Abstract
Chronic stress effects and sex differences were examined on conditioned fear extinction. Male and female Sprague-Dawley rats were chronically stressed by restraint (6h/d/21d), conditioned to tone and footshock, followed by extinction after 1h and 24h delays. Chronic stress impaired the recall of fear extinction in males, as evidenced by high freezing to tone after the 24h delay despite exposure to the previous 1h delay extinction trials, and this effect was not due to ceiling effects from overtraining during conditioning. In contrast, chronic stress attenuated the recall of fear conditioning acquisition in females, regardless of exposure to the 1h extinction exposure. Since freezing to tone was reinstated following unsignalled footshocks, the deficit in the stressed rats reflected impaired recall rather than impaired consolidation. Sex differences in fear conditioning and extinction were observed in nonstressed controls as well, with control females resisting extinction to tone. Analysis of contextual freezing showed that all groups (control, stress, male, female) increased freezing immediately after the first tone extinction trial, demonstrating contextual discrimination. These findings show that chronic stress and sex interact to influence fear conditioning, with chronic stress impairing the recall of delayed fear extinction in males to implicate the medial prefrontal cortex, disrupting the recall of the fear conditioning acquisition in females to implicate the amygdala, and nonstressed controls exhibiting sex differences in fear conditioning and extinction, which may involve the amygdala and/or corticosterone levels.
Keywords: Stress, Sex Difference, Prefrontal Cortex, Fear Conditioning, Extinction
1. Introduction
Chronic stress alters neuronal dendritic morphology and function in a number of brain regions involved in cognition. Chronic stress reduces dendritic arbors in the hippocampus (Baran, Campbell, Kleen, Foltz, Wright, Diamond, and Conrad, 2005; Magariños, McEwen, Flügge, and Fuchs, 1996; McLaughlin, Gomez, Baran, and Conrad, 2007a; Vyas, Mitra, Shankaranarayana Rao, and Chattarji, 2002; Watanabe, Gould, and McEwen, 1992) and prefrontal cortex (PFC, Brown, Henning, and Wellman, 2005; Cook and Wellman, 2004; Izquierdo, Wellman, and Holmes, 2006; Radley, Rocher, Janssen, Hof, McEwen, and Morrison, 2005), and these morphological alterations correspond with impaired hippocampal-dependent spatial ability (Kleen, Sitomer, Killeen, and Conrad, 2006; Luine, Villegas, Martinez, and McEwen, 1994; McLaughlin et al., 2007a; Park, Campbell, and Diamond, 2001; Wright and Conrad, 2005) and PFC-dependent recall of conditioned fear extinction (Garcia, Spennato, Nilsson-Todd, Moreau, and Deschaux, 2008; Miracle, Brace, Huyck, Singler, and Wellman, 2006), respectively. Chronic stress also enhances dendritic arborization in the amygdala (Vyas et al., 2002) and facilitates amygdala-dependent emotionally salient events such as acquisition of fear conditioning (Conrad, Magariños, LeDoux, and McEwen, 1999; Conrad, Mauldin-Jourdain, and Hobbs, 2001). These studies demonstrate that chronic stress influences dendritic morphology, which appears to impact function in a variety of brain regions.
The aforementioned studies used male subjects, but chronic stress influences dendritic morphology and function in females differently than in males. Compared to chronically stressed males, chronically stressed females show mild dendritic retraction in the CA3 region of the hippocampus (Galea, McEwen, Tanapat, Deak, Spencer, and Dhabhar, 1997), which is exacerbated by ovariectomy (McLaughlin, Baran, Wright, and Conrad, 2005). However, chronic stress fails to impair hippocampal-dependent spatial ability in females to the same extent as previously reported in males (Bowman, Zrull, and Luine, 2001; Conrad, Grote, Hobbs, and Ferayorni, 2003a). Specifically, chronically stressed females show delayed spatial navigation, which is maintained longer than nonstressed controls (Conrad et al., 2003a), and may involve interactions with novelty (Frye, 1995) and/or perseveration tendencies of sustained interest (Baran, Wright, Jackson, Kleen, Tsekhanov, Wise, Zachow, and Conrad, 2002; Conrad et al., 2003a). Perseveration is a disruption of behavioral inhibition and involves the PFC (Squire, Bloom, McConnell, Roberts, Spitzer, and Zigmond, 2003). Given the recent evidence that chronic stress decreases PFC dendritic complexity in females (Garrett and Wellman, 2006), chronic stress may influence cognition in females through PFC-mediated functions.
The current study examined the impact of chronic stress on PFC function in male and female rats using PFC-dependent conditioned fear extinction recall. Previous research shows medial PFC lesions in male rats impair conditioned fear extinction recall after a 24 hour delay (Quirk, Russo, Barron, and Lebron, 2000) and that chronic stress causes a similar deficit in male rats (Garcia et al., 2008; Miracle et al., 2006). However, it is unclear whether overtraining contributed to ceiling effects, which could have produced the previously described chronic stress deficit in males (Garcia et al., 2008; Miracle et al., 2006). The following study will be the first to utilize a modified version of the fear extinction recall task to test the effect of chronic stress on PFC function in both male and female rats and incorporating control groups to investigate potential ceiling and/or nonassociative effects. We hypothesized that chronic stress and sex differences will influence recall of conditioned fear extinction.
2. Materials and Methods
2.1. Subjects
One hundred four, female and male Sprague-Dawley rats (sixty females weighed approximately 250g upon arrival with fifty-four males age-matched to females; Charles River Laboratories) were housed in light and sound attenuating chambers on a 12:12 light cycle (lights off at 6AM) according to conditions specified by the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Science, National Research Council, 1996). Food and water were administered ad libitum. Behavioral testing occurred during the dark phase of the light cycle.
2.2. Chronic Stress by Restraint
After one week acclimation to the facilities at ASU, rats were weighed and placed into either a control or chronic stress group. Rats in the chronic stress group were then placed in wire mesh restrainers (16.5 cm diameter × 24.1 cm long; 19.1 cm diameter × 26.7 cm long as the rats grew) for 6 hours per day for 21 days. Rats were returned to their home cages during restraint, a procedure consistent with previous research (Conrad, Grote, Hobbs, and Ferayorni, 2003b; McLaughlin, Gomez, Baran, and Conrad, 2007b, Magariños and McEwen, 1995). Control rats were undisturbed during the restraint period. Restraint occurred during the dark phase of the light cycle.
2.3. Fear Conditioning
2.3.1. Apparatus
Rodent fear conditioning chambers (25 cm depth × 29 cm height × 26 cm width: Coulbourn Instruments, E10-18TC) were contained in sound-attenuating cubicles (Coulbourn, E10-23, white). A PC interface card (Coulbourn, L18-16S/C), a universal link (Coulbourn, L91-04S), and Winlinc software (v 1.1, Coulbourn, D91-04) controlled the stimulus presentation. A frequency generator (Coulbourn, E12-01) produced a tone (75 dB, ∼3.0 kHz) through a speaker located in the side panel of the conditioning chamber. The shock (500 ms, 0.5 mA, Coulbourn Animal Shock Generator, E13-14) was a current, equally distributed through a metal grid floor (Coulbourn, E10-18RF). Behavior was videotaped for analysis using a camera (Coulbourn, E27-01) mounted on the ceiling. Infrared lights (Coulbourn, E27-91) located on the side panels of the chamber denoted the onset and offset of the tone, since there was no audio on the videotaped recordings. The infrared lights were undetectable to the rats. A house light (Coulbourn, E11-01) mounted in the side panel illuminated the chamber. The fear conditioning chambers were cleaned with 95%-ethanol each time a rat was removed from the chamber.
