastric refluxate, can induce CDX-2 expression in esophageal squamous cells [68]. CDX2 expression has been found in esophageal squamous epithelium from patients with Barrett esophagus, suggesting that induction of CDX expression by gastric reflux in esophageal squamous cells may mediate their differentiation into the intestinal-type cells of Barrett metaplasia [67].
Another factor which may be involved in the squamous-to-columnar metaplasia of Barrett esophagus is bone morphogenetic protein (BMP)-4. In patients with GERD and Barrett esophagus, BMP-4 expression has been localized to the stromal tissue underlying inflamed esophageal squamous epithelium and specialized intestinal metaplasia, but not in the stroma underlying normal esophageal squamous epithelium [69]. When human esophageal squamous cells grown in culture are exposed to BMP-4, their gene expression patterns change to resemble those of Barrett metaplasia [69]. These data suggest that perhaps GERD may cause esophageal stromal cells to express BMP-4, which promotes the epithelium to change from a squamous to columnar cell type.
6. Stem Cells and Barrett-Associated Esophageal Adenocarcinoma
Regardless of whether Barrett metaplasia develops initially from the abnormal differentiation of esophageal or bone marrow-derived stem cells, or from the conversion of mature esophageal squamous cells, the maintenance of the metaplastic epithelium depends on a stem cell for its continual renewal. Likewise, it is now thought that cancer stem cells are responsible for maintaining and renewing tumors. Therefore, the cancer stem cells must be eliminated in order to eliminate the tumor. Recent data suggest that the stem cells of gastrointestinal tumors may express the same stem cell markers as the normal intestinal epithelium [70;71]. Presently, no markers of stem cells in metaplastic Barrett esophagus have been identified. However, since the epithelium of Barrett esophagus is a form of incomplete intestinal metaplasia, perhaps Lgr5 and DCAMKL-1, the recently identified markers for intestinal stem cells, may be of use in the search for the stem cells in Barrett esophagus and esophageal adenocarcinoma [70;71]. If this is the case, then it is conceivable that, in the future, pharmacologic agents or endoscopic ablative therapies could be targeted to eliminate the Barrett stem cells and thus prevent the development of esophageal adenocarcinoma.
7. Conclusion
Over the past several decades, there has been an alarming increase in the frequency of adenocarcinoma of the esophagus. The major risk factors for esophageal adenocarcinoma are GERD and its sequela, Barrett esophagus. Thus, it has become increasingly important to understand the pathogenesis of esophageal adenocarcinoma and Barrett esophagus at the molecular level in order to develop more targeted and effective cancer-preventive strategies. While only a fraction of the genetic alterations required for benign, metaplastic Barrett cells to progress to adenocarcinoma were reviewed in this report, it is hoped that the conceptual basis for evaluating studies on developing cancer treatments and preventive strategies has been established. It is also reasonable to assume that further elucidation of basic mechanisms underlying the development of Barrett esophagus and its progression to malignancy will lead to improved clinical strategies and outcomes for patients with Barrett adenocarcinomas.
Acknowledgments
Supported by the Office of Medical Research, Department of Veteran’s Affairs (R.F.S.) and the National Institutes of Health (DK63621 to R.F.S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lambert R, Hainaut P. The multidisciplinary management of gastrointestinal cancer. Epidemiology of oesophagogastric cancer. Best Pract Res Clin Gastroenterol. 2007;21:921–945. doi: 10.1016/j.bpg.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 4.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 5.Spechler SJ. Clinical practice. Barrett’s Esophagus. N Engl J Med. 2002;346:836–842. doi: 10.1056/NEJMcp012118. [DOI] [PubMed] [Google Scholar]
