Preface
The brain processes information by transmitting signals at synapses, which connect neurons into vast networks of communicating cells. In these networks, synapses not only transmit, but also process and refine information. Neurexins and neuroligins are synaptic cell-adhesion molecules that connect pre- and postsynaptic neurons at synapses, mediate trans-synaptic signaling, and shape neural network properties by specifying synaptic functions. In humans, alterations in neurexin or neuroligin genes are implicated in autism and other cognitive diseases, connecting synaptic cell adhesion to cognition and its disorders. Thus, neurexins and neuroligins are core components of the molecular machinery that controls synaptic transmission and enables neural networks to process complex signals.
Introductory paragraphs
The brain integrates and processes sensory inputs to generate motor outputs appropriate for the survival of the organism. Cascades of synapses, assembled into overlapping neural circuits, transform sensory inputs and generate motor outputs1. All information processing in the brain involves synapses, and virtually all abnormalities in brain function affect, directly or indirectly, synaptic function.
Synapses are specialized intercellular junctions dedicated to transfer information from a neuron to a target cell, usually another neuron (Figure 1a)1. Synaptic transmission of information is fast, dynamic, efficient, and tightly regulated (Box 1). Synapses share many properties with intercellular junctions found in other tissues, but differ from all other such junctions because they are inherently asymmetric, transmit information by an extremely fast mechanism, and are highly plastic. Moreover, synapses exhibit diverse properties that are specified by both the pre- and the postsynaptic neuron (e.g., see 2).
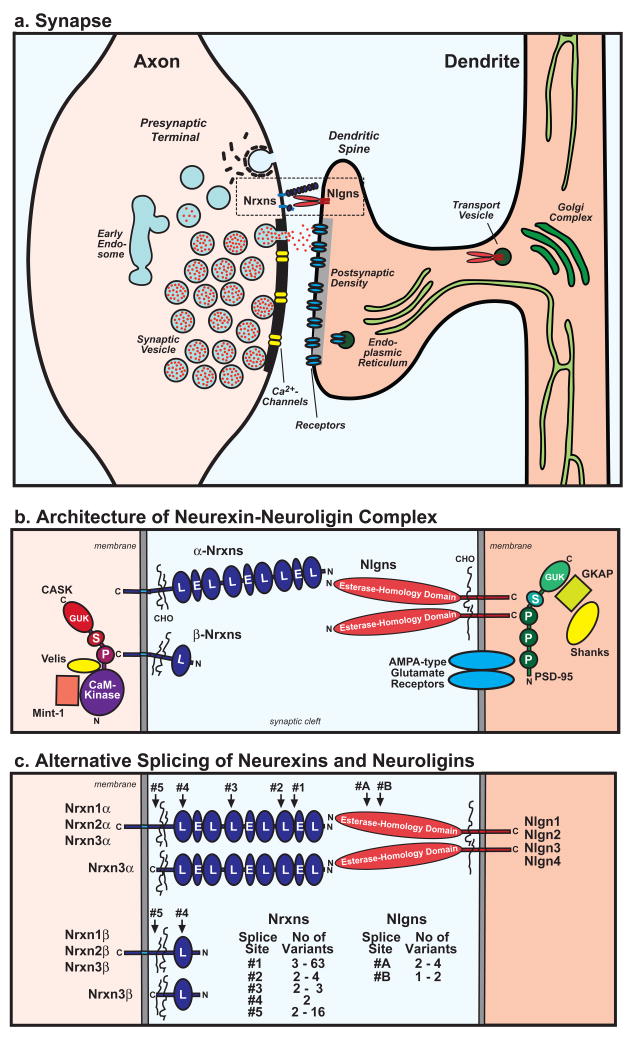
Figure 1. Architecture of the trans-synaptic neurexin/neuroligin complex.
a. Cartoon of the structure of an excitatory synapse and the putative locations of Nrxns and Nlgns in the synapse.
b. Schematic diagram of the Nrxn/Nlgn junction including selected pre- and postsynaptic binding proteins: CASK, Velis, and Mints on the presynaptic side62, and PSD-95 (which binds to AMPA-type glutamate receptors via its first PDZ domain, and to Nlgns via its third PDZ domain64), GKAP, and Shanks on the postsynaptic side. Note that Nrxns and CASK could be, at least in part, also postsynaptic, and that Shank may also be presynaptic (Abbreviations used: C and N = C- and N-termini; CHO = carbohydrate-attachment sequence; CaM Kinase = CaM kinase domain of CASK; E = EGF-like domain; GUK = guanylate-kinase domain; L = LNS-domain; P = PDZ-domain; S = SH3 domain).
c. Alternative splicing of Nrxns and Nlgns. α-Nrxns contain five canonical splice sites (#1 to #5), and β-Nrxns two (#4 and #5). Splice site #1 is C-terminal to the first EGF-like domain, #2, #3, and #4 are at similar positions in the second, fourth and sixth LNS-domain, respectively, and #5 is between the glycosylated CHO-sequence and the transmembrane region. Most alternative splicing involves insertions of small evolutionarily conserved sequences except for splice site #5 which in Nrxn2 involves a large insert (191 residues), and in Nrxn3 involves a at least 16 variants, some of which include stop codons and thus produce secreted Nrxn3 isoforms35. Nlgns contain only two sites of alternative splicing, of which site #B is only present in Nlgn1.
BOX 1. How a synapse works
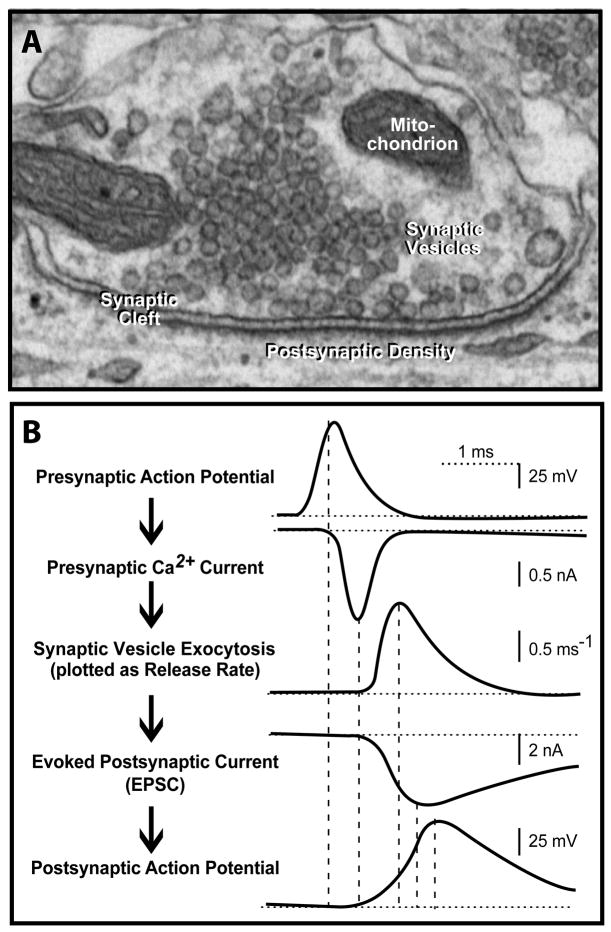
At a synapse, a presynaptic terminal containing abundant synaptic vesicles contacts a postsynaptic cell, usually a neuron (see electron micrograph [Figure a]). When an action potential invades the presynaptic terminal, Ca2+-channels open, and the inflowing Ca2+ triggers fusion of synaptic vesicles with the presynaptic plasma membrane, thereby emptying the neurotransmitters contained in the vesicles into the synaptic cleft90. The neurotransmitters then react with postsynaptic receptors to complete the information transfer. The overall process is incredibly rapid, with each of the major steps (presynaptic synaptic vesicle fusion, postsynaptic signal reception) initiating in <1 ms (Figure b). In addition to this classical mode of synaptic transmission, synapses exhibit other types of signaling that operate on a slower timeframe and serve to regulate the synaptic transmission. Structurally, synapses are characterized by coats that line the intracellular face of the presynaptic plasma membrane (referred to as the active zone because synaptic vesicles undergo fusion here) and the postsynaptic plasma membrane (referred to as the postsynaptic density). Pre- and postsynaptic plasma membranes are always precisely aligned, and are separated by a synaptic cleft of ~20 nm. The cleft contains an undefined proteinaceous material in the middle, and is presumably bridged by synaptic cell-adhesion molecules such as Nrxns and Nlgns that align the pre- and postsynaptic elements and mediate trans-synaptic signaling.