2.3.2. Procedure
This procedure was adapted from Quirk et al. (2000). In that study, both lever pressing and freezing to tone were utilized to assess fear extinction recall. Since both measures portrayed similar recall of fear extinction, we incorporated a paradigm that measured freezing only. A timeline of the study is presented in Table 1. Fear conditioning was conducted over three days beginning one day after the end of restraint. The transport of rats to the fear conditioning chamber occurred in the rats' home cages.
Table 1.
Timeline and Treatment Conditions
| Group | Week 1 | Week 2-4 | Week 5 | |
|---|---|---|---|---|
| Extinction Day 1 | Extinction Day 2 | |||
| Paired Control Chronic Stress | Arrival | Restraint | Habituation, Fear Conditioning (paired), & Extinction | Extinction |
| Unpaired Control Chronic Stress | Arrival | Restraint | Habituation, Fear Conditioning (unpaired), & Extinction | Extinction |
| Day 1 Extinction Naïve Control Chronic Stress | Arrival | Restraint | Habituation, Fear Conditioning (paired), & No Extinction | Extinction |
On day 0 (acclimation), rats were placed in the fear conditioning chamber for ten minutes. Following acclimation, the rats were returned to the colony room.
On day 1 (habituation, conditioning, 1-hr delay extinction), control and chronically stressed rats were placed in the fear conditioning chambers and given five habituation trials consisting of 30 second tones to slow acquisition during conditioning and to reduce the likelihood for chronic stress to potentiate acquisition, as we found previously (Conrad et al., 1999; Conrad et al., 2001). Immediately following the habituation trials, seven conditioning trials occurred in which the completion of a 30 second tone was immediately paired with a footshock. The habituation and conditioning trials lasted approximately one hour. After the conditioning trials, the rats were transported to the colony room for one hour before being returned to the chambers. Rats were then given 15 extinction trials consisting of 30 second tones without footshock. The extinction trials lasted approximately one hour. The average inter-trial interval (ITI) during each exposure to tone only (habituation, extinction) or tone and footshock pairings (training) was four minutes, with a range of two to six minutes.
On day 2 (24-hr delay extinction), rats were given another 15 extinction trials. Immediately following these trials, two unsignalled footshocks were administered to reinstate extinguished conditioned freezing responses, followed by another 15 extinction trials. Previous studies show that unsignalled shocks partially reinstate extinguished conditioned responses and that reinstatement quickly extinguishes, which suggests that reinstatement is due to a previously conditioned association instead of a sensitization effect (Quirk et al., 2000; Rescorla and Heth, 1975). The trials on day 2 lasted approximately 2 hours.
Two control groups of rats (unpaired and day 1 extinction-naïve) received modified fear conditioning procedures and served as additional controls for potential nonassociative and ceiling effects, respectively. For the unpaired group, the fear conditioning procedure was the same as described previously for the paired group except the footshock and tone were explicitly unpaired during the conditioning trials. The tones and footshocks were presented between one and three minutes apart with an average ITI of two minutes, and the total exposure to the chamber similar to the paired conditions. The day 1 extinction-naïve rats received the same conditioning as the paired group, but without extinction exposure to tone on day 1, although they were placed in the chamber during extinction to keep exposure to the environment consistent. Consequently, all groups spent the same amount of time in the fear conditioning chambers and received the same number of tones and footshocks during conditioning, albeit in different temporal parameters for paired and day 1 extinction-naïve compared to unpaired groups.
2.3.3. Dependent Variable
Behavior was videotaped for scoring later by three separate observers who were blind to the experimental conditions. The intra-rater reliability was 98.4% ± 1.5% and the inter-rater reliability was 95.5% ± 1.5%. The dependent variable measured was the number of seconds freezing during each 30 second tone presentation or freezing to context during selected periods that were 30 seconds prior to or immediately after a tone. For extinction trials, freezing to tone was averaged into blocks of 3 trials each. Freezing was defined as the absence of all movements except those associated with respiration (Blanchard and Blanchard, 1969; Conrad et al., 1999; Quirk et al., 2000). For graphical presentations, the freezing duration was converted to a percentage of tone duration.
2.4. Sensitivity to Footshock
Footshock sensitivity testing was conducted for rats in all groups one day after completion of all extinction trials. The rats were placed in the fear conditioning chambers and presented with unsignalled footshocks of increasing amplitudes beginning with 0.05 mA. The footshocks increased in amperage by 0.05 mA increments until two different responses were reached: flinch (orienting head movement or paws briefly raised off the bars) and jumping (Conrad et al., 1999; Quirk et al., 2000).
2.5. Data Analysis
Data were analyzed by analysis of variance (ANOVA) and when significant effects were detected at a p-value of 0.05 or less, Newman-Keuls posthocs were performed. Data are represented by means ± S.E.M. with 8 to 10 subjects per group. One rat from the control female paired condition was excluded from behavioral analyses because it froze to multiple tones during the habituation session.
3. Results
3.1. Fear Conditioning: Habituation
Freezing to tone was similar for all groups during the habituation session. A mixed-factor ANOVA for sex (male, female) and stress treatment (control, chronic stress) across the five habituation trials revealed a significant interaction among sex, stress treatment, and trial, F(4,132) = 3.32, p < 0.05 (Figure 1A and 1D). However, Newman-Keuls post hoc analyses failed to reveal significant differences among the conditions (p > 0.1), and so additional analyses were performed on habituation trials for the unpaired group. A mixed-factor ANOVA for sex (male, female) and stress treatment (control, chronic stress) across the five habituation trials showed no significant main effects or interactions, p > 0.2 (Figure 1C and 1F). Also, no significant differences were observed for freezing to tone during habituation when comparing paired and unpaired conditions, mixed-factor ANOVA for sex (male, female), stress treatment (control, chronic stress), and test type (paired, unpaired) across the five habituation trials (compare Figure 1A and 1C for males, Figure 1D and 1E for females). Therefore, tone presentation during habituation did not evoke robust freezing among sex and stress treatment conditions.
Figure 1.
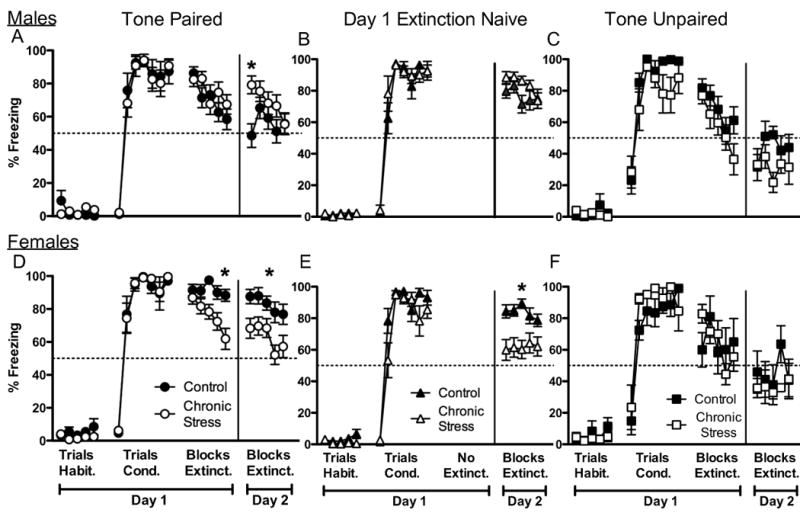
Percentage of freezing to tone during fear conditioning and extinction in male and females rats. A. Note the enhanced freezing to tone of the chronically stressed males during the first block of extinction on day 2, despite showing similar freezing to tone as control males during fear conditioning and extinction on day 1. B. When tone was paired with footshock, but no extinction trials were given on day 1, male rats (control and chronic stress) receiving extinction to tone on the second day maintained high and similar levels of freezing to tone. C. When tone and footshock were explicitly not paired (unpaired), freezing to tone decreased across blocks during extinction on day 1 for both control and chronically stressed males. Notably, the chronically stressed males showed freezing to tone near 20% on day 2, which never fell below 50% when tone and footshock were paired (compare to A and B). D. When tone was paired with footshock, chronically stressed female rats froze to tone significantly less than did the controls during extinction on day 1, and this pattern continued during extinction on day 2. E. When the tone was paired with footshock, but no extinction trials were given on day 1, chronically stressed females froze less to tone than did the controls. F. When tone and footshock were explicitly not paired (unpaired), female rats (control and chronic stress) froze less to tone than did rats in the paired conditioning paradigm (compared to D). Habit. = habituation trials; Cond. = conditioning trials; Extinct. = extinction trials. Data represent means ± S.E.M. * p < 0.05 compared to controls. Controls = black symbols; Chronic stress = white symbols. n = 8 to 10 rats/group.