- 6.Souza RF, Morales CP, Spechler SJ. Review article: a conceptual approach to understanding the molecular mechanisms of cancer development in Barrett’s oesophagus. Aliment Pharmacol Ther. 2001;15:1087–1100. doi: 10.1046/j.1365-2036.2001.01046.x. [DOI] [PubMed] [Google Scholar]
- 7.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:872–878. doi: 10.1158/1055-9965.EPI-05-0860. [DOI] [PubMed] [Google Scholar]
- 8.Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:352–358. doi: 10.1158/1055-9965.EPI-07-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Serag HB, Kvapil P, Hacken-Bitar J, Kramer JR. Abdominal obesity and the risk of Barrett’s esophagus. Am J Gastroenterol. 2005;100:2151–2156. doi: 10.1111/j.1572-0241.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 10.Frystyk J, Skjaerbaek C, Vestbo E, Fisker S, Orskov H. Circulating levels of free insulin-like growth factors in obese subjects: the impact of type 2 diabetes. Diabetes Metab Res Rev. 1999;15:314–322. doi: 10.1002/(sici)1520-7560(199909/10)15:5<314::aid-dmrr56>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Iijima K, Henry E, Moriya A, Wirz A, Kelman AW, McColl KE. Dietary nitrate generates potentially mutagenic concentrations of nitric oxide at the gastroesophageal junction. Gastroenterology. 2002;122:1248–1257. doi: 10.1053/gast.2002.32963. [DOI] [PubMed] [Google Scholar]
- 12.Spechler SJ. Carcinogenesis at the gastroesophageal junction: free radicals at the frontier. Gastroenterology. 2002;122:1518–1520. doi: 10.1053/gast.2002.33368. [DOI] [PubMed] [Google Scholar]
- 13.Freedman ND, Park Y, Subar AF, Hollenbeck AR, Leitzmann MF, Schatzkin A, Abnet CC. Fruit and vegetable intake and esophageal cancer in a large prospective cohort study. Int J Cancer. 2007;121:2753–2760. doi: 10.1002/ijc.22993. [DOI] [PubMed] [Google Scholar]
- 14.Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol. 2004;31:450–464. doi: 10.1053/j.seminoncol.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1413–1417. doi: 10.1016/j.cgh.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Corley DA, Kubo A, Levin TR, Block G, Habel L, Zhao W, Leighton P, Rumore G, Quesenberry C, Buffler P, Parsonnet J. Helicobacter pylori infection and the risk of Barrett’s oesophagus: a community-based study. Gut. 2008;57:727–733. doi: 10.1136/gut.2007.132068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedman ND, Abnet CC, Leitzmann MF, Mouw T, Subar AF, Hollenbeck AR, Schatzkin A. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol. 2007;165:1424–1433. doi: 10.1093/aje/kwm051. [DOI] [PubMed] [Google Scholar]
- 18.Tosh D, Slack JM. How cells change their phenotype. Nat Rev Mol Cell Biol. 2002;3:187–194. doi: 10.1038/nrm761. [DOI] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 20.Arber N, Lightdale C, Rotterdam H, Han KH, Sgambato A, Yap E, Ahsan H, Finegold J, Stevens PD, Green PH, Hibshoosh H, Neugut AI, Holt PR, Weinstein IB. Increased expression of the cyclin D1 gene in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 1996;5:457–459. [PubMed] [Google Scholar]
- 21.Sarbia M, Bektas N, Muller W, Heep H, Borchard F, Gabbert HE. Expression of cyclin E in dysplasia, carcinoma, and nonmalignant lesions of Barrett esophagus. Cancer. 1999;86:2597–2601. [PubMed] [Google Scholar]
- 22.Geddert H, Heep HJ, Gabbert HE, Sarbia M. Expression of cyclin B1 in the metaplasia-dysplasia-carcinoma sequence of Barrett esophagus. Cancer. 2002;94:212–218. doi: 10.1002/cncr.10152. [DOI] [PubMed] [Google Scholar]
- 23.Lao-Sirieix P, Lovat L, Fitzgerald RC. Cyclin A immunocytology as a risk stratification tool for Barrett’s esophagus surveillance. Clin Cancer Res. 2007;13:659–665. doi: 10.1158/1078-0432.CCR-06-1385. [DOI] [PubMed] [Google Scholar]
- 24.Jankowski J, Hopwood D, Wormsley KG. Flow-cytometric analysis of growth-regulatory peptides and their receptors in Barrett’s oesophagus and oesophageal adenocarcinoma. Scand J Gastroenterol. 1992;27:147–154. doi: 10.3109/00365529209165436. [DOI] [PubMed] [Google Scholar]