Enormous progress has been made in our understanding of synaptic transmission; much is known about the machinery and functional properties of synapses. However, the molecular mechanisms underlying synapse formation and the specification of synapse diversity are less clear, as are the processes mediating the assembly of synapses into neural circuits3. For neural circuit function, synapse formation and specification are immensely important. The input/output properties of a neural circuit depend on both its pattern of synaptic connectivity (referred to as its wiring diagram), and on the diverse properties of individual synapses in the circuit pattern4. The pattern of connectivity in a circuit is no more important than the properties of the individual synapses comprising the circuit. For example, use-dependent changes in synaptic strength (i.e., synaptic plasticity) can completely alter the relative contributions of different synapses in a circuit, thereby sometimes even reversing its input/output properties as a function of previous use without a change in the wiring diagram (e.g., see 5).
Synapse formation and the specification of synaptic diversity are intricately linked, and likely depend on the actions of synaptic cell-adhesion molecules3. The diversity of synapses is partly due to differences in the composition of their release and receptor machineries, but appears to be largely based on differences in the organization of these machineries. Synapse formation and specification likely involves three steps:
initial recognition of the target cell by the neural growth cone
formation of synaptic junctions with recruitment of synaptic components
maturation of synaptic junctions with specification of circuit-specific properties
Functional assays for synapse formation and tests of specific molecules are difficult (Box 2), hindering identification of the molecular mechanisms involved. These difficulties are confounded by the fact that many candidate molecules, such as cadherins and wnts, perform essential functions during earlier development besides their presumptive role in synapse formation6,7.
BOX 2. Analyzing synaptic cell-adhesion molecules and synapse formation
Gain-of-function approaches
Cell-adhesion assays employ non-neuronal cells expressing cell-adhesion molecules to test whether these molecules are capable of mediating stable cell-cell interactions (e.g., Nrxns/Nlgns).
Artificial synapse formation assays co-culture neurons with non-neuronal cells expressing a cell-adhesion molecule. The assay tests whether the cell-adhesion molecule induces the neurons to form stable junctions with synapse-like properties with the non-neuronal cells38,50,67,68. Many molecules are active in this assay.
Neuronal transfection assays utilize neurons overexpressing a cell-adhesion molecule, and measure the synapse density on the transfected neurons by microscopy53, and synapse function by electrophysiology12. The assay allows a better functional analysis of the effects of a cell-adhesion molecule than the artificial synapse formation assay, but neither assays directly measures synapse formation, and both assays are subject to overexpression artifacts.
Loss-of-function approaches
RNAi experiments in cultured neurons or cultured slices test whether a cell-adhesion molecule is essential for synapse formation or synapse function. When paired with rescue controls, RNAi is ideal, but suffers from three potential limitations. First, it is difficult to target multiple proteins simultaneously with RNAi, and thus hard to address redundancy. Second, for many targets RNAi is simply inefficient, i.e. achieves <75% suppression when measured quantitatively (and not by densitometry of blots). Even successful RNAi is never complete, i.e. achieves >95% suppression. Third, compensatory changes are as likely during RNAi as during KO experiments.
Constitutive genetic manipulations via gene targeting experiments permanently delete or alter expression of a gene to test its overall importance. In addition to the problems listed for RNAi, this approach suffers from the potential for developmental alterations, but allows for complete elimination of expression, and makes organismal analyses possible.
Conditional deletions via gene targeting allow spatially and/or temporally regulated deletion or changes of a cell-adhesion molecule usually involving cre-recombinase mediated genetic changes. A powerful approach that, however, is labor intensive, and limited by the paucity of mouse lines with reproducible, tight, and robust expression of cre-recombinase.
Pharmacological inhibition of a cell-adhesion molecule to cause an acute disruption of function. Potentially the best approach, it is limited by the unavailability of effective agents for almost all cell-adhesion molecules, and by the side effects of many of the agents that do exist.
Gain-of-function approaches for analyzing synapse formation are more sensitive, but harder to interpret. Loss-of-function approaches exhibit greater validity, but are technically more difficult, and can be limited by functional redundancy between multiple genes. Note that noth gain- and loss-of-function approaches, including RNAi and overexpression experiments, suffer from the problem of compensatory changes in the expression, localization, and/or stability of other proteins induced by the experimental manipulation.
Neurexins (Nrxns) and neuroligins (Nlgns) are arguably the best characterized synaptic cell-adhesion molecules, and the only ones for which a specifically synaptic function was established8,9. In the present review, we will describe the role of Nrxns and Nlgns as synaptic cell-adhesion molecules that act in an heretofore unanticipated fashion. We will show that they are required for synapse function, not synapse formation; that they affect trans-synaptic activation of synaptic transmission, but are not essential for synaptic cohesion of the pre- and postsynaptic specializations; and that their dysfunction impairs the properties of synapses and disrupts neural networks without completely abolishing synaptic transmission as10–12. As cell-adhesion molecules, Nrxns and Nlgns probably function by binding to each other and by interacting with intracellular proteins, most prominently PDZ-domain proteins, but the precise mechanisms involved and their relation to synaptic transmission remain unclear. The importance of Nrxns and Nlgns for synaptic function is evident from the dramatic deficits in synaptic transmission in mice lacking Nrxns or Nlgns.
As we will describe, the role of Nrxns and Nlgns in synaptic function almost predestines them for a role in cognitive diseases, such as schizophrenia and autism spectrum disorders (ASDs), that have been resistant to our understanding. One reason for the difficulties in understanding cognitive diseaseas is that they may arise from subtle changes in a subset of synapses in a neural circuit, as opposed to a general impairment of all synapses in all circuits. As a result, the same molecular alteration may produce different circuit changes and neurological symptoms that are then classified as distinct cognitive diseases. Indeed, recent studies have identified mutations in the genes encoding Nrxns and Nlgns as a cause for ASDs, Tourette syndrome, mental retardation, and schizophrenia, sometimes in patients with the same mutation in the same family13–27. Viewed as a whole, current results thus identify Nrxns and Nlgns as trans-synaptic cell-adhesion molecules that mediate essential signaling between pre- and postsynaptic specializations, signaling that performs a central role in the brain’s ability to process information and that is a key target in the pathogenesis of cognitive diseases.
Neurexins: polymorphic synaptic receptors
Black widow spider venom contains a vertebrate-specific toxin called α-latrotoxin. α-Latrotoxin is a large protein that binds to presynaptic receptors and induces massive neurotransmitter release28. Nrxns were originally discovered as receptors for α-latrotoxin29. Nrxns are type 1-membrane proteins that come in two flavors: larger α-Nrxns, and shorter β-Nrxns. α- and β-Nrxns contain different N-terminal extracellular sequences, but identical C-terminal transmembrane regions and cytoplasmic tails (Fig. 1b). Extracellularly, α-Nrxns have six LNS-domains [laminin/neurexin/sex hormone-binding globulin-domain] with three intercalated EGF-like domains, whereas β-Nrxns have a single LNS domain. In addition to α- and β-Nrxns, neurons express Nrxn-related proteins called CASPRs (contactin-associated proteins) that resemble α-Nrxns, but contain additional extracellular domains not found in α-Nrxns30. CASPRs also function as cell-adhesion molecules like Nrxns, but are primarily involved in neuron-glia interactions outside of synapses31. The mammalian genome harbors three Nrxn genes, each of which directs transcription of α- and β-Nrxns from independent promoters32. Furthermore, extensive alternative splicing at five canonical positions generates thousands of Nrxn isoforms (Fig. 1c)33. Conceptually, these isoforms could specify a ‘code’ of interactions at synapses. Consistent with this notion, alternative splicing of Nrxns is regionally regulated, and altered by activity in neurons33,34. Splice sites 1 to 4 (SS#1 to SS#4) involve relatively short sequences (≤30 residues), are located in or adjacent to LNS domains, and are conserved in all three Nrxns. Splice site 5 in Nrxn1 involves only three residues, but in Nrxn2 inserts 191 residues, and in Nrxn3 creates a baroque diversity of sequence inserts that include multiple variants with in-frame stop codons encoding secreted Nrxns33,35.