3.2. Fear Conditioning: Paired Tone-Footshock Conditions for Males and Females
All groups acquired freezing to tone similarly during the tone-footshock pairings. A mixed-factor ANOVA for sex (male, female) and stress treatment (control, chronic stress) across the seven conditioning trials revealed a significant effect of trial, F(6,198) = 162.2, p < 0.001, but no other significant main effects or interactions. Newman-Keuls post hoc analyses revealed that freezing to tone was low during the first trial and increased rapidly in the second and third trials, and stayed elevated for the remaining trials. Freezing to tone approached 100% of the tone duration for all groups during trials three through seven of the conditioning sequence (Figure 1A and 1D).
3.3. Fear Conditioning: Recall of Fear Extinction in the Paired Tone-Footshock Conditions in Males and Females
Previous research showed significant impairments in recall of fear extinction after a 24 hour delay (Miracle et al., 2006; Quirk et al., 2000), and so we compared freezing to tone in the last block of extinction on day 1 to the first block of extinction on day 2. A mixed-factor ANOVA for sex (male, female) and stress treatment (control, chronic stress) for block five of extinction on day 1 and block one of extinction on day 2 revealed a significant interaction between sex and stress treatment, F(1,33) = 6.55, p < 0.05, and a significant interaction between stress treatment and block, F(1,33) = 4.28, p < 0.05. Newman-Keuls post hoc analyses revealed that control males froze to the two blocks significantly less than did the control females, p < 0.05, suggesting that the control males extinguished freezing to tone faster than did control females. In contrast, the control females showed a different pattern of behavior that will be described more fully in the extinction sections (compare extinction day 1 in Figure 1A and 1D for males and females, respectively). The posthocs did not detect any other significant effects among chronic stress treatment and sex. Since males and females performed differently on this task, the subsequent analyses were conducted on each sex individually to fully characterize the influence of chronic stress on extinction.
3.4. Males: Extinction in the Paired Tone and Footshock Conditions
Male rats (control and chronic stress) showed reduced freezing to tone across the extinction blocks on day 1. A mixed-factor ANOVA for stress treatment (control, chronic stress) across the five extinction blocks in day 1 revealed a significant effect of block, F(4,64) = 4.54, p < 0.01. Post hoc tests showed that male rats froze less to tone after the second extinction block on day 1 compared to the first block on day 1 and continued to decrease freezing to tone with additional tone exposure (Figure 1A).
The extinction to tone on day 2 analysis revealed that chronic stress impaired recall of fear extinction in males. A mixed-factor ANOVA for stress treatment (control, chronic stress) on freezing to tone across the five blocks of extinction on day 2 revealed a significant effect across blocks, F(4,64) = 2.59, p < 0.05, and a significant interaction for stress treatment across blocks, F(1,64) = 2.68, p < 0.05. Posthoc analysis showed that chronically stressed males froze significantly more to tone than did control males during block one of extinction on day 2 (Figure 1A). No other significant effects were found.
Another cohort of control and chronically stressed rats that received extinction to tone on the second day only (i.e. day 1 extinction naïve) was tested to determine whether the differences in recall of fear extinction in males were potentially due to ceiling effects during the acquisition of the conditioning session rather than extinction exposure on day 1. For instance, overtraining during acquisition of fear conditioning could have produced high levels of freezing on extinction during day 2, which could be unrelated to extinction to tone on day 1. If this was the cause of the previously described effects, then day 1 extinction naïve rats (Figure 1B) would show freezing to tone on day 2 that would match the freezing pattern on day 2 of rats from Figure 1A. However, both control and chronically stressed male rats receiving extinction only on the second day maintained high and similar levels of freezing to tone, demonstrating that extinction to tone on day 1 was critical for the chronic stress-produced effect. A mixed-factor ANOVA for stress treatment (control, chronic stress) across the blocks of day 2 extinction revealed no significant main effects or interactions (p > 0.1; Figure 1B). Therefore, chronic stress impaired recall of fear extinction as opposed to facilitating acquisition of conditioning during the initial tone-footshock pairings.
3.5. Females: Extinction in the Paired Tone and Footshock Conditions
Chronically-stressed female rats decreased freezing to tone across blocks compared to controls during extinction on day 1, which was maintained during extinction on day 2. A mixed-factor ANOVA for stress treatment (control, chronic stress) across the five blocks of extinction on day 1, revealed a significant main effect of stress treatment, F(1,17) = 5.64, p < 0.05, and a significant effect across blocks, F(4,68) = 3.26, p < 0.05, without a significant interaction. As shown in Figure 1D, freezing to tone decreased across blocks on days 1 and 2 in chronically stressed females, whereas freezing to tone was maintained at high levels by the control females. Indeed, control females failed to show extinction to tone on day 1, as observed with the chronically stressed females.
This pattern of freezing to tone during extinction continued in extinction blocks on day 2 for the females. An ANOVA for extinction on day 2 revealed a significant effect across blocks, F(4,68) = 3.1, p < 0.05, and a marginally significant main effect of stress treatment, F(1,17) = 3.38, p = 0.08. Control females maintained higher levels of freezing to tone than did the chronically stressed females throughout extinction on day 2 (Figure 1D).
To determine whether this pattern of freezing was influenced by the extinction experience on day 1, another cohort of control and chronically stressed females was used whereby extinction to tones on day 1 was not given. Indeed, the data reveal that extinction exposure on day 1 failed to influence freezing to tone on day 2. A mixed-factor ANOVA for treatment (control, chronic stress) across the blocks of extinction on day 2 revealed a significant main effect of stress treatment, F(1,18) = 5.33, p < 0.05 (Figure 1E). Chronically-stressed female rats that received extinction on the second day only (day 1 extinction naïve) showed lower levels of freezing to tone than did the control females. Since the pattern of freezing to tone of the day 1 extinction naïve rats was similar to the pattern of freezing to tone during day 2 of the tone-paired groups, then these data indicate that control females may have exhibited ceiling effects during conditioning and/or that chronically stressed females failed to consolidate or recall the conditioning trials.
3.6. Unpaired Fear Conditioning: Comparison with Paired Groups for Males and Females
To determine whether nonassociative effects could have influenced performance, we compared freezing to tone in the paired and unpaired conditions. A mixed-factor ANOVA for sex (male, female), stress treatment (control, chronic stress), and test type (paired, unpaired) across the seven conditioning trials revealed a significant main effect of sex, F(1,68) = 4.67, p < 0.05, a significant effect of trial, F(6,408) = 212.15, p < 0.001, and a significant interaction for test type across trials, F(6,408) = 4.86, p < 0.001. While females froze to tone more than did the males during acquisition of fear conditioning, this effect appears to be carried mainly by the unpaired condition as differences in freezing to tone between the sexes in the paired condition were not quite distinct based on our earlier analyses. As expected, rats of both sexes and stress conditions froze to tone as conditioning trials progressed (Figure 1C & 1F). The critical finding was that the rats in the paired condition (males and females) froze less to the first tone compared to rats in the unpaired condition (males and females). Freezing to tone was similar between all groups in trials 3-7. Therefore, how rats froze to tone differed depending upon whether tone was paired or unpaired with the footshock.