- 25.Brito MJ, Filipe MI, Linehan J, Jankowski J. Association of transforming growth factor alpha (TGFA) and its precursors with malignant change in Barrett’s epithelium: biological and clinical variables. Int J Cancer. 1995;60:27–32. doi: 10.1002/ijc.2910600103. [DOI] [PubMed] [Google Scholar]
- 26.Brien TP, Odze RD, Sheehan CE, McKenna BJ, Ross JS. HER-2/neu gene amplification by FISH predicts poor survival in Barrett’s esophagus-associated adenocarcinoma. Hum Pathol. 2000;31:35–39. doi: 10.1016/s0046-8177(00)80195-1. [DOI] [PubMed] [Google Scholar]
- 27.Reichelt U, Duesedau P, Tsourlakis MC, Quaas A, Link BC, Schurr PG, Kaifi JT, Gros SJ, Yekebas EF, Marx A, Simon R, Izbicki JR, Sauter G. Frequent homogeneous HER-2 amplification in primary and metastatic adenocarcinoma of the esophagus. Mod Pathol. 2007;20:120–129. doi: 10.1038/modpathol.3800712. [DOI] [PubMed] [Google Scholar]
- 28.Lord RV, O’Grady R, Sheehan C, Field AF, Ward RL. K-ras codon 12 mutations in Barrett’s oesophagus and adenocarcinomas of the oesophagus and oesophagogastric junction. J Gastroenterol Hepatol. 2000;15:730–736. doi: 10.1046/j.1440-1746.2000.02163.x. [DOI] [PubMed] [Google Scholar]
- 29.Sommerer F, Vieth M, Markwarth A, Rohrich K, Vomschloss S, May A, Ell C, Stolte M, Hengge UR, Wittekind C, Tannapfel A. Mutations of BRAF and KRAS2 in the development of Barrett’s adenocarcinoma. Oncogene. 2004;23:554–558. doi: 10.1038/sj.onc.1207189. [DOI] [PubMed] [Google Scholar]
- 30.Barrett MT, Sanchez CA, Galipeau PC, Neshat K, Emond M, Reid BJ. Allelic loss of 9p21 and mutation of the CDKN2/p16 gene develop as early lesions during neoplastic progression in Barrett’s esophagus. Oncogene. 1996;13:1867–1873. [PubMed] [Google Scholar]
- 31.Wong DJ, Barrett MT, Stoger R, Emond MJ, Reid BJ. p16INK4a promoter is hypermethylated at a high frequency in esophageal adenocarcinomas. Cancer Res. 1997;57:2619–2622. [PubMed] [Google Scholar]
- 32.Wong DJ, Paulson TG, Prevo LJ, Galipeau PC, Longton G, Blount PL, Reid BJ. p16(INK4a) lesions are common, early abnormalities that undergo clonal expansion in Barrett’s metaplastic epithelium. Cancer Res. 2001;61:8284–8289. [PubMed] [Google Scholar]
- 33.Hamelin R, Flejou JF, Muzeau F, Potet F, Laurent-Puig P, Fekete F, Thomas G. TP53 gene mutations and p53 protein immunoreactivity in malignant and premalignant Barrett’s esophagus. Gastroenterology. 1994;107:1012–1018. doi: 10.1016/0016-5085(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 34.Galipeau PC, Prevo LJ, Sanchez CA, Longton GM, Reid BJ. Clonal expansion and loss of heterozygosity at chromosomes 9p and 17p in premalignant esophageal (Barrett’s) tissue. J Natl Cancer Inst. 1999;91:2087–2095. doi: 10.1093/jnci/91.24.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maley CC, Galipeau PC, Li X, Sanchez CA, Paulson TG, Blount PL, Reid BJ. The combination of genetic instability and clonal expansion predicts progression to esophageal adenocarcinoma. Cancer Res. 2004;64:7629–7633. doi: 10.1158/0008-5472.CAN-04-1738. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y, Peters CJ, Fitzgerald RC, Gjerset RA. Progressive silencing of p14ARF in oesophageal adenocarcinoma. J Cell Mol Med Epress. 2008 doi: 10.1111/j.1582-4934.2008.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh SP, Lipman J, Goldman H, Ellis FH, Jr, Aizenman L, Cangi MG, Signoretti S, Chiaur DS, Pagano M, Loda M. Loss or altered subcellular localization of p27 in Barrett’s associated adenocarcinoma. Cancer Res. 1998;58:1730–1735. [PubMed] [Google Scholar]
- 38.Kawakami K, Brabender J, Lord RV, Groshen S, Greenwald BD, Krasna MJ, Yin J, Fleisher AS, Abraham JM, Beer DG, Sidransky D, Huss HT, DeMeester TR, Eads C, Laird PW, Ilson DH, Kelsen DP, Harpole D, Moore MB, Danenberg KD, Danenberg PV, Meltzer SJ. Hypermethylated APC DNA in Plasma and Prognosis of Patients With Esophageal Adenocarcinoma. J Natl Cancer Inst. 2000;92:1805–1811. doi: 10.1093/jnci/92.22.1805. [DOI] [PubMed] [Google Scholar]
- 39.Clement G, Jablons DM, Benhattar J. Targeting the Wnt signaling pathway to treat Barrett’s esophagus. Expert Opin Ther Targets. 2007;11:375–389. doi: 10.1517/14728222.11.3.375. [DOI] [PubMed] [Google Scholar]