In situ hybridizations showed that different α- and β-Nrxns are co-expressed in the same class of neurons, but that each type of Nrxn is differentially distributed among different classes of neurons33. Immunofluorescence studies, subcellular fractionations, and the function of Nrxns as α-latrotoxin receptors indicate that Nrxns are located on presynaptic terminals29,36–38. The exact localization of Nrxns remains unclear, however, as deletion of α-Nrxns also causes postsynaptic effects39, and Nrxn is also partly present on postsynaptic sites40.
Neuroligins are neurexin ligands
Nlgns were identified as endogenous Nrxn ligands41. Nlgns are type-I membrane proteins like Nrxns, but exhibit a simpler domain structure and less diversity (Fig. 1). In addition to Nlgns, neurexophilins (neuropeptide-like proteins), and dystroglycan (a cell-adhesion molecule involved in many different types of junctions) are also Nrxn ligands42,43. Different from Nlgns, however, no functional effect of neurexophilin- or dystroglycan-binding to Nrxns has been observed.
The extracellular sequences of Nlgns are composed of a single domain that is homologous to acetylcholinesterases, but lacks critical residues in the active site which is thus disabled (Fig. 1). Nlgns form constitutive dimers via this domain, which is connected to the single transmembrane region by a glycosylated linker sequence. Mammals express four Nlgn genes, with the Nlgn3 and Nlgn4 gene in humans localized to the X-chromosome. In humans, the Nlgn4 gene is complemented on the Y-chromosome by a similar Nlgn5 gene. All Nlgns are alternatively spliced at a single canonical position (referred to as SS#A); in addition, Nlgn1 is alternatively spliced at a second position (called SS#B) 44,45. Most Nrxns and Nlgns are conserved evolutionarily in vertebrates, with more distant relatives in invertebrates32,46. Interestingly, Nlgn4 diverged rapidly in rodents, suggesting that at least some Nlgns are subject to a lesser evolutionary constraint47. Sequence comparisons indicate that Nlgn1, Nlgn3, and Nlgn4/5 are more similar to each other than to Nlgn2. All Nlgns are enriched in postsynaptic densities as judged by subcellular localizations. Immunocytochemistry revealed that Nlgn1 and Nlgn2 are exclusively localized to excitatory and inhibitory synapses, respectively, whereas Nlgn3 may be present in both48–51.
Nlgns bind to both α- and β-Nrxns with nanomolar affinities; binding involves the sixth LNS-domain of α-Nrxns which corresponds to the only LNS-domain of β-Nrxns52. The binding affinities differ characteristically between various pairs of Nlgns and Nrxns, and are controlled by alternative splicing of both Nrxns and Nlgns (Figure 1c)45,52,53. SS#B of Nlgn1 represents a master switch for Nrxn binding – inclusion of only 8 residues in this site restricts Nlgn1 binding to β-Nrxns lacking an insert in SS#4, whereas exclusion of these 8 residues allows binding of both α- and β-Nrxns independent of SS#4 (45). The Nlgn1 splice variant containing an insert in SS#B predominates, indicating that most Nlgn1is specific for β-Nrxns lacking an insert in SS#4, whereas all other Nlgns react with both α- and β-Nrxns. SS#A of all Nlgns also regulates Nrxn binding, but the effect is smaller52. In Nrxns, SS#4 (which is located in the last LNS-domain) not only controls binding of β-Nrxns to Nlgn1 containing an insert in SS#B (see above), but also modulates the affinity of α- andβ-Nrxns for Nlgns lacking an insert in SS#B. Thus, the current data suggest that Nrxn/Nlgn binding is governed by a hierarchical code that depends on which principal isoforms are expressed, and which splice variants are used.
The trans-synaptic neurexin/neuroligin complex
Nrxns and Nlgns are thought to form a trans-synaptic complex that is coated on both sides by PDZ-domain containing proteins (Fig. 1b). The crystal structure of the Nrxn1/Nlgn1 complex (without inserts in the Nrxn SS#4 and Nlgn1 SS#B) revealed that the Nrxn LNS-domain attaches with a large contact area to the lateral sides of the Nlgn esterase-homology domain, opposite to the position of the crippled active site (Fig. 2)54–56. In the structure of crystals that were grown in the presence of Ca2+, two fully occupied Ca2+-binding sites were found that are coordinated by ligands from both proteins55. Mapping of the alternative splicing sites into the structure shows that SS#B is included in the binding interface, and that SS#A of Nlgn1 and SS#4 of Nrxn1 are close by, providing an explanation for the effect of alternative splicing of these sites on the Nrxn/Nlgn binding affinity. Indeed, direct comparison of the crystal structures of β-Nrxn LNS domains containing and lacking inserts in SS#4 supports this conclusion by revealing major conformational changes induced by this alternative splicing event57,58.
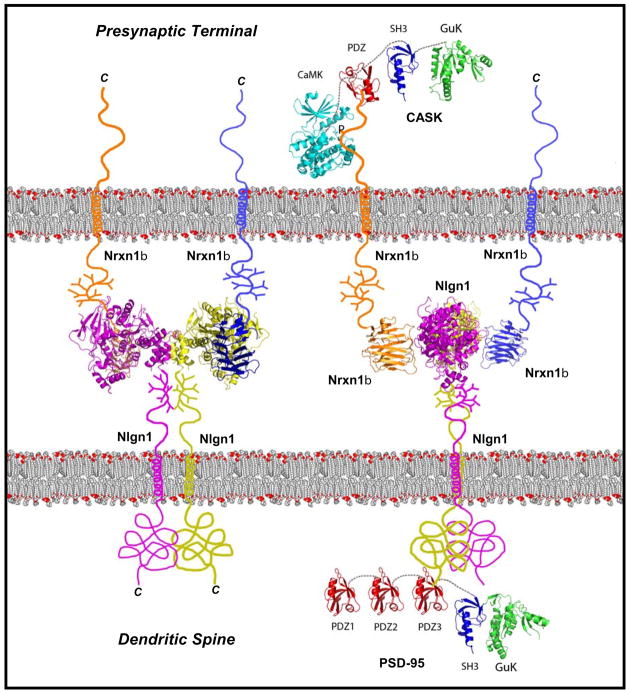
Figure 2. Atomic model of the trans-synaptic complex formed by neurexin-1β and Neuroligin-1.
The Nrxn1β/Nlgn1 complex is shown in two orientations: Left, en face with the Nrxn1β LNS-domain on top of the Nlgn1 esterase-domain to illustrate the Nlgn1 dimer; right, in a 90° rotation to illustrate the sideways attachment of the Nrxn1β LNS-domains onto the Nlgn1 esterase-domains (structures are from the following PDB entries: 3BIW Nrxn/Nlgn, 1JXO PSD95 SH3 GuK, 1BE9 PSD95 PDZ, 3C0I CASK CAMK, 1KGD CASK GuK, 1KWA CASK PDZ; abbreviations as in Fig. 1; courtesy of D. Arac and A. Brunger, Stanford U.). The two orientations of the Nrxn1β/Nlgn1 complex illustrates the spatial arrangement and relative sizes of the Nrxn LNS-domains and the Nlgn esterase domain in a synaptic cleft. Other Nrxn and Nlgn isoforms for which no full structure is available, including α-Nrxns, would presumably have a similar arrangement except that the additional LNS-domain in α-Nrxns would occupy a larger space in the synaptic cleft.