Extinction to tone on days 1 and 2 influenced freezing to tone differently when rats were previously exposed to the paired or unpaired tone-footshock conditions. A mixed-factor ANOVA for sex (male, female), stress treatment (control, chronic stress), and test type (paired, unpaired) across the five blocks of extinction on day 1 revealed a significant main effect of test type, F(1,68) = 13.51, p < 0.001, and a significant effect of trial, F(4,272) = 18.4, p < 0.001. While an overall effect for less freezing to tone across trials was observed for both paired and unpaired conditions, freezing to tone extinguished faster when tones were unpaired with footshock than when tone and footshock were paired (collapsed across sex and stress condition, Figure 1C & 1F). Again, these data demonstrate that how rats froze to tone differed depending upon whether the tone was paired or unpaired with footshock.
This pattern of greater freezing to tone by the paired condition continued into the extinction trials on day 2. A mixed-factor ANOVA for sex (male, female) and stress treatment (control, chronic stress) and test type (paired, unpaired) across the five extinction blocks on day 2 revealed a significant main effect of test type, F(1,68) = 31.91, p < 0.001 and a significant effect across blocks, F(4,272) = 2.69, p < 0.05. Again, rats in the paired condition froze to tone more than did the rats in the unpaired condition (collapsed across sex and stress condition), while freezing to tone continued to decrease across blocks (carried mainly by the paired group, Figure 1C & 1F). These results suggest differences seen between the sex and stress conditions in the paired group are unlikely attributed to nonassociative effects because significant differences in freezing to tone were detected between the paired and unpaired conditions.
3.7. Reinstatement of Freezing to Tone
Another test was performed to determine whether freezing to tone reflected associative effects as opposed to impaired consolidation or recall of the tone-footshock pairing. If extinguished responses are reflective of impaired consolidation of the tone-footshock pairing, then unsignalled footshocks would be ineffective at reinstating freezing to tone. In contrast, if extinguished responses illustrate impaired recall, then unsignalled footshocks should reinstate freezing to tone. Rats in the paired groups were given two unsignalled footshocks immediately after the last extinction trial on day 2. A mixed-factor ANOVA for sex (male, female) and stress treatment (control, chronic stress) across freezing to tone on the last block of extinction on day 2 and the first block following the two unsignalled footshocks revealed a significant effect of block, F(1,33) = 19.21, p < 0.001 (Figure 2), with no other significant effects. As seen in Figure 2, freezing to tone increased after the unsignalled footshocks. This effect was carried predominately by the males (control and stress) and chronically stressed females, as the control females showed high freezing in the last extinction trial on day 2, which provided very little room to increase further with this reinstatement paradigm.
Figure 2.
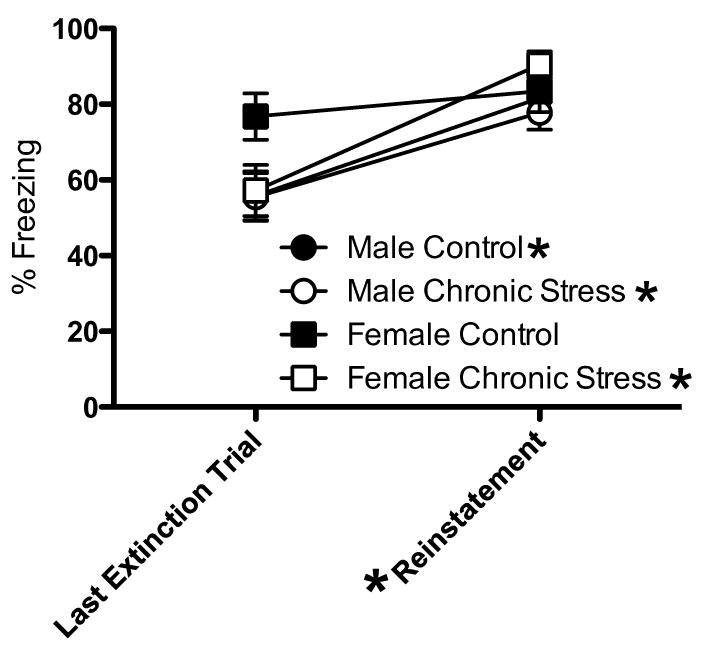
Percentage of freezing to tones following two unsignalled footshocks. Male rats (control and chronic stress) and chronically stressed females froze more to tone following the two unsignalled footshocks than compared to their own level of freezing to tone at the last extinction trial. The control females failed to show enhanced freezing to tone following the unsignalled footshock, in part, because they maintained high freezing during extinction. Data represented as mean ± S.E.M. *p < 0.05 for reinstatement compared to the last extinction trial from the same group.
3.8. Freezing to Context
Given the control female propensity to maintain high levels of freezing to tone despite two days of extinction, we investigated whether the control females were showing generalized sensitization effects. Consequently, we measured freezing to context at various time points during extinction. A diagram outlining the time points during which contextual freezing was quantified is illustrated in Figure 3A. These time points were selected to capture freezing levels to the context without tone presentation, but in close proximity to the first and last tone presentation during extinction as indicated. First, freezing to context was assessed 30 seconds before and 30 seconds immediately after the first tone presentation during extinction on day 1. Contextual freezing was enhanced in chronically stressed rats of both sexes immediately prior to and after extinction training on the first day. A mixed-factor ANOVA for sex (male, female) and stress treatment (control, chronic stress) for contextual freezing before and after the first extinction trial (context represented by A & B in Figure 3A) on day 1 revealed a significant main effect of stress treatment, F(1,33) = 5.02, p < 0.05, and a significant effect of context trial, F(1,33) = 189.05, p < 0.001 (Figure 3B). As seen in Figure 3B, chronically stressed rats (male and female) froze more to context than did controls (male and female) prior to and immediately after the presentation of the first tone of the extinction trial on day 1, p < 0.05. However, when the tone presentation was finished, all rats froze more to context than they did prior to the tone and these high freezing levels were similar across sex and stress treatment. Therefore, all conditions discriminated between the context that was less likely to have a footshock (see point A in Figure 3A) compared to the context that was most likely to have a footshock (see point B in Figure 3A).
Figure 3.