- 40.Hetts SW. To die or not to die: an overview of apoptosis and its role in disease. JAMA. 1998;279:300–307. doi: 10.1001/jama.279.4.300. [DOI] [PubMed] [Google Scholar]
- 41.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 42.Popnikolov NK, Gatalica Z, Adegboyega PA, Norris BA, Pasricha PJ. Downregulation of TNF-related apoptosis-inducing ligand (TRAIL)/Apo2L in Barrett’s esophagus with dysplasia and adenocarcinoma. Appl Immunohistochem Mol Morphol. 2006;14:161–165. doi: 10.1097/01.pai.0000157905.30872.9f. [DOI] [PubMed] [Google Scholar]
- 43.Younes M, Schwartz MR, Ertan A, Finnie D, Younes A. Fas ligand expression in esophageal carcinomas and their lymph node metastases. Cancer. 2000;88:524–528. [PubMed] [Google Scholar]
- 44.Souza RF, Shewmake K, Beer DG, Cryer B, Spechler SJ. Selective Inhibition of Cyclooxygenase-2 Suppresses Growth and Induces Apoptosis in Human Esophageal Adenocarcinoma Cells. Cancer Res. 2000;60:5767–5772. [PubMed] [Google Scholar]
- 45.Wilson KT, Fu S, Ramanujam KS, Meltzer SJ. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett’s esophagus and associated adenocarcinomas. Cancer Res. 1998;58:2929–2934. [PubMed] [Google Scholar]
- 46.Shirvani VN, Ouatu-Lascar R, Kaur BS, Omary MB, Triadafilopoulos G. Cyclooxygenase 2 Expression in Barrett’s Esophagus and Adenocarcinoma: Ex Vivo Induction by Bile Salts and Acid Exposure. Gastroenterology. 2000;118:487–496. doi: 10.1016/s0016-5085(00)70254-x. [DOI] [PubMed] [Google Scholar]
- 47.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 48.Morales CP, Lee EL, Shay JW. In situ hybridization for the detection of telomerase RNA in the progression from Barrett’s esophagus to esophageal adenocarcinoma. Cancer. 1998;83:652–659. [PubMed] [Google Scholar]
- 49.Saad RS, El Gohary Y, Memari E, Liu YL, Silverman JF. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in esophageal adenocarcinoma. Hum Pathol. 2005;36:955–961. doi: 10.1016/j.humpath.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 50.Bailey T, Biddlestone L, Shepherd N, Barr H, Warner P, Jankowski J. Altered cadherin and catenin complexes in the Barrett’s esophagus-dysplasia-adenocarcinoma sequence: correlation with disease progression and dedifferentiation. Am J Pathol. 1998;152:135–144. [PMC free article] [PubMed] [Google Scholar]
- 51.Washington K, Chiappori A, Hamilton K, Shyr Y, Blanke C, Johnson D, Sawyers J, Beauchamp D. Expression of beta-catenin, alpha-catenin, and E-cadherin in Barrett’s esophagus and esophageal adenocarcinomas. Mod Pathol. 1998;11:805–813. [PubMed] [Google Scholar]
- 52.Salmela MT, Karjalainen-Lindsberg ML, Puolakkainen P, Saarialho-Kere U. Upregulation and differential expression of matrilysin (MMP-7) and metalloelastase (MMP-12) and their inhibitors TIMP-1 and TIMP-3 in Barrett’s oesophageal adenocarcinoma. Br J Cancer. 2001;85:383–392. doi: 10.1054/bjoc.2001.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herszenyi L, Hritz I, Pregun I, Sipos F, Juhasz M, Molnar B, Tulassay Z. Alterations of glutathione S-transferase and matrix metalloproteinase-9 expressions are early events in esophageal carcinogenesis. World J Gastroenterol. 2007;13:676–682. doi: 10.3748/wjg.v13.i5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Riordan JM, Abdel-latif MM, Ravi N, McNamara D, Byrne PJ, McDonald GS, Keeling PW, Kelleher D, Reynolds JV. Proinflammatory cytokine and nuclear factor kappa-B expression along the inflammation-metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Gastroenterol. 2005;100:1257–1264. doi: 10.1111/j.1572-0241.2005.41338.x. [DOI] [PubMed] [Google Scholar]