The shape of the Nrxn/Nlgn complex suggests that it forms an interaction layer in the center of the synaptic cleft, with the C-terminal sequences emerging from the complex in opposite directions (Fig. 2). This interaction layer – which may contribute to the electron-dense material observed in the synaptic cleft by electron microscopy – is separated from the pre- and postsynaptic plasma membranes by the glycosylated linker sequences that are present in Nrxns and Nlgns just outside of the membrane. These glycosylated sequences could serve as a ‘cuff’ that creates a distance between the interaction layer and the plasma membranes, and forces the extracellular domains to project into the synaptic cleft away from the membrane.
The cytoplasmic sequence of Nrxns contains a C-terminal binding site for class-II PDZ-domains that binds to the PDZ-domain of CASK and related proteins, and a membrane-proximal binding site for protein 4.1 59,60. CASK is a MAGUK protein (for “membrane-associated guanylate-kinase protein”) containing a PDZ-, SH3- and guanylate kinase-domain. CASK is an unusual MAGUK, however, because the PDZ-, SH3- and guanylate kinase-domains account for only its C-terminal half; its N-terminal half is occupied by a Ca2+/calmodulin-dependent protein kinase (CaM Kinase) domain that is absent from other MAGUKs. The CASK CaM kinase domain contains substitutions in canonical residues that coordinate Mg2+ in CaM kinases, suggesting that it may be catalytically inactive. However, recent evidence indicates that CASK may be the first described Mg2+-independent kinase that phosphorylates Nrxn1 in vivo61. In addition, CASK nucleates the assembly of actin on the Nrxn cytoplasmic sequence by simultaneously binding to protein 4.1 60. Finally, CASK interacts with Velis/MALs proteins (homologs of C. elegans Lin-7) and with Mints/X11 proteins to form a tight trimeric complex62,63. Besides Nrxns, CASK binds to other cell-surface proteins including CASPRs, and likely performs analogous functions there. Deletion of CASK in mice causes a lethal phenotype that includes synaptic abnormalities, indicating that CASK is an important molecule64. It is likely that CASK acts as a component of a signal transduction cascade that translates extracellular interactions of cell-surface proteins into an intracellular response by modulating the actin cytoskeleton and phosphorylating target proteins.
Like Nrxns, Nlgns bind to intracellular PDZ-domain proteins, but different from Nrxns, Nlgns bind to class-I PDZ-domains such as those contained in PSD-95, a postsynaptic MAGUK protein65. PSD-95 and its homologs are centrally involved in recruiting glutamate receptors at postsynaptic sites66. Similar to CASK, PSD-95 binds to intracellular adaptor proteins, especially GKAP which in turn binds to Shank (Fig. 1b). A possible role of these interactions is to recruit postsynaptic adaptor proteins to the site of synaptic junctions. Due to their binding to PDZ-domain proteins, the junction formed by Nrxns and Nlgns resembles the architecture of tight junctions, but differs from them in that the Nrxn/Nlgn junction is asymmetric in all of its components.
Function of neuroligins and neurexins
Initial evidence that Nlgns function at synapses came from ingenious experiments demonstrating that Nlgns expressed in a non-neuronal cell can induce co-cultured neurons to form presynaptic specializations onto the non-neuronal cell (Box 2)67. This finding was amplified by complementary experiments demonstrating that Nrxns, when expressed in a non-neuronal cell, can induce formation of postsynaptic specializations in co-cultured neurons50,68. Moreover, direct overexpression of Nlgns in transfected neurons caused an increase in synapse numbers on these neurons69.
Together, these studies indicated that Nlgns and Nrxns may induce synapse formation. However, analysis of knockout (KO) mice surprisingly revealed that Nlgns and α-Nrxns are essential for synaptic function, not synapse formation10–12. Triple KO mice lacking Nlgn1, Nlgn2 and Nlgn3 die at birth, but exhibit relatively normal synapse numbers with an apparently normal ultrastructure. Electrophysiological analyses in acute brain slices showed that these mice display a severe impairment of synaptic transmission11. Although single Nlgn1 or Nlgn2 KO mice are viable and fertile, electrophysiological analysis also uncovered significant synaptic dysfunctions in these mice12. Consistent with the localizations of Nlgn1 and Nlgn2 to excitatory and inhibitory synapses, respectively, excitatory synapses exhibited impairments in NMDA-receptor mediated signaling in the Nlgn1 KO mice, whereas the Nlgn2 KO mice displayed deficits in inhibitory synaptic transmission12.
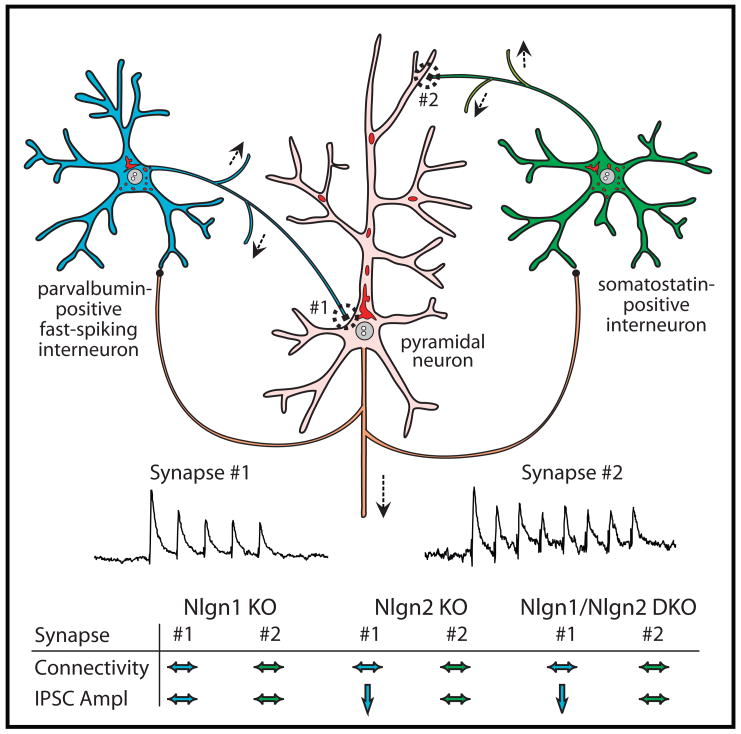
The KO analysis appears to contradict the in vitro assays showing that Nlgns induce synapses in the artificial synapse formation assay and the neuronal transfection assay (see Box 2 explaining the various approaches). However, the assays using cultured neurons do not directly measure synapse induction – rather, they measure an increase in synapse numbers after a particular manipulation. In these assays, the molecules tested could simply act by inducing signal transduction events that stabilize otherwise transient, tentative synaptic contacts. In support of this interpretation, and consistent with the KO results, the ability of Nlgns to increase the number of synapses in a transfected neuron can be decreased by inhibition of synaptic activity, which has no effect on the expression and localization of the transfected Nlgns12. More conclusively, paired recordings from inhibitory neurons in the somatosensory cortex of Nlgn KO mice demonstrated that deletion of Nlgns did not decrease the number of synaptic connections (Fig. 3). Instead, deletion of Nlgn2 (but not of Nlgn1) selectively lowered the strength of GABAergic synapses formed by fast-spiking, parvalbumin-containing interneurons, but not of GABAergic synapses formed by somatostatin-containing interneurons. Together, these data suggest that Nlgns function in the maturation of synaptic junctions with specification of circuit-specific properties, but not in the initial formation of synaptic junctions. This conclusion is consistent with the finding that a partial knockdown of Nlgns in cultured neurons produced a partial decrease in synapse numbers that could have been a secondary consequence of a decrease in synaptic function70.
Figure 3. Differential effects of neuroligin-1 and -2 deletions on inhibitory synapses in the somatosensory cortex.