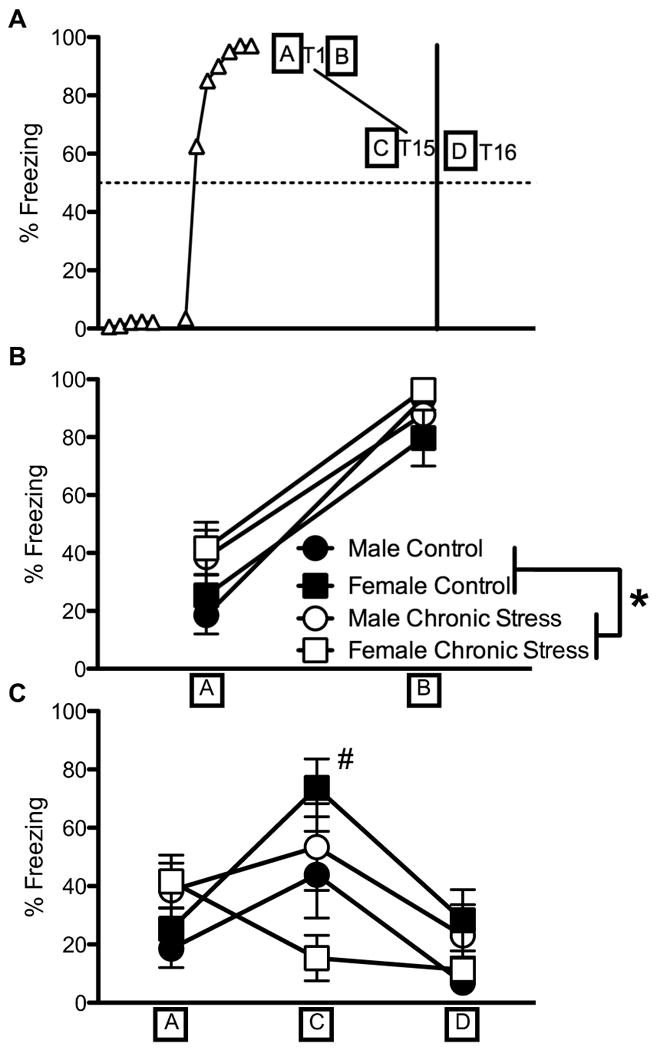
Percentage of freezing to context at designated periods during extinction to tone. A. Diagram outlining the different time points by which freezing to context was sampled. Triangles represent arbitrary data points of tone presentation. The boxed letter “A” represents the 30 seconds before the first tone (T1) presented during extinction on day 1. The boxed letters “B”, “C”, and “D” represent the 30 seconds immediately following the termination of T1, the last tone during extinction on day 1 (T15), and the first tone presentation on day 2 (T16), respectively. B. Chronically stressed rats (male and female) froze more to context than did the controls of both sexes (collapsed across contexts “A” and “B”). Once the first tone (T1) was presented, rats in all groups froze more to context B than to context A. C. As extinction trials progressed on day 1, control females significantly increased freezing to context, while the remaining rats (control and chronically stress males and chronically stressed females) froze similarly to context throughout extinction. Freezing to context was similar between all rats immediately prior to the extinction trials on day 2. Data represented as means ± S.E.M. * p < 0.05 for chronically stressed rats (male and female) compared to controls across contexts A and B. # p < 0.05 compared to freezing in context A for the same group.
We next investigated whether rats extinguished freezing to context during extinction. Freezing was measured for 30 seconds immediately prior to the presentation of the first and last tones of extinction on day 1. A mixed-factor ANOVA for sex (male, female) and stress treatment (control, chronic stress) for contextual freezing prior to the first and last extinction trials on day 1 (context represented by A & C in Figure 3A) revealed a significant effect of context trial, F(1,33) = 8.23, p < 0.01, and significant interactions for sex and stress treatment, F(1,33) = 7.62, p < 0.01, stress treatment and context trial, F(1,33) = 13.61, p < 0.001, and sex, stress treatment and context trial, F(1,33) = 8.7, p < 0.01 (Figure 3C). The notable finding is that control females not only failed to extinguish freezing to context by the end of extinction on day 1, but they significantly increased freezing to context (compare points A and C in Figure 3C). Post hoc analyses show that female controls froze more to context as trials progressed by the end of extinction on day 1 than did chronically stressed females and control males, p < 0.05, and marginally more than did chronically stressed males, p = 0.07. For males (control and chronically stressed) and chronically stressed females, freezing to context did not extinguish as freezing levels were statistically similar between the beginning and end of extinction on day 1 (Figure 3C). However, chronically stressed females showed a nonsignificant declining level of freezing to context (compare points A and C in Figure 3C), and even froze significantly less to context at the end of extinction on day 1 compared to chronically stressed males at the same time point (context C in Figure 3C; p < 0.05). Therefore sex differences were seen in how chronic stress influences freezing to context, with chronically stress females being the only group to extinguish to context compared to chronically stressed males, while freezing to context remained the same in males (control and chronically stressed) and increased in control females.
We also investigated whether rats froze less to context after a 24 hour delay. Freezing was measured for the 30 seconds immediately prior to the last tone of extinction on day 1 and the first tone of extinction on day 2 (see points C & D on Figure 3A). A mixed-factor ANOVA for sex (male, female) and stress treatment (control, chronic stress) for contextual freezing revealed a significant effect of context trial, F(1,33) = 24.00, p < 0.01, a significant interaction between sex and stress treatment, F(1,33) = 14.37, p < 0.01, and the interaction for stress treatment across context trial approached statistical significance, F(1,33) = 3.75, p = 0.06 (Figure 3C). Overall, all groups froze less to context on day 2 than on day 1, showing that freezing to context extinguished across the delay and that freezing was not generalized to the chambers on day 2. Moreover, chronically stressed females continued to show reduced freezing to context (collapsed across context C & D) compared to chronically stressed males (p < 0.05) and this sex difference was not observed for the controls.
3.9. Footshock Sensitivity
Females jumped to lower shock intensity than did males regardless of stress treatment. A mixed-factors ANOVA for sex (male, female) and stress treatment (control, chronic stress) across the different response types (flinch, jump) in the paired condition revealed a significant interaction between sex and response type, F(1,32) = 4.70, p < 0.05 (Table 2). Post hoc tests showed that female rats jumped in response to lower shock intensity than did the male rats, whereas threshold to flinch was statistically similar between the sexes. Sensitivity to footshock at both thresholds was similar for control and chronically stressed rats and so these data are collapsed across control and chronic stress conditions in Table 2.
Table 2.
Measures of Body Weight Gain and Footshock Detection Threshold
| Change in Body Weight Gain from Day 1 to Day 21 (gm) |
Shock Detection Threshold for “Flinching” (mA) |
Shock Detection Threshold for “Jumping” (mA) |
|
|---|---|---|---|
| Males | |||
| -Control | 81.0 ± 10.7 | 0.079 ± 0.006 | 0.251 ± 0.017 |
| -Chronic Stress | 1.8 ± 5.3 | 0.079 ± 0.003 | 0.252 ± 0.011 |
| Females | |||
| -Control | 24.4 ± 2.1 | 0.084 ± 0.004 | 0.209 ± 0.010 |
| -Chronic Stress | -3.8 ± 2.7 | 0.076 ± 0.000 | 0.211 ± 0.009 |
3.10. Body Weight
Chronic restraint reduced weight gain in both sexes. Individual one-way ANOVAs for stress treatment (control, chronic stress) on the difference in weight gain (weight in grams on day 21 of restraint minus the weight in grams on day 1 of restraint) revealed significant main effects of stress treatment in males, F(1,17) = 29.32, p < 0.001, and in females, F(1,16) = 16.69, p < 0.001 (Table 2). Restraint successfully reduced weight gain and was a stressor.
4. Discussion
These findings are the first to report sex differences in how chronic stress influences acquisition, extinction, and recall of extinction in a fear conditioning paradigm requiring the PFC. We supported our hypothesis that chronic stress impairs recall of fear extinction in males and provide novel data that ceiling effects during the conditioning trials did not cause this deficit. Moreover, additional controls demonstrated that freezing to tone represents associative effects. We provide novel findings that chronic stress alters fear extinction in females and facilitated extinction to tone and context compared to nonstressed females. Furthermore, nonstressed females appeared resistant to extinction under these conditions, suggesting that nonstressed females may have been over trained under conditions that do not cause overtraining in males, resulting in control females freezing to multiple cues, without generalized freezing (such as sensitization). These findings show a complex interaction between sex and stress, and these can be modulated by numerous mechanisms that may provide insight into psychiatric conditions such as depression, schizophrenia and post-traumatic stress disorder (PTSD).