- 55.Fitzgerald RC, Abdalla S, Onwuegbusi BA, Sirieix P, Saeed IT, Burnham WR, Farthing MJ. Inflammatory gradient in Barrett’s oesophagus: implications for disease complications. Gut. 2002;51:316–322. doi: 10.1136/gut.51.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliott M, Saeed IT, Burnham WR, Farthing MJ. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut. 2002;50:451–459. doi: 10.1136/gut.50.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tselepis C, Perry I, Dawson C, Hardy R, Darnton SJ, McConkey C, Stuart RC, Wright N, Harrison R, Jankowski JA. Tumour necrosis factor-alpha in Barrett’s oesophagus: a potential novel mechanism of action. Oncogene. 2002;21:6071–6081. doi: 10.1038/sj.onc.1205731. [DOI] [PubMed] [Google Scholar]
- 58.Chen X, Ding YW, Yang G, Bondoc F, Lee MJ, Yang CS. Oxidative damage in an esophageal adenocarcinoma model with rats. Carcinogenesis. 2000;21:257–263. doi: 10.1093/carcin/21.2.257. [DOI] [PubMed] [Google Scholar]
- 59.Wetscher GJ, Hinder RA, Bagchi D, Hinder PR, Bagchi M, Perdikis G, McGinn T. Reflux esophagitis in humans is mediated by oxygen-derived free radicals. Am J Surg. 1995;170:552–556. doi: 10.1016/s0002-9610(99)80014-2. [DOI] [PubMed] [Google Scholar]
- 60.Clemons NJ, McColl KE, Fitzgerald RC. Nitric oxide and acid induce double-strand DNA breaks in Barrett’s esophagus carcinogenesis via distinct mechanisms. Gastroenterology. 2007;133:1198–1209. doi: 10.1053/j.gastro.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 61.Cobbe SC, Scobie GC, Pohler E, Hayes JD, Kernohan NM, Dillon JF. Alteration of glutathione S-transferase levels in Barrett’s metaplasia compared to normal oesophageal epithelium. Eur J Gastroenterol Hepatol. 2003;15:41–47. doi: 10.1097/00042737-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Lee OJ, Schneider-Stock R, McChesney PA, Kuester D, Roessner A, Vieth M, Moskaluk CA, El Rifai W. Hypermethylation and loss of expression of glutathione peroxidase-3 in Barrett’s tumorigenesis. Neoplasia. 2005;7:854–861. doi: 10.1593/neo.05328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarosi G, Brown G, Jaiswal K, Feagins LA, Lee E, Crook TW, Souza RF, Zou YS, Shay JW, Spechler SJ. Bone marrow progenitor cells contribute to esophageal regeneration and metaplasia in a rat model of Barrett’s esophagus. Dis Esophagus. 2008;21:43–50. doi: 10.1111/j.1442-2050.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 64.Silberg DG, Sullivan J, Kang E, Swain GP, Moffett J, Sund NJ, Sackett SD, Kaestner KH. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689–696. doi: 10.1053/gast.2002.31902. [DOI] [PubMed] [Google Scholar]
- 65.Mutoh H, Sakurai S, Satoh K, Osawa H, Hakamata Y, Takeuchi T, Sugano K. Cdx1 induced intestinal metaplasia in the transgenic mouse stomach: comparative study with Cdx2 transgenic mice. Gut. 2004;53:1416–1423. doi: 10.1136/gut.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong NA, Wilding J, Bartlett S, Liu Y, Warren BF, Piris J, Maynard N, Marshall R, Bodmer WF. CDX1 is an important molecular mediator of Barrett’s metaplasia. Proc Natl Acad Sci USA. 2005;102:7565–7570. doi: 10.1073/pnas.0502031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moons LM, Bax DA, Kuipers EJ, van Dekken H, Haringsma J, Van Vliet AH, Siersema PD, Kusters JG. The homeodomain protein CDX2 is an early marker of Barrett’s oesophagus. J Clin Pathol. 2004;57:1063–1068. doi: 10.1136/jcp.2003.015727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu T, Zhang X, So CK, Wang S, Wang P, Yan L, Myers R, Chen Z, Patterson AP, Yang CS, Chen X. Regulation of Cdx2 expression by promoter methylation, and effects of Cdx2 transfection on morphology and gene expression of human esophageal epithelial cells. Carcinogenesis. 2007;28:488–496. doi: 10.1093/carcin/bgl176. [DOI] [PubMed] [Google Scholar]
- 69.Milano F, van Baal JW, Buttar NS, Rygiel AM, de Kort F, DeMars CJ, Rosmolen WD, Bergman JJ, VAn MJ, Wang KK, Peppelenbosch MP, Krishnadath KK. Bone morphogenetic protein 4 expressed in esophagitis induces a columnar phenotype in esophageal squamous cells. Gastroenterology. 2007;132:2412–2421. doi: 10.1053/j.gastro.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 70.Barker N, van Es JH, Kuipers J, Kujala P, van den BM, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 71.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26:630–637. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]