Connections of parvalbumin-positive fast-spiking interneurons (blue) and of somatostatin-positive interneurons (green) with exctitatory pyramidal neurons (pink) are shown schematically. The connectivity (as measured in paired recordings as % success) and amplitude (pA) of the inhibitory synapses of the interneurons onto the pyramidal neuron are shown for wild-type (WT), Nlgn1 KO, Nlgn2 KO, and Nlgn1/Nlgn2 double KO (DKO) mice (* = statistically significantly different from WT; modified from J. Gibson, K. Huber, and T.C. Südhof, unpublished).
The activities of Nrxns have been more difficult to characterize than those of Nlgns. The lack of high-affinity antibodies, the complexity of the Nrxn isoforms, and the challenges in analyzing presynaptic function have contributed to this difficulty. At present, it even remains uncertain whether Nrxns are exclusively presynaptic, or whether at least some Nrxns are postsynaptic. Analysis of KO mice lacking all α-Nrxns but still containing β-Nrxns uncovered a phenotype that is similar to that of Nlgn KO mice (note that β-Nrxn KO or α-/β-Nrxn KO mice were not yet analyzed). Deletions of individual α-Nrxns cause only moderate increases in mortality in mice, but deletions of two of the three α-Nrxns increase postnatal mortality dramatically, and deletions of all three α-Nrxns lead to invariable neonatal fatality in mice10. Again, synapse numbers and their ultrastructure are relatively normal in α-Nrxn KO mice, but synapse function is severely impaired. This impairment is both pre- and postsynaptic, but is most significantly observed in action potential-driven neurotransmitter release, which is severely depressed, largely due to a loss of presynaptic Ca2+-channel function71. Postsynaptically, deletion of α-Nrxns caused a decrease in NMDA- but not AMPA-receptor dependent synaptic responses, similar to the deletion of Nlgn1 39. The overall analysis of the α-Nrxn KO mice indicates that deletion ofα-Nrxns disorganizes synapses. These findings characterize α-Nrxns, like Nlgns, as synaptic cell-adhesion molecules essential for proper assembly of synapses into a fully functional unit, but not for the initial formation of synapses. Nrxns may also be globally required for the organization of secretory systems since α-Nrxn KO mice exhibit an additional major change in neuroendocrine neurons72.
How precisely do Nrxns and Nlgns function in synapses? A plausible hypothesis is that trans-synaptic cell adhesion mediated by Nrxns and Nlgns – either by binding to each other, or by binding to other ligands – triggers pre- and postsynaptic signal transduction events that activate synaptic function and specify synaptic properties. Without this activation, synapses assemble, but do not work properly. The activation is clearly not a simple yes-or-no switch. Instead, Nrxns and Nlgns shape synaptic efficacy and plasticity. Moreover, a synaptic transmission-specific element is involved at least for Nlgns. How this synapse activation may occur is unclear. Nlgn binding to Nrxns does not induce dimerization of Nrxns analogous to other receptor dimerization-dependent signaling cascades because the crystal structure reveals that the two Nrxns bound to a Nlgn dimer are distant monomers (Fig. 2)54–56. The most parsimonious mechanism for this activation would be that Nrxns and Nlgns recruit ‘coats’ to the junction, coats that may consist of PDZ-domain proteins, actin filaments, and/or involve other types of interactions. For example, Nlgn-binding to Nrxns may stimulate CASK-dependent phosphorylation of Nrxns and other substrates, but no direct evidence for this mechanism exists.
Neuroligins and neurexins in autism
ASDs are common and enigmatic diseases. ASDs comprise classical idiopathic autism, Asperger’s syndrome, Rett syndrome, and pervasive developmental disorder not otherwise specified73,74. Moreover, several other genetic disorders, such as Down syndrome, Fragile-X Mental Retardation, and tuberous sclerosis, are frequently associated with autism. Such syndromic forms of autism and Rett syndrome are usually more severe due to the nature of the underlying diseases. The key features of ASDs are difficulties in social interactions and communication, language impairments, a restricted pattern of interests, and/or stereotypic and repetitive behaviors. Mental retardation (~70% of cases) and epilepsy (~30% of cases) are frequently observed; in fact, the observation of epilepsy in patients with ASDs has fueled speculation that autism may be caused by an imbalance of excitatory vs. inhibitory synaptic transmission. In rare instances, idiopathic autism is associated with specialized abilities, for example in music, mathematics, or memory. The relation of ASDs to other cognitive diseases such as schizophrenia and Tourette’s syndrome is unclear. As we will see below with the phenotypes caused by mutations in Nlgns and Nrxns, the boundaries between the various disorders may not be as real as the clinical manifestations suggest.
A key feature of ASDs is that they typically develop before 2–3 years of age73,74. ASDs thus affect brain development relatively late, during the time of human synapse formation and maturation. Consistent with this time course, few anatomical changes are associated with ASDs75. An increase in brain size was repeatedly reported76, but is not generally agreed upon75. Thus, similar to other cognitive diseases, ASDs are not a disorder of brain structure but of brain function. Among cognitive diseases, ASDs are the most heritable (~ 80%), suggesting that they are largely determined by genes and not the environment. ASDs exhibit a male:female ratio of approximately 4:1, indicating that ASDs involve the X-chromosome directly, or that the penetrance of pathogenic genes is facilitated in males73,74.
Mutations in many genes have been associated with familial ASDs. A consistent observation emerging from recent studies is the discovery of mutations in the genes encoding Nrxn1, Nlgn3, and Nlgn4. Specifically, seven point mutations, two distinct translocation events, and four different large-scale deletions in the Nrxn1 gene were detected in autistic patients13–18. Ten different mutations in the Nlgn4 gene were observed (2 frameshifts, 5 missense mutations, and 3 internal deletions), and a single mutation in the Nlgn3 gene (the R451C substitution)21–24. Besides these mutations, five different larger deletions of X-chromosomal DNA that includes the Nlgn4 locus (referred to as copy-number variations) were detected in autism patients18,25–27.
In addition to the Nrxn/Nlgn complex, mutations in the gene encoding Shank3 – an intracellular scaffolding protein that binds indirectly to Nlgns via PSD-95 and GKAP (Fig. 1)66 – may also be a relatively frequent occurrence in ASDs. An astounding 18 point mutations were detected in the Shank3 gene in autistic patients, in addition to several cases containing CNVs that cover the gene18,77–82. Indeed, the so-called terminal 22q deletion syndrome is a relatively frequent occurrence that exhibits autistic features, which have been correlated with the absence of the Shank3 gene normally localized to this chromosome section. Shank3 is particularly interesting because it not only indirectly interacts with Nlgns, but also directly binds to CIRL/Latrophilins which in turn constitute α-latrotoxin receptors similar to Nrxns, suggesting a potential functional connection between Shank3 and Nrxns83.
Overall, the description of the various mutations in the Nrxn/Nlgn/Shank3 complex appears to provide overwhelming evidence for a role of this complex in ASDs, given the fact that in total, these mutations account for a significant proportion of autism patients. It should be noted, however, that two issues give rise to skepticism to the role of this complex in ASDs.
First, at least for some of the mutations in this complex, non-symptomatic carriers were detected in the same families in which the patients with the mutations were found. Whereas the Nlgn3 and Nlgn4 mutations appear to be almost always penetrant in males, and even female carriers with these mutations often have a phenotype, the Shank3 point mutations in particular were often observed in non-symptomatic siblings77,78. Thus, these mutations may only increase the chance of autism, but not actually cause autism.
Second, the same mutations can be associated with quite different phenotypes in different people. For example, a microdeletion in Nlgn4 was found to cause severe autism in one brother, but Tourette’s syndrome in the other26. This raises the issue whether the ‘autism’ observed in patients with mutations in these genes is actually autism, an issue that could also be rephrased as the question of whether autism is qualitatively distinct from other cognitive diseases, as opposed to a continuum of cognitive disorders. In support of the latter idea, two different deletions of Nrxn1α have also been observed in families with schizophrenia19,20, indicating that there is a continuum of disorders that involves dysfunctions in synaptic cell adhesion and manifests in different ways. Conversely, very different molecular changes may produce a similar syndrome, as exemplified by the quite different mutations that are associated with ASDs84.