4.1. Chronic Stress Produces Sex Differences in the Fear Conditioning Paradigm
Chronic stress impaired fear extinction recall in males, corroborating previous research (Garcia et al., 2008; Miracle et al., 2006) and providing new evidence that the deficit was not caused by overtraining during conditioning or nonassociative effects. Overtraining can lead to ceiling effects that evoke persistent high freezing during extinction. Consequently, we included a group of rats that were not exposed to day 1 extinction. We found that control and chronically stressed day 1 extinction naïve rats showed similar high levels of freezing to tone during day 2 extinction, indicating that chronic stress impaired recall of extinction when day 1 extinction was presented, rather than producing ceiling effects. Nonassociative factors were excluded as a potential interpretation by incorporating unsignalled footshocks, another cohort of rats exposed to unpaired tone and footshock, and contextual conditioning measures. Unsignalled footshocks partially reinstate extinguished conditioned responses (Quirk et al., 2000; Rescorla and Heth, 1975), which was observed in our study for both the control and stressed males. Moreover, the unpaired male rats (control, chronically stressed) decreased freezing to tone across extinction trials faster than paired males, showing that tone paired with footshock has different significance than when they are explicitly unpaired. Contextual freezing also illustrated that paired males discriminated between context before and immediately after tone, as freezing significantly increased following the first tone in extinction. Taken together, the changes in freezing to tone in the paired group were attributed to associations made between the tone and footshock rather than non-associative factors and indicate that chronic stress impaired fear extinction retrieval in males.
In contrast to males, chronic stress facilitated fear extinction in females. Since freezing to tone during extinction on day 2 was similar regardless of extinction exposure on day 1, chronic stress impaired consolidation and/or recall of the acquisition of fear conditioning. The unsignalled footshocks rapidly reinstated freezing to tone, demonstrating that an association was made between tone and footshock. In addition, unpaired females decreased freezing to tone across extinction trials faster than paired females, suggesting that differences in the paired condition were due to tone/footshock associations rather than non-associative factors. Therefore, chronic stress impaired recall (and not consolidation) of fear conditioning acquisition in females.
Chronic stress and sex differences in fear conditioning were not observed during the acquisition of fear conditioning. Previous research shows that chronic stress or corticosterone enhances acquisition of fear conditioning in male rats (Conrad, MacMillan II, Tsekhanov, Wright, Baran, and Fuchs, 2004c; Conrad et al., 1999; Conrad et al., 2001; Cordero, Merino, and Sandi, 1998); however, Quirk and colleagues (2000) incorporated habituation trials to slow and maintain similar acquisition rates across groups in order to test extinction effects without potential confounds arising from group differences during acquisition. In the current study, the habituation trials successfully kept acquisition rates similar among groups with respect to freezing to tone, but chronic stress still increased freezing to context.
4.2. Sex Differences in Fear Conditioning and Extinction
Surprisingly, control females were resistant to extinction and maintained high levels of freezing to tone during extinction on day 2, despite extinction on day 1. These findings suggest that control females were over trained or exhibited sensitization effects. Importantly, control females exhibited context discrimination by showing high levels of freezing to context immediately after, but not prior to, the first extinction tone presentation. Moreover, the control females decreased contextual freezing by day 2 when freezing to tone remained elevated. These data exclude nonassociative effects such as sensitization for the failure of control females to extinguish freezing to tone, and instead show that control females most likely associate multiple cues with footshock.
Sex differences in fear conditioning were unlikely caused by sensitivity to footshock exclusively. Females are generally more sensitive to noxious stimuli than males (for review, see Wiesenfeld-Hallin, 2005), and so studying sex differences may be difficult in aversive training paradigms with shock. In the current study, both control and chronically stressed females jumped at a lower footshock threshold than did the males, which may have contributed to some of the sex differences. However, enhanced freezing to tone and context was observed in control females and not in chronically stressed females, despite both groups showing similar footshock sensitivities. Moreover, this enhanced freezing to tone was not observed in the control and chronically stressed females in the unpaired condition even though footshock number and intensity were similar. Therefore, footshock sensitivity alone cannot explain the divergent outcomes of control females maintaining high freezing to tone and context compared to control males.
An important consideration for the observed sex differences in fear conditioning involves latent inhibition. Latent inhibition occurs when pre-exposure to a conditioned stimulus (tone) interferes with subsequent associations between tone and unconditioned stimulus (footshock), thereby weakening the conditioned response (freezing to tone). In our paradigm, the chronically stressed females may have exhibited latent inhibition because freezing to tone decreased during extinction, but not in control females because freezing to tone remained elevated. A recent study found that ovariectomized female rats expressed latent inhibition that was more like the males than the estrogen-replaced females (Nofrey, Ben-Shahar, and Brake, 2008), showing that latent inhibition can be modified by factors that include hormonal state. Perhaps the chronic stress in our study caused latent inhibition. Another issue is that control females in our study maintained high freezing to tone during extinction when control males began to decrease freezing. This finding is consistent with a study reporting that male rats, but not estrogen-replaced female rats, were not distracted by a tone even though the tone was previously paired with footshock (Nofrey et al., 2008). An interpretation is that the estrogen-replaced rats in the Nofrey and colleagues (2008) study and the control females in the current paradigm attend to many cues, including irrelevant cues (Toufexis, Myers, Bowser, and Davis, 2007). However, the females in the current study clearly froze more to context at the termination of a tone than before tone onset. Perhaps nonstressed females were impaired in their ability to inhibit fear responses, which was reversed by chronic stress, while male rats (control, chronic stress) were able to show appropriate inhibition of fear responses.
This study is the first to show that chronic stress differentially alters recall of fear conditioning acquisition and extinction depending upon sex, corroborating previous research suggesting that each sex is influenced by different mechanisms, including brain circuitry and stress hormones (Conrad et al., 2003a; Conrad, Jackson, Wieczorek, Baran, Harman, Wright, and Korol, 2004a; Conrad, Jackson, and Wise, 2004b; Conrad, Leone, Nemivant, and Roy, 1997; Lin, Westenbroek, Bakker, Termeer, Liu, Li, and Ter Horst, 2008; Shors and Wood, 1995; Wood, Beylin, and Shors, 2001). The amygdala is a critical region involved in fear conditioning and memory processing of emotionally salient events (Cahill, Babinsky, Markowitsch, and McGaugh, 1995; LeDoux, 2000a; LeDoux, 2000b) and extinction depends upon the inhibition of the amygdala (Hefner, Whittle, Juhasz, Norcross, Karlsson, Saksida, Bussey, Singewald, and Holmes, 2008; Lin, Yeh, Lu, and Gean, 2003). Inactivation of the amygdala can reverse sex differences observed in response to acute stress on aversive classical conditioning (Waddell, Bangasser, and Shors, 2008). The level of amygdala activation could have contributed to some of the sex differences in this study. The hippocampus also plays a role in the contextual components of fear conditioning and extinction (Kim and Fanselow, 1992; Phillips and LeDoux, 1992; Wilson, Brooks, and Bouton, 1995), and the degree of hippocampal activation could have influenced sex differences in this study (Conrad et al., 2004c; Lin et al., 2003; Milad, Wright, Orr, Pitman, Quirk, and Rauch, 2007). Sex differences in corticosterone levels may influence fear conditioning following chronic stress as females have higher corticosterone levels than males (Atkinson and Waddell, 1997; Dalla, Antoniou, Drossopoulou, Xagoraris, Kokras, Sfikakis, and Papadopoulou-Daifoti, 2005). Corticosterone influences a number of behaviors associated with fear conditioning in males, including increasing footshock-induced inactivity (Báez, Siriczman, and Volosin, 1996) and enhancing contextual fear conditioning (Conrad, 2005; Conrad et al., 2004c; Conrad et al., 2001; Marchand, Barbelivien, Seillier, Herbeaux, Sarrieau, and Majchrzak, 2007). If corticosterone mediates some of these outcomes in females as it does in males, then corticosterone may contribute to some of the fear conditioning effects in females of the current study.