At present, the relation between the Nrxn/Nlgn synaptic cell-adhesion complex and ASDs is tenuous. On one hand, many of the mutations observed in familial ASD are clearly not polymorphisms but deleterious, as evidenced by the effect of these mutations on the structure or expression of the corresponding genes, and by the severe autism-like phenotypes observed in Nlgn3 and Nlgn4 mutant mice85–87. On the other hand, the nonlinear genotype/phenotype relationship in humans, evident from the only 70–80% heritability and from the occasional presence of mutations in non-symptomatic individuals, requires explanation. Elucidating the underlying mechanisms for this incomplete genotype/phenotype relationship is a promising avenue to insight into the genesis of autism. Furthermore, in addition to the link of Nrxn1α mutations to schizophrenia19,20, linkage studies have connected Nrxn3 to different types of addiction88,89. It is possible that because of the nature of their function, mutations in genes encoding Nrxns and Nlgns constitute hotspots for human cognitive diseases.
Dissecting autism in mouse models
One way to address the question whether the mutations in Nrxns and Nlgns observed in human patients are directly related to ASDs is to test whether the same mutations elicit a significant phenotype in an animal. Such experiments were performed in mice for two Nlgn mutations, the Nlgn3 R451C substitution and the Nlgn4 loss-of-function mutation86,87.
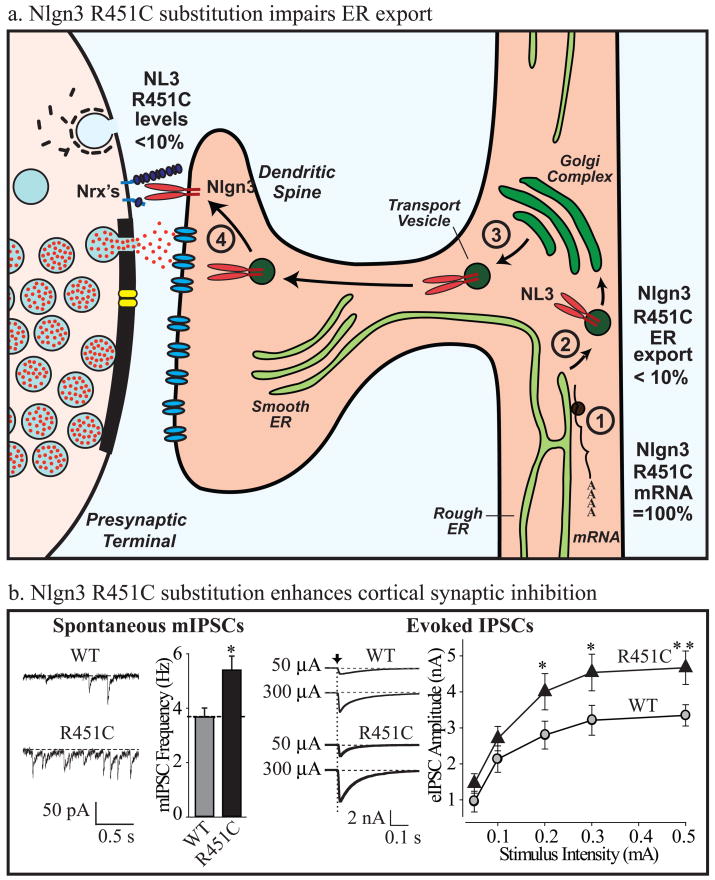
The R451C knockin mouse exhibits a striking phenotype that shares some, but not all features – as far as analyzable – with human ASD patients. Behaviorally, the mice display normal motor and anxiety behaviors, exhibit a modest impairment in social interactions, and demonstrate a large increase in spatial learning capability86. Although this behavioral phenotype is somewhat satisfying because it is reminiscent of the ‘savant’ variant of autism and indicates that the R415C substitution did not impair cognitive function in the mice, this phenotype is also puzzling because the human patients with the R451C substitution suffer from mental retardation21. Electrophysiologically, the R451C mutant mice displayed an increase in inhibitory synaptic transmission in the somatosensory cortex, consistent with the notion that a change in the excitatory/inhibitory balance contributes to the phenotype (Fig. 4). Interestingly, the R451C mutation appears to be a gain-of-function and not a loss-of-function mutation because Nlgn3 KO mice did not exhibit any of the phenotypes associated with the R451C knockin mice86. This is surprising because the R451C mutation depressed the Nlgn3 protein levels in the knockin mice by ~90%; thus, it is the remaining 10% of the mutant protein that produced a dramatic change in synaptic transmission (Fig. 4).
Figure 4. The R451C substitution in Nlgn3 impairs Nlgn3 synthesis but enhances inhibitory synapses.
a. Schematic illustration of the effect of the R451C mutation on Nlgn3 synthesis. The mutation does not alter Nlgn3 mRNA levels (1), but decreases the export of Nlgn3 from the endoplasmic reticulum (2)85. As a result, the concentration of R451C-mutant Nlgn3 that is exported from the Golgi complex (3) and inserted into synapses (4) is <10% of the wild-type Nlgn3 concentration86.
b. Despite decreasing the Nlgn3 concentration, the R451C mutation produces a synaptic gain-of-function effect in inhibitory synapses in the somatosensory cortex. The figure illustrates in two examples increased inhibitory synaptic activity in R451C mutant mice: by measurements of spontaneous ‘miniature’ synaptic events (left), or by measurements of evoked synaptic responses (right). Each example depicts representative electrophysiology traces on the left, and summary graphs on the right (modified from ref. 86; * = statistically significantly different from WT). Note that Nlgn3-deficient synapses from KO mice do not exhibit this phenotype86.
The gain-of-function action of the R451C mutation differs from that of the Nlgn4 deletion which also caused an autism-like phenotype in KO mice87, but clearly represents a loss-of-function mutation. These observations may provide an explanation for the finding of multiple Nlgn4 mutations in autism patients, but only a single Nlgn3 mutation, despite the fact that both genes are X-chromosomal. It seems likely that only a loss-of-function of Nlgn4 but not of Nlgn3 produces autistic symptoms, and that the R451C mutation in Nlgn3 was a accidental gain-of-function mutation that occurred only in a single family. Moreover, these observations provide further support for the notion that Nlgns and Nrxns are activators of synapse function, not simply building blocks of synapses, where small changes in Nlgn function can induce massive changes in the neural network.
Perspective
Discovery of the Nrxn/Nlgn cell-adhesion system opened up new avenues to the understanding of synapses and cognitive disease, but also raised many new questions. For example, do Nrxns and Nlgns only act by binding to each other – in fact, do they actually function by binding to each other at all? Do different Nrxns - either different principal isoforms, or different splice variants - perform distinct functions? α- and β-Nrxns cannot be functionally redundant because the α-Nrxn deletion causes a massive phenotype that cannot be compensated for by the remaining β-Nrxns10, so what else do α-Nrxns do? Uncovering answers to these and many other questions will provide insight not only into the fundamental mechanisms of synaptic cell-adhesion, but also into the molecular determinants of neural circuit properties. Moreover, the apparent involvement of Nrxns and Nlgns in different cognitive diseases begs the question whether these diseases represent truly distinct entities, or a continuum of mental dysfunctions. With the emerging findings on the genetics of cognitive diseases, a molecular nosology of cognitive disesases may become possible. Furthermore, if a participation of Nrxns and Nlgns in cognitive diseases is confirmed in more extensive studies, new diagnostic and therapeutic possibilities may emerge, for example by selectively modulating the Nrxn/Nlgn interaction. Again, much more work will be required to explore these possibilities, but the present results are encouraging in this direction as well.
Figure 5.
a, electron micrograph of a synapse (courtesy of Dr. X. Liu, UT Southwestern)
b, time course of synaptic transmission as measured electrophysiologically. The five sequential steps are indicated, as deduced from measurements in the Calyx of Held synapse (modified from ref. 90).