4.3. Significance
The interaction between stress and sex differences on fear conditioning and extinction are modulated by interactions between the PFC, hippocampus, and amygdala, perhaps via corticosterone levels. Stress contributes to human disorders, such as depression (Diamond, Campbell, Park, and Vouimba, 2004; Kessler, 1997; Kessler, McGonagle, Swartz, Blazer, and Nelson, 1993; Nolen-Hoeksema, 1987), and schizophrenia (Gispen-de Wied, 2000). Symptoms of depression include changes in mood/anhedonia and impaired cognition. Our current paradigm is consistent with the literature showing that chronic stress in rats impairs memory on fear conditioning, albeit differently for males and females. Moreover, one of the most robust sex differences in psychiatry is that women are more likely than men to be diagnosed with depression (Bebbington, 1996; Heller, 1993; Kessler et al., 1993; Troisi, 2001), and our paradigm observed striking sex differences with chronically stressed females showing deficits for memory of fear conditioning acquisition and males exhibiting impairments for memory of fear conditioning extinction. Stress and the hypothalamic-pituitary-adrenal axis are theorized to be one of the primary biological factors contributing to depression (Jans, Riedel, Markus, and Blokland, 2007; Urani, Chourbaji, and Gass, 2005), with both depression and schizophrenia manifesting changes in the PFC and PFC-dependent behaviors, such as perseveration and behavioral flexibility (Anisman and Matheson, 2005; Ellenbroek, Budde, and Cools, 1996; Fitzgerald, Laird, Maller, and Daskalakis, 2008; Fossati, Amar, Raoux, Ergis, and Allilaire, 1999; Murphy, Sahakian, Rubinsztein, Michael, Rogers, Robbins, and Paykel, 1999; Steele, Currie, Lawrie, and Reid, 2007; Wible, Anderson, Shenton, Kricun, Hirayasu, Tanaka, Levitt, O'Donnell, Kikinis, Jolesz, and McCarley, 2001). Another link with human conditions is that women are more susceptible than men to PTSD (Kessler, Sonnega, Bromet, Hughes, and Nelson, 1995), which is characterized by hyperarousal and episodes of reliving intrusive memories (Stam, 2007a; b). Interestingly, the control females in our study did not extinguish their conditioned responses to tone under conditions that males extinguished, and a similar maintenance of memory in females and not males was found in another study using a single episode of predator stress on an escape paradigm (Park, Zoladz, Conrad, Fleshner, and Diamond, 2008). PTSD is thought to involve a dysregulated stress response (Pervanidou, Kolaitis, Charitaki, Lazaropoulou, Papassotiriou, Hindmarsh, Bakoula, Tsiantis, and Chrousos, 2007; Schelling, 2002; Simeon, Knutelska, Yehuda, Putnam, Schmeidler, and Smith, 2007) and altered amygdala function (Karl, Schaefer, Malta, Dörfel, Rohleder, and Werner, 2006), and these may be putative targets for sex differences (see Cahill, 2003; Cahill, Haier, White, Fallon, Kilpatrick, Lawrence, Potkin, and Alkire, 2001). The findings in the current study illustrate that males and females with and without chronic stress exposure express fear conditioning differently and emphasize the need to understand the underlying mechanism(s) in both sexes.
Acknowledgments
Supported by NIMH 64727 and a grant from the Institute for Mental Health Research (Conrad) and funds from ASU School of Life Sciences and the Howard Hughes Medical Institute through the Undergraduate Science Education Program (Armstrong and Niren). We gratefully acknowledge the following individuals: Heather Bimonte-Nelson, Krystal Dorathy, Mariam El-Ashmawy, Thu Huynh, Katie McLaughlin, Ryan Meyers, Janet Neisewander, Federico Sanabria, Michelle Shiota, Michelle Sparks, Dawn St. John, Ryan Wright, Matthew Young.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anisman H, Matheson K. Stress, depression, and anhedonia: Caveats concerning animal models. Neuroscience and Biobehavioral Reviews. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology. 1997;138:3842–3848. doi: 10.1210/endo.138.9.5395. [DOI] [PubMed] [Google Scholar]
- Báez M, Siriczman I, Volosin M. Corticosterone is involved in foot shock-induced inactivity in rats. Physiology & Behavior. 1996;60:795–801. doi: 10.1016/0031-9384(96)00025-x. [DOI] [PubMed] [Google Scholar]
- Baran SE, Campbell AM, Kleen JK, Foltz CH, Wright RL, Diamond DM, Conrad CD. Synergy between high fat diet and chronic stress retracts apical dendrites in CA3. NeuroReport. 2005;16:39–43. doi: 10.1097/00001756-200501190-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran SE, Wright RL, Jackson JL, Kleen JK, Tsekhanov S, Wise L, Zachow KA, Conrad CD. Ovariectomized female rats demonstrate enhanced spatial memory on the Y-maze following chronic stress while acute estrogen treatment may attenuate performance. Society for Neuroscience Abstracts. 2002;28:370.312. [Google Scholar]
- Bebbington P. The origins of sex differences in depressive disorder: Bridging the gap. International Review of Psychiatry. 1996;8:295–332. [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. Journal of Comparative and Physiological Psychology. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Research. 2001;904:279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cerebral Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Cahill L. Sex- and hemisphere-related influences on the neurobiology of emotionally influenced memory. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:1235–1241. doi: 10.1016/j.pnpbp.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, Potkin SG, Alkire MT. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiology of Learning and Memory. 2001;75:1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Conrad CD. The relationship between acute glucocorticoid levels and hippocampal function depends upon task aversiveness and memory processing stage. Nonlinearity in Biology, Toxicology and Medicine. 2005;3:57–78. doi: 10.2201/nonlin.003.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. The Neurobiology of Learning and Memory. 2003a;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiology of Learning and Memory. 2003b;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Acute restraint stress impairs spatial memory in male but not female rats: Influence of estrous cycle. Pharmacology Biochemistry and Behavior. 2004a;78:569–579. doi: 10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wise L. Chronic stress enhances ibotenic acid-induced damage selectively within the hippocampal CA3 region of male, but not female rats. Neuroscience. 2004b;125:759–767. doi: 10.1016/j.neuroscience.2004.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Leone D, Nemivant RR, Roy EJ. Long-term adrenalectomy can increase or decrease hippocampal dentate gyrus volumes. Journal of Neuroendocrinology. 1997;9:355–361. doi: 10.1046/j.1365-2826.1997.00584.x. [DOI] [PubMed] [Google Scholar]
- Conrad CD, MacMillan DD, II, Tsekhanov S, Wright RL, Baran SE, Fuchs RE. Influence of chronic corticosterone and glucocorticoid receptor antagonism in the amygdala on fear conditioning. Neurobiology of Learning and Memory. 2004c;81:185–199. doi: 10.1016/j.nlm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Magariños AM, LeDoux JE, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behavioral Neuroscience. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Mauldin-Jourdain ML, Hobbs RJ. Metyrapone reveals that previous chronic stress differentially impairs hippocampal-dependent memory. Stress. 2001;4:305–318. doi: 10.3109/10253890109014754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. The Journal of Neurobiology. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Merino JJ, Sandi C. Correlational relationship between shock intensity and corticosterone secretion on the establishment and subsequent expression of contextual fear conditioning. Behavioral Neuroscience. 1998;112:885–891. doi: 10.1037//0735-7044.112.4.885. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell A, Park CR, Vouimba RM. Preclinical research on stress, memory, and the brain in the development of pharmacotherapy for depression. European Neuropsychopharmacology. 2004;14 5:S491–S495. doi: 10.1016/j.euroneuro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Budde S, Cools AR. Prepulse inhibition and latent inhibition: the role of dopamine in the medial prefrontal cortex. Neuroscience. 1996;75:535–542. doi: 10.1016/0306-4522(96)00307-7. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati P, Amar G, Raoux N, Ergis AM, Allilaire JF. Executive functioning and verbal memory in young patients with unipolar depression and schizophrenia. Psychiatry Research. 1999;89:171–187. doi: 10.1016/s0165-1781(99)00110-9. [DOI] [PubMed] [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiology and Behavior. 1995;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiology of Learning and Memory. 2008;89:560–566. doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Wellman CL. Chronic stress alters dendritic morphology in medial prefrontal cortex in female rats. Society for Neuroscience Abstracts. 2006;32:357–357. [Google Scholar]