References
- 1.Cowan WM, Südhof TC, Stevens CF, editors. Synapses. Johns Hopkins University Press; 2000. [Google Scholar]
- 2.Rozov A, Burnashev N, Sakmann B, Neher E. Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J Physiol. 2001;531(Pt 3):807–826. doi: 10.1111/j.1469-7793.2001.0807h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dityatev A, El-Husseini A, editors. Molecular Mechanisms of synaptogenesis. Springer Verlag; New York: 2006. [Google Scholar]
- 4.Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- 5.Linkenhoker BA, von der Ohe CG, Knudsen EI. Anatomical traces of juvenile learning in the auditory system of adult barn owls. Nat Neurosci. 2005;8:93–98. doi: 10.1038/nn1367. [DOI] [PubMed] [Google Scholar]
- 6.Arikkath J, Reichardt LF. Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends Neurosci. 2008 doi: 10.1016/j.tins.2008.07.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salinas PC, Zou Y. Wnt Signaling in Neural Circuit Assembly. Annu Rev Neurosci. 2008;31:339–358. doi: 10.1146/annurev.neuro.31.060407.125649. [DOI] [PubMed] [Google Scholar]
- 8.Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29:21–29. doi: 10.1016/j.tins.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Missler M, et al. α-Neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. Shows that deletion of α-Nrxns in mice causes a lethal presynaptic release phenotype and a loss of presynaptic Ca2+-channel function. [DOI] [PubMed] [Google Scholar]
- 11.Varoqueaux F, et al. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. Describes mice lacking neuroligin-1 to -3, demonstrating that deletion of Nlgns is lethal because of impaired synaptic transmisison, not a decrease in synapse numbers. [DOI] [PubMed] [Google Scholar]
- 12.Chubykin AA, et al. Activity-dependent validation of excitatory vs. inhibitory synapses by neuroligin-1 vs. neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. Demonstrates that the synapse-enhancing activity of overexpressed neuroligin-1 depends on NMDA-receptor signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng J, et al. High frequency of neurexin 1β signal peptide structural variants in patients with autism. Neurosci Lett. 2006;409:10–13. doi: 10.1016/j.neulet.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Szatmari P, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HG, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan J, et al. Neurexin 1α structural variants associated with autism. Neurosci Lett. 2008 doi: 10.1016/j.neulet.2008.04.074. [DOI] [PubMed] [Google Scholar]
- 17.Zahir FR, et al. A patient with vertebral, cognitive and behavioural abnormalities and a de novo deletion of NRXN1alpha. J Med Genet. 2008;45:239–243. doi: 10.1136/jmg.2007.054437. [DOI] [PubMed] [Google Scholar]
- 18.Marshall CR. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirov G. Comparative genome hybridization suggests a role for NRXN1 and APBA2 in schizophrenia. Hum Mol Genet. 2008;17:458–465. doi: 10.1093/hmg/ddm323. [DOI] [PubMed] [Google Scholar]
- 20.Walsh T, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 21.Jamain S, et al. Mutations of the X-linked genes encoding Nlgns NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. Describes the first mutations in neuroligin genes in patients with familial ASD, initiating a search for mutations in other families in Nlgns or their associated molecules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laumonnier F, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–527. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan J, et al. Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Mol Psychiatry. 2005;10:329–332. doi: 10.1038/sj.mp.4001629. [DOI] [PubMed] [Google Scholar]
- 24.Talebizadeh Z, et al. Novel splice isoforms for NLGN3 and NLGN4 with possible implications in autism. J Med Genet. 2006;43:e21. doi: 10.1136/jmg.2005.036897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chocholska S, Rossier E, Barbi G, Kehrer-Sawatzki H. Molecular cytogenetic analysis of a familial interstitial deletion Xp22.2–22.3 with a highly variable phenotype in female carriers. Am J Med Genet A. 2006;140:604–610. doi: 10.1002/ajmg.a.31145. [DOI] [PubMed] [Google Scholar]
- 26.Lawson-Yuen A, Saldivar JS, Sommer S, Picker J. Familial deletion within NLGN4 associated with autism and Tourette syndrome. Eur J Hum Genet. 2008;16:614–618. doi: 10.1038/sj.ejhg.5202006. [DOI] [PubMed] [Google Scholar]
- 27.Macarov M, et al. Deletions of VCX-A and NLGN4: a variable phenotype including normal intellect. J Intellect Disabil Res. 2007;51:329–333. doi: 10.1111/j.1365-2788.2006.00880.x. [DOI] [PubMed] [Google Scholar]
- 28.Ushkaryov YA, Rohou A, Sugita S. α-Latrotoxin and its receptors. Handb Exp Pharmacol. 2008;184:171–206. doi: 10.1007/978-3-540-74805-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ushkaryov YA, Petrenko AG, Geppert M, Südhof TC. Neurexins: Synaptic cell surface proteins related to the α-latrotoxin receptor and laminin. Science. 1992;257:40–56. doi: 10.1126/science.1621094. Reports the discovery of Nrxns as presynaptic α-latrotoxin receptors. [DOI] [PubMed] [Google Scholar]
- 30.Missler M, Südhof TC. Neurexins: three genes and 1001 products. Trends in Genetics. 1998;14:20–25. doi: 10.1016/S0168-9525(97)01324-3. [DOI] [PubMed] [Google Scholar]
- 31.Peles E, Salzer JL. Molecular domains of myelinated axons. Curr Opin Neurobiol. 2000;10:558–565. doi: 10.1016/s0959-4388(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 32.Tabuchi K, Südhof TC. Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics. 2002;79:849–859. doi: 10.1006/geno.2002.6780. [DOI] [PubMed] [Google Scholar]
- 33.Ullrich B, Ushkaryov YA, Südhof TC. Cartography of neurexins: More than 1000 isoforms generated by alternatice splicing and expressed in distinct subsets of neurons. Neuron. 1995;14:497–507. doi: 10.1016/0896-6273(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 34.Rozic-Kotliroff G, Zisapel N. Ca2+-dependent splicing of neurexin IIα. Biochem Biophys Res Commun. 2007;352:226–230. doi: 10.1016/j.bbrc.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Ushkaryov YA, Südhof TC. Neurexin IIIα: Extensive alternative splicing generates membrane-bound and soluble forms in a novel neurexin. Proc Natl Acad Sci USA. 1993;90:6410–6414. doi: 10.1073/pnas.90.14.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugita S, Khvotchev M, Südhof TC. Neurexins are functional α-latrotoxin receptors. Neuron. 1999;22:489–496. doi: 10.1016/s0896-6273(00)80704-7. [DOI] [PubMed] [Google Scholar]
- 37.Berninghausen O, et al. Neurexin Iβ and neuroligin are localized on opposite membranes in mature central synapses. J Neurochem. 2007;103:1855–1863. doi: 10.1111/j.1471-4159.2007.04918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chubykin AA, Liu X, Comoletti D, Tsigelny I, Taylor P, Südhof TC. Dissection of synapse induction by neuroligins: Effect of a neuroligin mutation associated with autism. J Biol Chem. 2005;280:22365–22374. doi: 10.1074/jbc.M410723200. [DOI] [PubMed] [Google Scholar]
- 39.Kattenstroth G, Tantalaki E, Südhof TC, Gottmann K, Missler M. Postsynaptic N-methyl-D-aspartate receptor function requires α-neurexins. Proc Natl Acad Sci USA. 2004;101:2607–2612. doi: 10.1073/pnas.0308626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taniguchi H, et al. Silencing of neuroligin function by postsynaptic neurexins. J Neurosci. 2007;27:2815–2824. doi: 10.1523/JNEUROSCI.0032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ichtchenko K, et al. Neuroligin 1: A splice-site specific ligand for β-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. Identification of Nlgns as postsynaptic neurexin ligands. [DOI] [PubMed] [Google Scholar]
- 42.Petrenko AG, et al. Structure and evolution of neurexophilin. J Neurosci. 1996;16:4360–4369. doi: 10.1523/JNEUROSCI.16-14-04360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugita S, et al. A stoichiometrix complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154:435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ichtchenko K, Nguyen T, Südhof TC. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem. 1996;271:2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- 45.Boucard A, Chubykin AA, Comoletti D, Taylor P, Südhof TC. A splice-code for trans-synaptic cell adhesion mediated by binding of Neuroligin 1 to α- and β-Neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 46.Rissone A, et al. Comparative genome analysis of the neurexin gene family in Danio rerio: insights into their functions and evolution. Mol Biol Evol. 2007;24:236–252. doi: 10.1093/molbev/msl147. [DOI] [PubMed] [Google Scholar]