- Gispen-de Wied CC. Stress in schizophrenia: an integrative view. European Journal of Pharmacology. 2000;405:375–384. doi: 10.1016/s0014-2999(00)00567-7. [DOI] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. The Journal of Neuroscience. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W. Gender differences in depression: Perspectives from neuropsychology. Journal of Affective Disorders. 1993;29:129–143. doi: 10.1016/0165-0327(93)90028-i. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. The Journal of Neuroscience. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans LA, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry. 2007;12:522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dörfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neuroscience and Biobehavioral Reviews. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kessler DC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity study. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. Journal of Affective Disorders. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kleen JK, Sitomer MT, Killeen PR, Conrad CD. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behavioral Neuroscience. 2006;120:842–851. doi: 10.1037/0735-7044.120.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The amygdala and emotion: A view through fear. In: Aggleton JP, editor. Amygdala: A functional Analysis. New York, NY: Oxford University Press; 2000a. pp. 289–310. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000b;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu HY, Gean PW. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. The Journal of Neuroscience. 2003;23:8310–8317. doi: 10.1523/JNEUROSCI.23-23-08310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Westenbroek C, Bakker P, Termeer J, Liu A, Li X, Ter Horst GJ. Effects of Long-Term Stress and Recovery on the Prefrontal Cortex and Dentate Gyrus in Male and Female Rats. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhn035. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Research. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons. Comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. The Journal of Neuroscience. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand AR, Barbelivien A, Seillier A, Herbeaux K, Sarrieau A, Majchrzak M. Contribution of corticosterone to cued versus contextual fear in rats. Behavioural Brain Research. 2007;183:101–110. doi: 10.1016/j.bbr.2007.05.034. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: Possible involvement of CA1 neurons. Neuroscience. 2005;135:1045–1054. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: An evaluation of chronic restraint paradigms. Brain Research. 2007a;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007b;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biological Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. The Neurobiology of Learning and Memory. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Nofrey BS, Ben-Shahar OM, Brake WG. Estrogen abolishes latent inhibition in ovariectomized female rats. Brain Cogn. 2008;66:156–160. doi: 10.1016/j.bandc.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Sex differences in unipolar depression: evidence and theory. Psychological Bulletin. 1987;101:259–282. [PubMed] [Google Scholar]
- Park CR, Campbell AM, Diamond DM. Chronic psychosocial stress impairs learning and memory and increases sensitivity to yohimbine in rats. Biological Psychiatry. 2001;50:994–1004. doi: 10.1016/s0006-3223(01)01255-0. [DOI] [PubMed] [Google Scholar]
- Park CR, Zoladz PR, Conrad CD, Fleshner M, Diamond DM. Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learning & Memory. 2008;15:271–280. doi: 10.1101/lm.721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervanidou P, Kolaitis G, Charitaki S, Lazaropoulou C, Papassotiriou I, Hindmarsh P, Bakoula C, Tsiantis J, Chrousos GP. The natural history of neuroendocrine changes in pediatric posttraumatic stress disorder (PTSD) after motor vehicle accidents: progressive divergence of noradrenaline and cortisol concentrations over time. Biological Psychiatry. 2007;62:1095–1102. doi: 10.1016/j.biopsych.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. The Journal of Neuroscience. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Experimental Neurology. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. Journal of Experimental Psychology and Animal Behavioral Processes. 1975;1:88–96. [PubMed] [Google Scholar]
- Schelling G. Effects of stress hormones on traumatic memory formation and the development of posttraumatic stress disorder in critically III patients. Neurobiology of Learning and Memory. 2002;78:596–609. doi: 10.1006/nlme.2002.4083. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Wood GE. Contribution of stress and gender to exploratory preferences for familiar versus unfamiliar conspecifics. Physiology & Behavior. 1995;58:995–1002. doi: 10.1016/0031-9384(95)00153-a. [DOI] [PubMed] [Google Scholar]
- Simeon D, Knutelska M, Yehuda R, Putnam F, Schmeidler J, Smith LM. Hypothalamic-pituitary-adrenal axis function in dissociative disorders, post-traumatic stress disorder, and healthy volunteers. Biological Psychiatry. 2007;61:966–973. doi: 10.1016/j.biopsych.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Bloom FE, McConnell SK, Roberts JL, Spitzer NC, Zigmond MJ. Fundamental Neuroscience. Second. San Diego: Academic Press; 2003. [Google Scholar]
- Stam R. PTSD and stress sensitisation: A tale of brain and body Part 1: Human studies. Neuroscience and Biobehavioral Reviews. 2007a;31:530–557. doi: 10.1016/j.neubiorev.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Stam R. PTSD and stress sensitisation: A tale of brain and body Part 2: Animal models. Neuroscience and Biobehavioral Reviews. 2007b;31:558–584. doi: 10.1016/j.neubiorev.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Steele JD, Currie J, Lawrie SM, Reid I. Prefrontal cortical functional abnormality in major depressive disorder: a stereotactic meta-analysis. Journal of Affective Disorders. 2007;101:1–11. doi: 10.1016/j.jad.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Bowser ME, Davis M. Estrogen disrupts the inhibition of fear in female rats, possibly through the antagonistic effects of estrogen receptor alpha (ERalpha) and ERbeta. The Journal of Neuroscience. 2007;27:9729–9735. doi: 10.1523/JNEUROSCI.2529-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi A. Gender differences in vulnerability to social stress: A Darwinian perspective. Physiology & Behavior. 2001;73:443–449. doi: 10.1016/s0031-9384(01)00459-0. [DOI] [PubMed] [Google Scholar]
- Urani A, Chourbaji S, Gass P. Mutant mouse models of depression: candidate genes and current mouse lines. Neuroscience and Biobehavioral Reviews. 2005;29:805–828. doi: 10.1016/j.neubiorev.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Bangasser DA, Shors TJ. The basolateral nucleus of the amygdala is necessary to induce the opposing effects of stressful experience on learning in males and females. The Journal of Neuroscience. 2008;28:5290–5294. doi: 10.1523/JNEUROSCI.1129-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Wible CG, Anderson J, Shenton ME, Kricun A, Hirayasu Y, Tanaka S, Levitt JJ, O'Donnell BF, Kikinis R, Jolesz FA, McCarley RW. Prefrontal cortex, negative symptoms, and schizophrenia: an MRI study. Psychiatry Research. 2001;108:65–78. doi: 10.1016/s0925-4927(01)00109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z. Sex differences in pain perception. Gender Medicine. 2005;2:137–145. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]
- Wilson A, Brooks DC, Bouton ME. The role of the rat hippocampal system in several effects of context in extinction. Behavioral Neuroscience. 1995;109:828–836. doi: 10.1037//0735-7044.109.5.828. [DOI] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behavioral Neuroscience. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Chronic stress leaves novelty-seeking intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8:151–154. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]