- 47.Bolliger MF, et al. Usually rapid evolution of Neuroligin-4 in mice. Proc Natl Acad Sci USA. 2008;105:6421–6426. doi: 10.1073/pnas.0801383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song J-Y, Ichtchenko K, Südhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci USA. 1999;96:1100–1125. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- 50.Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via Nlgns. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Budreck EC, Scheiffele P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur J Neurosci. 2007;26:1738–1748. doi: 10.1111/j.1460-9568.2007.05842.x. [DOI] [PubMed] [Google Scholar]
- 52.Comoletti D, et al. Gene selection, alternative splicing, and post-translational processing regulate neuroligin selectivity for β-neurexins. Biochemistry. 2006;45:12816–12827. doi: 10.1021/bi0614131. [DOI] [PubMed] [Google Scholar]
- 53.Chih B, Gollan L, Scheiffele P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51:171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Fabrichny IP, et al. Neuron. Vol. 56. 2007. Structural analysis of the synaptic protein neuroligin and its β-neurexin complex: determinants for folding and cell adhesion; pp. 979–991. References 54–56 report the first atomic structures of the neuroligin-neurexin complex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arac D, et al. Structures of Neuroligin-1 complex reveal specific protein-protein and protein-Ca2+ interactions. Neuron. 2007;56:992–1003. doi: 10.1016/j.neuron.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 56.Chen X, Liu H, Shim AH, Focia PJ, He X. Structural basis for synaptic adhesion mediated by neuroligin-neurexin interactions. Nat Struct Mol Biol. 2008;15:50–56. doi: 10.1038/nsmb1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen KC, et al. Regulation of neurexin 1β tertiary structure and ligand binding through alternative splicing. Structure. 2008;16:422–431. doi: 10.1016/j.str.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koehnke J, et al. Crystal structures of β-neurexin 1 and β-neurexin 2 ectodomains and dynamics of splice insertion sequence 4. Structure. 2008;16:410–421. doi: 10.1016/j.str.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hata Y, Butz S, Südhof TC. CASK: A novel dlg/PSD95 homologue with an N-terminal CaM kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biederer T, Südhof TC. CASK and protein 4.1 support F-actin nucleation on neurexins. J Biol Chem. 2001;276:47869–47876. doi: 10.1074/jbc.M105287200. [DOI] [PubMed] [Google Scholar]
- 61.Mukherjee K, et al. CASK functions as a neurexin-kinase by an unusual mechanism. Cell. 2008;133:328–339. doi: 10.1016/j.cell.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Butz S, Okamoto M, Südhof TC. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94:773–782. doi: 10.1016/s0092-8674(00)81736-5. [DOI] [PubMed] [Google Scholar]
- 63.Borg JP, et al. Molecular analysis of the X11-mLin-2/CASK complex in brain. J Neurosci. 1999;19:1307–1316. doi: 10.1523/JNEUROSCI.19-04-01307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atasoy D, et al. Deletion of CASK in mice is lethal and impairs synaptic function. Proc Natl Acad Sci USA. 2007;104:2525–2530. doi: 10.1073/pnas.0611003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Irie Ml, et al. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. Together with the finding that Nrxns bind to the MAGUK CASK (ref. 48), this paper reveals a quasi-symmetric design of the neurexin-neuroligin junction that contains PSD-95 bound to Nlgns postsynaptically. [DOI] [PubMed] [Google Scholar]
- 66.Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- 67.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. Describes the first evidence that Nlgns are not only localized to synapses, but function there by showing that overexpressed neuroligin-1 or -2 in a non-neuronal cell can induce co-cultured neurons to form synapses onto that cell. [DOI] [PubMed] [Google Scholar]
- 68.Nam CI, Chen L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc Natl Acad Sci USA. 2005;102:6137–6142. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chih B, Afridi SK, Clark L, Scheiffele P. Disorder-associated mutations lead to functional inactivation of neuroligins. Hum Mol Genet. 2004;13:1471–1477. doi: 10.1093/hmg/ddh158. [DOI] [PubMed] [Google Scholar]
- 70.Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- 71.Zhang W, et al. Extracellular domains of α-neurexins participate in regulating synaptic transmission by selectively affecting N-and P/Q-type Ca2+ -channels. J Neurosci. 2005;25:4330–4342. doi: 10.1523/JNEUROSCI.0497-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dudanova I, et al. Important contribution of α-neurexins to Ca2+-triggered exocytosis of secretory granules. J Neurosci. 2006;26:10599–10613. doi: 10.1523/JNEUROSCI.1913-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000;28:355–363. doi: 10.1016/s0896-6273(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 74.Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathol. 2007;7:434–447. doi: 10.1111/j.1750-3639.2007.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmitz C, Rezaie P. The neuropathology of autism: where do we stand? Neuropathol Appl Neurobiol. 2008;34:4–11. doi: 10.1111/j.1365-2990.2007.00872.x. [DOI] [PubMed] [Google Scholar]
- 76.Courchesne E, et al. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 77.Durand CM, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–37. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moessner R, et al. Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet. 2007;81:1289–1297. doi: 10.1086/522590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okamoto N, et al. 22q13 Microduplication in two patients with common clinical manifestations: a recognizable syndrome? Am J Med Genet A. 2007;143A:2804–2809. doi: 10.1002/ajmg.a.31771. [DOI] [PubMed] [Google Scholar]
- 80.Manning MA, et al. Terminal 22q deletion syndrome: a newly recognized cause of speech and language disability in the autism spectrum. Pediatrics. 2004;114:451–457. doi: 10.1542/peds.114.2.451. [DOI] [PubMed] [Google Scholar]
- 81.Jeffries AR, et al. Molecular and phenotypic characterization of ring chromosome 22. Am J Med Genet A. 2005;137:139–147. doi: 10.1002/ajmg.a.30780. [DOI] [PubMed] [Google Scholar]
- 82.Wilson HL, et al. Molecular characterisation of the 22q13 deletion syndrome supports the role of haploinsufficiency of SHANK3/PROSAP2 in the major neurological symptoms. J Med Genet. 2003;40:575–584. doi: 10.1136/jmg.40.8.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tobaben S, Südhof TC, Stahl B. The G protein-coupled receptor CL1 interacts directly with proteins of the Shank family. J Biol Chem. 2000;275:36204–36210. doi: 10.1074/jbc.M006448200. 2000. [DOI] [PubMed] [Google Scholar]
- 84.Morrow EM, et al. Science. Vol. 321. 2008. Identifying autism loci and genes by tracing recent shared ancestry; pp. 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Comoletti D, et al. The Arg451Cys-neuroligin-3 mutation associated with autism reveals a defect in protein processing. J Neurosci. 2004;24:4889–4893. doi: 10.1523/JNEUROSCI.0468-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tabuchi K, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. 2007. Describes a mouse model of ASD in which a point mutation found in two brothers with ASD (R451C in Nlgn3) was introduced into mice by homologous recombination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jamain S, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci USA. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hishimoto A, et al. Neurexin 3 polymorphisms are associated with alcohol dependence and altered expression of specific isoforms. Hum Mol Genet. 2007;16:2880–2891. doi: 10.1093/hmg/ddm247. [DOI] [PubMed] [Google Scholar]
- 89.Lachman HM, et al. Genomewide suggestive linkage of opioid dependence to chromosome 14q. Hum Mol Genet. 2007;16:1327–1334. doi: 10.1093/hmg/ddm081. [DOI] [PubMed] [Google Scholar]
- 90.Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. 2004. [DOI] [PubMed] [Google Scholar]